Abstract
This study aimed to investigate the effect of ozone on hypoxia, hemolysis and morphological change of blood from aortic dissection (AD) patients for providing preliminary evidence of application of ozonated autohemotherapy (Ozone-AHT) in AD patients. 20 AD patients and 20 healthy volunteers were consecutively included, and blood samples were collected from all participants and ozonized in vitro. PO2, SO2, malondialdehyde (MDA), superoxide dismutase (SOD), malformation percentage, morphology change and spatial distribution of filamentous actin (F-actin) in erythrocytes at different ozone concentrations were evaluated. After ozonation of whole blood, the median levels of PO2 and SO2 increased under Ozone concentrations at 40 μg/mL, 80 μg/mL and 160 μg/mL compared with samples exposed to 0 μg/mL in both AD group and control group. The MDA level was similar in samples exposed to 0 μg/mL, 40 μg/mL and 80 μg/mL ozone, while the levels of SOD increased in samples exposed to 40 μg/mL and 80 μg/mL in both AD group and control group. Compared with the samples exposed to 0 μg/mL ozone, FHb level only increased in samples exposed to 80 μg/mL and 160 μg/mL Ozone in both AD group and control group. In addition, overdosed ozone (160 μg/mL) but not therapeutic ozone concentrations (0 μg/mL, 40 μg/mL and 80 μg/mL) increased malformation percentage and morphology change of erythrocytes in both AD group and control group. In conclusion, Ozone improves oxygen content and reduces oxidative damage in blood from AD patients, and therapeutic dose ozone do not induce hemolysis and morphology change of erythrocytes.
Keywords: Ozonated autohemotherapy (Ozone-AHT), aortic dissection, oxygenation, antioxidant, morphological change, erythrocytes
Introduction
Aortic dissection (AD), caused by the tear of intima of the aorta, is a fatal disease with high mortality in patients both before and after hospital admission [1]. AD patients always suffer from unbearable chest or back pain, ischemia and systemic symptoms. The treatment options mainly include open surgery and endovascular management, between which the open surgery might be the most efficient treatment for patients, however, it is also one of the most complicated operations in cardiac surgery, with extremely high fatality rate and severe postoperative complications, such as hypoxemia and neurological complications (delirium, confusion, et al) [2].
Ozone (O3) therapy has been applied and extensively researched for more than a century, despite of a few severe side effects, it is believed that the O3 therapy is effective in treating multiple diseases such as heart failure and cervical spondylodiscitis [3-5]. O3, a strong oxidant that is 10-fold more soluble than oxygen, is capable of providing sufficient energy and oxygen supply to the damaged tissues. Accumulating studies illustrate that O3 might improve the ischemia of injured tissue via delivering the oxygen to tissues, activating the pentose phosphate pathway, elevating 2,3-DPG content in erythrocyte and stimulating the erythrocyte oxygen metabolism [6]. Furthermore, O3 also can protect the blood cell from oxidative stress by inducing the expression of antioxidant enzymes [7]. Ozonated autohemotherapy (Ozone-AHT) is firstly applied in clinical practice in 1968, and now is widely used in various clinical disciplines, specifically in systemic disease and ischemic disease [8,9]. In light of the benefit in other systemic and ischemic diseases, we assumed that Ozone-AHT could change the hypoxemia of AD, however there is no report about Ozone-AHT on treating AD patients.
Thus, our study aimed to investigate the effect of ozone on hypoxia, hemolysis and morphological changes of blood from AD patients for providing preliminary evidence of the application of Ozone-AHT in AD patients.
Materials and methods
Collection
Twenty patients with AD (AD group) and 20 healthy volunteers (control group) with no smoking and treatment histories were consecutively included in the First Affiliated Hospital of Harbin Medical University from March 2016 to Aug 2016. Blood samples of 20 ml were drawn from vein from AD patients and healthy volunteers, and subsequently stored in the polyvinyl chloride (PVC) bags (20 U/mL blood). In addition, the blood samples from AD patients were collected right after the arrival in the emergency department, and AD patients did not receive any medication, including oxygen administration at the time of the sample collection. Approval from the Ethics Committee of the First Affiliated Hospital of Harbin Medical University was obtained and informed consents were collected from all participants.
Ozone generation and measurement
Ozone was generated from medical-grade oxygen (O2) through electrical corona arc discharge using the O3 generator (Model Ozonosan PM 100K, Hansler GmbH, Iffezheim, Germany), which allows the gas flow rate and O3 concentration to be controlled in real time. The procedure was conducted under standard condition according to the Standardisation Committee of the International O3 Association (IOA). The ozone flow-rate was kept constant at 3 L/min in all machines. Tygon polymer tubes and single-use silicon treated syringes (ozone-resistant) were used throughout the procedure to ensure containment and concentrations.
Gas delivery to blood samples
According to the method described by Valter Travagli et al [10], gas delivery was carried out with a single dose of ozone (concentration per volume) as follows: a predetermined volume of gas mixture composed of O2 (95%) and O3 (5%) with ozone concentrations at 0, 40, 80, 160 μg/mL was collected by a second syringe and immediately introduced into the blood samples. The optimal blood sample/gas ratio was 1:1. The syringes were in a monodirectional oscillator (60 cycles/min) for 20 min until the foaming became minimal, which indicated the liquid and gas were mixed completely.
Erythrocyte membrane preparation
Erythrocyte membranes were obtained according the method modified by Dodge et al [11]. Erythrocytes were lysed using 4°C hypotonic Tris-HCL (PH=8.0, 10 mM) solution with 0.02 mM phenylmethanesulfonyl fluoride (PMSF) (PMSF was dissolved with glycerol) at 4°C for 12 h with the volume ratio 1:5. And then, the erythrocyte membranes were centrifuged at 15,000×g for 20 min, after the supernatant was extracted, the remaining part was washed three times with the same above-mentioned hemolysis buffer until the purified white membranes (ghost protein) were obtained, which only contains the erythrocyte membranes. The final membrane was resuspended in 250 μL of cold Tris-HCL (PH=8.0, 10 mM) buffer and stored. The protein concentration at the membranes was determined by BCA Protein Assay Kit (Beyotime Biotechnology Co., Guangzhou, China).
Physicochemical determinations
The effect of ozone on improving the hypoxia of blood obtained from AD patients and controls were determined by evaluating PO2, SO2, which were examined by an ABL800 blood gas analyser (Radiometer, Danmark).
Enzyme-linked immuno sorbent assay (ELISA)
Free hemoglobin (FHb) concentration, malondialdehyde (MDA) and superoxide dismutase (SOD) of erythrocyte membrane were evaluated by ELISA kits (YuanMu Biological technology Co., Shanghai, China) following the manufacturer’s protocol.
Morphological determinations
Scanning electron microscopy (SEM) sample preparation
To begin with, the ozone preconditioned samples were centrifuged at 3000 rpm for 10 mins, after which the supernatant (plasma, white blood cells and platelets) was discarded. Then one drop of each sample was added into EP tubes containing 500 μL 2.5% glutaraldehyde DPBS with pH of 7.4 and stand for 120 mins. This was followed by rinsing twice for 3 mins before being fixed for 15 min with 1% osmium tetraoxide (OsO4). The samples were rinsed thrice with dulbecco’s phosphate buffered saline (DPBS) for 2 mins and were dehydrated serially 3 times with 100% ethanol, about 30 μL of each sample were placed on glass cover slips, air-dried at room temperature, gold coated for 3 min at 13.3 Pa in a sputter device (Edwards S 150, Sussex, England) and examined in a scanning electron microscope (JEOL JSM-6380LV, Japan).
Atomic force microscope (AFM) sample preparation
All the ozone preconditioned samples were centrifuged (3000 rpm×5 min), after which the supernatant was discarded and the erythrocytes was suspended in 2.5% glutaraldehyde for 60 mins, and rinsed with DPBS and post-fixed with OsO4 to protect membrane phospholipids. The samples were then dehydrated with ethanol and dried on a glass cover slip.
Cell morphology of erythrocyte were obtained from AFM (Dimension Edge, Bruker, Germany) tapping mode. 0.01-0.025 Ohm-cm Antimony (n) doped Si tips (RTESP, Bruker, Germany) with a spring constant of 40 N/m, a resonant frequency of 300 kHz and a nominal tip radius of 8 nm were employed. Six cells were scanned randomly in the air using tapping mode of each sample at the following fields: 20×20 μm2, 10 ×10 μm2, 1×1 μm2. NanoScope Analysis (Bruker, Germany) was used to filter out any noise, subtract the plane of average inclination and perform all measurements. The following parameters were measured: the diameter, height and concave depth of the erythrocyte. The parameters for evaluating cell surface roughness are arithmetic average roughness (Ra) and root-mean-square roughness (Rq). Membrane roughness was measured by 1×1 μm2 erythrocyte edge bulge.
Confocal sample preparation
Ozone preconditioned samples were centrifuged and supernatant was extracted, afterwards 100 μL of pellets of each sample were transferred to EP tubes containing 5 ml of 2.5% glutaraldehyde DPBS with pH of 7.4 and stand at room temperature for 12 h. Then 1 ml of cell suspension was taken after vortex and centrifuged at 3000 rpm for 5 mins, after which around 7×106 cells were collected, which were rinsed twice with DPBS (3000 rpm×5 min), and then the supernatant was discarded. The cells were suspended in 200 μL DPBS containing 0.1% TritonX-100 and subsequently incubated at room temperature for 5 mins, rinsed twice with DPBS (3000 rpm×5 min), and then extracted the supernatant. Subsequently, the cells were suspended in 200 μL DPBS containing 1% BSA, incubated at room temperature for 30 min, rinsed twice with DPBS (3000 rpm×5 min), then the supernatants was discarded. And then the cells were suspended in 200 μL DPBS containing 10 μL phalloidin-rhodamine (Sigma, St. Louis, MO) (500 μL/mL, dissolved in methanol); incubation for 60 min at 37°C in darkness, rinsed thrice with DPBS (3000 rpm×5 min), and then the supernatants was discarded. Finally, the cells were suspended in 4 ml of DPBS, preserved at 4°C in darkness. Cell suspension of 100 μL was used to observe cytoskeleton of erythrocytes under laser confocal microscope.
Results
Characteristics of AD patients and controls
As listed in Table 1, the mean age of patients in AD group was older than that in the control group (P=0.005). No difference of gender and weight was found between two groups (P=0.288 and P=0.143, respectively). The number of patients with history of hypertension and coronary heart disease were 12 (60.0%) and 6 (30.0%), and no patient had those histories in control group (P<0.001 and P=0.007, respectively). In addition, patients in the AD group had reduced median level of hematocrit (HCT) compared with control group (P<0.001). In the meantime, the median levels of FHb and MDA in AD group were increased than that in the control group (P<0.001 and P=0.028, respectively). Additionally the median value of two erythrocyte membrane surface parameters (Rq and Ra) were notably greater in the AD group compared with control group (all P<0.001). No difference of other characteristics was found between two groups.
Table 1.
Characteristics of all participants
| Parameter | AD group (N=20) | Control group (N=20) | P value |
|---|---|---|---|
| Age (years) | 50.10±10.74 | 41.35±7.65 | 0.005 |
| Gender (male/female) | 16/4 | 13/7 | 0.288 |
| Weight (kg) | 66.10±9.50 | 70.90±10.76 | 0.143 |
| History of hypertension (n/%) | 12 (60.0) | 0 (0.0) | <0.001 |
| History of coronary heart disease (n/%) | 6 (30.0) | 0 (0.0) | 0.007 |
| History of drink (n/%) | 9 (45.0) | 4 (20.0) | 0.176 |
| HCT (%) | 33.50 (30.75-35.00) | 36.50 (35.00-44.00) | <0.001 |
| PO2 (mmHg) | 49.40 (45.13-53.30) | 49.98 (46.75-55.05) | 0.499 |
| SO2 (%) | 72.95 (69.33-77.38) | 69.41 (64.64-74.16) | 0.088 |
| FHb (μg/mL) | 111.59 (107.27-113.44) | 23.89 (22.27-25.00) | <0.001 |
| MDA (nmol/mg prot-1) | 7.53 (6.68-8.11) | 6.71 (5.13-7.87) | 0.028 |
| SOD (U/mg prot-1) | 12.50 (11.83-13.33) | 13.10 (12.48-14.35) | 0.110 |
| Malformation percentages of erythrocyte (%) | 3.78 (2.87-5.21) | 5.23 (2.72-6.45) | 0.425 |
| Erythrocyte membrane surface parameters | |||
| D (μm) | 7.76 (7.72-7.79) | 7.67 (7.50-7.79) | 0.078 |
| H (μm) | 1.57 (1.50-1.64) | 1.64 (1.54-1.73) | 0.208 |
| Depth (μm) | 1.31 (1.29-1.33) | 1.33 (1.25-1.45) | 0.378 |
| Rq (nm) | 17.15 (12.55-19.53) | 8.45 (7.73-10.60) | <0.001 |
| Ra (nm) | 9.80 (9.30-14.48) | 5.58 (3.63-7.87) | <0.001 |
Data was presented as mean ± standard deviation, median (25th-75th) or count (%). Comparison between two groups was determined by t test, Chi-Square test or Wilcoxon rank sum test. P<0.05 was considered significant. AD, aortic dissection; HCT, hematocrit; PO2, partial pressure O2; SO2, oxygen saturation; FHb, free hemoglobin; MDA, malondialdehyde; SOD, superoxide dismutase; D, diameter; H, height; Depth, concave depth; Rq, root-mean-square roughness; Ra, roughness.
The effect of ozone on oxygenation
After ozonation of whole blood, the median levels of PO2 and SO2 were increased in samples with ozone concentrations at 40 μg/mL, 80 μg/mL and 160 μg/mL in both AD group and control group (all P<0.05) compared with samples exposed to 0 μg/mL ozone (Table 2). In addition, the median PO2 levels increased in samples exposed to 80 μg/mL and 160 μg/mL compared with that of samples exposed to 40 μg/mL ozone in both AD group and control group (all P<0.05). No difference of PO2 median levels in samples was disclosed between two groups at different ozone concentrations.
Table 2.
Levels of PO2 and SO2 in human blood samples after exposure to different Ozone concentrations
| Concentrations | 0 μg/mL | 40 μg/mL | 80 μg/mL | 160 μg/mL | P value |
|---|---|---|---|---|---|
| PO2 (mmHg) | |||||
| AD group | 49.40 (45.13-53.30) | 426.80 (395.60-474.93)a | 576.51 (517.28-664.95)a,b | 602.45 (538.69-654.53)a,b | <0.001 |
| Control group | 49.98 (46.75-55.05) | 405.37 (361.24-526.32)a | 584.54 (535.39-640.37)a,b | 608.68 (534.10-658.76)a,b | <0.001 |
| P value | 0.499 | 0.589 | 0.871 | 0.935 | |
| SO2 (%) | |||||
| AD group | 72.95 (69.33-77.38) | 99.58 (99.23-99.78)a | 99.90 (99.80-100.00)a | 100.16 (99.82-100.77)a,b | <0.001 |
| Control group | 69.41 (64.64-74.16) | 99.95 (99.44-100.08)a | 99.70 (99.28-99.95)a | 99.76 (99.33-100.31)a | <0.001 |
| P value | 0.088 | 0.013 | 0.046 | 0.042 |
Data was presented as median (25th-75th). Comparison among different concentrations was determined by Kruskal-Wallis H rank sum test, and a Dunn-Bonferroni test for post hoc comparisons. Comparison between AD groups and C group was determined by Wilcoxon rank sum test. P<0.05 was considered significant.
Ozone concentrations 0 μg/mL vs 40 μg/mL, 80 μg/mL or 160 μg/mL are significantly different (P<0.05);
Ozone concentrations 40 μg/mL vs 80 μg/mL or 160 μg/mL are significantly different (P<0.05).
AD, aortic dissection; PO2, partial pressure O2; SO2, oxygen saturation.
As to the median SO2 level, it was elevated only in the 160 μg/mL samples when compared with the 40 μg/mL samples (P<0.05) in AD group. In addition, the SO2 median level was notably lower in 40 μg/mL samples but higher in 80 μg/mL and 160 μg/mL samples in AD group compared with control group (all P<0.05).
The effect of ozone-AHT on antioxidant effect
As presented in Table 3, the MDA median levels were similar in samples exposed to 0 μg/mL, 40 μg/mL and 80 μg/mL ozone in both two groups (all P>0.05). However, when exposed to 160 μg/mL, the MDA median level was obviously elevated compared to that in samples with other ozone concentrations (all P<0.05). In addition, the MDA median level was increased in AD group when exposed to 0 μg/mL and 80 μg/mL ozone compared with control group (P=0.028 and P=0.012, respectively).
Table 3.
Levels of MDA and SOD in human blood samples after exposure to different Ozone concentrations
| Concentrations | 0 μg/mL | 40 μg/mL | 80 μg/mL | 160 μg/mL | P value |
|---|---|---|---|---|---|
| MDA (nmol/mg prot-1) | |||||
| AD group | 7.53 (6.68-8.11) | 7.10 (6.09-8.02) | 7.36 (6.38-7.96) | 9.37 (8.51-10.01)a,b,c | <0.001 |
| Control group | 6.71 (5.13-7.87) | 6.46 (5.42-7.46) | 6.08 (5.39-7.45) | 8.93 (8.23-9.55)a,b,c | <0.001 |
| P value | 0.028 | 0.152 | 0.012 | 0.310 | |
| SOD (U/mg prot-1) | |||||
| AD group | 12.50 (11.83-13.33) | 14.45 (13.45-15.71)a | 15.33 (13.71-16.36)a | 10.85 (10.25-11.96)b,c | <0.001 |
| Control group | 13.10 (12.48-14.35) | 16.38 (15.38-17.67)a | 16.88 (15.88-18.49)a | 12.13 (10.67-12.67)b,c | <0.001 |
| P value | 03e.110 | 0.006 | 0.012 | 0.055 |
Data was presented as median (25th-75th). Comparison among different concentrations was determined by Kruskal-Wallis H rank sum test, and a Dunn-Bonferroni test for post hoc comparisons. Comparison between AD group and C group was determined by Wilcoxon rank sum test. P<0.05 was considered significant.
Ozone concentrations 0 μg/mL vs 40 μg/mL, 80 μg/mL or 160 μg/mL are significantly different (P<0.05);
Ozone concentrations 40 μg/mL vs 80 μg/mL or 160 μg/mL are significantly different (P<0.05);
Ozone concentrations 80 μg/mL vs 160 μg/mL are significantly different (P<0.05).
AD, aortic dissection; MDA, malondialdehyde; SOD, superoxide dismutase.
The median levels of SOD in both two groups increased in samples exposed to 40 μg/mL, 80 μg/mL and 160 μg/mL compared to that in samples exposed to 0 μg/mL (all P<0.05). However, the SOD median level in 160 μg/mL samples was markedly higher when compared that in the 40 μg/mL and 80 μg/mL samples in both two groups (all P<0.001). In addition, the SOD median level was declined in AD group than control group at 40 μg/mL and 80 μg/mL ozone concentrations (P=0.006 and P=0.012, respectively).
The effect of ozone-AHT on FHb level
The FHb level in AD group and control group only increased in samples at 80 μg/mL and 160 μg/mL ozone concentrations compared to samples at 0 μg/mL ozone concentration (all P<0.001). Additionally, in AD group, the FHb level in samples with 160 μg/mL ozone concentration was higher compared with that in samples with 40 μg/mL and 80 μg/mL ozone concentrations (all P<0.001). And in control group, the FHb level increased in 160 μg/mL samples compared with that in the 40 μg/mL samples (P<0.001). In addition, FHb levels were elelvated in AD group compared with that in control group at all ozone concentrations (all P<0.001) (Table 4).
Table 4.
Levels of FHb in human blood samples after exposure to different Ozone concentrations
| Concentrations | 0 μg/mL | 40 μg/mL | 80 μg/mL | 160 μg/mL | P value |
|---|---|---|---|---|---|
| FHb (μg/mL) | |||||
| AD group | 111.59 (107.27-113.44) | 115.81 (113.03-118.22) | 119.89 (118.21-125.62)a | 186.98 (183.80-189.58)a,b,c | <0.001 |
| Control group | 23.89 (22.27-25.00) | 23.89 (22.27-25.00) | 28.48 (24.51-30.47)a | 30.99 (27.71-35.00)a,b | <0.001 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 |
Data was presented as median (25th-75th). Comparison among different concentrations was determined by Kruskal-Wallis H rank sum test, and a Dunn-Bonferroni test for post hoc comparisons. Comparison between AD groups and C group was determined by Wilcoxon rank sum test. P<0.05 was considered significant.
Ozone concentrations 0 μg/mL vs 40 μg/mL, 80 μg/mL or 160 μg/mL are significantly different (P<0.05);
Ozone concentrations 40 μg/mL vs 80 μg/mL or 160 μg/mL are significantly different (P<0.05);
Ozone concentrations 80 μg/mL vs 160 μg/mL are significantly different (P<0.05).
AD, aortic dissection; FHb, free hemoglobin.
The malformation of erythrocyte under different ozone concentrations
As shown in Table 5, the malformation percentage of the erythrocyte increased when samples were exposed to ozone with concentration 160 μg/mL compared with that in samples at 0 μg/mL and 40 μg/mL ozone concentration (all P<0.001). No difference was found between AD group and control group in regard to different ozone concentrations (P=0.425, P=0.482 and P=0.223, respectively).
Table 5.
The malformation percentages of the erythrocyte in different Ozone concentrations
| Concentrations | 0 μg/mL | 80 μg/mL | 160 μg/mL | P value |
|---|---|---|---|---|
| Malformation percentages of erythrocyte (%) | ||||
| AD group | 3.78 (2.87-5.21) | 5.65 (4.62-8.96) | 13.24 (10.43-15.26)a,b | <0.001 |
| Control group | 5.23 (2.72-6.45) | 5.36 (3.62-7.53) | 10.32 (7.97-16.24)a,b | <0.001 |
| P value | 0.425 | 0.482 | 0.223 |
Data was presented as median (25th-75th). Comparison among different concentrations was determined by Kruskal-Wallis H rank sum test, and a Dunn-Bonferroni test for post hoc comparisons. Comparison between AD group and Control group was determined by Wilcoxon rank sum test. P<0.05 was considered significant.
Ozone concentrations 0 μg/mL vs 80 μg/mL or 160 μg/mL are significantly different (P<0.05);
Ozone concentrations 80 μg/mL vs 160 μg/mL are significantly different (P<0.05).
AD, aortic dissection.
As shown in Figure 1, in control group, the healthy erythrocytes presented uniform and circular biconcave shapes (Figure 1A), and there was no malformation percentage increase when the cells exposed to the ozone concentration at 80 μg/mL (Figure 1B), while the malformation percentages increased when the cells exposed to the ozone concentration at 160 μg/mL compared to that of cells exposed to 0 μg/mL and 80 μg/mL ozone concentration (Figure 1C) (P<0.05).
Figure 1.
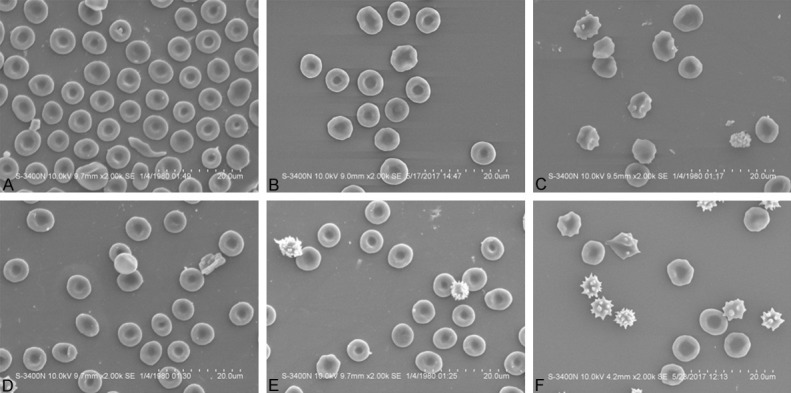
The malformation percentages of the erythrocyte when exposed to the different ozone concentrations by SEM. In control Healthy erythrocytes presented uniform and circular biconcave shapes at 0 μg/mL ozone concentration (A), and the malformation percentage did not change when the cells exposed to the ozone concentration at 80 μg/mL (B). The malformation percentage was elevated when the cells exposed to the ozone concentration at 160 μg/mL (C). In AD group, the malformation percentage slightly increased compared with control group at 0 μg/mL ozone concentration (D). However, no malformation percentage change was observed when the blood exposed to 80 μg/mL ozone (E), the malformation percentage was rised when the blood exposed to 160 μg/mL ozone (F) (P<0.05). Bar =10 μm, original magnfication =×2000. Comparison between two groups was determined by t test. P<0.05 was considered significant.
In AD group, the malformation percentage was slightly increased compared with control group (P>0.05) at 0 μg/mL ozone concentration (Figure 1D). However, there was no malformation percentage change when the blood exposed to 80 μg/mL ozone (Figure 1E), the malformation percentage was rised when the blood exposed to 160 μg/mL ozone (Figure 1F) (P<0.05).
The morphology change of erythrocyte under different ozone concentrations
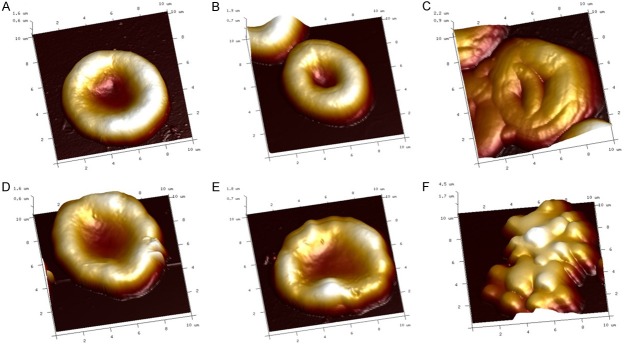
As presented in Figure 2A, in the control group, the erythrocyte was a biconcave shape and with regular cell surface lattice structure, there was no morphological change when the erythrocyte exposed to 80 μg/mL ozone (Figure 2B), however, the change happened when the ozone concentration reached 160 μg/mL compared to that of samples exposed to 0 μg/mL ozone concentration (Figure 2C). In the AD-group, although some abnormal changes were observed on the cells, the shape remained to exhibit double concave disc (Figure 2D). There was no worse change when cell exposed to ozone concentration at 80 μg/m (Figure 2E), but the morphology changed largely when the cell exposed to 160 μg/mL ozone (Figure 2F).
Figure 2.
The morphological change of the erythrocyte when blood exposed to the ozone by AFM 3D image. In the control group, the erythrocyte was a biconcave shape and with regular cell surface lattice structure (A), there was no morphological change when the erythrocyte exposed to 80 μg/mL ozone (B). However, the morphology changed when the ozone concentration reached 160 μg/mL compared to that at 0 μg/mL ozone concentration (C). In the AD-group, the shape remained to exhibit double concave disc (D). There was no worse change of erythrocyte exposed to ozone concentration at 80 μg/m (E), but the morphology changed greatly when the cell exposed to 160 μg/mL ozone (F). Scanning area: 10 μm×10 μm. Comparison between two groups was determined by t test. P<0.05 was considered significant.
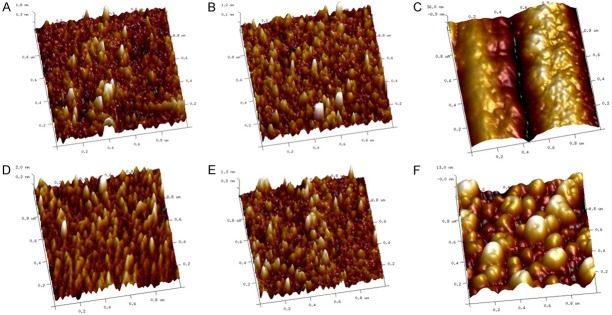
As presented in Figure 3, the cell membrane was relatively smooth and intact, which was formed by strengthening different sizes of concave and convex surfaces, like ears of corn with uniform density, and the gaps between uplifts of concave depth were relatively equally distributed under ozone concentration at 0 μg/mL and 80 μg/mL (Figure 3A, 3B). While, different sizes of protrusions appeared on cell membrane surface, the surface concavity and convexity were enhanced and disoriented, and the surface roughness was increased under ozone concentration at 160 μg/mL (Figure 3C). Similar 3D images of erythrocytes under ozone concentrations at 0 μg/mL and 80 μg/mL were observed in AD group (Figure 3D, 3E). However, as presented in Figure 3F obvious protrusions appeared on cell membrane surface under ozone concentration at 160 μg/mL.
Figure 3.
The morphological change of the erythrocyte when blood exposed to the ozone by AFM 3D image. The cell membrane was smooth and intact, like ears of corn with uniform density under ozone concentration at 0 μg/mL (A) and 80 μg/mL (B). However, the surface concavity and convexity were enhanced and disoriented, and the surface roughness was increased under ozone concentration at 160 μg/mL (C). Similar 3D images of erythrocytes under ozone concentrations at 0 μg/mL (D) and 80 μg/mL (E) were observed in AD group. However, obvious protrusions appeared on cell membrane surface under ozone concentration at 160 μg/mL (F). Scanning area: 1 μm×1 μm. Comparison between two groups was determined by t test. P<0.05 was considered significant.
As listed in Table 6, no morphological parameter changed in both AD group and control group when samples exposed to 80 μg/mL ozone. However, when samples exposed to 160 μg/mL ozone, the diameter (D) in AD group, root-mean-square roughness (Rq) and roughness (Ra) in both two groups predominantly changed compared with samples under 0 μg/mL ozone (all P<0.001). Additionally, the D and H in AD group, as well as depth (concave depth), Rq and Ra in both two groups changed under 160 μg/mL ozone compared to 0 μg/mL ozone (all P<0.001). Meanwhile, the D under 80 μg/mL ozone, Depth under 160 μg/mL ozone, Rq and Ra under 0 μg/mL and 80 μg/mL ozone were different in AD group compared to control group (all P<0.05).
Table 6.
Erythrocyte membrane surface parameters measurement results
| Concentrations | 0 μg/mL | 80 μg/mL | 160 μg/mL | P value |
|---|---|---|---|---|
| D (μm) | ||||
| AD group | 7.76 (7.72-7.79) | 7.79 (7.76-7.91) | 7.51 (7.41-7.61)a,b | <0.001 |
| Control group | 7.67 (7.50-7.79) | 7.65 (7.58-7.84) | 7.47 (7.27-7.72) | 0.121 |
| P value | 0.078 | 0.006 | 0.597 | |
| H (μm) | ||||
| AD group | 1.57 (1.50-1.64) | 1.69 (1.56-1.73) | 1.51 (1.48-1.54)b | <0.001 |
| Control group | 1.64 (1.54-1.73) | 1.68 (1.54-1.76) | 1.54 (1.29-1.78) | 0.290 |
| P value | 0.208 | 0.871 | 0.935 | |
| Depth (μm) | ||||
| AD group | 1.31 (1.29-1.33) | 1.35 (1.29-1.41) | 1.27 (1.25-1.29)b | <0.001 |
| Control group | 1.33 (1.25-1.45) | 1.43 (1.33-1.46) | 1.33 (1.25-1.36)b | 0.026 |
| P value | 0.378 | 0.081 | 0.033 | |
| Rq (nm) | ||||
| AD group | 17.15 (12.55-19.53) | 17.20 (12.78-20.98) | 71.75 (65.45-76.40)a,b | <0.001 |
| Control group | 8.45 (7.73-10.60) | 9.39 (8.27-10.73) | 68.59 (64.75-72.48)a,b | <0.001 |
| P value | <0.001 | <0.001 | 0.256 | |
| Ra (nm) | ||||
| AD group | 9.80 (9.30-14.48) | 10.77 (9.52-14.17) | 41.75 (37.38-45.55)a,b | <0.001 |
| Control group | 5.58 (3.63-7.87) | 5.03 (3.78-7.40) | 41.58 (39.26-44.96)a,b | <0.001 |
| P value | <0.001 | <0.001 | 0.957 |
Data was presented as median (25th-75th). Comparison among different concentrations was determined by Kruskal-Wallis H rank sum test, and a Dunn-Bonferroni test for post hoc comparisons. Comparison between AD groups and C group was determined by Wilcoxon rank sum test. P<0.05 was considered significant.
Ozone concentrations 0 μg/mL vs 80 μg/mL or 160 μg/mL are significantly different (P<0.05);
Ozone concentrations 80 μg/mL vs 160 μg/mL are significantly different (P<0.05).
AD, aortic dissection; D, diameter; H, height; Depth, concave depth; Rq, root-mean-square roughness; Ra, roughness.
The spatial distribution of F-actin in erythrocytes
The erythrocytes had obvious red fluorescence rings under laser scanning confocal microscope, and the erythrocyte membrane was relatively complete under ozone concentration at 0 μg/mL and 80 μg/mL (Figure 4A, 4B, 4D, 4E). In addition, erythrocyte membrane was not complete, some cells did not form a complete red fluorescence ring, and the area of fluorescent dye in each erythrocyte was increased in the cell cytoplasm in samples exposed to ozone concentration at 160 μg/mL (Figure 4C, 4F).
Figure 4.
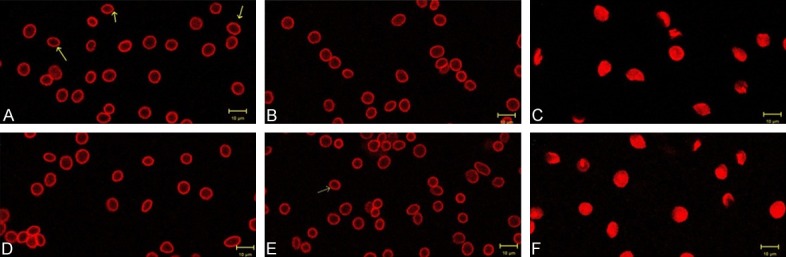
The spatial distribution of F-actin in erythrocytes revealed by fluorescent probes of phalloidin-rhodamine when cells exposed to different concentration ozone. The erythrocyte membrane was relatively complete under ozone concentration at 0 μg/mL and 80 μg/mL in the control goup (A, B) and the AD group (D, E). Erythrocyte membrane was not complete, complete red fluorescence ring were not seen in cells, and the area of fluorescent dye in each erythrocyte was increased in samples exposed to ozone concentration at 160 μg/mL in control group (C) and AD group (F). Bar =10 μm, original magnfication =×2000. Comparison between two groups was determined by t test. P<0.05 was considered significant.
Discussion
Ozone-AHT, as an emerging type of ozone therapy, is a very popular and controversial therapeutic technology using the ozonated blood for the treatment of patients in recent years. As one of the most newly developed ozone therapies, the procedure of Ozone-AHT is conducted through transferring the ozonated blood, which is obtained from the same patient and prepared by the injection of different concentrations of ozone, to patients with various diseases such as gout and acute cerebral infarction [8,12,13]. In order to explore the usefulness of Ozone-AHT in AD patients, we conducted this in vitro experiment as a preliminary study, in which the effect of ozone on the hypoxia, hemolysis and morphological changes of erythrocytes of blood samples obtained from AD patients and healthy controls.
In our study, the Ozone-AHT markedly increased the PO2 and SO2 of blood samples obtained from AD patients, which was in consistent with a previous study [13]. Moreover, in our study, the results showed that samples were more accessible to PO2 of 400 mmHg when the ozone concentration was 40 μg/mL, which satisfied the requirement of clinical needs, indicating the oxygenation effect of ozone was powerful in vitro. Bocci et al demonstrate that the correlation of oxygenation effect of ozone on human blood is dose-response association, which means that ozone is benefit to human blood at low level but harmful at high level [14]. An in vivo experiment conducted in rat models reveals that ozone-AHT protects kidney from ischemia and an improvement of the oxygen consumption of the rat kidney is reported [15]. However, no study has been done for exploring the mechanism of ozone-AHT for AD patients or for verifying its clinical efficacy and safety in clinical practice, as a preliminary in vitro experiment we firstly discovered good effect of ozone-AHT on increasing the oxygenation in AD blood samples. And this effect observed in our study largely came from that ozone was a strong oxidizing agent and has been found to be capable of supplying energy and oxygen to blood and tissues. As another aspect of efficacy evaluated in our study, the MDA level did not change in blood samples exposed to 40 μg/mL and 80 μg/mL ozone, however the SOD level was greatly improved under ozone concentrations at 40 μg/mL and 80 μg/mL. Emerging number of studies have clearly shown that erythrocytes are protected by plasmatic antioxidants when exposed to ozone concentrations at therapeutic doses, which is claimed to be the doses between 10 μg/mL and 80 μg/mL [16,17]. Furthermore, the elucidation of the mechanisms might be that ozone may be active in antioxidant system [18,19]. In view that oxidative stress contributes to the progression of AD, the effects of ozone administration on SOD and MDA levels may indicate that ozone-AHT has the potential of preventing progression of AD [20]. Although the age of AD patients is older than the control group, which may result in the PO2, SO2 and antioxidant effect in the elderly is poorer than the younger, due to that aging is correlated with cardiovascular and cerebral vascular degeneration, which may lead to relatively reduced oxygen saturation. However, it is reported that the oxygen saturation and antioxidant in the elderly compared with younger population may be limited [21,22].
Additionally, recent studies indicate that the cytokines are stimulated in AD patients, which may lead to the progression of AD [23]. Interestingly, ozone is reported to be correlated with increased levels of inflammatory cytokines in other diseases, however, those are most inflammation related diseases [24,25]. Although the association of inflammatory cytokines with AD is not well established yet, the association of ozone with inflammatory cytokines in AD might need further investigation.
For the assessment of hemolysis, the FHb levels at different ozone concentrations were evaluated, and the data showed that the FHb slightly increased when the blood exposed to different ozone concentrations, but in the control group, all the values were in normal range (10~50 mg/L). This means that the slight hemolysis caused by therapeutic ozone doses is acceptable. The dissection itself may induce red cells damage due to disorder of blood coagulation system and hemodynamic, which could explain why the basic value of FHb of AD-group was higher than control group [26].
Most previous studies aims to investigate the physicochemical and oxidative stress mechanisms of the effect of ozone on multiple diseases, while the studies which aim to observe the microscopic morphological changes of erythrocyte are few. According to previous reports, erythrocyte membrane surface ultrastructure changes when the cell is damaged under non-physiological situation [27,28]. In particular, membrane surface roughness (Ra and Rq) is more sensitive to changes in membrane-skeleton integrity which could be conjectured that the damage of erythrocyte can be detected at the nanometer level. In our study, the cell membranes of erythrocytes under therapeutic ozone doses (10-80 μg/mL) were relatively smooth and intact, and the surface roughness did not obviously change, therefore therapeutic ozone seemed to be unharmful to erythrocytes. In addition, some cell shapes and ultrastructure parameters changed when the erythrocyte exposed to the overdosed ozone (160 μg/mL), and heterogeneous features of size, shape were presented, such as acanthocytes, echinocytes, leptocyte et al, implying that the overdosed ozone could cause damage to erythrocytes. So far, some researchers suggest that it might be resulted from that the membrane proteins, skeleton construction and cell mechanical stability are damaged by excessive oxidative stress [29,30].
The erythrocyte membrane skeleton mainly composes of actin and spectrin, presenting a flexible discoid shape in three-dimensional network [31]. It plays a critical role in diverse physiological processes including cell morphological changes, motility and migration. Our data showed that ozone at concentration of 160 μg/mL promoted the F-action to be in the depolymerization state, destroyed the integrity of cytoskeleton protein. And the erythrocyte membrane was not complete and couldn’t form a complete fluorescent ring, therefore, fluorescent dyes entered in the cytoplasm. Fortunately, although the integrity of the erythrocyte membrane was destroyed at over dosed ozone concentration, we didn’t find the similar change in the cells at therapeutic ozone concentrations. However, the damage mechanism remains unclear.
There were some limitations in this study: (1) the sample size was relatively small, further study should enlarge the sample size; (2) this was a preliminary study, which only investigated the effect of ozone on the blood samples obtained from AD patients, thus a clinical study using the ozone-AHT treating AD patients should be performed to further validate our results; (3) the effect of ozone on the levels of inflammatory cytokines in the blood samples from AD patients was not investigated in our study, therefore further studies could evaluate the effect of ozone on the inflammatory cytokine expression in AD patients to further explore their correlations.
In conclusion, Ozone improves oxygen content and reduces oxidative damage in blood from AD patients, and therapeutic dose ozone do not induce hemolysis and morphology change of erythrocytes.
Acknowledgements
This research was supported by National Clinical Key Specialty Construction Project.
Disclosure of conflict of interest
None.
References
- 1.Nienaber CA, Clough RE. Management of acute aortic dissection. Lancet. 2015;385:800–811. doi: 10.1016/S0140-6736(14)61005-9. [DOI] [PubMed] [Google Scholar]
- 2.Sheng W, Yang HQ, Chi YF, Niu ZZ, Lin MS, Long S. Independent risk factors for hypoxemia after surgery for acute aortic dissection. Saudi Med J. 2015;36:940–946. doi: 10.15537/smj.2015.8.11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buyuklu M, Kandemir FM, Set T, Bakirci EM, Degirmenci H, Hamur H, Topal E, Kucukler S, Turkmen K. Beneficial effects of ozone therapy on oxidative stress, cardiac functions and clinical findings in patients with heart failure reduced ejection fraction. Cardiovasc Toxicol. 2017;17:426–433. doi: 10.1007/s12012-017-9400-8. [DOI] [PubMed] [Google Scholar]
- 4.Andres-Cano P, Vela T, Cano C, Garcia G, Vera JC, Andres-Garcia JA. Cervical spondylodiscitis after oxygen-ozone therapy for treatment of a cervical disc herniation: a case report and review of the literature. HSS J. 2016;12:278–283. doi: 10.1007/s11420-016-9500-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng W, Xu Y, Li D, Zhu E, Deng L, Liu Z, Zhang G, Liu H. Ozone protects rat heart against ischemia-reperfusion injury: a role for oxidative preconditioning in attenuating mitochondrial injury. Biomed Pharmacother. 2017;88:1090–1097. doi: 10.1016/j.biopha.2017.01.151. [DOI] [PubMed] [Google Scholar]
- 6.Giunta R, Coppola A, Luongo C, Sammartino A, Guastafierro S, Grassia A, Giunta L, Mascolo L, Tirelli A, Coppola L. Ozonized autohemotransfusion improves hemorheological parameters and oxygen delivery to tissues in patients with peripheral occlusive arterial disease. Ann Hematol. 2001;80:745–748. doi: 10.1007/s002770100377. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Chen H, Liu XH, Chen ZY, Weng XD, Qiu T, Liu L. The protective effect of ozone oxidative preconditioning against hypoxia/reoxygenation injury in rat kidney cells. Ren Fail. 2014;36:1449–1454. doi: 10.3109/0886022X.2014.950934. [DOI] [PubMed] [Google Scholar]
- 8.Wu XN, Zhang T, Wang J, Liu XY, Li ZS, Xiang W, Du WQ, Yang HJ, Xiong TG, Deng WT, Peng KR, Pan SY. Magnetic resonance diffusion tensor imaging following major ozonated autohemotherapy for treatment of acute cerebral infarction. Neural Regen Res. 2016;11:1115–1121. doi: 10.4103/1673-5374.187046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molinari F, Simonetti V, Franzini M, Pandolfi S, Vaiano F, Valdenassi L, Liboni W. Ozone autohemotherapy induces long-term cerebral metabolic changes in multiple sclerosis patients. Int J Immunopathol Pharmacol. 2014;27:379–389. doi: 10.1177/039463201402700308. [DOI] [PubMed] [Google Scholar]
- 10.Re L, Rowen R, Travagli V. Ozone therapy and its use in medicine: further comments. Cardiology. 2017;136:269. doi: 10.1159/000452618. [DOI] [PubMed] [Google Scholar]
- 11.Dodge JT, Mitchell C, Hanahan DJ. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- 12.Li LY, Ma RL, Du L, Wu AS. Ozonated autohemotherapy modulates the serum levels of inflammatory cytokines in gouty patients. Open Access Rheumatol. 2017;9:159–165. doi: 10.2147/OARRR.S119749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molinari F, Rimini D, Liboni W, Acharya UR, Franzini M, Pandolfi S, Ricevuti G, Vaiano F, Valdenassi L, Simonetti V. Cerebrovascular pattern improved by ozone autohemotherapy: an entropy-based study on multiple sclerosis patients. Med Biol Eng Comput. 2017;55:1163–1175. doi: 10.1007/s11517-016-1580-z. [DOI] [PubMed] [Google Scholar]
- 14.Bocci VA, Zanardi I, Travagli V. Ozone acting on human blood yields a hormetic dose-response relationship. J Transl Med. 2011;9:66. doi: 10.1186/1479-5876-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foglieni C, Fulgenzi A, Belloni D, Sciorati C, Ferrero E, Ferrero ME. Ozonated autohemotherapy: protection of kidneys from ischemia in rats subjected to unilateral nephrectomy. BMC Nephrol. 2011;12:61. doi: 10.1186/1471-2369-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bocci V, Zanardi I, Travagli V. Oxygen/ozone as a medical gas mixture. A critical evaluation of the various methods clarifies positive and negative aspects. Med Gas Res. 2011;1:6. doi: 10.1186/2045-9912-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Campos C, Lara-Padilla E, Bobadilla-Lugo RA, Kross RD, Villanueva C. Effects of exercise on oxidative stress in rats induced by ozone. ScientificWorldJournal. 2012;2012:135921. doi: 10.1100/2012/135921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sagai M, Bocci V. Mechanisms of Action Involved in Ozone Therapy: is healing induced via a mild oxidative stress? Med Gas Res. 2011;1:29. doi: 10.1186/2045-9912-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larini A, Bianchi L, Bocci V. The ozone tolerance: I) Enhancement of antioxidant enzymes is ozone dose-dependent in Jurkat cells. Free Radic Res. 2003;37:1163–1168. doi: 10.1080/10715760310001604170. [DOI] [PubMed] [Google Scholar]
- 20.Liao M, Liu Z, Bao J, Zhao Z, Hu J, Feng X, Feng R, Lu Q, Mei Z, Liu Y, Wu Q, Jing Z. A proteomic study of the aortic media in human thoracic aortic dissection: implication for oxidative stress. J Thorac Cardiovasc Surg. 2008;136:65–72. 72.e1–3. doi: 10.1016/j.jtcvs.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Molinero A, Narvaiza L, Ruiz J, Galvez-Barron C. Normal respiratory rate and peripheral blood oxygen saturation in the elderly population. J Am Geriatr Soc. 2013;61:2238–2240. doi: 10.1111/jgs.12580. [DOI] [PubMed] [Google Scholar]
- 22.Gradinaru D, Margina D, Borsa C, Ionescu C, Ilie M, Costache M, Dinischiotu A, Prada GI. Adiponectin: possible link between metabolic stress and oxidative stress in the elderly. Aging Clin Exp Res. 2017;29:621–629. doi: 10.1007/s40520-016-0629-z. [DOI] [PubMed] [Google Scholar]
- 23.Sano M, Anzai J. The molecular mechanisms contributing to the pathophysiology of systemic inflammatory response after acute aortic dissection. Nihon Rinsho Meneki Gakkai Kaishi. 2016;39:91–95. doi: 10.2177/jsci.39.91. [DOI] [PubMed] [Google Scholar]
- 24.Mathews JA, Krishnamoorthy N, Kasahara DI, Cho Y, Wurmbrand AP, Ribeiro L, Smith D, Umetsu D, Levy BD, Shore SA. IL-33 drives augmented responses to ozone in obese mice. Environ Health Perspect. 2017;125:246–253. doi: 10.1289/EHP272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie TY, Yan W, Lou J, Chen XY. Effect of ozone on vascular endothelial growth factor (VEGF) and related inflammatory cytokines in rats with diabetic retinopathy. Genet Mol Res. 2016:15. doi: 10.4238/gmr.15027558. [DOI] [PubMed] [Google Scholar]
- 26.Guan X, Li J, Gong M, Lan F, Zhang H. The hemostatic disturbance in patients with acute aortic dissection: a prospective observational study. Medicine (Baltimore) 2016;95:e4710. doi: 10.1097/MD.0000000000004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Lu L, Li J. Topological structures and membrane nanostructures of erythrocytes after splenectomy in hereditary spherocytosis patients via atomic force microscopy. Cell Biochem Biophys. 2016;74:365–371. doi: 10.1007/s12013-016-0755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girasole M, Pompeo G, Cricenti A, Congiu-Castellano A, Andreola F, Serafino A, Frazer BH, Boumis G, Amiconi G. Roughness of the plasma membrane as an independent morphological parameter to study RBCs: a quantitative atomic force microscopy investigation. Biochim Biophys Acta. 2007;1768:1268–1276. doi: 10.1016/j.bbamem.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Putter JS, Seghatchian J. Cumulative erythrocyte damage in blood storage and relevance to massive transfusions: selective insights into serial morphological and biochemical findings. Blood Transfus. 2017;15:348–356. doi: 10.2450/2017.0312-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gyawali P, Richards RS, Bwititi PT, Nwose EU. Association of abnormal erythrocyte morphology with oxidative stress and inflammation in metabolic syndrome. Blood Cells Mol Dis. 2015;54:360–363. doi: 10.1016/j.bcmd.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Goncharov EI, Pinaev GP. [The cytoskeletal proteins of erythrocytes] . Tsitologiia. 1988;30:5–18. [PubMed] [Google Scholar]