Abstract
The aim of this post-mortem ultrastructural and immunohistochemical study is to explore the characteristics of acute myocardial ischemia in the context of sudden death, using the combination of two different methods, both more insightful than ordinary histology. Transmission electron microscopy and immunohistochemistry, in addition to the traditional histology, were applied to study human heart specimens collected during forensic autopsies. The whole series was sub-grouped into cases (n=17) and controls (N=10). The control group consisted of unnatural death with a short agonal period (immediately lethal injuries). Heart samples of the two cohorts of subjects were prepared for electron microscopy. On the other hand, each specimen, formalin fixed and paraffin embedded, was stained with haematoxylin and eosin and immunoreacted with the following primary antibodies: anti-Fibronectin, anti- Connexin-43, anti-npCx43 (dephosphorylated form of Connexin43), anti-Zonula occludens-1. Immunopositivity for each marker in the myocardium was semi-quantitatively graded. Electron microscopy revealed a number of interesting differences, statistically significant, between acute myocardial ischemia and controls, regarding the morphology of nucleus, mitochondria and intercellular junctions. By immunohistochemistry, fibronectin was found to be increased in the extracellular matrix of the acute myocardial ischemia cases, with a statistically significant difference compared to the controls. Connexin 43 staining disclosed a slight increase (not statistically significant) in the cytoplasm of acute myocardial ischemia cases compared to the controls, whereas no significant differences were seen between cases and controls at intercellular junctions. npCx43 showed an evident difference of intensity and pattern (even though not statistically significant) in cases compared to controls and overall this difference was more evident in the cytoplasm. Zonula occludens 1, described as an important marker for functional modification of cardiac muscle fibers, resulted negative or very weak in the vast majority of both cases and controls. The present study attempts to simultaneously apply electron microscopy and immunohistochemistry, in order to figure out the morphological changes that might lead to pathological processes underlying the sudden, unexpected death due to acute myocardial ischemia, and consequently to find useful diagnostic markers of very early ischemic injury. Both methods showed significant differences between acute myocardial ischemia and controls, regarding, overall nuclei, mitochondria, and intercellular junctions.
Key words: Sudden death, early myocardial ischemia, ultrastructure, immunohistochemistry, Connexin 43
Introduction
Sudden cardiac death is a major health problem. Almost 85% of all the sudden deaths are due to cardiac causes.1 Moreover, it has been reported that in subjects over than 55 years old, 80% of sudden cardiac deaths are due to coronary heart disease. 1 The present study is focused on ischemic heart disease, that represents the most frequent cause of sudden cardiac death in industrialized countries.
In many cases of sudden cardiac death, the examination of the heart shows a number of chronic alterations (stenosing coronary atherosclerosis, fibrosis of the myocardium), consequent to previously occurred ischemic insults. Even though these findings can be suggestive of an ischemic aetiology of the sudden death, the only evidence of stenosing coronary atherosclerosis cannot allow the pathologist to determine the cause of death. In fact, in such cases the pathologist is not able, using the classic tools, to identify the effects of an acute myocardial damage. Therefore, often the medico-legal diagnosis is made per exclusionem. In other words, in presence of atherosclerotic coronary sclerosis determining stenosis of various entity, the death is often identified as ischemic, although the heart shows only chronic injuries and scars of previous ischemic episodes. Yet, such chronic alterations cannot be regarded as the cause of the death, considering that the subject, until very shortly before the event, had sufficient cardiac function. There is a lack of knowledge about the changes that occur shortly after the starting of the ischemia in the human cardiomyocytes that can quickly lead to death. In fact, in early ischemia (less than 4-6 hours) an actual myocardial damage detectable by ordinary histological methods2 is absent, because the ischemic lesion had not time enough to evolve. Sometimes, at light microscopy level, early myocardial ischemia (around 4-6 hours) is characterized by non-specific changes, such as “wavy fibers” and contraction bands, but these findings are well known to be present in many other conditions (electrocution, defibrillation, cocaine and amphetamine abuse, massive adrenaline release etc.) and therefore do not allow to attribute the cause of the death to an acute myocardial ischemia. The specific gross and microscopic picture of heart infarction appears after 6 to 12 hours, and it is the consequence of the myocardiocytes necrosis due to prolonged ischemia. In cardiac ischemic deaths, the typical macroscopic findings revealed at autopsy are focal coronary atheromas, remote or healing infarcts, or the absence of any gross abnormality. Thus, the careful examination of the coronary arteries is crucial.3
A severe stenosis caused by an atherosclerotic plaque or an occlusion by a thrombus, the age of which may range from early platelet-fibrin composition to organised, often vascularised tissue, may be observed.4
Coronary atherosclerosis is the underlying substrate for the majority of cases of ischemic heart disease. An acute coronary syndrome recognizes at least two mechanisms: it may be triggered by a disrupted atherosclerotic plaque with a superimposed thrombosis (with the rapid and complete occlusion of a coronary branch) or can be the result of a chronic critical stenosis that leads to an acute heart failure, often during a condition of increased demand of oxygen, such as physical exercise. In the first hypothesis, that is a atheromasic plaque, the simple endothelial thickening develops to involve the media and usually becomes infiltrated with lipids. Whilst the covering endothelium remains intact, the danger to life is confined to the luminal reduction from the bulge of the enlarging plaque.4 When the fibroendothelial cap begins to break down under the pressure and erosion of the central necrosing process, the plaque may rupture into the lumen. This has several consequences, which may precipitate acute symptoms or even death. Thus, mural thrombus may completely block or severely narrow the residual lumen, with all the consequences of reduced blood flow to the distal myocardium.5
The myocardial ischemia can lead the patient to death very quickly, before the appearance of the necrosis. In the majority of such cases, death is caused by acute ventricular arrhythmias, which represents the consequence of the ischemia and might be unrelated with specific structural anomalies (both macroscopic and microscopic) of the heart appreciable with common methods, due to the short time between the ischemic insult and the death.
Thus, the certain attribution of the cause of death to an acute myocardial ischemia (AMI) represents one of the most hard challenges faced by the pathologist, especially considering that such deaths often occur in a person previously thought to be healthy.6
The morbidity and death which result from AMI are due to mechanical and electrical complications that finally lead to ventricular tachyarrhythmia and sudden death. In animal models, the progression from reversible to irreversible ischemic injury has been proved to develop after 15 min (up to 30 min according to Jennings et al.7 in the most profoundly ischemic sub-endocardium of the left ventricle and then extends in a wave-front pattern from sub-endocardium to sub-epicardium throughout the ischemic bed-at-risk.8 The process of cell death remains reversible until the dying cell goes through the first irreversible step, or the “point-of-no-return”: that step represents the key concept that can allow to understand the physiopathology of sudden cardiac death due to AMI.
A lot of research has been performed in the context of cardiac diseases, in particular in heart failure, and possibly some of the latest findings may be applied to sudden cardiac death cases too. In particular, the main component of cardiac gap junctions (GJ), Connexin 43 (Cx43) and Zonula Occludens 1 (ZO-1) appears to be involved.9-14
The Cx43 is the main component of GJ, the structures that mediate electrical coupling between cardiomyocytes, responsible of the synchronous contraction of the heart.9 Focal disorganization of GJ distribution and down regulation of Cx43 at intercalary discs (ID) level are typical features of myocardial remodelling in failing heart due to various diseases,15 among which myocardial ischemia. Namely, in the normal heart, Cx43 is phosphorylated and localized at ID; stimuli such as ischemia, hypoxia and hypothermia induce de-phosphorylation and redistribution of npCx43 protein into the cytoplasm and/or lateral cell border of cardiomyocytes.16 Particularly, the electrical uncoupling induced by ischemia (and therefore the rhythm alteration responsible for sudden death) is related to alterations in phosphorylation of Cx43.17 In animal models, decreased Cx43 and increased npCx43 at ID were detected 15 min after the beginning of ischemia and became more evident with prolonged ischemia.17 So, the increased positivity of npCx43, at ID and at cytoplasm, in the ischemic hearts is likely to indicate the progressive de-phosphorylation of Cx43 and its redistribution from the cell junction to the cytoplasm due to the ischemic insult.
Regarding the ZO-1, it is known it binds and regulates the Cx43, having a mechanical function.18 So, it might be another marker of the junction disruption during very early ischemia. In literature there are only a few reports about this phenomenon in autoptic material,15,16 whereas it was extensively studied in animal models. 14,19-21 Although the mechanisms underlying the ischemic changes has been so deeply investigated, the diagnosis of AMI is still one of the most difficult for the pathologist. In fact, using conventional histochemical staining procedures, acute ischemic injury can only be detected 4-6 hours after the onset of ischemia.2
Therefore, the aim of the presents study is to observe the ultrastructure of ischemic cardiomyocytes, compared to controls, as well as the expression of a selected group of immunohistochemical markers such as, Fibronectin, Cx43, npCx43 and ZO-1. The purpose is to find useful markers of early myocardial ischemia, that can allow to detect the acute myocardial damage when it is so initial that it is not accompanied by lesions, observable through the traditional methods (light microscope), that can be regarded as specifically related to ischemia. This investigation, on the other hand, has the goal to go deeper into the physiopathology of the sudden death due to heart ischemia in human. Overall, this work is likely to provide results that can be important also from a clinical point of view, in terms of prevention and therapy of AMI.
Materials and Methods
Subjects and specimens
We retrospectively studied 27 heart specimens, selected among heart samples collected during forensic autopsies performed between 2012 and 2016. The subjects’ profiles are shown in Supplementary Table 1 and 2.
Table 1.
Scoring method chosen for the evaluation of immunohistochemistry.
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|---|
| Fibronectin (cytoplasm) | Negative | Weak, focal | Weak, diffuse | Strong, diffuse |
| CX43 (cytoplasm) | Negative | Scattered positivity | Patchy positivity | Diffuse positivity |
| CX43 (ID and along LCB) | Negative | Thin linear and intermittent positivity | Dense but intermittent positivity | Dense band-like positivity |
| NPCX43 (cytoplasm) | Negative | Scattered positivity | Patchy positivity | Diffuse positivity |
| CX43 (ID and along LCB) | Negative | Thin linear and intermittent positivity | Dense but intermittent positivity | Dense band-like positivity |
| ZO1 (cytoplasm) | Negative | Weak and patchy | Dense but patchy positivity | Diffuse positivity |
ID, intercalated discs; LCB, longitudinal cell membrane.
The cases of AMI were selected on the basis of anamnesis and circumstantial data, as well as the macroscopic and traditional microscopy observation. In each case, after having excluded the other possible causes, the pathologist had finally attributed the death to an AMI, even though the heart did not present any specific alteration that could demonstrate the acute ischemic injury. In most cases, chronic ischemic alteration could be detected (coronary stenosis, myocardiosclerosis).
The control group, chosen considering homogeneous characteristics in respect of the cases, consisted of unnatural deaths with a short agonal period (immediately lethal injuries). In each case the samples submitted for the study were obtained from the left ventricular wall or septum, according to the supply regions of the damaged coronaries (where coronary lesions were present. The ischemic area was identified according to the coronary lesions (described in Supplementary Table 1): the myocardium for investigation was sampled from the area perfused by the occluded or suboccluded areas, avoiding any regions of complete fibrosis. In each case, the coronaries and the myocardium were carefully examined both macroscopically and microscopically. As mentioned in Supplementary Table 1, only one subject showed thrombosis.
Altogether, 27 cases were examined, 8 females and 19 males, ranging from 39 to 82-year-old subjects. The whole series was sub-grouped as follows: AMI (N=17; 29.41% females) and controls (N=10; 30% females). The post-mortem intervals (PMI) varied from 11 to 195 hours. (mean PMI: 53.25 hs; SD: 47.33). The mean weight of the heart was 447.88 g (±96.56) in AMI group, compared to 326.1 g (±68.82) in controls.
The study has been performed, according to the Italian Laws, on archived biological samples which had been taken from cadavers during forensic autopsies and already examined for diagnostic purposes. The subjects of the study have been keeping anonymous and identified by a code.
Transmission electron microscopy
In order to perform TEM study, the heart samples conserved in formalin were retrieved. A 2 mm3 large fragment was taken according to the supply regions of the damaged coronaries. After a thorough washing with phosphate buffer, the samples were post-fixed in glutaraldehyde for two hours and then immersed in a solution composed of osmium tetroxide (2%) and iron cyanide (3%) for 2 h. Then, after another thorough washing with phosphate buffer, specimens were dehydrated in increasing concentration alcohol solutions, and embedded in epon-araldite mixture. Semithin section were cut and stained with toluidine blue; after the observation at light microscopy, to make sure the fibers are longitudinally oriented, the ultrathin sections were cut at 70 nm thickness, placed on Cu/Rh grids, stained with lead citrate and observed with Morgagni 268D electron microscope (Philips).
Immunohistochemistry
To carry out the histochemical and immunohistochemical investigation, paraffin blocks were retrieved from the archive of our Department. Four μm-thick sections were cut. Each section had been previously routinely stained with Hematoxylin-Eosin. Immunostaining was performed with the following primary antibodies: antifibronectin (rabbit monoclonal antibody, ab32419, diluted 1:200; Abcam, Cambridge, UK), anti-Cx43 (Abcam; rabbit monoclonal antibody, ab11370, diluted 1:2000), anti-npCx43 (Invitrogen, mouse monoclonal antibody, CX-1B1, diluted 1:50), anti-ZO-1 (Invitrogen, Carlsbad, CA, USA; mouse monoclonal antibody, ZO1-1A12, diluted 1:50). Tissue sections were blocked by 3% hydrogen peroxide for 12 min. Then, heat induced antigen retrieval was obtained. Following overnight incubation with the primary antibody at 4°C, immunoreactions were visualized using the avidin-biotin method, followed by color development with diaminobenzidine. Immunostaining of each marker in the myocardium were semi-quantitatively graded and the staining pattern of each marker was described as stated in Table 1, adapted from Kawamoto’s work.16 The evaluation was performed by two independent observers (SV and AF).
Statistical analysis
The summary statistics are expressed as means and standard deviations, medians and Interquartile range (IQR) or absolute frequencies and percentages, as appropriate. We used chi-square or Fisher’s exact test to analyse the association between ultrastructural findings, indicated as present or not. Non-parametric Mann-Witney test was performed to compare findings which were graded as quantitative discrete variables (immunohistochemistry features described in Table 1).
Results
Macroscopic observations and conventional microscopy
Regarding the controls, the myocardium did not show any alteration, neither macroscopically nor at light microscopy observation.
Among the AMI group, in 14 out of 17 subjects the coronary stenosis, together with chronic changes due to previous ischemic insults, were identified. The observation at light microscopy (H&E staining) of the 17 AMI cases showed non-specific alterations, such as contraction bands and cardiomyocyte fragmentation, that can be regarded as morphologic signs of cardiac distress, and can be seen in many other conditions (not shown). In 10 out 17 subjects some areas of coagulative necrosis were also observed.
Transmission electron microscopy
Firstly, it is noteworthy that the technique used in the present study, that consisted in retrieving specimens conserved in formalin for a long period of time and treating them as previously described (see Materials and Methods), proved to be efficient and to provide good-quality material for observation at TEM level. This is a remarkable result by itself, as it is widely believed that autoptic material is not suitable for this purpose and that a very accurate and immediate fixation of the specimens after death of the subject is absolutely necessary. Conversely, the present results suggest that, as long as the specimens are properly prepared, the ultrastructural evaluation of such samples can provide a great amount of information.
The ultrastructural TEM study was possible, overall, in 20 out of 27 cases, including 10 deaths due to AMI and 10 controls (deaths due to traumatic causes with a short agonic period), because the formalin fixed heart samples were available for this number of subjects. In fact, when an autopsy is performed, the samples to be embedded in paraffin for the ordinary histology are firstly taken. For each specimen, the exact site of sampling was registered. Then, other fragments of each organ are formalin fixed and stored (as judiciary trial records). Yet, the latter were not always extensive enough to make sure about the exact localization where the specimen was taken. Therefore, it was decided not to consider such cases for TEM examination and to limit the investigation to the specimens for which the precise site of sampling was well known.
The most remarkable differences concerning the ultrastructural characteristics detected between cases and controls regarded the morphology of mitochondria, the nuclei, the sarcolemmas and intercellular junctions. The following alteration were identified as the most significant and qualitatively assessed.
Mitochondria abnormalities
Four different grades of mitochondrial damage were observed (and compared between AMI and controls in Figure 2): swelling (observed in the totality of the specimens examined, regardless the cause of death; Figure 1A, black arrowhead); fragmentation or lysis of cristae (Figure 1A, white arrowheads; present in 4/10 controls compared to 9/10 AMI, P=0.019); the decrease of the matrix density (Figure 1A, asterisks; in 1/10 controls and 4/10 AMI) and finally the disruption of external membrane (figure not shown, in 2/10 controls and 5/10 AMI).
Figure 2.
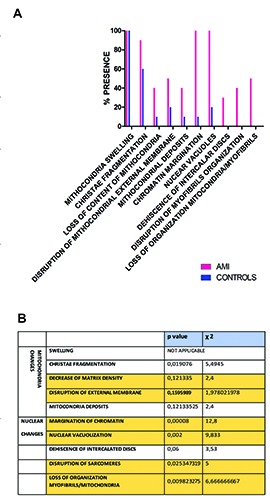
Graphical representation of qualitative and quantitative differences in the ultrastructural features described in AMI vs control. A) In the x axis, the ultrastructural changes considered are listed. For each of them, the number of case in which the characteristic is present is represented by the column (pink for cases and blue for controls). B) Results of the statistical analysis of the myocardiocyte pathologic conditions observed at electron microscope, described qualitatively as present or absent.
Figure 1.
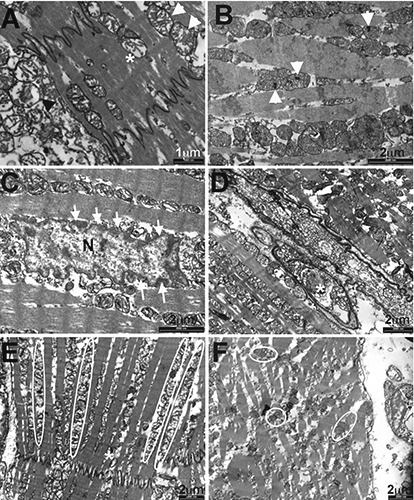
Ultrastructural features by TEM analysis of myocardiocytes of AMI vs control. A) Mitochondrial swelling, in some mitochondria (arrows) associated with cristae fragmentation; disruption of the external membrane together with loss of matrix density (subject 7). B) Mitochondrial deposits (arrows) associated with the widely represented mitochondrial swelling (subject n. 8). C) Margination of chromatin (subject n. 8). D) Nuclear vacuoles (subject n. 10). E) The normal organization of the myofibrils in respect of the mitochondria. F) The loss of organization of the myofibril in respect of the mitochondria. (Subject n. 5).
Electron-dense deposits (Figure 1B, arrowheads) within the matrix were detected in 4/10 AMI and 1/10 of the controls.
Nuclear changes
They represent one of the most striking results, considered that they seem not to be influenced by post-mortem autolysis. The normal myocardiocyte nucleus is bounded by a double membrane, nucleoplasm is homogeneously distributed, without any aggregate, as already reported.22 Nuclear changes mainly observed included margination of chromatin (Figure 1C, serial white arrows at the periphery of nucleus, N) and intranuclear vacuolization (Figure 1D, white asterisk). The rarefaction of nucleoplasm with chromatin margination was observed in 1/10 control compared to 9/10 AMI; similarly, nuclear vacuolization was present in 2/10 controls in respect of 9/10 AMI, showing a significant difference between the two groups (Figure 2, P<0.0001 and P=0.002, respectively).
Damage of the intercalated discs and sarcomers disruption
ID are structures resistant to the ischemic insult; therefore, the dehiscence of ID is a late sign of ischemia. Notwithstanding, also considering the immunohistochemistry (IHC) results illustrated later, we hypothesize that the ultrastructural observation of this site might be of interest. Yet, the oedematous separation or widening of ID, together with the loss of their integrity, was detected in only 3/10 cases and in none of the controls. It does not represent a statistically significant result (Figure 2; P=0,06). Conversely, the disruption of the sarcomeres (considered resistant) was appreciated in 4/10 cases and none of controls, and it was associated to a statistically significant difference between groups (Figure 2; P=0.025).
Organization of the relationship between mitochondria and myofibrils
Usually mitochondria are located throughout the heart muscle fibres (Figure 1E, mitochondria in regular pattern circled by white lines), about the nucleus at either pole, in long chain between bundles of myofilaments).9 The loss of this organization was seen in 4/10 AMI (Figure 1F, white circle pointing randomly organized mitochondria) and in none of the x controls (Figure 2; P=0.0098).
In sum, on the basis of the results described above, it is possible to state that the most meaningful connection was observed between acute myocardial ischemia and nuclear changes (which were present in the totality of the AMI compared to none or few of the controls) and the loss of organization between mitochondria and myofibrils (present in the 50% of AMI compared to none of the controls). The mitochondrial changes (except the cristae fragmentation) appeared to be more dependent on the post-mortem autolysis, and therefore not significantly different between the two groups (Figure 1A). The quantification of these abnormalities and qualitative results are summarized in Figure 1 A,B.
The relationship between the ultrastructural alterations observed and the postmortem interval and the resuscitation was also investigated. A significant association was found only between sarcomers disruption and resuscitation. In particular, 3 out 16 subjects who did not show the sarcomers disruption underwent the resuscitation, whereas 3 out 4 subjects that showed sarcomers disruption underwent the resuscitation. Yet, we do not think that this isolated result is meaningful by itself in such a small number of subjects.
Immunohistochemistry
Fibronectin
In almost the totality of the controls fibronectin staining was graded as 1 (based on elements reported in Table 1; representative image in Figure 3A). Conversely, in the AMI group, in 6 out of 17 cases, fibronectin showed a weak and diffuse pattern (grade 2; not shown); 6 cases were evaluated as a grade 3 (strong and diffuse pattern, representative image Figure 3B); 5 showed a weak diffuse pattern (grade 1; not shown), whereas none of them were negative. Collectively, the differences between the two groups evaluated on the basis of the staining pattern resulted statistically significant (Table 2; P=0.019).
Figure 3.
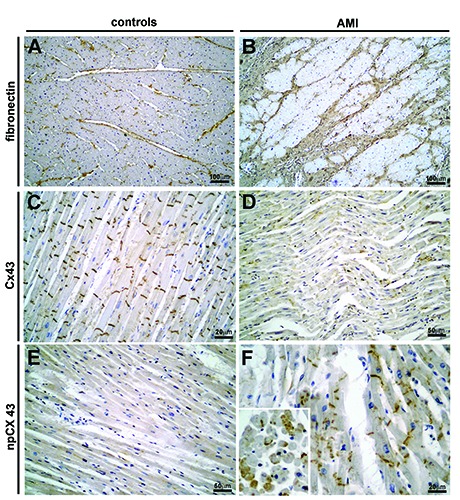
Immunohistochemistry performed against several potential clinical markers on control and AMI. A) Subject n. 27; fibronectin staining was graded as 1. B) Subject n. 13; fibronectin staining was graded as 3. C) Subject n. 22 (control); Cx43 resulted positive (grade 2) at ID, whereas it was negative in the cytoplasm. D) Subject n. 12; Cx43 resulted weakly positive (grade 1) at ID, whereas it was appreciably positive in the cytoplasm (grade 1). E) Subject n. 26 (control); npCx43 staining resulted weakly positive in the ID (grade 1), negative in the cytoplasm. F) Subject n. 11; npCx43 staining resulted strongly positive in the cytoplasm (grade 3).
Connexin 43 and npCx43
In intercalated discs, the physiological localisation of Cx43 showed a slightly higher positive staining in the controls (representative image in Figure 3C) compared to the heart ischemia cases, (more cases graded as 2 or 3, representative image in Figure 3D). Besides, in the cytoplasm the positivity was higher in the group of acute ischemic heart disease compared to controls (not shown). On the other hand, npCx43 was positive in a higher number of AMI (representative image in Figure 3F) in respect of the controls (Figure 3E); this difference was more marked in the cytoplasm of myocardial cells, as expected (representative image in the inset of Figure 3F showing cross section of muscle fibres of AMI). The potential difference in the staining pattern did not give significant results (Table 2), even though a trend has been observed for npCx43 in the cytoplasm (Table 2; P=0.0731).
Table 2.
P-value estimation from the Mann-Whitney U test.
| AMI Median (IQR) | Controls Median (IQR) | P-value | |
|---|---|---|---|
| Fibronectin | 2(1-3) | 1(1-2) | 0.019 |
| Connexin (Cx43) ID localisation | 2(1-2) | 2(1-3) | 0.451 |
| cytoplasmatic | 0(0-1) | 0(0-2) | 0.990 |
| Connexin (npCx43) ID localisation | 1(1-2) | 1(0-1) | 0.138 |
| cytoplasmatic | 1(0-2) | 0(0-1) | 0.073 |
| ZO-1 | 0(0-1) | 0(0-0) | 0.093 |
AMI, acute myocardial ischemia; ID, intercalated discs.
ZO-1
Overall, ZO-1 did not show any significant difference in the intensity and in the pattern of staining between cases and controls. Moreover, the positivity, in both groups, even though undoubtedly present at the ID, was weak, graded mostly as grade 1 (representative images for both groups Figure 4 and Table 2).
Figure 4.
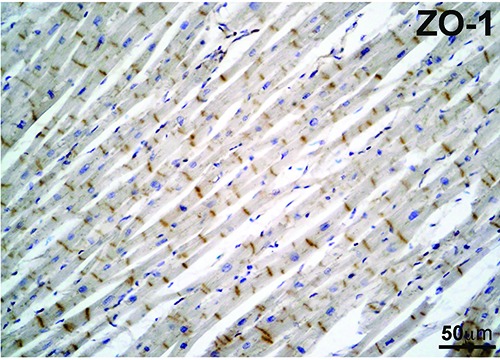
Representative image of ZO- 1staining in an AMI subject. ZO-1 did not showed any significant difference neither in the intensity nor in the pattern of staining between control and AMI subjects. The longitudinal section of cardiac muscle in the image was obtained from an AMI subject.
The relationship of the immunohistochemical features with the post-mortem interval and the resuscitation was also investigated. A significant difference was found only comparing ID Cx43 positivity levels in 7 subjects late post-mortem interval with respect to 7/20 subjects with early post-mortem interval (P=0.0037).
Based on these evidences it is, in our opinion, possible to state that neither the post-mortem interval nor the resuscitation are confounding factors in the expression of the immunohistochemical markers here considered.
Discussion
This study attempts to simultaneously apply, in combination to light microscopy, either TEM and IHC and compare and combine the findings, in order to better understand the changes in morphology that occur early in cardiac muscle and might be at the basis of the physiopathology of early myocardial ischemia. Moreover, another potential important aim is to identify useful markers for the post-mortem diagnosis for early onset of AMI.
In view of the previous studies on animal models,22-24 it was felt that structural alterations, not observable using the light microscope, might be demonstrated using TEM, as it provides a much deeper insight into the morphology of cells and tissues.
Firstly, it must be said that any consideration about ultrastructure made on autoptic material must take into account the factor of post-mortem autolysis. A number of autolytic changes mimic early ischaemic changes quite closely and include loss of glycogen, swelling of mitochondria, elongation of sarcomeres, clumping of nuclear chromatin and the appearance of lipid droplets in the sarcoplasm.25
Such autolytic alterations have been extensively studied. Interestingly, it was seen that, despite the autolytic changes evolve rapidly in about the first 2-3 h, they show the tendency not to evolve substantially in the following hours.26,27 Moreover, the present study strongly suggests that these changes still permit worthwhile ultrastructural observations to be made in postmortem samples of human myocardium, in contrast with previous reports, which stated that autolytic changes hinder a reliable morphological assessment of very early ischaemic injury at TEM level.25 The hypothetical association between post-mortem interval and the ultrastructural changes were deeply investigated; no significant relationship was seen, suggesting that the post-mortem interval do not interfere with the ultrastructural features that appeared to be significantly related to early ischemia. In order to reduce the number of potential confounding factors, the choice of the controls was particularly accurate, all of them consisting in extremely rapid traumatic deaths in which such changes had no time to develop. Agonal period, in fact, might be a confounding factor when it is necessary to evaluate the exact evolution of autolytic changes.
Concerning the ischemia-induced changes, the most striking results regard the nuclear abnormalities, that consist in chromatin margination and vacuolization. These characteristics were observed in almost the totality of AMI subjects and in none of the controls, in which the chromatin showed a homogenous distribution. Other findings, especially the disruption of sarcomers and the loss of organization between mitochondria and myofibrils, even though not statistically significant, seem to be more advanced signs of myocardial damage, as they were present in a few AMIs (without a clear tendency) but in none of the controls. Also, the mitochondria showed interesting features, even though they are more vulnerable to autolytic changes, first swelling, which was observed in the totality of the subjects. More advanced abnormalities, like the decrease of matrix density and the disruption of the external membrane, were detected in very few AMI and, respectively, in 2 and 1 control. Therefore, it is possible to state that the mitochondrial alteration are, generally, not of great relevance in the diagnosis of the early ischemia, because, consistently with what had been previously pointed out by literature,26 they are particularly susceptible to the post-mortem changes. The only exception regarded the fragmentation of cristae, which was observed in almost all the AMIs and in the majority of controls and showed a statistically significant difference. Yet, due to the relatively small group of subjects, this finding requests further investigations.
The presence of deposits within the mitochondrial matrix has been reported in literature to be significantly higher in AMI compared to controls,28-30 due to the role of mitochondria as intracellular buffer to eliminate the increased calcium content in the sarcoplasm.31 Interestingly, the presence of mitochondrial deposits did not show a significant difference in the present cohort of subjects, being present in only 4 out of 10 AMI cases and in 1 control. This finding do not confirm the data reported in the literature regarding ultrastructural changes observed in AMI.29,30
A mandatory addition to the present discussion should include possible weaknesses of the application of this method to the forensic practice. The use of qualitative or semi-quantitative method to evaluate the ultrastructural severity of diseased myocardium requires a high level of interpretative skill and special knowledge by the observer. Variations due to the subjective nature of the interpretative process make it difficult to obtain an objective estimate of the severity of myocardial lesions. For the assessment and for reproducibility, the assessment of the semi quantitative data or descriptive parameters by two independent observers is recommended.
Moreover, EM (a consolidated technique for morphologic examination) is not easy to introduce in forensic practice, especially considering that it is time-consuming and expensive, especially in terms of human resources; the results are much difficult to evaluate, giving the wide range of alterations and the various grades they can show. Yet, as it was demonstrated by the present work, it provides a deep insight into the morphology of cardiomyocytes and we believe that its role in forensic pathology (which was neglected so far) has to be upgraded.
Regarding IHC, the present study pointed out interesting findings about all the markers tested. Firstly, it showed a significant difference of grading for fibronectin between cases and controls. Interestingly, the marker did not stain the cytoplasm in any case, in contrast to what reported in literature. 32 Even though expression of fibronectin showed an increase due to the ischemia in comparison to controls, as widely known from previous studies, the results obtained suggest that it is not specific enough to be used as a marker of early myocardial ischemia for forensic purposes. In fact, it is undeniable that the staining was positive, even if weak, in controls that are healthy subjects dead from traumatic causes. In addition, this study pointed out, according to the literature,33,34 that the immunostaining for fibronectin is influenced by too many factors for being considered a reliable marker of early myocardial ischemia. Among the mentioned confounding factors, conditions of global hypoxia/ischemia, that influence the expression of fibronectin, can occur in may causes of death often encountered in the forensic practice, for example asphyxia. It is crucial being able to differentiate between a sudden death due to ischemic causes from violent deaths, for example drowning.
The immunostaining for the two forms of Cx43 (phosphorylated and non-phosphorylated) provided the most interesting results, showing, in AMI, a decrease of the phosphorylated form in ID and an increase of the non-phosphorylated form in the ID. To note, this difference of pattern distribution was more evident in the cytoplasm.
Collectively, the present study, using human autoptic heart samples, shows that the de-phosphorylation of Cx43 and its redistribution from ID to the cytoplasm can be detected, using IHC, in most of the examined cases of sudden cardiac death. These results indicate that the remodelling of ventricular GJ, that corresponds with the dephosphorylation of Cx43 and its dislocation from the ID to the lateral membrane and the cytoplasm of myocardiocytes, represents a key factor in myocardial heart ischemia, contributing the terminal arrhythmia responsible for sudden death. This phenomenon seems to be a very quick reaction to the ischemic insults; therefore, it may be used to diagnose early myocardial ischemia when it is too early to be detected with common methods. Concerning the ZO-1, this investigation pointed out a weak staining for ZO-1, either in AMI and the controls. Given the absence of any significant difference between the two groups, it is likely that the downregulation of the ZO-1, that is indicated in literature as another early consequence of ischemia,16,18 can be influenced from other factors (maybe post-mortem phenomena). This evidence suggests the need of further investigation.
Finally, it must be stated that all of the markers of AMI here investigated, despite the interesting findings about their distribution, have the disadvantage that they are positive also in the controls and, therefore, the grade of positivity and its location are conclusive for the outcome of the analysis. So, a correct scoring method is crucial. It must be taken into account that many factors, such as technical issues (fixation, postmortem interval) could potentially alter the results, and therefore a strict standardization of the methods used is absolutely necessary.
In conclusion, the present study using human autoptic material suggests that the ultrastructural observation with TEM, combined and integrated with IHC against the proteins that compose the GJ, can provide a complete overview of the structural changes due to early myocardial ischemia in order to achieve the diagnosis in samples devoid of any pathological alteration detectable with common methods (gross examination and light microscopy). In particular, nuclear abnormalities seem to be the most specific marker regarding ultrastructure, whereas Cx43, as well as npCx43, provided the most significant immunohistochemical results. The integration of the two methods provides useful information about the physiopathology of early myocardial ischemia that may be relevant from a clinical point of view.
References
- 1.Campuzano O, Allegue C, Partemi S, Iglesias A, Oliva A, Brugada R. Negative autopsy and sudden cardiac death. Int J Legal Med 2014;128:599-606. [DOI] [PubMed] [Google Scholar]
- 2.Ortmann C, Pfeiffer H, Brinkmann B. A comparative study on the immunohistochemical detection of early myocardial damage. Int J Legal Med 2000; 13:215-20. [DOI] [PubMed] [Google Scholar]
- 3.Basso C, Aguilera B, Banner J, Cohle S, d’Amati G, de Gouveia RH, et al. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch 2017;471:691-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thim T, Hagensen MK, Bentzon JF, Falk E. From vulnerable plaque to atherothrombosis. J Intern Med 2008; 263:506-16. [DOI] [PubMed] [Google Scholar]
- 5.Virmani R, Burke AP, Farb A. Sudden cardiac death. Cardiovasc Pathol 2001;10:211-8. [DOI] [PubMed] [Google Scholar]
- 6.Cohle SD, Sampson BA. The negative autopsy: Sudden cardiac death or other? Cardiovasc Pathol 2001;10:219-22. [DOI] [PubMed] [Google Scholar]
- 7.Herdson PB, Kaltenbach JP, Jennings RB. Fine structural and biochemical changes in dog myocardium during autolysis. Am J Pathol 1969;57:539-57. [PMC free article] [PubMed] [Google Scholar]
- 8.Reimer KA, Ideker RE. Myocardial ischemia and infarction: Anatomic and biochemical substrates for ischemic cell death and ventricular arrhythmias. Hum Pathol. 2017;18:462-75. [DOI] [PubMed] [Google Scholar]
- 9.Michela P, Velia V, Aldo P, Ada P., Pecoraro M, Verrilli V, Pinto A, Popolo A. Role of connexin 43 in cardiovascular diseases. Eur J Pharmacol 2015; 768:71-6. [DOI] [PubMed] [Google Scholar]
- 10.Goodenough D, Goliger J, Paul DL. Connexins, connexons, and Intercellular Communication. Annu Rev Biochem 1996;65:475-502. [DOI] [PubMed] [Google Scholar]
- 11.Barker RJ, Price RL, Gourdie RG. Increased association of ZO-1 With connexin43 during remodeling of cardiac gap junctions. Circ Res 2002; 90:317-24. [DOI] [PubMed] [Google Scholar]
- 12.Hesketh GG, Shah MH, Halperin VL, Cooke CA, Akar FG, Yen TE, et al. Ultrastructure and regulation of lateralized connexin43 in the failing heart. Circ Res 2010;106:1153-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dupont E, Matsushita T, Kaba RA, Vozzi C, Coppen SR, Khan N, et al. Altered connexin expression in human congestive heart failure. J Mol Cell Cardiol 2001;33:359-71. [DOI] [PubMed] [Google Scholar]
- 14.Bruce AF, Rothery S, Dupont E, Severs NJ. Gap junction remodelling in human heart failure is associated with increased interaction of connexin43 with ZO-1. Cardiovasc Res 2008;77:757-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue Y, Zhao R, Du S-H, Zhao D, Li DR, Xu J-T, et al. Decreased mRNA levels of cardiac Cx43 and ZO1 in sudden cardiac death related to coronary atherosclerosis: a pilot study. Int J Legal Med 2016;130:915-22. [DOI] [PubMed] [Google Scholar]
- 16.Kawamoto O, Michiue T, Ishikawa T, Maeda H. Immunohistochemistry of connexin43 and zonula occludens-1 in the myocardium as markers of early ischemia in autopsy material. Histol Histopathol 2014;29:767-75. [DOI] [PubMed] [Google Scholar]
- 17.Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, et al. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res 2000;87:656-62. [DOI] [PubMed] [Google Scholar]
- 18.Palatinus JA, Quinn MPO, Barker RJ, Harris BS, Jourdan J, Gourdie RG, et al. ZO-1 determines adherens and gap junction localization at intercalated disks. Am J Physiol Heart Circ Physiol 2011;300:H583-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beardslee MA, Laing JG, Beyer EC, Saffitz JE. Rapid Turnover of connexin43 in the adult rat heart. Circ Res 1998;83:629-35. [DOI] [PubMed] [Google Scholar]
- 20.Turner MS, Haywood GA, Andreka P, You L, Martin PE, Howard W, et al. Reversible connexin 43 dephosphorylation during hypoxia and reoxygenation is linked to cellular ATP levels. Circ Res 2004;95:726-33. [DOI] [PubMed] [Google Scholar]
- 21.Matsushita S, Kurihara H, Watanabe M, Okada T, Sakai T, Amano A. Alterations of Phosphorylation state of connexin 43 during hypoxia and reoxygenation are associated with cardiac function. J Histochem Cytochem 2006;54:343-53. [DOI] [PubMed] [Google Scholar]
- 22.Caulfield J, Klionsky B. Myocardial ischemia and early infarction; an electron microscopic study. Am J Pathol 1959;35:489-523. [PMC free article] [PubMed] [Google Scholar]
- 23.Hegstad AC, Ytrehus K, Lindal S, Jørgensen L. Ultrastructural alterations during the critical phase of reperfusion: A stereological study in bufferperfused isolated rat hearts. Cardiovasc Pathol 1999;8:279-89. [DOI] [PubMed] [Google Scholar]
- 24.Spinale FG, Schulte B a, Crawford F a. Demonstration of early ischemic injury in porcine right ventricular myocardium. Am J Pathol 1989;134:693-704. [PMC free article] [PubMed] [Google Scholar]
- 25.Heggtveit HA. Contributions of electron microscopy to the study of myocardial ischaemia. Bull World Health Organ 1969;41:865-72. [PMC free article] [PubMed] [Google Scholar]
- 26.Muñoz DR, Almeida M De, Lopes EA, Iwamura ESM. Potential definition of the time of death from autolytic myocardial cells: A morphometric study. Forensic Sci Int 1999;104:81-9. [DOI] [PubMed] [Google Scholar]
- 27.Hibbs RG, Ferrans VJ, Black WC, Weilbaecher DG, Burch GE. Alcoholic cardiomyopathy; An electron microscopic study. Am Heart J 1965;69:766-79. [DOI] [PubMed] [Google Scholar]
- 28.Begieneman MP V, van de Goot FRW, Fritz J, Rozendaal R, Krijnen PAJ, Niessen HWM. Validation of ultrastructural analysis of mitochondrial deposits in cardiomyocytes as a method of detecting early acute myocardial infarction in humans. J Forensic Sci 2010; 55:988-92. [DOI] [PubMed] [Google Scholar]
- 29.Buja LM, Dees JH, Harling DF, Willerson JT. Analytical electron microscopic study of mitochondrial inclusions in canine myocardial infarcts. J Histochem Cytochem 1976; 24:508-16. [DOI] [PubMed] [Google Scholar]
- 30.Jennings RB, Shen AC, Hill ML, Ganote CE, Herdson PB. Mitochondrial matrix densities in myocardial ischemia and autolysis. Exp Mol Pathol 1978;29:55-65. [DOI] [PubMed] [Google Scholar]
- 31.Somogyi E, Balogh I, Sótonyi P, Kerényi NA. Comparative electronmicroscopic investigation of postmortem human heart muscle biopsy. Am J Forensic Med Pathol 1983;4:7-13. [DOI] [PubMed] [Google Scholar]
- 32.Hu B, Chen Y, Zhu J. Immuno - histochemical study of fibronectin for postmortem diagnosis of early myocardial infarction. Forensic Sci Int 1996; 78:209-17. [DOI] [PubMed] [Google Scholar]
- 33.Fracasso T, Pfeiffer H, Köhler H, Wieseler S, Hansen SD, Jentgens L, et al. Immunohistochemical expression of fibronectin and C5b-9 in the myocardium in cases of fatal ethanol intoxication. Int J Legal Med 2011; 125:537-42. [DOI] [PubMed] [Google Scholar]
- 34.Fracasso T, Pfeiffer H, Michaud K, Köhler H, Sauerland C, Schmeling A. Immunohistochemical expression of fibronectin and C5b-9 in the myocardium in cases of carbon monoxide poisoning. Int J Legal Med 2011;125: 377-84. [DOI] [PubMed] [Google Scholar]


