Abstract
Objectives
Direct-acting antiviral (DAA) therapy for hepatitis C virus (HCV) has excellent cure rates and minimal side effects. Despite the high burden of disease, strategies to ultimately eradicate HCV are being developed. However, the delivery of care in regional settings is challenging and the efficacy of decentralised models of care is incompletely defined.
Methods
A prospective cohort study of patients whose treatment was initiated or supervised by Cairns Hospital, a tertiary hospital which provides services to a culturally diverse population across a 380,748 km2 area in regional Australia. Patients' demographics, clinical features, DAA regimens and outcomes were recorded and correlated with their ensuing clinical course.
Results
Over 22 months, 734 patients were prescribed DAA therapy for HCV. No patients were prescribed interferon. Sofosbuvir/ledipasvir (n=371, 50.5%) and sofosbuvir/daclatasvir (n=287, 39.1%) were the most commonly prescribed regimens. No patients ceased treatment due to adverse effects. There were 612/734 (83.4%) patients with complete results, with 575 (94%) cured. At the end of the study period, there were 50 (6.8%) patients lost to follow-up and 72 (9.8%) awaiting SVR12 testing. The presence of cirrhosis (n=147/612, 24.1%) did not impact significantly on SVR12 rates, this being achieved in 136/147 (92.5%) cirrhotic patients versus 440/465 (94.6%) in non-cirrhotic patients (p=0.34). Treatment-experienced patients (95/612, 18.3%) were more likely to be non-responders than treatment-naïve patients (10/95 (10.5%) versus 26/517 (5%), p=0.04). Strategies to facilitate treatment included a dedicated clinical nurse consultant, education to primary health care providers, specialist outreach clinics to regional communities and shared care with general practitioners. SVR12 rates were similar amongst gastroenterologists (283/306, 92.5%), general practitioners (152/161, 94.4%), sexual health physicians (104/106, 98.1%) and other prescribers (37/39, 94.9%).
Conclusions
This study confirms that decentralised, multidisciplinary models of care can provide HCV treatment in regional and remote settings with excellent outcomes.
Keywords: hepatitis C, direct-acting antiviral therapy, regional Australia, model of care, service delivery
Introduction
Chronic hepatitis C virus infection (HCV) is a leading cause of chronic liver disease and the commonest indication for liver transplantation in Australia [1]. In 2015, there were 800 deaths attributable to the disease in Australia, and it was estimated that 227,000 people were living with HCV; approximately 17,000 had cirrhosis [2].
In Queensland, Australia's second-largest state, half the state's population reside in regional areas and have limited access to both primary and specialist care [3,4]. To eradicate HCV, health services must overcome the challenges of delivering care to these regional and remote areas, as this has previously limited HCV treatment uptake [5].
The safety and efficacy of direct-acting antivirals (DAAs) has heralded a new era of HCV treatment [6]. DAAs were subsidised on the Pharmaceutical Benefit Scheme from March 2016, facilitating access. Further barriers to treatment were removed when the Pharmaceutical Benefits Advisory Committee recommended that DAAs could be prescribed by any medical practitioner experienced in treatment of HCV, or working in consultation with an appropriate specialist or nurse practitioner [7]. This had the potential to improve access to HCV treatment in regional and remote centres significantly.
This study describes a decentralised model of DAA treatment delivery to a culturally diverse population in a regional Australian population with limited access to specialist care, and the associated treatment outcomes.
Methods
This is a prospective cohort study of patients in regional Australia with HCV infection and receiving treatment with DAAs between 1 February 2016 and 31 December 2017. Patients were identified by referral to, or discussion with, the Cairns and Hinterland Hospital and Health Service located in Far North Queensland, Australia. Far North Queensland has a geographic area of 380,748 km2, and a population of approximately 280,000, 9% of whom identify as Aboriginal and/or Torres Strait Islanders [8].
Patient demographics (age, gender, indigenous status) were collected to accurately describe the population characteristics. Clinical data (HCV viral load, HCV genotype, presence of cirrhosis and prior HCV treatment) were collected to analyse outcomes and variables influencing differences in outcomes. The presence of cirrhosis was determined by ultrasound or transient elastography (defined as a value kPa ≥12.5).
Data were entered into an electronic database (Microsoft Excel, Washington, USA) and analysed using statistical software (SPSS, IBM Software, New York, USA). Differences between groups were analysed using the chi-squared test.
Ethical approval for the study was provided by the Human Research Ethics Committee of St Vincent's Hospital, Sydney. As the collected data were retrospective, aggregated and de-identified, a requirement for patient consent was waived. The study received no dedicated funding.
Model of care
Referrals were triaged by staff from the liver clinic, the sexual health service or the addiction medicine service.
Patients with decompensated cirrhosis, prior treatment, significant comorbidities or complex drug–drug interactions were seen in the liver clinic. The sexual health and addiction medicine services and general practitioners cared for their patients with uncomplicated disease. The provision of treatment in regional settings was facilitated through specialist visits to remote communities and the delivery of DAAs to these areas by outreach pharmacy services. A clinical nurse and sexual health physician visited the local prison regularly to educate health workers and to assess and treat patients [9]. Patient care was facilitated by having dedicated clinical nurses or other staff collating required information or performing transient elastography prior to the patient seeing a prescriber.
General practitioners' referrals of uncomplicated cases were managed by providing the referring clinician with a standardised document that provided guidance regarding the recommended treatment regimen and monitoring. Additional phone or email support was provided as required. Sessions to promote awareness and provide education on hepatitis C treatment were provided to primary health providers in an effort to increase the number of potential prescribers. These education sessions were facilitated by local gastroenterologists, general and sexual health physicians, and supported by the Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine (ASHM), as well as pharmaceutical companies.
Promoting awareness has been a key priority in reaching and treating patients with HCV. The Cairns Hospital Sexual Health Service launched a campaign ‘Cairns Hep C Free by 2020’ and has a local volunteer group called ‘CHAT’ (Cairns Hepatitis Action Team) that promotes awareness of hepatitis C treatment in the community. The campaign is staffed by two health promotion officers who visit and provide education and promote awareness to general practice clinics, needle exchange centres, addiction medicine support groups and homeless shelters. Public awareness has been further increased by advertising on highway billboards, on public buses and in local newspapers.
Results
Over the 22-month study period, 734 consecutive patients were prescribed DAAs for HCV.
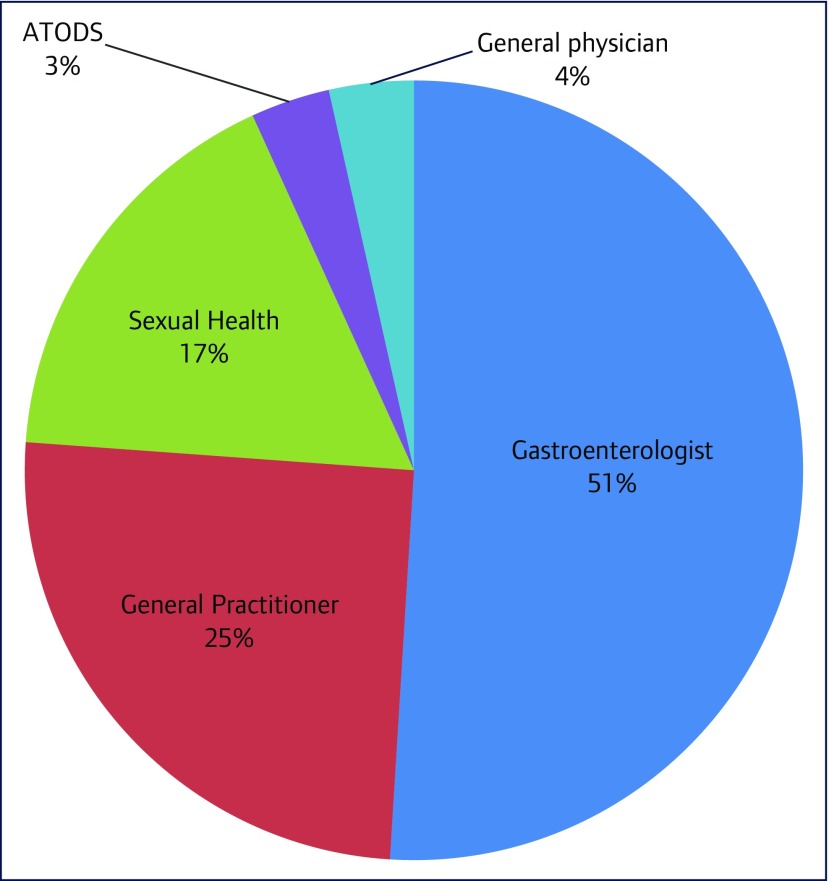
Approximately half (49%) of the cohort had treatment prescribed by general practitioners and non-gastroenterologist specialists (Figure 1).
Figure 1.
Distribution of prescribers. ATODS: Alcohol, Tobacco and Other Dugs Service
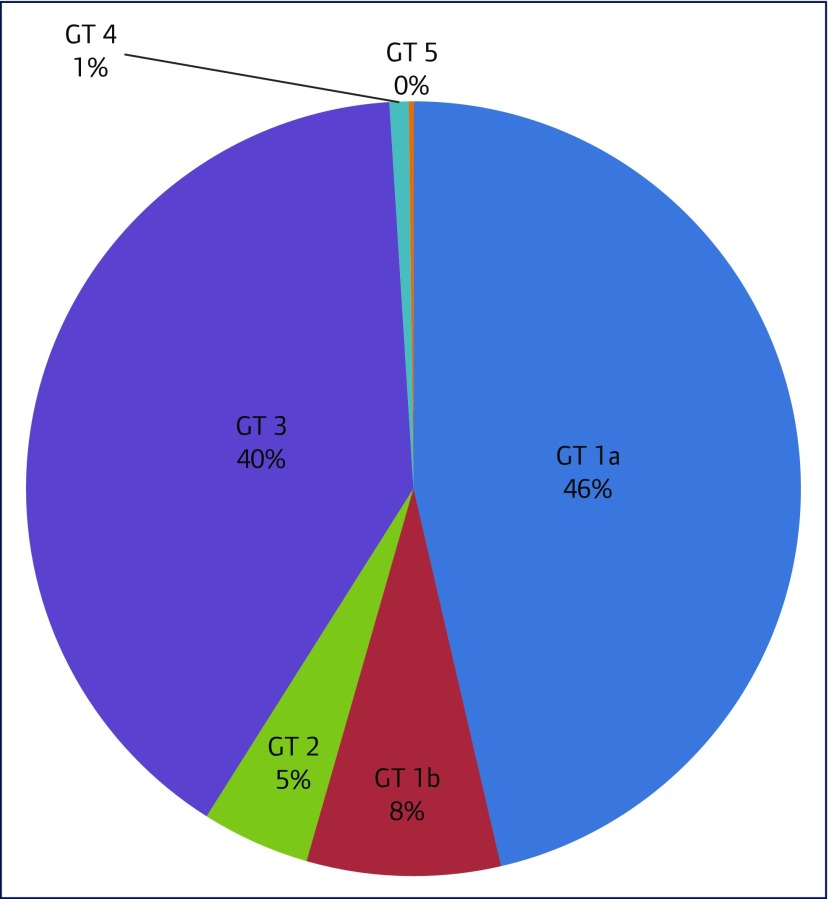
The most common genotypes were genotype 1a (340/734, 46.3%), genotype 3 (294/734, 40.1%) and genotype 1b (59/734, 8%) (Figure 2)
Figure 2.
Distribution of genotype. GT: genotype
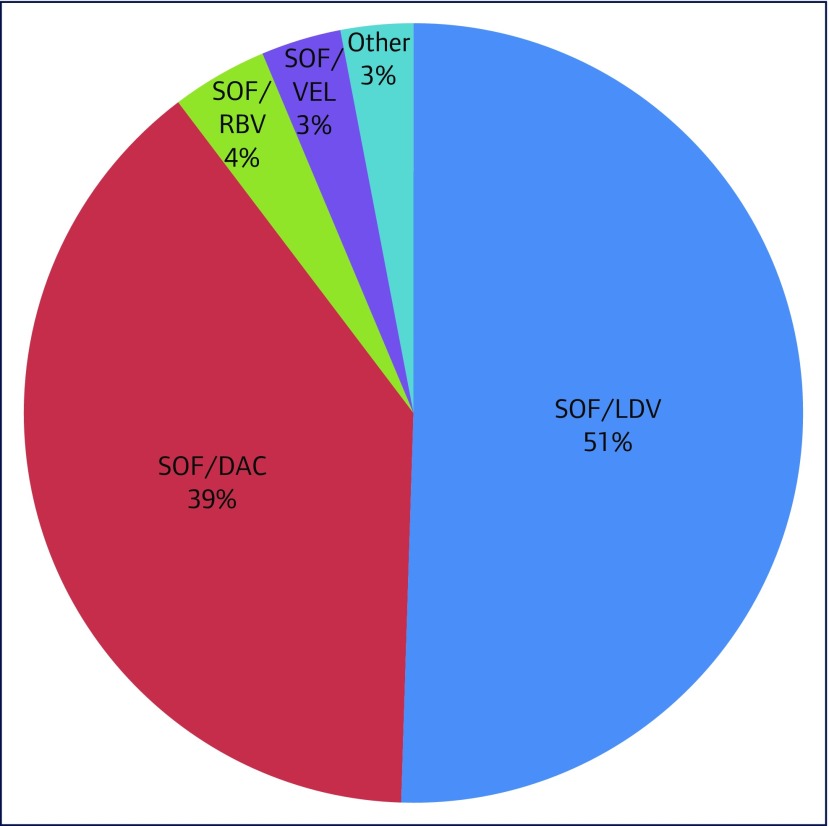
The most frequently prescribed DAA regimens were sofosbuvir/ledipasvir (371/734, 50.5%), sofosbuvir/daclatasvir (287/734, 39.1%) and sofosbuvir/ribavirin (29/734, 4.0%) (Figure 3). No patients were prescribed interferon. Other treatment regimens included sofosbuvir/velpatasvir, elbasvir/grazoprevir, paritaprevir/ritonavir/ombitasvir/dasabuvir, glecaprevir/pibrentasvir and sofosbuvir/simeprevir. No patients ceased treatment due to adverse effects.
Figure 3.
Direct-acting antiviral regimen prescriptions. SOF/LDV: sofosbuvir/ledipasvir; SOF/DAC: sofosbuvir/daclatasvir; SOF/RBV: sofosbuvir/ribavirin; SOF/VEL: sofosbuvir/velpatasvir
The cohort was predominantly male (507/734, 69.1%) and the mean age was 51.3 (range 19–77) years. Thirty-nine patients (5.3%) identified as Aboriginal or Torres Strait Islander, while 1.6% (12/734) of the cohort did not provide information on indigenous status. Seventy-six patients (10.4%) were participating in an opioid substitution programme and 34 (4.6%) patients had used injectable drugs within the preceding 6 months; six (0.8%) patients had HIV co-infection.
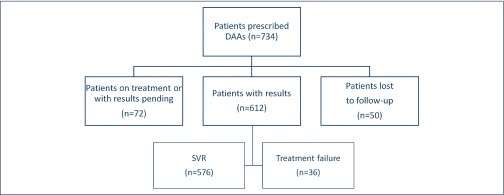
SVR12 results were available for 612 (83.4%) patients. These patients had an SVR12 rate of 94%. Fifty patients (6.8%) were lost to follow-up and 72 (9.8%) patients were awaiting SVR12 testing at the time of the study's completion (Figure 4).
Figure 4.
Patient prescriptions and outcomes. DAAs: direct-acting antivirals; SVR: sustained virological response at 12 weeks
Presence of cirrhosis (147/612, 24.1%) did not independently impact significantly on SVR12 rates (136/147 [92.5%] vs 440/465 [94.6%], p=0.34). Most cirrhotic patients were Child–Pugh A (92.1%) rather than Child–Pugh B (7.3%) or Child–Pugh C (0.6%).
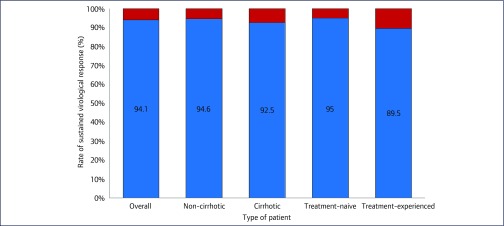
Treatment-experienced patients (95/612, 18.3% of the cohort) were more likely to be non-responders compared to treatment-naïve patients (10/95 [10.5%] vs 26/517 [5%], p=0.04) (Figure 5). The treatment-experienced patients included four patients who had failed DAA therapy (4/95, 4.2%); three (75%) of these four patients were non-responders to second-line DAA therapy.
Figure 5.
Rates (%) of sustained virological response at 12 weeks
Treatment outcomes were similar amongst gastroenterologists (SVR 283/306, 92.5%), general practitioners (SVR 152/161, 94.4%), sexual health physicians (SVR 104/106, 98.1%) and other prescribers (SVR 37/39, 94.9%).
Discussion
This study demonstrates that HCV can be treated in a regional setting by non-specialist physicians with outcomes equivalent to those from published clinical trials [10], with limited additional resources and funding. The multidisciplinary, decentralised approach used in this cohort was able to reach vulnerable populations in one of the most remote regions of Australia. This approach would be expected to decrease HCV-related morbidity and mortality and reduce transmission, increasing the likelihood of HCV elimination [7,11].
The health service used several strategies to decentralise treatment to increase DAA uptake. Specific models of care provided access to treatment for marginalised populations, including persons who inject drugs and persons in correctional facilities, in a culturally appropriate manner. The fact that the patients' existing health care workers were able to prescribe the DAA therapy permitted prescription of DAA therapy within an existing therapeutic relationship, improving engagement and treatment adherence [12,13].
The current study demonstrates that with appropriate education and support, primary care providers can treat the vast majority of patients eligible for direct-acting antivirals and play an integral role in HCV treatment. Their role is likely to increase with the simplicity of new pan-genotypic regimens with shorter lengths of treatment. Primary care providers were empowered through education programmes and shared-care models to prescribe community-based treatment, enhancing their skill mix and job satisfaction [14]. An additional benefit is that these providers, through their long-term involvement with the patients, are better able to manage other major comorbidities – including alcohol consumption and obesity – which affect liver and general health as much as, if not more than, HCV [15]. Competent primary care prescribers also minimise the need for specialist referral, which can interrupt the cascade of care and delay therapy, and can also disadvantage patients averse to attending hospitals. These collaborative and multidisciplinary approaches are critical to achieving the goal of HCV elimination and are recognised in federal and state government strategies [16,17], as well as other recently published real-world examples [18]. They can foster collaborative care amongst clinicians and may be a model for other medical conditions or health issues [19].
Dedicated nurse practitioners and clinical nurses can play an integral role with triaging, assessing, educating and coordinating DAA treatment of patients with HCV, thereby increasing the accessibility and throughput of services [4,7]. Although our model did not allow for nurse prescribers, this strategy has been used elsewhere in treatment of HCV with similar cure rates to medical officers [19].
Further specialist care was expanded by increasing tertiary clinic throughput in hospital clinic settings, and the delivery of specialist care to remote areas via outreach visits in a ‘hub and spoke’ manner [3]. Gastroenterologist and other specialist-led treatment will continue to be important in managing patients with HCV who have cirrhosis, are treatment experienced, or who have complex care needs. However, this type of service does not have the capacity to treat every patient with HCV, particularly in remote centres. A decentralised model is necessary to ensure that all patients have access to this very safe, effective and well tolerated DAA therapy.
The experience from this study is in keeping with the growing body of evidence surrounding models of care for HCV treatment, and other health care in rural and remote Australia. These include integrated/shared-care models between specialists and general practitioners, outreach services (hub and spoke type delivery with periodic visiting specialists), use of telehealth services, nurse practitioner models and educational programmes [5,20,21]. By using these strategies, this study's outcomes of HCV treatment are consistent with the high benchmark set in clinical trials and confirmed in recently published real-world experience [10,22,23].
There are several weaknesses in this study. Data collection was incomplete, particularly because not all patients treated by general practitioners independent of Cairns and Hinterland Hospital and Health Service may have been captured. Even so, this study has demonstrated that a significant number of patients have been treated in primary care with excellent outcomes.
Strengths of this study include its prospective design, with relatively large and consecutive numbers capturing data from multiple models of care. Furthermore, although the North Queensland population is a relatively mobile one, we were able to access the vast majority of patient data.
Future studies might examine treatment strategies to access individuals who are continuing to transmit the infection, particularly those who continue to inject drugs. Although not captured in this study, data detailing country of birth and ethnicity could provide insights into the local epidemiology of the infection and the individuals who are and, as significantly, who are not accessing treatment. This might inform specific culturally appropriate strategies to reach these populations. It will be interesting to see whether similar or even better results can be achieved with new shorter regimens. It will also be important to optimise the therapy of patients who have failed DAA treatment, and special populations such as persons with chronic kidney disease [24].
In conclusion, non-specialist prescribers can effectively and quickly treat large numbers of patients using a decentralised, multidisciplinary model of care that reaches geographically isolated, socially marginalised and other vulnerable patient populations. This has the potential to significantly reduce the burden of HCV-related disease and is an excellent model for other chronic health conditions affecting these populations.
Acknowledgements
We are grateful to A/Prof Gail Matthews, Prof Greg Dore, Pip Marks and Jasmine Yee from the Kirby Institute for their support. We acknowledge the many clinicians from the Cairns Hospital involved in delivering this model of care including Dr Geogry Peter Kini, Dr John Ombiga, Dr Trent Yarwood and Dr Simon Smith.
Declaration of interests
The authors have no declarations of interest.
Funding
The study received no dedicated funding.
References
- 1. McCaughan GW, Munn SR. Liver transplantation in Australia and New Zealand. Liver Transpl 2016; 22: 830– 838. [DOI] [PubMed] [Google Scholar]
- 2. Kirby Institute HIV, viral hepatitis and sexually transmissible infections in Australia Annual Surveillance Report 2016. Sydney: UNSW Australia; 2016. Available at: https://kirby.unsw.edu.au/report/annual-surveillance-report-hiv-viral-hepatitis-stis-2016 ( accessed June 2018).
- 3. Wakerman J, Humphreys JS, Wells R et al. Primary health care delivery models in rural and remote Australia: a systematic review. BMC Health Serv Res 2008; 8: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Keogh K, Clark P, Valery PC et al. Use of telehealth to treat and manage chronic viral hepatitis in regional Queensland. J Telemed Telecare 2016; 22: 459– 464. [DOI] [PubMed] [Google Scholar]
- 5. Cheng W, Nazareth S, Flexman JP. Statewide hepatitis C model of care for rural and remote regions. J Gastroenterol Hepatol 2015; 30 Suppl 2: 1– 5. [DOI] [PubMed] [Google Scholar]
- 6. Manns MP, Buti M, Gane E et al. Hepatitis C virus infection. Nat Rev Dis Primers 2017; 3: 17006. [DOI] [PubMed] [Google Scholar]
- 7. Hepatitis C Virus Infection Consensus Statement Working Group Australian recommendations for the management of hepatitis C virus infection: a consensus statement (August 2017). Melbourne: Gastroenterological Society of Australia; 2017. Available at: www.hepcguidelines.org.au ( accessed June 2018).
- 8. Australian Bureau of Statistics Population distribution, Aboriginal and Torres Strait Islander Australians (cat. no. 4705.0). Australia: 2007. Available at: www.abs.gov.au/ausstats/abs@.nsf/mf/4705.0 ( accessed June 2018).
- 9. Bartlett SR, Fox P, Cabatingan H et al. Demonstration of near-elimination of hepatitis C virus among a prison population: the Lotus Glen Correctional Centre hepatitis C treatment project. Clin Infect Dis 2018. [DOI] [PubMed] [Google Scholar]
- 10. Younossi ZM, Park H, Gordon SC et al. Real-world outcomes of ledipasvir/sofosbuvir in treatment-naive patients with hepatitis C. Am J Manag Care 2016; 22: SP205– 211. [PubMed] [Google Scholar]
- 11. Backus LI, Belperio PS, Shahoumian TA, Mole LA. Direct-acting antiviral sustained virologic response: impact on mortality in patients without advanced liver disease. Hepatology 2018. [DOI] [PubMed] [Google Scholar]
- 12. Read P, Lothian R, Chronister K et al. Delivering direct acting antiviral therapy for hepatitis C to highly marginalised and current drug injecting populations in a targeted primary health care setting. Int J Drug Policy 2017. [DOI] [PubMed] [Google Scholar]
- 13. Scott N, Iser DM, Thompson AJ et al. Cost-effectiveness of treating chronic hepatitis C virus with direct-acting antivirals in people who inject drugs in Australia. J Gastroenterol Hepatol 2016; 31: 872– 882. [DOI] [PubMed] [Google Scholar]
- 14. Lambert SM, Page AN, Wittmann J et al. General practitioner attitudes to prescribing hepatitis C antiviral therapy in a community setting. Aust J Prim Health 2011; 17: 282– 287. [DOI] [PubMed] [Google Scholar]
- 15. Innes H, McAuley A, Alavi M et al. The contribution of health risk behaviors to excess mortality in American adults with chronic hepatitis C: A population cohort-study. Hepatology 2018; 67: 97– 107. [DOI] [PubMed] [Google Scholar]
- 16. Australian Government Department of Health Fourth National Hepatitis C Strategy 2014–2017. Canberra: Commonwealth of Australia; 2014. Available at: www.health.gov.au/internet/main/publishing.nsf/content/ohp-bbvs-hepc ( accessed June 2018).
- 17. Queensland Health Queensland Hepatitis C Action Plan 2016–2021. Brisbane: State of Queensland (Queensland Health); 2016. Available at: www.health.qld.gov.au/__data/assets/pdf_file/0024/601890/qh-hepc-action-plan.pdf ( accessed June 2018). [Google Scholar]
- 18. Capileno YA, Van den Bergh R, Donchunk D et al. Management of chronic hepatitis C at a primary health clinic in the high-burden context of Karachi, Pakistan. PLoS One 2017; 12: e0175562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kattakuzhy S, Gross C, Emmanuel B et al. Expansion of treatment for hepatitis C virus infection by task shifting to community-based nonspecialist providers: a nonrandomized clinical trial. Ann Intern Med 2017; 167: 311– 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arora S, Thornton K, Murata G et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med 2011; 364: 2199– 2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nazareth S, Kontorinis N, Muwanwella N et al. Successful treatment of patients with hepatitis C in rural and remote Western Australia via telehealth. J Telemed Telecare 2013; 19: 101– 106. [DOI] [PubMed] [Google Scholar]
- 22. Bhattacharya D, Belperio PS, Shahoumian TA et al. Effectiveness of all-oral antiviral regimens in 996 human immunodeficiency virus/hepatitis C virus genotype 1-coinfected patients treated in routine practice. Clin Infect Dis 2017; 64: 1711– 1720. [DOI] [PubMed] [Google Scholar]
- 23. Wu CJ, Roytman MM, Hong LK et al. Real-world experience with sofosbuvir-based regimens for chronic hepatitis C, including patients with factors previously associated with inferior treatment response. Hawaii J Med Public Health 2015; 74: 3– 7. [PMC free article] [PubMed] [Google Scholar]
- 24. Andreoni M, Babudieri S, Bruno S et al. Current and future challenges in HCV: insights from an Italian experts panel. Infection 2018; 46: 147– 163. [DOI] [PubMed] [Google Scholar]