Dear Editor,
The incidence of male infertility is approximately 10%;1 however, the causes of nonobstructive azoospermia (NOA) remain elusive.2,3 Recent studies into this type of idiopathic male infertility have focused on meiotic prophase I.4,5,6 Here, we report an NOA patient with anomalies in spermatogenesis.
This patient was a 35-year-old man and had unsuccessfully tried to conceive for 5 years. He showed a normal lymphocyte karyotype, normal testes, and epididymides, and had no Y chromosome microdeletion or cystic fibrosis. The circulating levels of follicle-stimulating hormone, luteinizing hormone, estradiol, progesterone, prolactin, and testosterone were normal. Three consecutive semen analyses and pathological examination of testicular tissue indicated that this patient exhibited NOA. Testicular tissues from five control donors with proven fertility (at least one child for each, aged 50–65 years old) were obtained through prostatectomy. Testicular tissue from this NOA patient was obtained by fine-needle biopsy.
This study was approved by the Institutional Review Board approval from the first Affiliated Hospital of Zhengzhou University. Informed consents were obtained from all patients for the use of their tissues and data writing. Immunofluorescence staining of testicular tissue using antibodies for synaptonemal complex protein 3 (Proteintech, Wuhan, China), γ-H2A histone family, member X (γ-H2AX) (Milipore, Temecula, CA, USA), and mutL homolg 1 (MLH1) (BD Pharmingen Biosciences, San Diego, CA, USA) was performed to identify the synaptonemal complex (SC), double-strand breaks (DSB), repair, and recombination, as described in previous studies.1,5
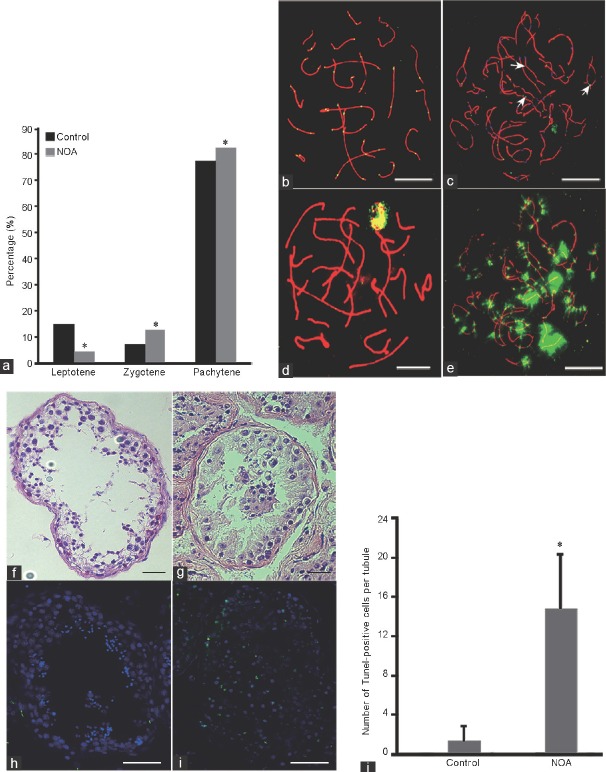
Analysis of prophase I progression showed that there were no significant differences for percentages of leptotene (range: 12.04%–16.10%, P = 0.35), zygotene (range: 6.63%–8.23%, P = 0.59), and pachytene (range: 75.64%–78.82%, P = 0.74) stages among the controls; however, a significantly higher proportion of zygotene and pachytene stage spermatocyte were observed in this patient compared with controls (Figure 1a). In addition, there was a remarkably large number of synaptonemal complexes with gaps (discontinuities of SCs) and splits (forming loop-like structures in the SCs, indicating unpaired chromosome regions)7 compared with controls (44.11% vs 16.14%, P = 0.013; 58.82% vs 2.40%, P < 0.001, respectively; Figure 1b and 1c). The recombination frequency in pachytene spermatocytes was significantly reduced, with a mean of 40.43 MLH1 foci per cell (range: 3–54, n = 100) in this patient compared with 48.97 MLH1 foci per cell (range: 25–61, n = 100 for each sample) in controls. There was no significant difference in MLH1 foci per cell among the controls (P = 0.78). Furthermore, the number of XY bodies with an MLH1 focus was significantly decreased in this patient compared with controls (41.18% vs 67.70%, P = 0.006).
Figure 1.
Analysis of meiotic progression and recombination in this NOA patient. (a) The percentages of leptotene, zygotene, and pachytene cells in the NOA patient and controls in meiosis prophase I. (b) Characteristic appearance of pachytene stages of germ cells from the controls, visualized using antibodies against synaptonemal complexes (red), MLH1 (green). (c) Abnormal recombination and γ-H2AX staining of pachytene stages from the NOA patient, MLH1 foci were observed in the chiasmata between homologous chromosomes (white arrow), visualized using antibodies against synaptonemal complexes (red), MLH1 (green). (d) γ-H2AX staining of pachytene cells from the controls, visualized using antibodies against γ-H2AX (green). (e) Amount of γ-H2AX foci occurred in the same cell with MLH1 in this NOA patient. (f) HE staining of testis tissue from the controls. (g) HE staining of testis tissue from the azoospermic man. (h) TUNEL staining of testis tissue from the controls. (i) TUNEL staining of testis tissue from the azoospermic man. (j) The average TUNEL-positive cell (green) number per seminiferous tubule of the patient and controls. Scale bars = 5 μm in b–e and 50 μm in f–i. *A significant difference with P < 0.05. NOA: nonobstructive azoospermia; HE: hematoxylin–eosin; MLH1: mutL homolg 1; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling; γ-H2AX: γ-H2A histone family, member X.
In some cells, MLH1 foci were observed in the chiasmata between homologous chromosomes (Figure 1c). We conducted a second round of immunofluorescence to detect whether DSB repair was affected in this patient, and the results showed that most of the cells (66/100) had many γ-H2AX foci on autosomes, which was significantly higher than that in the five controls (8/100, 11/100, 12/100, 6/100, and 9/100, respectively) (Figure 1d and 1e), indicating impaired DSB repair or abnormal gene inactivation in pachytene cells. Hematoxylin–eosin staining showed that there was no sperm in this patient, who had an obvious reduction in meiotic cells compared with controls (Figure 1f and 1g). Apoptosis analysis by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining showed that the average TUNEL-positive cell number per se miniferous tubule in the patient was significantly higher than that in controls (Figure 1h-1j).
In summary, we report a patient with a meiotic defect in prophase I, exhibiting abnormalities in the synapsis and recombination between homologous chromosomes. Meiotic prophase I is an important phase of spermatogenesis. In this patient, although chromosomal crossovers formed in pachytene cells, many DSB persisted in this stage of prophase I. There is limited knowledge about the genetic basis of meiotic defects in patients with NOA. Our findings indicate that impaired DSB repair or abnormal gene inactivation in pachytene cells ultimately led to germ cell apoptosis and defective spermatogenesis in this NOA patient. The presented phenotype of this patient enhances our understanding of the pathogenic mechanisms of NOA. Future work should focus on the accurate classification of meiotic defects and employ genotyping techniques to identify mutated genes contributing to spermatogenetic dysfunction in infertility.
AUTHOR CONTRIBUTIONS
YPS conceived of this study, participated in its design, and helped draft the manuscript. XYL and QLY participated in its design, performed the statistical analysis, and drafted the manuscript. HX, FFZ, RB, FYL, and JZ handled the recruitment of patients, sample collection, and partially carried out the analysis. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This research was supported by the National Science Foundation for Young Scientists of China (Grant 31401274 to QLY), the National Natural Science Foundation of China (Grants 31471404 to YPS), and the Youth Innovation Fund of the First Affiliated Hospital of Zhengzhou University (to QLY).
REFERENCES
- 1.Codina-Pascual M, Oliver-Bonet M, Navarro J, Campillo M, Garcia F, et al. Synapsis and meiotic recombination analyses: MLH1 focus in the XY pair as an indicator. Hum Reprod. 2005;20:2133–9. doi: 10.1093/humrep/dei023. [DOI] [PubMed] [Google Scholar]
- 2.Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathways. Nat Cell Biol. 2002;4 Suppl:s41–9. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- 3.Nuti F, Krausz C. Gene polymorphisms/mutations relevant to abnormal spermatogenesis. Reprod Biomed Online. 2008;16:504–13. doi: 10.1016/s1472-6483(10)60457-9. [DOI] [PubMed] [Google Scholar]
- 4.Leng M, Li G, Zhong L, Hou H, Yu D, et al. Abnormal synapses and recombination in an azoospermic male carrier of a reciprocal translocation t (1;21) Fertil Steril. 2009;91:1293e17–22. doi: 10.1016/j.fertnstert.2008.12.049. [DOI] [PubMed] [Google Scholar]
- 5.Sun F, Turek P, Greene C, Ko E, Rademaker A, et al. Abnormal progression through meiosis in men with nonobstructive azoospermia. Fertil Steril. 2007;87:565–71. doi: 10.1016/j.fertnstert.2006.07.1531. [DOI] [PubMed] [Google Scholar]
- 6.Sun F, Greene C, Turek PJ, Ko E, Rademaker A, et al. Immunofluorescent synaptonemal complex analysis in azoospermic men. Cytogenet Genome Res. 2005;111:366–70. doi: 10.1159/000086913. [DOI] [PubMed] [Google Scholar]
- 7.Sun F, Kozak G, Scott S, Trpkov K, Ko E, et al. Meiotic defects in a man with non-obstructive azoospermia: case report. Hum Reprod. 2004;19:1770–3. doi: 10.1093/humrep/deh335. [DOI] [PubMed] [Google Scholar]