Abstract
The present study aims to analyze sperm concentration trends among young and healthy Chinese adults in Wuhan, Central China, from 2010 to 2015. Semen analysis data from 9357 participants were collected and analyzed using a general linear model and the Cochran–Armitage trend test. A significant decline was observed in sperm concentration (β [standard deviation]: −1.53 [0.16]; P < 0.001). In addition, a decline in sperm density was observed by stratifying student versus nonstudent sperm donors and by analyzing the year of birth or birth year cohort of the participants. Furthermore, the percentage of participants with sperm densities of over 40 × 106 ml−1 significantly decreased with year. Notably, a dramatic decline in sperm density was recorded over the first 5 years of study. This research reported a decline in sperm concentration among young adults in Wuhan, Central China, in 2010–2015.
Keywords: healthy men, semen concentration, sperm donors, temporal trends
INTRODUCTION
Semen analysis results based on clinical practice are used to assess male reproductive health and determine semen parameters, particularly sperm concentration, which is closely related to male fertility potential.1 However, a comprehensive review by Carlsen et al.2 reported a pronounced decline in sperm density since the 1930s after analyzing data from 14 947 healthy men worldwide. This conclusion was verified by Swan et al.3 approximately 10 years later. Despite such results, the decrease in sperm density remains a subject of longstanding debate4 because the decline was observed among men in Boston,5 Paris,6 and Scotland7 but not among men in Copenhagen,8 Malmö,9 and Sydney.10
The major reason for the dispute is the small sample used in most previous studies.11,12 Although some studies sampled a large population, the men included in these studies generally belonged to infertile couples who were undergoing assisted reproductive technologies13 or those visiting clinics for fertility examinations.14 In addition, data were collected from multiple centers, thereby leading to participant recruitment and semen analysis biases because of nonuniformity in recruitment criteria, laboratory technicians, and instruments used.15 Other reasons include lifestyle differences and environmental aspects in earlier studies16,17 and ethnic differences.18
China has the largest population in the world, and many recent studies have shown that a high proportion of healthy reproductive-aged Chinese adults have semen parameter values that are below the reference ranges established by the World Health Organization (WHO).19,20,21,22 In addition, two recent studies showed a decline in most of the semen parameters of sperm donor samples collected from sperm banks in Shandong and Hunan, China.23,24 Wuhan, which is the largest city in Central China, has a population of over 10 million with approximately 1.3 million university students. The city also has unique geographic and climate characteristics compared with other cities in China. Reproduction is a fundamental process among all species; therefore, determining whether sperm concentration is changing in Wuhan is a significant issue. The present study retrospectively reviewed the data of the first semen specimens collected from a large population of young adults who applied as sperm donors at the same facility that employed the same technicians and used the same equipment in 2010–2015.
PATIENTS AND METHODS
Study subjects
The participants were volunteer donors who provided semen samples to the Hubei Province Human Sperm Bank from March 1, 2010, to December 31, 2015. Donors were recruited using various means, such as through posters, newspapers, websites, widely used social software, and personal contacts with existing donors. Moreover, the recruitment method used was consistent. The screening of sperm donors was conducted strictly in accordance with the standards published by the Chinese Ministry of Health in 2003, which adopted the WHO 1999 criteria for semen analysis.22,23,24 In addition, the criteria for screening sperm donors have not been modified over time. The guidelines were described in our previous study.22 In summary, all donors must be 22–44 years old, have a college degree or higher, and are in good health based on physical examinations and psychological evaluations performed by qualified doctors and on thorough laboratory testing. When donors met the fundamental criteria, their semen samples were collected and analyzed. The demographic information of each donor and his semen analysis results were documented in a specialized system. Donor information included age, educational background, donation date, days of abstinence, and occupation. In general, three to five semen samples were assessed before a volunteer was either accepted into or rejected from the sperm donor program. We collected the analysis results of the first semen samples of the sperm donors because these specimens were not selected based on proven fertility or on factors associated with impaired semen quality and relevant demographic information, such as date of birth, date of submission of first sample, age, educational level, and abstinence time.
All donors provided informed consent forms during their first visit to the Hubei Province Sperm Bank. They agreed that their semen samples or the data gathered from their analysis could be used for scientific research. This retrospective study was approved by the Ethics Committee of the Reproductive Medicine Center, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Semen collection and analysis
The donors were instructed to collect semen samples in a sterile and wide-mouthed plastic container labeled with an anonymous serial number by masturbating in a private room at the reproductive health facility. The samples were immediately delivered to the adjacent laboratory. They were shaken thoroughly and then incubated in a water bath at 37°C for 30 min before analysis. All samples were analyzed within 60 min of collection.
After the recommended period of sexual abstinence (2–7 days), the semen samples were analyzed in a laboratory according to the recommendations of the WHO Laboratory Manual for the Examination of Human Semen and Semen–Cervical Mucus Interaction.25 Each semen sample was manually evaluated for appearance, odor, viscosity, liquefaction time, pH level, seminal volume, sperm concentration, and sperm motility. Semen volume was calculated based on semen weight under the assumption that semen density was 1.0 g ml−1. The pH level was determined using pH paper. Sperm concentration and motility were evaluated by aspirating 10 μl of thoroughly mixed semen into a clean Makler chamber (Sefi Medical Instruments, Haifa, Israel), which was maintained at 37°C. Furthermore, the sample was covered slightly with a coverslip and then immediately examined under a total magnification of ×400. Then, 10 of the 100 squares in the microscope field were randomly scanned, and sperm count was recorded using a cytometer. Only sperms with tails were counted. The percentage in each of the four motility categories was determined using an ocular grid. The sperms were categorized as (a) fast progressive, (b) slow progressive, (c) nonprogressive, and (d) immotile. The total sperm count was calculated by multiplying sperm density with seminal volume. All assessments were repeated twice. All analyses were performed using the same instruments and methods by the same three well-trained technicians during the entire duration of the study. Inter- and intra-technician comparisons were performed twice annually to ensure the accuracy of the measurement results.
Statistical analyses
For the demographic information, mean and standard deviation (s.d.) were obtained for continuous variables, whereas frequency and percentage were reported for categorical variables. With the exception of semen volume, the values of the other semen parameters in participants with azoospermia (the diagnosis of azoospermia was made based on two semen analyses) were set to 0.1 for statistical analyses. The general linear model and the Cochran–Armitage trend test were used to evaluate annual trends. In case of sperm parameters, a generalized linear model was used to evaluate sperm quality with adjustments in age, days of abstinence, and education degree. The median and the 5th and 95th percentiles of each parameter were calculated. In addition, donors were divided into three birth cohorts according to year of birth: ≤1985, 1986–1990, and 1991–1993. The percentages of donors with sperm densities below 15 × 106 ml−1, between 15 × 106 ml−1 and 40 × 106 ml−1, between 40 × 106 ml−1 and 60 × 106 ml−1, between 60 × 106 ml−1 and 80 × 106 ml−1, and above 80 × 106 ml−1 were analyzed using the Cochran–Armitage trend test with adjustments in age, days of abstinence, and education degree. A generalized additive model was used to check the “smoothing spline” curves of semen quality with years of donation to identify inflection points at any time trend. Subgroup analyses were stratified by student versus nonstudent donors. All P values were two-sided with P < 0.05 considered as statistically significant. Analyses were performed using SAS (Version 9.2; SAS Institute Inc., Cary, NC, USA), and figures were drawn using R software (Version 3.2.3, available from: www.bioconductor.org).
RESULTS
Participant characteristics
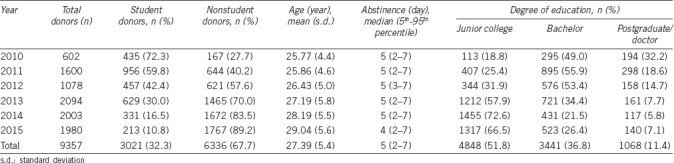
Over a period of 6 years, 9357 healthy men who underwent a recommended period of sexual abstinence with median (5th–95th percentile) of 5 (2–7) days were screened for inclusion into the data analysis in this study. All participants belong to the Han race. The general characteristics of the participants are provided in Table 1. The numbers of donors in 2013 (22.4%), 2014 (21.4%), and 2015 (21.2%) were more than those in 2010 (6.4%), 2011 (17.1%), and 2012 (11.5%). In addition, the donors consisted of university students (32.3%) and nonstudents (67.7%). A total of 112 participants with azoospermia (1.2%) were identified over the study period. In general, the age of the participants was 27.39 ± 5.44 years (expressed as mean ± s.d., with range of 22–44 years). Nearly half of the participants (48.2%) has a bachelor's degree or higher. During the study period, the mean age of the donors and the percentage of nonstudent donors exhibited an increasing trend (P < 0.001), whereas educational level and abstinence time presented a decline over time (P < 0.001).
Table 1.
Demographic data for all study participants
Trend in semen concentration
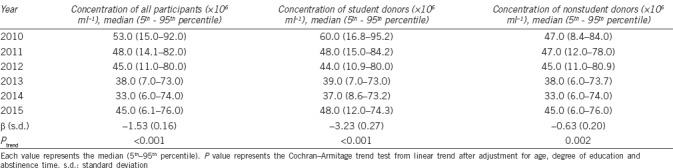
We observed a statistically significant decrease in sperm concentration among all the study participants (the value of the median dropped from 53.0 × 106 ml−1 in 2010 to 45.0 × 106 ml−1 in 2015; Table 2). For example, a decline of (−1.53 ± 0.16) × 106 ml−1 per year was also noted in sperm concentration. That is, we noted a 2.9% decline in sperm concentration in this population. However, the sperm density tendency observed from 2010 to 2014 appears contrary to that in 2015. Thus, the trends in the first 5 years were reanalyzed. Unexpectedly, a more evident decline in sperm density was observed ([–3.76± 0.20] × 106 ml−1 per year).
Table 2.
Semen concentration of participants by year of donation
Trend in semen concentration of student versus nonstudent donors
After stratifying the donors based on their occupation, the data for students and nonstudents were further analyzed. From 2010 to 2015, the sperm concentration of both students and nonstudents statistically and significantly decreased (P < 0.001 and P = 0.002, respectively). However, the decline in the sperm concentration of students ([−3.23± 0.27] × 106 ml−1 per year) was more evident compared with that of nonstudents ([−0.63± 0.20] × 106 ml−1 per year) (Table 2). Similarly, a more evident declining trend in the semen concentration of student (−4.52 × 106 ml−1 per year; 95% CI [−5.14, −3.90]; P < 0.001) and nonstudent (−3.19 × 106 ml−1 per year; 95% CI [−3.74, −2.65]; P < 0.001) donors was observed during the first 5 years of study.
Trend in semen concentration analyzed by birth year and birth year cohort
The data were also analyzed based on the year of birth of the participants. The results demonstrated a significant decline in concentration (P < 0.001; β [s.d.]: −0.26 [0.04]). Moreover, similar trends were observed in the student versus nonstudent subgroups (data not shown). Figure 1 presents the box plots of sperm concentrations based on birth year cohort (<1985, 1986–1990, and 1991–1993). Among all the participants, a significant decline ([−2.53±0.33] × 106 ml−1) in sperm concentration was noted (P < 0.001; Figure 1a). A significant decline ([−7.70±0.67] × 106 ml−1) in sperm concentration was observed for all students (P < 0.014; Figure 1b), whereas an insignificant decline ([−3.09±0.41] × 106 ml−1) in sperm concentration was noted for all nonstudents (P = 0.457; (Figure 1c).
Figure 1.
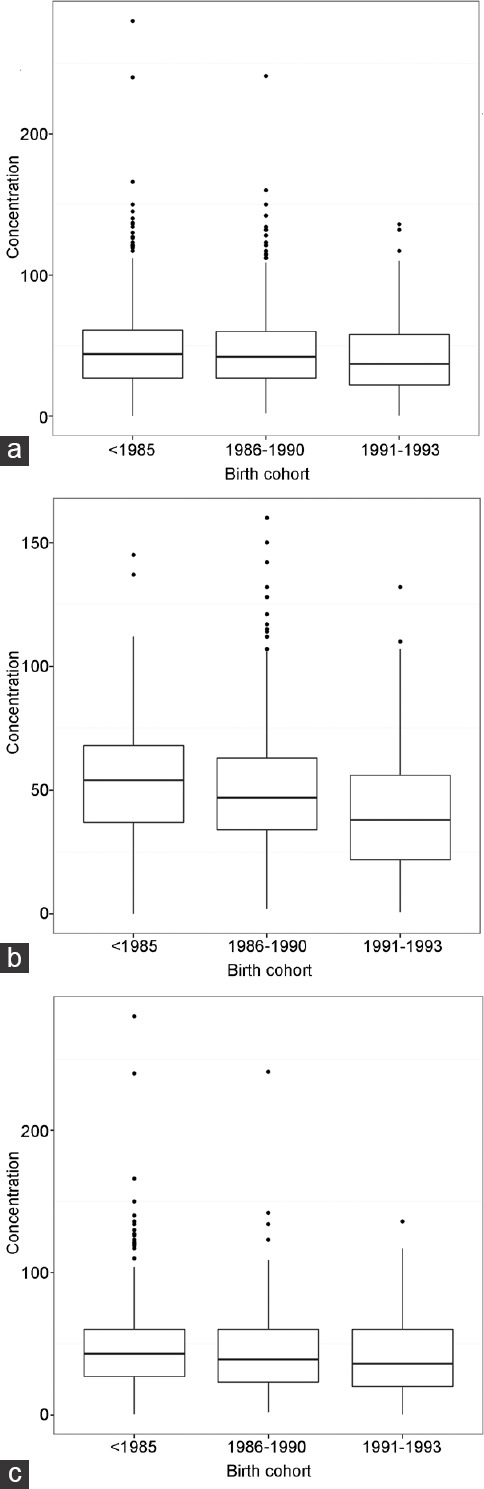
Box plot that demonstrates the decrease in sperm concentration (×106 ml−1) by birth cohort. (a) A significant decline ([−2.53±0.33] × 106 ml−1) in sperm concentration (Ptrend < 0.0001) was recorded for all participants. (b) A significant decline ([−7.70±0.67] × 106 ml−1) in sperm concentration (Ptrend < 0.014) was noted for all students. (c) An insignificant decline ([−3.09±0.41] × 106 ml−1) in sperm concentration (Ptrend = 0.4565) was observed for all nonstudents.
Distribution of the percentage of donors with various sperm concentrations in five density bands
To determine the distribution of donors with various sperm concentrations, the participants were categorized according to density band: <15 × 106 ml−1, 15 × 106 ml−1–40 × 106 ml−1, 40 × 106 ml−1–60 × 106 ml−1, 60 × 106 ml−1–80 × 106 ml−1, and >80 × 106 ml−1. Then, the trend for the distribution of sperm concentration was analyzed. Regardless of whether the participants were students or nonstudents, the results showed that the percentage of both groups with sperm densities <15 × 106 ml−1 and 15 × 106 ml−1–40 × 106 ml−1 significantly increased over time (P < 0.001), whereas that of donors with sperm concentrations higher than 40 × 106 ml−1 significantly decreased over the study period (P < 0.001) (Figure 2).
Figure 2.
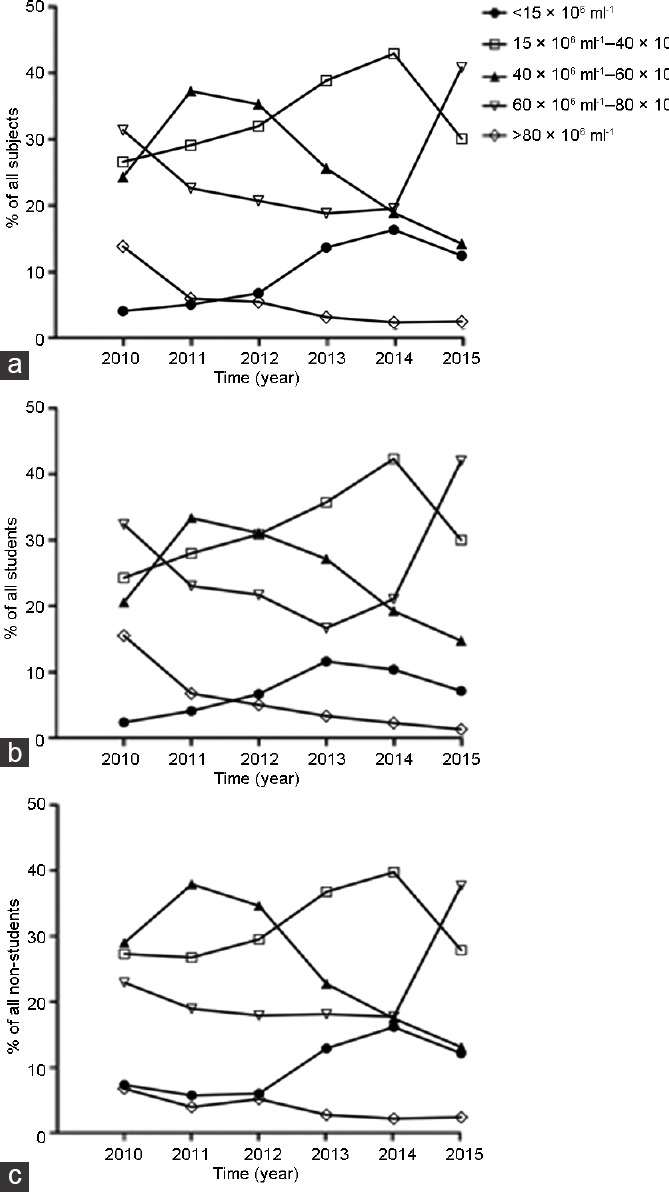
The distribution of percentage of (a) all participants, (b) students, and (c) nonstudents with various sperm concentrations in five density bands: below 15 × 106 ml−1, 15 × 106 ml−1–40 × 106 ml−1, 40 × 106 ml−1–60 × 106 ml−1, 60 × 106 ml−1–80 × 106 ml−1, and above 80 × 106 ml−1.
DISCUSSION
The major finding of our study was a trend toward sperm concentration decline, which was observed among reproductive-aged healthy Chinese males from 2010 to 2015 in Wuhan, Central China. Our data clearly demonstrated a statistically significant decline in sperm density regardless of whether the analysis was conducted by year of donation, by stratifying students versus nonstudents, by birth year, or by birth year cohort. In addition, we determined that the percentage of donors with sperm densities <40 × 106 ml−1 significantly increased over time, whereas that of donors with sperm densities >40 × 106 ml−1 decreased significantly. Furthermore, the extent of declining sperm concentration is more evident in students than that in nonstudents. Analyses were conducted after adjustments were made for age, education degree, and abstinence time based on the first semen samples provided by 9357 participants. All semen analyses were performed by three well-trained technicians using the same instruments at a laboratory and following the standardized WHO guidelines, thereby ensuring continuous quality control during the entire study period. These data suggest that a continuous decline in the sperm concentration of the study population occurred during the observation period of 6 years.
The sample size is the most important variable of the present study. With a sample of 9357 healthy males, this study is one of the largest on secular trend in semen quality worldwide. To our knowledge, this study is the first to address the issue of secular trend in semen parameters of well-defined, healthy, young men in Central China. The same recruitment procedures for sperm donors and standardized questionnaires for collecting personal information were utilized. Furthermore, the methods (the 1999 WHO criteria), as described in previous studies,23,24 used for the semen analysis of each participant were constant over the entire study period. In addition, the results represent real-life conditions to a certain extent because donors were not selected based on proven fertility or the absence of factors associated with the impaired quality of semen before the submission of their first sample.
However, our study has certain limitations. For example, we did not explore other possible risk factors, such as body mass index, alcohol use, and tobacco use. Nevertheless, some studies have demonstrated that the prevalence of these lifestyle risk factors has remained constant among healthy Chinese males over the past several years,14,23,24 and their effects on semen parameters were diverse. Hence, further study is necessary to resolve these controversial issues. Using the information from one semen sample of each participant has been considered insufficient because of the significant intra-individual variation. However, a study has indicated that a single ejaculate is fairly representative of the overall semen quality of healthy men in China.26 Although our findings may not be based on a community population, the donor recruitment methods, including posters, newspapers, websites, widely used social software, and personal contact with existing donors, are diverse. In addition, the recruitment covered all districts in Wuhan. Thus, the study population can represent the entire Wuhan area in Central China. In addition, the percentage of normal sperm morphology was not reported in this study because it mostly depends on the subjectivity of the observer, and the WHO reference value was very low (4%).
A recent comprehensive review that involved 23 126 healthy men across China showed that sperm concentration declined from 1985 to 2009.27 These results are in line with our findings. However, two previous studies did not report any time-related change in the semen parameters of healthy Chinese males.28,29 The discrepancy may be due to study period difference, selection, measure bias among various reports, and other unknown factors. The change in sperm concentration in our study is in accordance with two recent studies from sperm banks in China.23,24 Moreover, the prevalence of infertility, which is closely associated with the reduction in sperm density, particularly over 40 × 106 ml−1,30 has been increasing globally.31 Our data indicated a significant decline not only in sperm concentration as a whole but also in the percentage of participants with sperm densities above 40 × 106 ml−1. Notably, a sudden increase in all the semen parameters in 2015 apparently contradicts the evolution between 2010 and 2014. The reasons for this contradictory phenomenon may be the greater mean age of the participants in 2015, shorter sexual abstinence, and other unknown factors. Thus, further study should be conducted to analyze the cause of exceptional re-increase.
Although a statistically significant increase in semen volume over time (i.e., a 0.15 ml increase in volume per year [s.d.: 0.01 ml] or approximately 6% in the annual semen volume) was observed, the dilution effect was negligible because an evident decline of the total sperm count from 2010 to 2014 (the value of the median dropped from 129.3 × 106 ml−1 in 2010 to 88.4 × 106 ml−1 in 2014, and [−4.23±0.80] × 106 ml−1 per year) was recorded and the declining tendency of semen density was also indicated by analyzing groups by birth year and birth year cohort. Therefore, the causes of decline in sperm concentration are unknown based on the present study. However, these causes were extensively investigated in other studies.32,33 The decreasing sperm density may be a result of the continuously deteriorating environmental quality.34 As the most populated city in Central China, Wuhan constitutes an important part of the economic zone along the Yangtze River, which suffers from severe environmental pollution, including air,35 water,36 and heavy metal pollution.37 Exposure to the aforementioned pollutants has been reported to have a wide range of adverse influences on male reproductive health.38,39,40 Therefore, we speculate that pollutants may play a pivotal role in impairing human spermatogenesis. In addition, considerable literature has linked the decrease in human sperm concentration to a concomitant increase in the incidence of genitourinary abnormalities, such as germ cell tumor and cryptorchidism.41 However, no relevant datum is available for the Wuhan area.
Our results demonstrated a positive correlation between lower sperm concentration and later year of birth. The results, which may be related to exposure to endocrine disruptors present in the environment, particularly during the sensitive fetal and prenatal stage, are in accordance with the findings of two studies performed in the United Kingdom7 and the United States.5 The Yangtze River is the source of drinking water of the citizens of Wuhan. However, the river is heavily contaminated with endocrine-disrupting compounds, such as bisphenol A.42 Evidence from animal studies clearly indicated that bisphenol A exposure directly leads to impairment of the genital system.43,44
No published research has compared the temporal trend in semen quality between university students and nonstudents. Interestingly, our study found that the sperm density rate decreased faster in students than in nonstudents. Possible explanations for this discrepancy may include the higher prevalence of sedentary behavior,45 sleep deficiency,46 and psychosocial stress47 among university students. These lifestyle factors are negatively associated with sperm production.48,49,50 Moreover, the use of smartphones and the internet is more frequent among the student cohort than in the nonstudent group, and both technologies have detrimental effects on semen quality.51
CONCLUSION
We observed a continuous significant decrease in semen concentration among a sample of 9357 healthy Chinese males of reproductive age from Wuhan, Central China, after performing analyses by year of donation, stratification of students versus nonstudents, birth year, and birth year cohort between 2010 and 2015. In particular, the decline mainly occurred among donors with sperm densities of over 40 × 106 ml−1. The etiologies of the decreasing trend of sperm concentration remain unclear; therefore, further studies are warranted to confirm, understand, and, hopefully, reverse the trend of sperm decline.
AUTHOR CONTRIBUTIONS
HTG, CLX, and HFY designed the work. TQM, HFSG, and HTG performed data collection. HFY, HFSG, and YZ analyzed and interpreted the data and drafted this work. All authors revised it critically and finally approved the version to be published.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
The authors are thankful to all sperm donors. This study was supported by the National Science and Technology Support Program of the Ministry of Science and Technology (Grant No. 2012BAI32B03).
REFERENCES
- 1.van der Merwe FH, Kruger TF, Oehninger SC, Lombard CJ. The use of semen parameters to identify the subfertile male in the general population. Gynecol Obstet Invest. 2005;59:86–91. doi: 10.1159/000082368. [DOI] [PubMed] [Google Scholar]
- 2.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–13. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934-1996. Environ Health Perspect. 2000;108:961–6. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonde JP, Ramlau-Hansen CH, Olsen J. Trends in sperm counts: the saga continues. Epidemiology. 2011;22:617–9. doi: 10.1097/EDE.0b013e318223442c. [DOI] [PubMed] [Google Scholar]
- 5.Centola GM, Blanchard A, Demick J, Li S, Eisenberg ML. Decline in sperm count and motility in young adult men from 2003 to 2013: observations from a US sperm bank. Andrology. 2016;4:270–6. doi: 10.1111/andr.12149. [DOI] [PubMed] [Google Scholar]
- 6.Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med. 1995;332:281–5. doi: 10.1056/NEJM199502023320501. [DOI] [PubMed] [Google Scholar]
- 7.Irvine S, Cawood E, Richardson D, MacDonald E, Aitken J. Evidence of deteriorating semen quality in the United Kingdom: birth cohort study in 577 men in Scotland over 11 years. BMJ. 1996;312:467–71. doi: 10.1136/bmj.312.7029.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gyllenborg J, Skakkebaek NE, Nielsen NC, Keiding N, Giwercman A. Secular and seasonal changes in semen quality among young Danish men: a statistical analysis of semen samples from 1927 donor candidates during 1977-1995. Int J Androl. 1999;22:28–36. doi: 10.1046/j.1365-2605.1999.00137.x. [DOI] [PubMed] [Google Scholar]
- 9.Axelsson J, Rylander L, Rignell-Hydbom A, Giwercman A. No secular trend over the last decade in sperm counts among Swedish men from the general population. Hum Reprod. 2011;26:1012–6. doi: 10.1093/humrep/der045. [DOI] [PubMed] [Google Scholar]
- 10.Handelsman DJ. Sperm output of healthy men in Australia: magnitude of bias due to self-selected volunteers. Hum Reprod. 1997;12:2701–5. doi: 10.1093/humrep/12.12.2701. [DOI] [PubMed] [Google Scholar]
- 11.Ginsburg J, Okolo S, Prelevic G, Hardiman P. Residence in the London area and sperm density. Lancet. 1994;343:230. doi: 10.1016/s0140-6736(94)91012-x. [DOI] [PubMed] [Google Scholar]
- 12.Bujan L, Mansat A, Pontonnier F, Mieusset R. Time series analysis of sperm concentration in fertile men in Toulouse, France between 1977 and 1992. BMJ. 1996;312:471–2. doi: 10.1136/bmj.312.7029.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolland M, Le Moal J, Wagner V, Royere D, De Mouzon J. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum Reprod. 2013;28:462–70. doi: 10.1093/humrep/des415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang M, Chen X, Yue H, Xu W, Lin L, et al. Semen quality evaluation in a cohort of 28213 adult males from Sichuan area of South-West China. Andrologia. 2014;46:842–7. doi: 10.1111/and.12168. [DOI] [PubMed] [Google Scholar]
- 15.Younglai EV, Collins JA, Foster WG. Canadian semen quality: an analysis of sperm density among eleven academic fertility centers. Fertil Steril. 1998;70:76–80. doi: 10.1016/s0015-0282(98)00118-6. [DOI] [PubMed] [Google Scholar]
- 16.Van Waeleghem K, De Clercq N, Vermeulen L, Schoonjans F, Comhaire F. Deterioration of sperm quality in young healthy Belgian men. Hum Reprod. 1996;11:325–9. doi: 10.1093/humrep/11.2.325. [DOI] [PubMed] [Google Scholar]
- 17.Zorn B, Virant-Klun I, Verdenik I, Meden-Vrtovec H. Semen quality changes among 2343 healthy Slovenian men included in an IVF-ET programme from 1983 to 1996. Int J Androl. 1999;22:178–83. doi: 10.1046/j.1365-2605.1999.00167.x. [DOI] [PubMed] [Google Scholar]
- 18.Fisch H, Goluboff ET, Olson JH, Feldshuh J, Broder SJ, et al. Semen analyses in 1,283 men from the United States over a 25-year period: no decline in quality. Fertil Steril. 1996;65:1009–14. doi: 10.1016/s0015-0282(16)58278-8. [DOI] [PubMed] [Google Scholar]
- 19.Gao J, Gao ES, Yang Q, Walker M, Wu JQ, et al. Semen quality in a residential, geographic and age representative sample of healthy Chinese men. Hum Reprod. 2007;22:477–84. doi: 10.1093/humrep/del383. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Lin H, Ma M, Li L, Cai M, et al. Semen quality of 1346 healthy men, results from the Chongqing area of Southwest China. Hum Reprod. 2009;24:459–69. doi: 10.1093/humrep/den399. [DOI] [PubMed] [Google Scholar]
- 21.Tang YG, Tang LX, Wang QL, Song G, Jiang YJ, et al. The reference values for semen parameters of 1213 fertile men in Guangdong province in China. Asian J Androl. 2015;17:298–303. doi: 10.4103/1008-682X.143251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao M, Meng TQ, Hu SH, Guan HT, Wei QY, et al. Evaluation of semen quality in 1808 university students, from Wuhan, Central China. Asian J Androl. 2015;17:111–6. doi: 10.4103/1008-682X.135984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Zhang L, Song XH, Zhang HB, Xu CY, et al. Decline of semen quality among Chinese sperm bank donors within 7 years (2008-2014) Asian J Androl. 2017;19:521–5. doi: 10.4103/1008-682X.179533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C, Li B, Xu K, Liu D, Hu J, et al. Decline in semen quality among 30,636 young Chinese men from 2001 to 2015. Fertil Steril. 2017;107:83–88e2. doi: 10.1016/j.fertnstert.2016.09.035. [DOI] [PubMed] [Google Scholar]
- 25.WHO. 4th ed. New York: Cambridge University Press; 1999. Laboratory Manual for the Examination and Processing of Human Semen. [Google Scholar]
- 26.Zhu QX, Gao ES, Pathak N, Wu JQ, Zhou WJ. Single or double semen samples: the dilemma in epidemiological studies on semen quality. Hum Reprod. 2016;31:511–7. doi: 10.1093/humrep/dev326. [DOI] [PubMed] [Google Scholar]
- 27.Huang LP, Li YF, Xiong HY, Cao J. [Changing tendency analysis of Chinese normal male's semen quality in recent 25 years: samples from Chinese documents] J Reprod Contracept. 2010;21:229–41. [Article in Chinese] [Google Scholar]
- 28.Zhu XQ, Shen Y. [Trend analysis of sperm quality during the past 13 years in China] J Zhejiang Univ. 2000;4:173–6. [Article in Chinese] [Google Scholar]
- 29.Wen RQ, Ma C, Tang LX, Wang QL, Liu MY, et al. [No declining semen parameters from Guangdong: an analyses comparing with 18 years ago] Zhongguo Nan Ke Xue Za Zhi. 2006;20:3–5. [Article in Chinese] [Google Scholar]
- 30.Bonde JP, Ernst E, Jensen TK, Hjollund NH, Kolstad H, et al. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet. 1998;352:1172–7. doi: 10.1016/S0140-6736(97)10514-1. [DOI] [PubMed] [Google Scholar]
- 31.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9:e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharpe RM. Environmental/lifestyle effects on spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1697–712. doi: 10.1098/rstb.2009.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gianotten J, Lombardi MP, Zwinderman AH, Lilford RJ, van der Veen F. Idiopathic impaired spermatogenesis: genetic epidemiology is unlikely to provide a short-cut to better understanding. Hum Reprod Update. 2004;10:533–9. doi: 10.1093/humupd/dmh045. [DOI] [PubMed] [Google Scholar]
- 34.Bao J, Yang X, Zhao Z, Wang Z, Yu C, et al. The spatial-temporal characteristics of air pollution in China from 2001-2014. Int J Environ Res Public Health. 2015;12:15875–87. doi: 10.3390/ijerph121215029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian Z, Liang S, Yang S, Trevathan E, Huang Z, et al. Ambient air pollution and preterm birth: a prospective birth cohort study in Wuhan, China. Int J Hyg Environ Health. 2016;219:195–203. doi: 10.1016/j.ijheh.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Lu Q, Yang Z, Wu L, Ruan X, Yang W. The temporal distribution, source and potential toxicity of polycyclic aromatic hydrocarbons in a sediment core from an urban lake in Wuhan, China. Environ Sci Process Impacts. 2015;17:825–34. doi: 10.1039/c4em00698d. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Wu S, Xiang Y, Liang X. An investigation of outpatient children's blood lead level in Wuhan China. PLoS One. 2014;9:e95284. doi: 10.1371/journal.pone.0095284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lafuente R, Garcia-Blaquez N, Jacquemin B, Checa MA. Outdoor air pollution and sperm quality. Fertil Steril. 2016;106:880–96. doi: 10.1016/j.fertnstert.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 39.Thakur JS, Prinja S, Singh D, Rajwanshi A, Prasad R, et al. Adverse reproductive and child health outcomes among people living near highly toxic waste water drains in Punjab, India. J Epidemiol Community Health. 2010;64:148–54. doi: 10.1136/jech.2008.078568. [DOI] [PubMed] [Google Scholar]
- 40.Sinawat S. The environmental impact on male fertility. J Med Assoc Thai. 2000;83:880–5. [PubMed] [Google Scholar]
- 41.Jorgensen N, Joensen UN, Jensen TK, Jensen MB, Almstrup K, et al. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-000990. pii: e000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Z, Shen Z, Gao F, Tang Z, Niu J. Occurrence and possible sources of polychlorinated biphenyls in surface sediments from the Wuhan reach of the Yangtze River, China. Chemosphere. 2009;74:1522–30. doi: 10.1016/j.chemosphere.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 43.Tian J, Ding Y, She R, Ma L, Du F, et al. Histologic study of testis injury after bisphenol a exposure in mice. Toxicol Ind Health. 2017;33:36–45. doi: 10.1177/0748233716658579. [DOI] [PubMed] [Google Scholar]
- 44.Quan C, Wang C, Duan P, Huang W, Yang K. Prenatal bisphenol a exposure leads to reproductive hazards on male offspring via the Akt/mTOR and mitochondrial apoptosis pathways. Environ Toxicol. 2017;32:1007–23. doi: 10.1002/tox.22300. [DOI] [PubMed] [Google Scholar]
- 45.Wu X, Tao S, Zhang Y, Zhang S, Tao F. Low physical activity and high screen time can increase the risks of mental health problems and poor sleep quality among Chinese college students. PLoS One. 2015;10:e0119607. doi: 10.1371/journal.pone.0119607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Q, Yang H, Zhou N, Sun L, Bao H, et al. Inverse U-shaped association between sleep duration and semen quality: longitudinal observational study (MARHCS) in Chongqing, China. Sleep. 2016;39:79–86. doi: 10.5665/sleep.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gollenberg AL, Liu F, Brazil C, Drobnis EZ, Guzick D, et al. Semen quality in fertile men in relation to psychosocial stress. Fertil Steril. 2010;93:1104–11. doi: 10.1016/j.fertnstert.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 48.Priskorn L, Jensen TK, Bang AK, Nordkap L, Joensen UN, et al. Is sedentary lifestyle associated with testicular function? A cross-sectional study of 1,210 men. Am J Epidemiol. 2016;184:284–94. doi: 10.1093/aje/kwv338. [DOI] [PubMed] [Google Scholar]
- 49.Alvarenga TA, Hirotsu C, Mazaro-Costa R, Tufik S, Andersen ML. Impairment of male reproductive function after sleep deprivation. Fertil Steril. 2015;103:1355–62e1. doi: 10.1016/j.fertnstert.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Jurewicz J, Hanke W, Sobala W, Merecz D, Radwan M. [The effect of stress on the semen quality] Med Pr. 2010;61:607–13. [Article in Polish] [PubMed] [Google Scholar]
- 51.Zhang G, Yan H, Chen Q, Liu K, Ling X, et al. Effects of cell phone use on semen parameters: results from the MARHCS cohort study in Chongqing, China. Environ Int. 2016;91:116–21. doi: 10.1016/j.envint.2016.02.028. [DOI] [PubMed] [Google Scholar]


