Abstract
The objective of this study was to investigate the effects of chronic stress on the testes of prepubertal and adult rats and to evaluate whether any alterations could be reversed when stress induction is ended. Seventy-six male rats were assigned to eight groups depending on the type of treatment (control or stressed), the age at which stress was initiated (prepubertal or adult), and the time of evaluation (immediate or late). Stress stimuli were applied for 6 weeks. Stressed prepubertal and adult rats evaluated immediately after the last stress stimulus were included in SP-I and SA-I groups, respectively. The late prepubertal (SP-L) and adult (SA-L) groups of stressed rats were evaluated 6 weeks after the last stress stimulus. Age-matched rats were used as controls (CP-I, CA-I, CP-L, and CA-L groups). Application of stress stimuli to rats in the SP-I group resulted in body weight and seminiferous tubule diameter reduction. The rats in the SA-I group also showed several functional (testosterone level and sperm parameter) and morphological (testicular weight and seminiferous tubule diameter) reductions. The rats in the SP-L group showed increased body weight and intertubular compartment volumetric and absolute densities and reduced tubular compartment volumetric density. The rats in the SA-L group presented only reduced sperm viability. Stress stimuli promoted changes in the rats in all the study groups. The testes of the adult rats were the most affected by chronic stress. However, the stressed adult rats recovered well from the testicular alterations.
Keywords: chronic stress, morphometry, rat, testis
INTRODUCTION
Stress may be characterized as a reactive process that triggers systemic and behavioral responses related to a set of physiological changes. This condition is important as it enables the organism to respond to different situations.1 However, prolonged stress has a destructive effect on tissues, inhibiting several bodily activities and negatively influencing cellular proliferation and differentiation.2
Several studies have demonstrated that chronic stress leads to morphological and functional alterations in the testes. Rats subjected to chronic stress stimuli showed impaired sperm production and reduced serum testosterone levels.3,4 Experimental studies have also demonstrated reduced testicular weight,3,4 with several histological alterations4,5,6 in stressed rats.
The influence of psychosocial stress on testosterone levels and semen quality has been also demonstrated in men. Guay et al.7 found a strong association between work stress and low testosterone level. Furthermore, psychosocial stress was associated with decreased semen quality in fertile men.8 Male occupational stress is also associated with sperm damage9 and decreased number of conceptions.10 Healthy men were examined to evaluate several socio-psycho-behavioral factors in China, and psychological stress is associated with semen quality.11
Despite several reports of the influence of stress stimuli on the testes, one important point has not been addressed, which is whether recovery from testicular damage after exposure to stress stimuli is possible. Furthermore, some studies focused on prepubertal individuals, while others investigated adult individuals, but no study has compared prepubertal and adult animals subjected to the same stress stimuli.
Thus, the objectives of the present study were to evaluate the testicular alterations in prepubertal and adult Wistar rats subjected to chronic stress and to investigate whether the potential chronic stress-induced testicular alterations could be reversed after termination of the stress stimuli.
MATERIALS AND METHODS
Animals
Seventy-six male Wistar rats were used in the experiments. The animals were allocated into two groups as follows: prepubertal rats (n = 40, 4 weeks old) and adult rats (n = 36, 10 weeks old), with mean weights of 84.8 g and 283.4 g, respectively. The rats were kept in a temperature-controlled room (22 ± 1°C) with an artificial dark-light cycle (lights on from 7:00 a.m. to 7:00 p.m.) and fed standard rat chow and water ad libitum. The Animal Care and Use Committee of the State University of Rio de Janeiro (Brazil) approved the handling of the animals and the study design (protocol number CEUA/004/2015).
Experimental design
Four groups of age-matched (prepubertal and adult) rats categorized by age were used to evaluate the immediate effects of stress stimuli at the beginning of the experiment. The SP-I group (n = 10) was composed of 4-week-old rats (stressed prepubertal animals killed immediately after 10 weeks of life) and the SA-I group (n = 9) was composed of 10-week-old rats (stressed adult animals killed immediately after 16 weeks of life); both groups were subjected to the same stress stimuli. We compared each of these groups with age-matched control groups: CP-I group (n = 10, control prepubertal animals killed immediately after 10 weeks of life) and CA-I group (n = 9, control adult animals killed immediately after 16 weeks of life) (Table 1).
Table 1.
Experimental groups
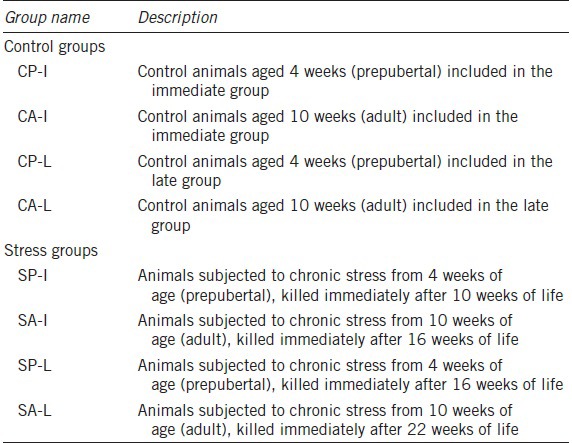
The SP-I and SA-I groups were subjected to chronic stress using the immobilization method.12 Each animal was kept in a rigid opaque plastic tube for 2 h daily to restrain its movements during the 6-week period. The plastic-restraint tubes with different diameters and lengths were adjusted weekly depending on the rats' size (Supplementary Figure 1 (1.6MB, tif) ). Meanwhile, the control groups (CP-I and CA-I) were kept under normal conditions and not subjected to any stresses. Control groups, as well as stressed groups when under stress stimuli, were deprived of food and water for 2 h per day. All the animals were killed 24 h after the last day of stress stimuli application when the rats were 10 (SP-I and CP-I) and 16 weeks old (SA-I and CA-I).
(a) Rigid opaque plastic tubes of different diameters and lengths used to restraint rats to induce chronic stress by the immobilization method. (b) Animals inside the tubes (with tails outside). Each animal was kept in these tubes for 2 h daily to restrain its movements leading to a chronic stress.
Furthermore, to investigate whether the potential changes caused by the stress stimuli were transitory or permanent, we studied four other groups. These groups were categorized by the same age used previously and subjected to the same stress stimuli protocol. However, they were evaluated much later, at 6 weeks after the end of the stress stimuli (what represents the same period that they were subjected to stress). Thus, we had the rats in the SP-L prepubertal (n = 10) and SA-L adult groups (n = 9) subjected to stress stimuli during the 6-week period (i.e., until their 10th and 16th weeks of age, respectively). These groups of rats were killed at 16 and 22 weeks of age, respectively. The two age-matched control groups, CP-L (n = 10) and CA-L (n = 9), kept under normal conditions throughout the study were used for comparison. All the animals were killed using isoflurane (Forane, Abbott Laboratories, Buenos Aires, Argentina) inhalation until cardiac arrest.
Testosterone and sperm analyses
Just before death, blood sample was collected by heart puncture for the determination of testosterone levels using a commercially available enzyme-linked immunosorbent assay kit (Cat. ADI-900-065, Enzo, New York, NY, USA; sensitivity of 5.67 pg ml−1).13
During testicular harvest, the epididymal tail was sectioned in five fragments and homogenized in 5 ml of phosphate-buffered saline with 0.5% bovine serum albumin (A9647, bovine serum albumin, Sigma, Frederick, MD, USA) under 37°C. This homogenized solution was denominated the spermatic solution. This solution was observed in a Neubauer chamber to determine spermatozoa concentrations and motilities. In addition, sperm viability was assessed using the hypo-osmotic test;14 200 spermatozoa per rat were evaluated blindly in different fields.13
Biometrical and morphometrical analyses
The rats were weighed before they were killed; this was considered as the final body weight, expressed in grams. Both testes were dissected from their appendages, and their weights and volumes were measured by Scherle's method.15 The right testis was fixed in Bouin solution by immersion for 24 h, followed by immersion in 3.7% formaldehyde (Formaldeído, Isofar, Duque de Caxias, Brazil) for at least 48 h. Furthermore, the samples were sliced transversely and processed for paraffin embedding to obtain 5-μm-thick histological sections. Morphometric analyses were performed in hematoxylin and eosin (H&E)-stained sections using a microscope (BX51, Olympus, Tokyo, Japan) coupled with a digital camera (DP70, Olympus). All images were saved in the tagged image file format (.tiff) at a resolution of 2040 × 1536 pixels.16
For each rat, the diameters of 125 cross sections of seminiferous tubules were measured on images obtained at ×100 magnification. At least 25 fields were captured for this analysis using the straight-line tool of the ImageJ software (National Institutes of Health, Bethesda, MD, USA). Only round-section tubules were considered, and the straight line always crossed through the center of the tubules. The seminiferous epithelium height was also measured in 125 tubules per rat using images obtained at ×200 magnification. For this analysis, three equidistant lines were drawn from the tunica propria of the seminiferous tubules to the last germinative cell, thus excluding the spermatozoa. The mean of these three lines was considered as the height of that seminiferous tubule.13
The volumetric density (Vv) of each testicular structure was assessed using the point counting method.13 For each rat, 25 fields randomly captured were evaluated. Briefly, using the ImageJ software, a 99-point grid was superimposed over images at ×400 magnification. However, to overlay the grid, the area of the image was previously measured using the “measure” tool of the ImageJ software. Each structure touched by the grid point was counted using the “cell counter” tool of ImageJ. The density was determined as a percentage of the analyzed field. In this way, we quantified the Vv of the tunica propria, seminiferous epithelium, tubular lumen, and intertubular compartment. The sum of the Vv (tunica propria), Vv (seminiferous epithelium), and Vv (tubular lumen) was considered the Vv (tubular compartment). The points on the interstitial space were considered the Vv (intertubular compartment). For each parameter, the result was expressed as a percentage and calculated from the average of the results of each analyzed image. Furthermore, the absolute volume (Av) of each of the aforementioned structures was calculated by multiplying the testicular volume by the Vv of each structure. This parameter was expressed in milliliters. For this purpose, the following formula was used: Y (analyzed structure) volume = (Vv [Y]/100 × testicular volume)
The protocols used for morphometrical analyses are presented in a Supplementary Document 1 (442.9KB, pdf) and illustrated in Supplementary Figure 2 (1.9MB, tif) .
Testicular histological field, captured under ×400 magnification, with a 99-point grid superimposed to assess the volumetric density of each testicular structure.
Statistical analysis
All parametric values were analyzed using the Kolmogorov–Smirnov normality test, and all sample data were normally distributed (Gaussian distribution). The mean values in each group of rats subjected to stress stimuli were compared with those of the corresponding control group using the Student's t-test. All analyses were performed with the GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA, USA). Mean differences were considered significant when P < 0.05. All results are presented as mean ± standard deviation.
RESULTS
Immediate effects of stress stimuli on the testes
Serum testosterone levels were altered only in the animals subjected to chronic stress in adulthood. The SA-I group showed a decrease of 21% in testosterone levels in comparison with the CA-I group (8.83 ± 1.53 ng ml−1 vs 11.25 ± 2.14 ng ml−1, P = 0.04). These data are represented in Figure 1. No significant difference in serum testosterone level was observed between the SP-I and CP-I groups (11.93 ± 1.21 ng ml−1 vs 13.00 ± 1.67 ng ml−1; P = 0.23).
Figure 1.
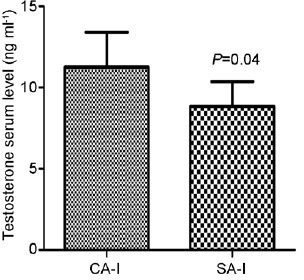
The effects of chronic stress stimuli on the serum testosterone levels in the adult group, showing alterations immediately after the end of stress stimuli. The stressed adult group evaluated immediately after the end of stress stimuli showed a decrease of 21% in testosterone levels in comparison with the control adult group. Data are expressed as the mean ± standard deviation. CA-I: adult rats of control group immediately analyzed; SA-I: adult rats of stress group immediately analyzed.
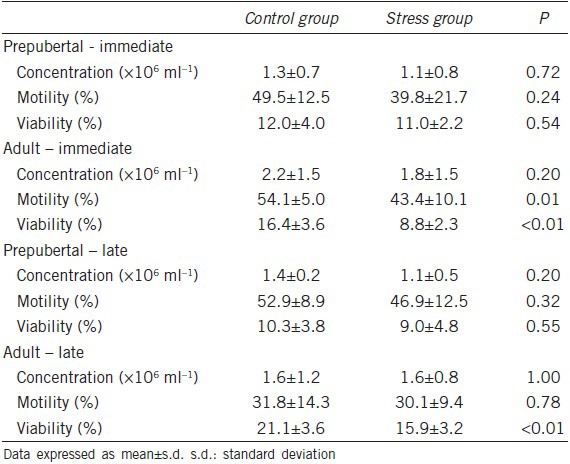
Spermatic changes were found only in the animals subjected to stress in adulthood. Sperm motility decreased by 19% in the SA-I group (43.4% ± 10.1%) as compared with that in the CA-I group (54.1% ± 5.0%). Furthermore, sperm viability was reduced by 46% in the SA-I group (8.8% ± 2.3%) as compared with that in the CA-I group (16.4% ± 3.6%). No significant differences in the evaluated sperm parameters were observed between the SP-I and CP-I groups. All sperm-related analysis results are listed in Table 2.
Table 2.
Effect of chronic stress on sperm concentration and motility and viability of rats stressed before puberty or in the adulthood
The final body weight was reduced by 14% in the SP-I group (177 ± 15.8 g) as compared with that in the CP-I group (206 ± 11.0 g), different from that of the animals subjected to chronic stress in adulthood, where the final body weights did not show a significant difference between the groups SA-I and CA-I. On the other hand, the testicular weights and volumes both reduced by 12% in the SA-I group (1.65 ± 0.17 g and 1.69 ± 0.17 ml, respectively) as compared with those in the CA-I group (1.87 ± 0.23 g and 1.93 ± 0.10 ml, respectively), different from those in the SP-I and CP-I groups, where no significant difference in the testicular weights and volumes was found.
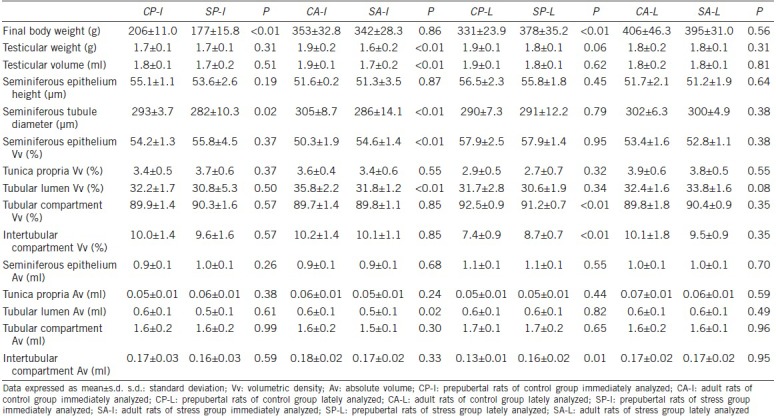
The SP-I group showed a seminiferous tubule diameter of 282 ± 10.3 μm, which was reduced by 4%, when compared with that in the CP-I group (293 ± 3.7 μm, Figure 2). However, no significant differences in all the other morphometric parameters analyzed were found between the groups. All the biometrical and morphometrical data of the rats in the SP-I and CP-I groups are listed in Table 3. The immediate effects of chronic stress on testicular morphology induced more changes when it occurred in adulthood. The seminiferous tubule diameter was reduced by 6% in the SA-I group (286 ± 14.1 μm) as compared with that in the CA-I group (305 ± 8.7 μm). The seminiferous epithelium Vv of the rats in the SA-I group (54.6% ± 1.4%) increased by 9% as compared with that of the rats in the CA-I group (50.3% ± 1.9%). The tubular lumen Vv (31.8% ± 1.2%) and Av (0.5 ± 0.1 ml) in the SA-I group decreased by 11% and 17%, respectively, as compared with those (35.8% ± 2.2% and 0.6 ± 0.1 ml, respectively) in the control groups. No significant differences in the other morphometrical parameters analyzed were found between the groups. All biometrical and morphometrical data of the SA-I and CA-I groups are listed in Table 3.
Figure 2.
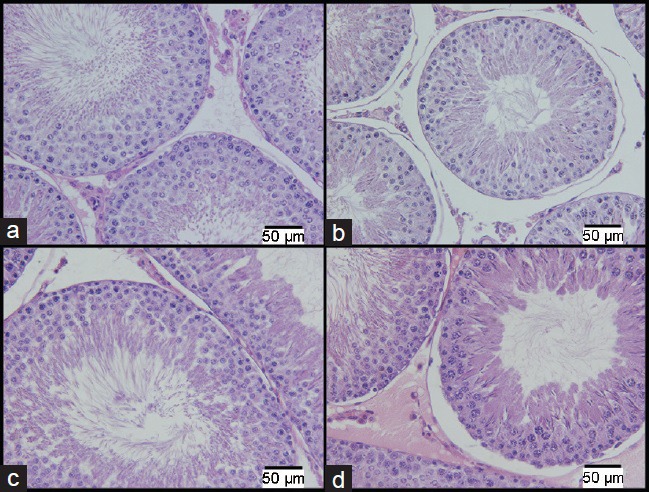
Photomicrographs of the cross section of seminiferous tubules. The images on the top row (a and b) are from the prepubertal group and those on the bottom row (c and d) are from the adult group, the analysis results of both samples were collected immediately after the end of stress stimuli. The images (a) and (c) are from the control groups. The images (b) and (d) are from the stressed groups (H&E: ×400). Scale bars = 50 μm.
Table 3.
Biometrical and morphometrical data of prepubertal and adult rats submitted to chronic stress
Late effects of stress stimuli on the testes
The animals subjected to chronic stress before puberty and in adulthood did not show significant changes in serum testosterone level, when assessed 6 weeks after the end of the stress stimuli. The serum testosterone levels of the SP-L and the CP-L groups were 11.85 ± 1.91 and 12.03 ± 2.59 ng ml−1, respectively (P = 0.89). The SA-L and CA-L groups presented serum testosterone levels of 12.59 ± 2.17 and 10.08 ± 2.43 ng ml−1, respectively (P = 0.10).
As in the SP-I and CP-I groups, the animals of groups SP-L and CP-L also did not show any significant changes in semen. However, sperm viability was reduced by 24% in the SA-L group (15.9% ± 3.2%) as compared with that in the CA-L group (21.1% ± 3.6%). All sperm-related analysis results are listed in Table 2.
The final body weight increased by 14% in the SP-L group (378 ± 35.2 g) as compared with that in the CP-L group (331 ± 23.9 g). These data are listed in Table 3. No significant differences were found in the final body weight analyses between the SA-L and CA-L groups. All biometrical and morphometrical data of the SA-L and CA-L groups are listed in Table 3. Regarding the testicular weights and volumes, no significant differences were observed between the groups.
Different from the rats evaluated immediately after the stress period, the animals assessed 6 weeks after the last stress stimulus showed significant morphological changes only between the SP-L and the CP-L groups. Tubular compartment Vv decreased by 2% in the stressed group (91.2% ± 0.7%) as compared with the control group (92.5% ± 0.9%). By contrast, the Vv and Av of the intertubular compartment increased by 18% and 23%, respectively, in the SP-L group (8.7% ± 0.7% and 0.16 ± 0.02 ml, respectively) as compared with those in the CP-L group (7.4% ± 0.9% and 0.13 ± 0.01 ml, respectively). No significant difference in testicular morphology was observed between the SA-L and CA-L groups. Histological fields are presented in Figure 3.
Figure 3.
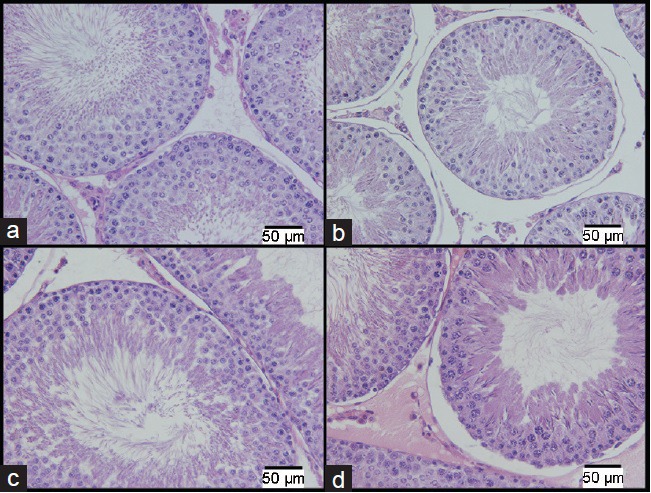
Photomicrographs of a cross section of seminiferous tubules. The images on the top row (a and b) are from the prepubertal groups and those on the bottom row (c and d) are from the adult groups, both showing alterations occurring 6 weeks after the end of the stress stimuli. The images (a) and (c) are from the control groups. The images (b) and (d) are from the stressed groups (H&E: ×400). Scale bars = 50 μm.
DISCUSSION
Testicular morphology and function depend on adequate microenvironment-related conditions such as adequate temperature, blood supply, and hormonal stimulation.17,18 Stress, especially when it is repeated or chronic, activates the hypothalamic–pituitary–adrenal axis, which in turn affects the function of the hypothalamic–pituitary–gonadal axis. Consequently, this mechanism reduces the amount of sex hormones (testosterone and estrogen) in stressed individuals.19
As the testes are the main producers of testosterone, which is essential to maintain regular testicular function, morphological and functional alterations are expected in the testes of stressed individuals. This is the first study to assess the immediate and late effects of chronic stress in the testes of prepubertal and adult rats.
In this study, chronic stress was more harmful when it occurred in adulthood and was evaluated immediately. For example, serum testosterone levels decreased only in the adult rats subjected to stress stimuli, but in a long-term evaluation, the serum testosterone levels were normal. Chronic stress increases glucocorticoid levels,20 thereby suppressing the release of gonadotropins and acting directly on Leydig cell receptors, inhibiting testosterone biosynthesis.21 This could explain the low levels of the male sex hormone in the stressed adult rats and the recovery of the serum testosterone levels in the adult animals when testosterone biosynthesis was not inhibited anymore.
Changes in such sperm parameters as viability and motility were found only in the rats subjected to chronic stress stimuli in adulthood. This could be explained by their low serum testosterone levels, as the hormone is important for spermatogenesis and spermiogenesis.22 By contrast, the study by Nirupama et al.23 demonstrated alterations in sperm concentrations in adult rats subjected to stress for 60 days. Probably, changes in sperm concentration only become conspicuous after longer periods of stress. Although the stress induced in the adult rats in this study was not enough to reduce sperm concentration, it reduced sperm motility (19%) and viability (46%) in SA-I group when evaluated immediately after the last stress stimulus was applied, enough to impair the reproductive function.
In addition, the greatest morphological change in the seminiferous tubules was found in the SA-I group. Besides the altered parameters of the seminiferous tubules, the testicular weight and volume were reduced, suggesting atrophy of the organ. This clearly demonstrated that the effects of chronic stress were more pronounced in the adult rats.
On the other hand, the prepubertal rats subjected to chronic stress and evaluated immediately after the end of the stress stimuli showed a significant decrease in body weight. Although the rats subjected to stress stimuli were unable to access food and water for 2 h per day, the control groups were also subjected to this restriction during the same period. The weight loss in the stressed rats during puberty was demonstrated in earlier studies.12 The higher energy expenditure in stress situations may explain the weight loss.24 The weight loss indicated that other organs could be affected by the chronic stress, as shown by the reduced diameters of the seminiferous tubules in the rats subjected to chronic stress.
Chronic stress conditions during childhood can lead to depression and anxiety, as well as obesity and metabolic syndrome.25 In addition, children and adolescents with increased hypothalamic–pituitary–adrenal axis activity have greater weight gain in adulthood.25,26 Chronic stress causes different and permanent (systemic and testicular) changes in adulthood when applied before puberty. These rats had elevated body weights, indicating a tendency for obesity in the future.
The chronic stress applied before puberty resulted in important testicular changes, with late alterations in adulthood. Morphological changes were found in the assessment performed 6 weeks after the end of the stress stimuli, but no alteration was found immediately after the last stress stimulus was applied. For example, the space occupied by the interstices increased, while the space occupied by the tubular compartments decreased only when these parameters were evaluated later.
The results of the present study highlight the extreme importance of avoiding stress stimuli in prepubertal rats, as rats exposed to chronic stress before puberty developed a significant reduction in the tubular compartment Vv and an increase in the intertubular compartment Vv when evaluated 6 weeks after the last stress stimulus was applied. As the testes did not present volume and weight alterations, the tubular compartment of the animals seemed reduced, suggesting that a late effect of chronic stress could cause a functional impairment in the future. No study has investigated the effects of chronic stress on reproductive function in prepubescent men. However, Almeida et al.27 showed that rats subjected to stress stimuli by immobilization before puberty had reduced fertility. Although stress is more intense than that applied in our work, our results reinforce the idea that changes caused by chronic stress before puberty may be more permanent. Based on the morphological alterations in the testes of the prepubertal rats, we can suppose that testicular damage would be more permanent if chronic stresses occur in childhood.
The rats subjected to chronic stress in adulthood showed several changes in testicular morphology, spermatic parameters, and serum testosterone levels when evaluated just after the end of the stress stimuli. However, only sperm viability was reduced when the rats were assessed late. Moreover, the reduction in sperm viability was higher (46%) in the rats evaluated immediately after termination of the stress stimuli than in the rats evaluated late (24%). Therefore, the effects of chronic stress stimuli in adult rats were almost completely reversible after interruption of the stimulus. Some changes in spermatozoa production have been reported even 4 months after the interruption of the stress stimulus.23
Although chronic stress promoted significant alterations in the testicular parameters in the adult rats, the testes recovered almost completely after the end of the stress stimulus. Such results could stimulate a change of routine activities in men with fertility problems who live a stressful life. Although the stress in rats and men are not the same, men with stressful lifestyles are more prone to fertility problems.28 A report indicated that paternal stress at work is associated with a decrease in the number of conceptions.10 Our result suggests that withdrawal of stress factors in men may improve fertility, and this could be an important factor to consider in the treatment of infertility.
More studies on the subject area are necessary, as the present study had a few limitations. The study was performed in an animal model within controlled experimental conditions, so the results of this study may not represent the events that occur in stressed men. Furthermore, testicular alterations may vary according to the type, intensity, and duration of the stress stimulus, besides the age of the individual. Thus, the differences related to these factors may explain the divergences presented between our results and other reports from the literature.
CONCLUSION
The results of the present study showed that chronic stress induced before or after puberty led to testicular alterations in rats. Nevertheless, the changes were more significant when the stress stimuli were induced in adulthood; however, the alterations identified in the rats stressed during adulthood may almost completely reverse after the end of the stress-inducing stimuli. On the other hand, in the rats stressed before puberty, testicular alterations appeared to be more permanent and seemed to increase even after the end of the stress stimuli.
AUTHOR CONTRIBUTIONS
CTR contributed to the research design; performed the experiments, data acquisition, analysis, and interpretation of data and drafted the manuscript. DBDS contributed to the research design; performed the analysis and interpretation of data and drafted the manuscript. WSC and FJBS contributed to the research design and revised the manuscript. MAPS contributed to the research design; performed the analysis and interpretation of data and drafted the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by grants from the Foundation for Research Support of Rio de Janeiro (FAPERJ), the Coordination for the Improvement of Post-Graduate Students (CAPES), and the National Council for Scientific and Technological Development (CNPq). These foundations had no involvement in the study design, data collection, analysis, and interpretation, drafting of the manuscript, and decision to submit for publication.
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Benchimol de Souza D, Silva D, Marinho Costa Silva C, Barcellos Sampaio FJ, Silva Costa W, et al. Effects of immobilization stress on kidneys of Wistar male rats: a morphometrical and stereological analysis. Kidney Blood Press Res. 2011;34:424–9. doi: 10.1159/000328331. [DOI] [PubMed] [Google Scholar]
- 2.Rosmond R, Bjorntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J Intern Med. 2000;247:188–97. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Diaz EC, Gomez-Quiroz LE, Arenas-Rios E, Aragon-Martinez A, Ibarra-Arias JA, et al. Oxidative status in testis and epididymal sperm parameters after acute and chronic stress by cold-water immersion in the adult rat. Syst Biol Reprod Med. 2015;61:150–60. doi: 10.3109/19396368.2015.1008071. [DOI] [PubMed] [Google Scholar]
- 4.Priya PH, Reddy PS. Effect of restraint stress on lead-induced male reproductive toxicity in rats. J Exp Zool A Ecol Genet Physiol. 2012;317:455–65. doi: 10.1002/jez.1738. [DOI] [PubMed] [Google Scholar]
- 5.Hou G, Xiong W, Wang M, Chen X, Yuan TF. Chronic stress influences sexual motivation and causes damage to testicular cells in male rats. J Sex Med. 2014;11:653–63. doi: 10.1111/jsm.12416. [DOI] [PubMed] [Google Scholar]
- 6.Wang FF, Wang Q, Chen Y, Lin Q, Gao HB, et al. Chronic stress induces ageing-associated degeneration in rat Leydig cells. Asian J Androl. 2012;14:643–8. doi: 10.1038/aja.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guay A, Seftel AD, Traish A. Hypogonadism in men with erectile dysfunction may be related to a host of chronic illnesses. Int J Impot Res. 2010;22:9–19. doi: 10.1038/ijir.2009.46. [DOI] [PubMed] [Google Scholar]
- 8.Gollenberg AL, Liu F, Brazil C, Drobnis EZ, Guzick D, et al. Semen quality in fertile men in relation to psychosocial stress. Fertil Steril. 2010;93:1104–11. doi: 10.1016/j.fertnstert.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Jurewicz J, Radwan M, Merecz-Kot D, Sobala W, Ligocka D, et al. Occupational, life stress and family functioning: does it affect semen quality? Ann Hum Biol. 2014;41:220–8. doi: 10.3109/03014460.2013.849755. [DOI] [PubMed] [Google Scholar]
- 10.Lee MS, Paek D, Eum KD, Siegrist J, Li J, et al. Paternal work stress and prolonged time to pregnancy. Int Arch Occup Environ Health. 2009;82:209–16. doi: 10.1007/s00420-008-0324-2. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Zhou N, Han X, Ma M, Li L, et al. Socio-psycho-behavioural factors associated with male semen quality in China: results from 1346 healthy men in Chongqing. J Fam Plann Reprod Health Care. 2013;39:102–10. doi: 10.1136/jfprhc-2011-100276. [DOI] [PubMed] [Google Scholar]
- 12.de Souza DB, Silva D, Cortez CM, Costa WS, Sampaio FJ. Effects of chronic stress on penile corpus cavernosum of rats. J Androl. 2012;33:735–9. doi: 10.2164/jandrol.111.014225. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro CT, Milhomem R, De Souza DB, Costa WS, Sampaio FJ, et al. Effect of antioxidants on outcome of testicular torsion in rats of different ages. J Urol. 2014;191:1578–84. doi: 10.1016/j.juro.2013.09.066. [DOI] [PubMed] [Google Scholar]
- 14.Jeyendran RS, Van der Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJ. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil. 1984;70:219–28. doi: 10.1530/jrf.0.0700219. [DOI] [PubMed] [Google Scholar]
- 15.Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970;26:57–60. [PubMed] [Google Scholar]
- 16.Ribeiro CT, De Souza DB, Costa WS, Pereira-Sampaio MA, Sampaio FJ. Effects of testicular transfixation on seminiferous tubule morphology and sperm parameters of prepubertal, pubertal, and adult rats. Theriogenology. 2015;84:1142–8. doi: 10.1016/j.theriogenology.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Damber JE, Janson PO. The influence of scrotal warming on testicular blood flow and endocrine function in the rat. Acta Physiol Scand. 1978;104:61–7. doi: 10.1111/j.1748-1716.1978.tb06251.x. [DOI] [PubMed] [Google Scholar]
- 18.Shalet SM. Normal testicular function and spermatogenesis. Pediatr Blood Cancer. 2009;53:285–8. doi: 10.1002/pbc.22000. [DOI] [PubMed] [Google Scholar]
- 19.Toufexis D, Rivarola MA, Lara H, Viau V. Stress and the reproductive axis. J Neuroendocrinol. 2014;26:573–86. doi: 10.1111/jne.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin H, Yuan KM, Zhou HY, Bu T, Su H, et al. Time-course changes of steroidogenic gene expression and steroidogenesis of rat Leydig cells after acute immobilization stress. Int J Mol Sci. 2014;15:21028–44. doi: 10.3390/ijms151121028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Donnell L, McLachlan RI, Wreford NG, Robertson DM. Testosterone promotes the conversion of round spermatids between stages VII and VIII of the rat spermatogenic cycle. Endocrinology. 1994;135:2608–14. doi: 10.1210/endo.135.6.7988449. [DOI] [PubMed] [Google Scholar]
- 23.Nirupama M, Devaki M, Nirupama R, Yajurvedi HN. Chronic intermittent stress-induced alterations in the spermatogenesis and antioxidant status of the testis are irreversible in albino rat. J Physiol Biochem. 2013;69:59–68. doi: 10.1007/s13105-012-0187-6. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein DS. Adrenal responses to stress. Cell Mol Neurobiol. 2010;30:1433–40. doi: 10.1007/s10571-010-9606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pervanidou P, Chrousos GP. Stress and obesity/metabolic syndrome in childhood and adolescence. Int J Pediatr Obes. 2011;6(Suppl 1):21–8. doi: 10.3109/17477166.2011.615996. [DOI] [PubMed] [Google Scholar]
- 26.Anderson SE, Cohen P, Naumova EN, Must A. Association of depression and anxiety disorders with weight change in a prospective community-based study of children followed up into adulthood. Arch Pediatr Adolesc Med. 2006;160:285–91. doi: 10.1001/archpedi.160.3.285. [DOI] [PubMed] [Google Scholar]
- 27.Almeida SA, Kempinas WG, Lamano Carvalho TL. Sexual behavior and fertility of male rats submitted to prolonged immobilization-induced stress. Braz J Med Biol Res. 2000;33:1105–9. doi: 10.1590/s0100-879x2000000900019. [DOI] [PubMed] [Google Scholar]
- 28.Nordkap L, Jensen TK, Hansen AM, Lassen TH, Bang AK, et al. Psychological stress and testicular function: a cross-sectional study of 1,215 Danish men. Fertil Steril. 2016;105:174–87. doi: 10.1016/j.fertnstert.2015.09.016. e1-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Rigid opaque plastic tubes of different diameters and lengths used to restraint rats to induce chronic stress by the immobilization method. (b) Animals inside the tubes (with tails outside). Each animal was kept in these tubes for 2 h daily to restrain its movements leading to a chronic stress.
Testicular histological field, captured under ×400 magnification, with a 99-point grid superimposed to assess the volumetric density of each testicular structure.


