Abstract
We evaluated whether LIM-kinase 2 inhibitor (LIMK2i) could improve erectile function by suppressing corporal fibrosis through the normalization of the Rho-associated coiled-coil protein kinase 1 (ROCK1)/LIMK2/Cofilin pathway in a rat model of cavernous nerve crush injury (CNCI). Sixty 11-week-old male Sprague-Dawley rats were divided equally into five groups: sham surgery (S), CNCI (I), and CNCI treated with low-dose (L), medium-dose (M), and high-dose (H) LIMK2i. The L, M, and H groups were treated with a daily intraperitoneal injection of LIMK2i (2.5, 5.0, and 10.0 mg kg−1 body weight, respectively) for 1 week after surgery. The erectile response was assessed using electrostimulation at 1 week, postoperatively. Penile tissues were processed for Masson's trichrome staining, double immunofluorescence, and Western blot assay. Erectile responses in the H group improved compared with the I group, while the M group showed only partial improvement. A significantly decreased smooth muscle/collagen ratio and an increased content of fibroblasts positive for phospho-LIMK2 were noted in the I group. The M and H groups revealed significant improvements in histological alterations and the dysregulated LIMK2/Cofilin pathway, except for LIMK2 phosphorylation in the M group. The inhibition of LIMK2 did not affect the ROCK1 protein expression. The content of fibroblasts positive for phospho-LIMK2 in the H group returned to the level found in the S group, whereas it did not in the M group. However, the L group did not exhibit such improvements. Our data suggest that the inhibition of LIMK2, particularly with administration of 10.0 mg kg−1 body weight LIMK2i, can improve corporal fibrosis and erectile function by normalizing the LIMK2/Cofilin pathway.
Keywords: erectile dysfunction, fibrosis, LIM kinase, penis, prostatectomy
INTRODUCTION
Despite the continuous optimization of nerve-sparing techniques during radical prostatectomy (RP), the prevalence of post-RP erectile dysfunction (ED) has been reported to range from 20% to 90%.1,2,3,4 A previous study showed that even visualization of the cavernous nerve (CN) without direct injury to CN resulted in rats' impaired erectile responses, indicating that even the most minimal damage to CNs might lead to at least short-term erectile impairment.5 Thus, neuropraxia caused by traction, compression, and minimal manipulation during RP can have adverse effects on recovery of erectile function in a short-term period after RP. A temporary block of neurotransmission due to CN injuries leads to persistence of a flaccid penis and, thereby, low oxygen supply to the penis during the early postoperative period after RP, which results in structural alterations of the corpus cavernosum such as corporal fibrosis through the TGF-β-driven fibrotic pathway.6,7,8,9 Thus, CN injury is a detrimental factor in the process of corporal fibrosis,10 where the latter plays a critical role in the development of corporal veno-occlusive dysfunction (CVOD) which is a main pathophysiological factor of ED after RP. However, little is known about the molecular mechanisms leading to corporal fibrosis.
Recently, we demonstrated that the RhoA/Rho-associated coiled-coil protein kinase 1 (ROCK1)/LIM-kinase 2 (LIMK2)/Cofilin pathway was involved in corporal fibrosis with a loss of smooth muscle (SM) through coordination with TGF-β/sphingosine-1-phosphate signaling after CN injuries (Figure 1).11,12 Furthermore, early inhibition of Rho-kinase, an upstream molecule of LIMK2 in the ROCK1/LIMK2/Cofilin pathway, after CN crush injury (CNCI) could prevent corporal apoptosis and fibrosis by suppressing the Akt-driven and ROCK1/LIMK2/Cofilin pathways, preventing both CVOD and ED.13 However, given the risk for significant adverse effects of the ROCK inhibitor, inhibition of LIMK2, a downstream target of Rho-kinase, could be a reasonable strategy for the treatment of corporal fibrosis after CN injury. Selectively inhibiting a downstream pathway of ROCK such as LIMK2/Cofilin would be better than targeting ROCK itself, analogous to a surgeon's scalpel being better than a chisel.14 Thus, the aim of this study was to determine whether inhibition of LIMK2 could improve erectile functions through normalizing the dysregulated ROCK1/LIMK2/Cofilin pathway related to corporal fibrosis after RP in a rat model of CNCI.
Figure 1.
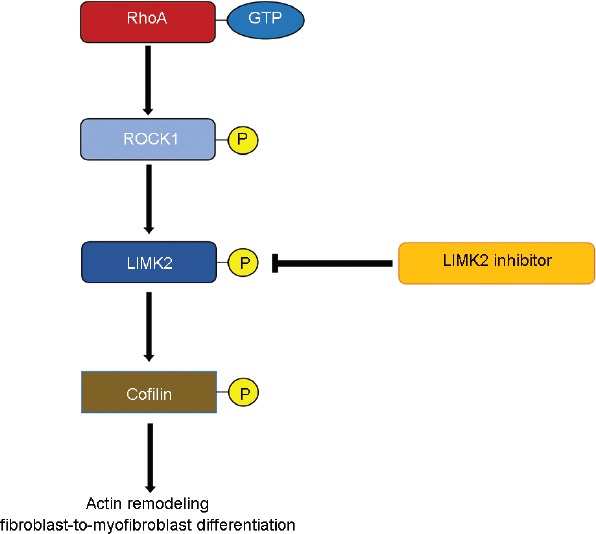
The role of RhoA/ROCK1/LIMK2/Cofilin signaling pathway in the corporal fibrosis after CN injury. CN: cavernous nerve; LIMK2: LIM-kinase 2; ROCK1: Rho-associated coiled-coil protein kinase 1.
MATERIALS AND METHODS
Study design
The experiments were performed in accordance with the Institutional Animal Care and Use Committee at the Clinical Research Institute, Seoul National University Hospital, an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited facility. All rats were cared for in accordance with the National Research Council guidelines for the care and use of laboratory animals. To determine treatment effects of LIMK2 inhibitor (LIMK2i) on CNCI-induced corporal fibrosis and ED, sixty 11-week-old male Sprague Dawley rats, weighing 300–350 g, were categorized into five equal experimental groups (n = 12 each): sham surgery, bilateral CNCI, and bilateral CNCI treated with intraperitoneal injection of low-dose (2.5 mg kg−1 [body weight] bwt), medium-dose (5.0 mg kg−1 bwt), and high-dose (10.0 mg kg−1 bwt) LIMK2i (LX-7101, Cellagen Technology, San Diego, CA, USA), respectively.15,16 The low-dose (L), medium-dose (M), and high-dose (H) LIMK2i groups were treated with once-daily intraperitoneal injections of LIMK2i for 1 week beginning on the day following CNCI. The sham (S) and crush injury (I) groups were treated with intraperitoneal administration of vehicle only (saline). The rats were anesthetized with an intraperitoneal injection of zoletil (10 mg kg−1 bwt; Vibac Laboratories, Carros, France) and an isoflurane inhalation (Abbott Laboratories, North Chicago, IL, USA). In the C group, which mimicked a clinical context of nerve-sparing RP, the CNs were crushed by applying a microsurgical vascular clamp (Solco, Pyeongtaek, Korea) at a location 2–3 mm distal to the major pelvic ganglion for 70 s, and then re-applied for an additional 70 s after a 35-s rest period.13 Both CNs were just exposed by an identical procedure of pelvic dissection in the S group, without any direct nerve damage.13 The CNCIs were produced by the same trained surgeon. Six rats in each group were evaluated for erectile function. The remaining six rats in each group were sacrificed without nerve stimulation or intracavernous cannulation to avoid inadvertent changes in histological staining or Western blot assay.
Assessment of erectile function
After a 48 h washout period, in vivo erectile function was assessed in anesthetized rats using erectile responses to electrical stimulation of the CNs at 1 week after surgery, as described previously.13 In brief, the carotid artery was cannulated using a 24G angiocatheter (Becton Dickinson, Seoul, Korea) for continuous monitoring of the mean arterial pressure (MAP). A 26G needle (Becton Dickinson) connected to a pressure transducer (Harvard Apparatus, Holliston, MA, USA) was introduced into the corpus cavernosum for continuous monitoring of intracavernous pressure (ICP). After identification of the CN, it was stimulated using a platinum bipolar electrode (SD 9 stimulator, Grass Instrument Company, Quincy, MA, USA), and the erectile responses were evaluated by increasing voltage settings. The stimulation parameters included 1.0 V, 2.5 V, and 4.0 V at 15 Hz with a square wave duration of 0.2 ms for 30 s. Evaluation parameters were ICP and the area under the curve (AUC) corresponding to the duration of electrical stimulation, normalized with MAP.
After completion of the functional studies in six rats in each group, the whole penis of the remaining six rats in each group was harvested for histological studies and Western blot assay. The middle part of the skin-denuded penile shaft was maintained overnight in a 10% formaldehyde solution (HT501128; Sigma-Aldrich, St. Louis, MO, USA) and wax-embedded paraffin (327204; Sigma-Aldrich). The remaining penile tissues were rapidly frozen in liquid nitrogen and stored at −80°C until further processing.
Massonsintrichrome staining
To calculate the SM/collagen ratio, the specimens were stained for Masson's trichrome (ab150686; Abcam, Cambridge, UK) according to a standard protocol, as described previously.13 For each stained slide, a ×40 magnification image of the penis comprising the corpora cavernosum half was analyzed for SM cells (stained in red) and collagen (blue), using a Nikon Eclipse Ci-L microscope (Nikon Corporation, Tokyo, Japan) and the Image-Pro Plus 4.5 software (Medica Cybernetics; Silver Spring, MD, USA). Six rats from each group were evaluated, and two tissue sections per animal were reviewed. The slides were evaluated by an independent observer who was blinded to the group allocation.
Double immunofluorescence for confocal laser microscopy
To assess the content of fibroblasts positive for phosphorylated LIMK2 (co-localization of vimentin with phosphorylated LIMK2), to identify whether LIMK2 phosphorylation in the fibroblasts could contribute to corporal fibrosis, and to evaluate the efficacy of LIMK2 inhibition in the cavernous tissue, double immunofluorescence microscopy (Leica TCS SP8; Leica Microsystems, Mannheim, Germany) was performed using paraffin-embedded sections (2.5 μm) of penile tissues, as described before.13 They were incubated overnight with primary antibodies to phospho-LIMK2 (phospho T505, 1:50; Abcam) and vimentin (1:100; Dako, Carpinteria, CA, USA), a fibroblast marker. After PBS washing, the sections were incubated with two secondary antibodies (goats' anti-rabbit IgG 594, ab150080, Abcam, and anti-mouse IgG 488, ab150113, Abcam) in 1% BSA for 1 h at room temperature. Digital images were obtained using a confocal microscope (Leica TCS SP8; Leica Microsystems). Six rats from each group (two sections per animal) were analyzed. The slides were evaluated by an independent observer who was blinded to the group allocation.
Western blot analysis
Western blot analyses were performed as described previously.11,12,13 Primary antibodies included anti-ROCK1 (1:2000; Cell-Signaling Technology, Danvers, MA, USA), anti-phospho-LIMK2 (phospho T505, 1:1000; Abcam), anti-LIMK2 (1:2000; Abcam), anti-phospho-Cofilin (1:500; Cell-Signaling Technology), and anti-Cofilin (1:1000; Cell-Signaling Technology). Densitometry results were normalized to β-actin expressions.
Statistical analysis
All data are presented as mean ± standard error of the mean (s.e.m.). Statistical comparisons among groups were performed using the Mann–Whitney U-test or the Kruskal–Wallis test. The P values were two sided with P < 0.05 considered to be statistically significant. The Statistical Package for the Social Sciences, Version 20.0 (IBM Corp. Released 2011; IBM SPSS Statistics for Windows, Armonk, NY, USA), was used for analysis.
RESULTS
Effect of bilateral CNCI on erectile response, corporal fibrosis, and the ROCK/LIMK2/Cofilin pathway
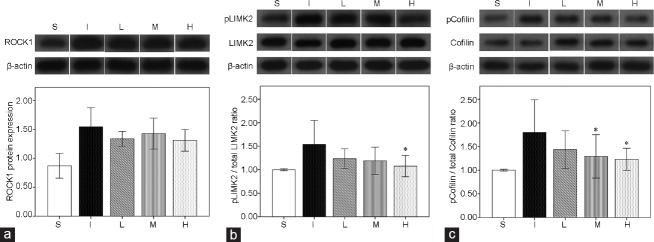
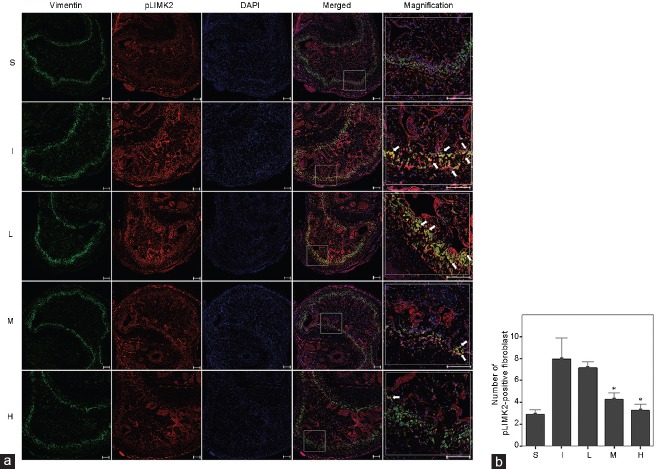
There was no significant difference in baseline MAP and changes of body weight among the experimental groups. Compared with the S group, the I group showed both lower ICP/MAP (1.0 V, P = 0.004; 2.5 V, P = 0.004; and 4.0 V, P = 0.004) and AUC/MAP (1.0 V, P = 0.002; 2.5 V, P = 0.004; and 4.0 V, P = 0.002) at all stimulation voltages 1 week after surgery (Figure 2), which was also the case for the SM/collagen ratio compared with the S group (P = 0.008), according to Masson's trichrome staining (Figure 3). The densitometry revealed that ROCK1 protein expression (P = 0.004), LIMK2 phosphorylation (P < 0.001), and Cofilin phosphorylation (P < 0.001) in the I group were significantly increased compared with those in the S group (Figure 4). The double immunofluorescent staining of cavernous tissue with antibodies to phospho-LIMK2 and vimentin demonstrated that the content of fibroblasts positive for phospho-LIMK2 in the I group was also significantly higher than that in the S group (P < 0.001; (Figure 5).
Figure 2.
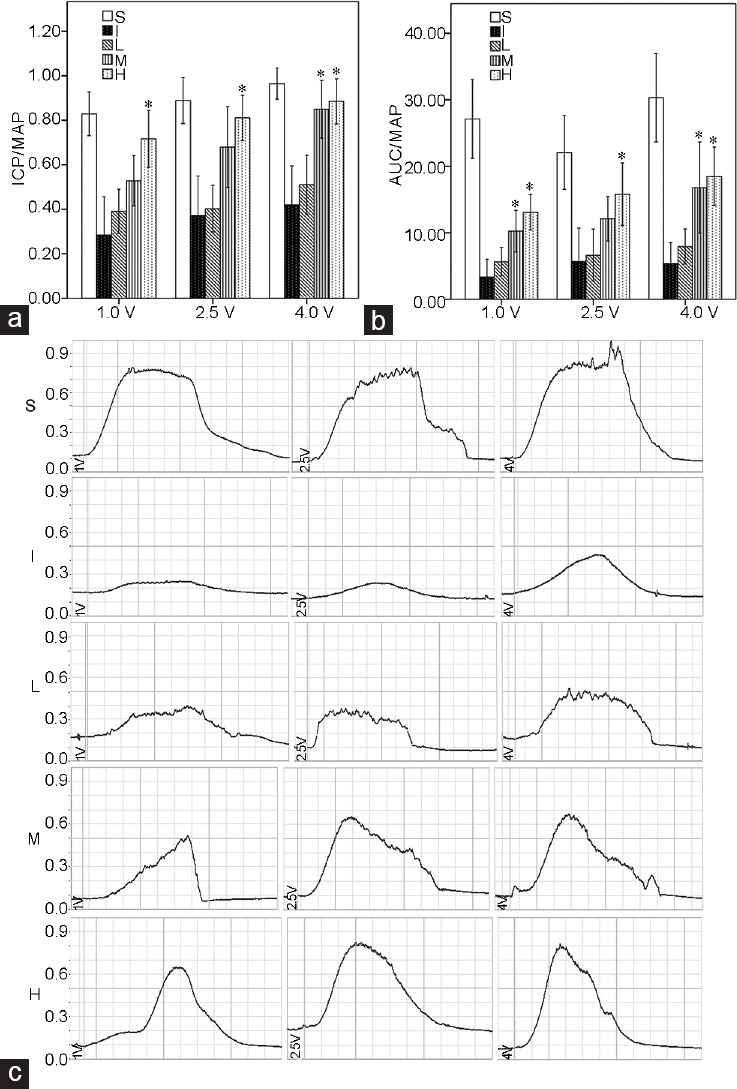
Bar graphs showing the comparison in erectile responses to electrostimulation at 1 week after bilateral CNCI among the five groups (S, I, L, M, and H). The erectile responses were evaluated by increasing voltage settings. (a) ICP/MAP, (b) AUC/MAP, (c) representative trace of ICP/MAP. Six rats in each group were evaluated for erectile function. CNCI: cavernous nerve crush injury; ICP: intracavernous pressure; AUC: area under the curve corresponding to the duration of electrical stimulation; MAP:mean arterial pressure; LIMK2: LIM-kinase 2; S: sham surgery group; I: bilateral CNCI group; L: bilateral CNCI group treated with low-dose LIMK2 inhibitors; M: bilateral CNCI group treated with medium-dose LIMK2 inhibitors; H: bilateral CNCI group treated with high-dose LIMK2 inhibitors. *P < 0.05 when compared to bilateral CNCI (I) group.
Figure 3.
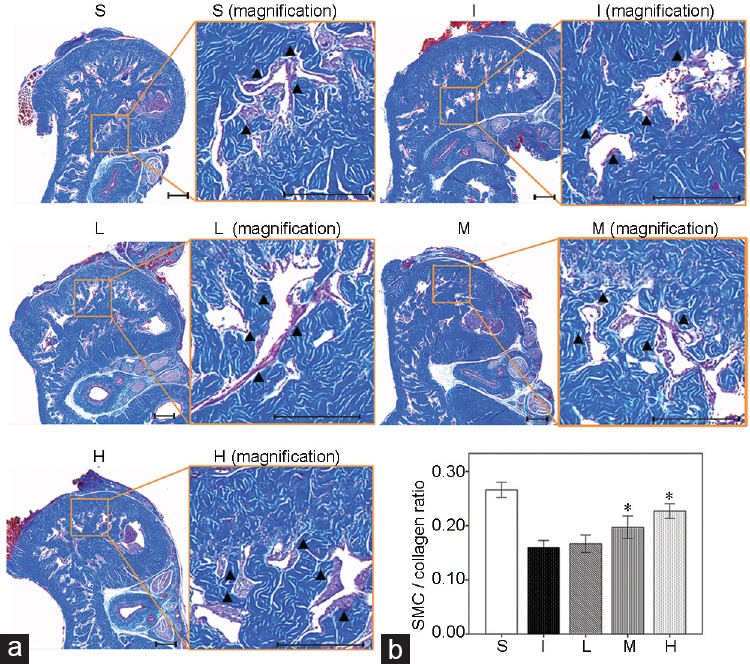
Effect of bilateral CNCI and LIMK2 inhibition on corporal fibrosis after 1 week of surgery, as evaluated by smooth muscle cell (SMC)/collagen ratio. (a) Representative images for Masson's trichrome staining and (b) bar graphs showing the smooth muscle cell/collagen ratio (mean ± s.e.m.) among the five groups at 1 week after surgery. Smooth muscle and collagen fibers were stained in red and blue, respectively. Magnification images and black short arrows indicate the smooth muscle contents. Six rats in each group were analyzed for smooth muscle cell/collagen ratio. CNCI: cavernous nerve crush injury; LIMK2: LIM-kinase 2; S: sham surgery group; I: bilateral CNCI group; L: bilateral CNCI group treated with low-dose LIMK2 inhibitors; M: bilateral CNCI group treated with medium-dose LIMK2 inhibitors; H: bilateral CNCI group treated with high-dose LIMK2 inhibitors; s.e.m.: standard error of the mean. Scale bars = 200 μm in (a). *P < 0.05 when compared to bilateral CNCI (I) group.
Figure 4.
Representative immunoblot images and bar graphs (mean ± s.e.m.) showing the comparison in the protein expression of (a) ROCK, (b) phosphorylated LIMK2 (pLIMK2)/total LIMK2, (c) phosphorylated Cofilin (pCofilin)/total Cofilin from the cavernosal tissues among the five groups using densitometry. The results were normalized by β-actin expression and presented as fold changes over controls. Six rats in each group were analyzed by densitometry. LIMK2: LIM-kinase 2; S: sham surgery group; I: bilateral CNCI group; L: bilateral CNCI group treated with low-dose LIMK2 inhibitors; M: bilateral CNCI group treated with medium-dose LIMK2 inhibitors; H: bilateral CNCI group treated with high-dose LIMK2 inhibitors; CNCI: cavernous nerve crush injury; ROCK1: Rho-associated coiled-coil protein kinase 1; s.e.m.: standard error of the mean. *P < 0.05 when compared to bilateral CNCI (I) group.
Figure 5.
Effect of bilateral CNCI and LIMK2 inhibition on the content of fibroblasts positive for phosphorylated LIMK2 (pLIMK2) after 1 week of surgery. Six rats in each group were analyzed by confocal laser scanning. (a) Representative images for double immunofluorescent staining of cavernosal tissue with anti-vimentin and anti-phospho-LIMK2 in the five groups using confocal microscope. White arrow indicates significant expression of phosphorylated LIMK2 in cavernosal fibroblasts (yellow color in merged or magnified image). (b) Bar graphs showing the comparison in fibroblasts positive for phosphorylated LIMK2 (mean ± s.e.m.) among the five groups. LIMK2: LIM-kinase 2; S: sham surgery group; I: bilateral CNCI group; L: bilateral CNCI group treated with low-dose LIMK2 inhibitors; M: bilateral CNCI group treated with medium-dose LIMK2 inhibitors; H: bilateral CNCI group treated with high-dose LIMK2 inhibitors; s.e.m.: standard error of the mean. Scale bars = 200 μm in (a). *P < 0.05 when compared to bilateral CNCI (I) group.
Daily administration of LIMK2i improves the erectile response in a rat model of bilateral CNCI
To verify the physiological relevance of administration of LIMK2i, in vivo erectile responses to electrical stimulation of the CNs were determined. In the L group, daily intraperitoneal administration of 2.5 mg kg−1 bwt LIMK2i did not improve the erectile responses, which were not different from those in the I group at any stimulation voltage. In the M group, ICP/MAP obtained with 4.0 V stimulation (P = 0.017) and AUC/MAP with electrical stimulation (1.0 V, P = 0.009; 4.0 V, P = 0.009) were significantly increased compared with the I group, but AUC/MAP with 1.0 V or 4.0 V stimulation did not recover to the level determined in the S group. In addition, ICP/MAP obtained with 1.0 V or 2.5 V stimulation and AUC/MAP with 2.5 V stimulation in the M group were not significantly different from those in the I group. In the H group, daily intraperitoneal administration of 10.0 mg kg−1 bwt LIMK2i significantly increased the ICP/MAP (1.0 V, P = 0.004; 2.5 V, P = 0.015; and 4.0 V, P = 0.004) and AUC/MAP (1.0 V, P = 0.004; 2.5 V, P = 0.041; and 4.0 V, P = 0.004) at all stimulation voltages compared with the I group. However, the values of AUC/MAP at all stimulation voltages in the H group did not completely return to those found in the S group, the age-matched control.
Inhibition of LIMK2 alleviates corporal fibrosis through normalization of ROCK1/LIMK2/Cofilin pathway
At 1 week after surgery, the SM/collagen ratios in the M and H groups were significantly higher than that in the I group (M group, P = 0.016; H group, P = 0.008), but lower than that in the S group (M group, P = 0.008; H group, P = 0.016) (Figure 3). In addition, the content of fibroblasts positive for phospho-LIMK2 in the M (P = 0.026) and H groups (P = 0.001) was significantly reduced compared with the I group (Figure 5). On the contrary, daily administration of 2.5 mg kg−1 bwt LIMK2i in the L group did not improve the SM/collagen ratio or the extent of LIMK2 phosphorylation in fibroblasts.
According to the densitometry, LIMK2 phosphorylation in the H group was significantly reduced compared with that in the I group (P = 0.035), while Cofilin phosphorylation was lowered in both the M (P = 0.027) and H groups (P = 0.043). However, LIMK2 and Cofilin phosphorylation in the L group did not significantly differ from those in the I group. Inhibition of LIMK2 in the L, M, and H groups did not reduce the protein expression of ROCK1 compared with the I group (Figure 4).
DISCUSSION
To our knowledge, the present study is the first to demonstrate that the inhibition of LIMK2, particularly with administration of 10.0 mg kg−1 bwt LIMK2i, could improve erectile function by suppression of corporal fibrosis via normalization of the LIMK2/Cofilin pathway in a rat model of CNCI. The main findings of this study are summarized as follows. (1) It was confirmed that the ROCK1/LIMK2/Cofilin signaling pathway could contribute to corporal fibrosis after CNCI. (2) Inhibition of LIMK2 with 10.0 mg kg−1 bwt LIMK2i from the immediate post-injury period significantly alleviated corporal fibrosis by normalizing the ROCK1/LIMK2/Cofilin signaling pathway and the content of fibroblasts positive for phosphorylated LIMK2. The inhibition of LIMK2 with 5.0 mg kg−1 bwt LIMK2i partially improved the dysregulated LIMK2/Cofilin pathway and the increased content of fibroblasts positive for phosphorylated LIMK2. (3) Daily administration of 10.0 mg kg−1 bwt LIMK2i significantly mended erectile functions compared with the vehicle treatment, while the administration of 5.0 mg kg−1 bwt LIMK2i partially rectified erectile functions. In addition, the present study demonstrated, to some degree, dose-escalation effects of early treatment with LIMK2i on corporal fibrosis and EDs caused by CNCI.
Although corporal fibrosis caused by CN injury plays a critical role in the development of post-RP ED, there has been a scarcity of studies about specific pathways responsible for the fibrosis of cavernosal tissues after CN injury or whether the restoration of specific dysregulated pathways can improve erectile functions by suppression of corporal fibrosis. Thus, there is still room for improving the alleviation of post-RP ED through suppression of specific target molecules that contribute to corporal fibrosis after CN injury. The ROCK pathway is important in the progression of TGF-β-induced vascular fibrosis in cardiovascular disease.17 Activation of LIMK2, a downstream effector of ROCK, induces cytoskeletal rearrangements through Cofilin phosphorylation, which results in a fibroblast-to-myofibroblast differentiation, a pathophysiological feature of fibrosis.18 In this context, we considered the ROCK1/LIMK2/Cofilin pathway as a potential candidate that can cause corporal fibrosis. Recently, we also revealed that fibroblasts positive for ROCK1 or phosphorylated LIMK2 significantly increased in cavernosal tissue from the early post-CNCI period.12 Furthermore, the present study confirmed that the ROCK1/LIMK2/Cofilin pathways might play a critical role in corporal fibrosis caused by CNCI, which corroborated our previous findings.11,12
A fibrogenic response may have adaptive features during a very short period.19 However, when it progresses over a prolonged period of time, parenchymal scarring and, ultimately, cellular dysfunction or organ failure can ensue.19 Previous reports using rat models on bilateral CN injuries showed that corporal fibrosis developed from early postinjury period and progressed over time.11,12,20 Therefore, after structural alterations such as the progressive fibrosis of cavernosal tissues are developed, erectile functions are unlikely to recover despite an improvement from neuropraxia, a finding possibly supported by previous research showing that corporal fibrosis was not alleviated by a single sildenafil administration at various time points after CN injury.21 One of our previous studies demonstrated that an inhibition of the Rho-kinase/LIMK2/Cofilin pathway with daily administration of Rho-kinase inhibitors at an early post-CNCI period improved corporal fibrosis and CVOD.13 However, for improvement in erectile function through alleviation of corporal fibrosis, the specific inhibition by a downstream effector of a fibrosis-related pathway such as LIMK2 may have benefits in terms of both efficacy and safety. Thus, we hypothesized that early intervention with LIMK2 inhibitors beginning from the immediate post-CNCI period would increase the chance of improvement in erectile functions by corporal fibrosis suppression. In accordance with our hypothesis, the present study noted that early LIMK2 inhibition could improve erectile functions through the alleviation of corporal fibrosis. Similarly, a prior in vitro investigation revealed that downregulation of LIMK by direct application of LIMK siRNA in corneal fibroblasts suppressed TGF-β-mediated ocular fibrosis.22 In addition, a previous report by Morin et al.14 showed that selective inhibition of LIMK effectively blocked TGF-β-induced cell motility or invasion and indicated that LIMK represented an attractive therapeutic target in TGF-β-induced organ fibrosis. Preclinical research utilizing a mouse model of glaucoma revealed that LIMK2i (LX7101) significantly reduced intraocular pressure by inducing depolymerization of actin filaments in the trabecular meshwork, resulting in tissue relaxation.23
Rho-kinase inhibitors are a series of compounds that interfere with the RhoA/ROCK pathway.24 Fasudil, a potent Rho-kinase inhibitor, is used for improvement and prevention of cerebral vasospasm and cerebral ischemia after subarachnoid hemorrhage surgery in China and Japan.25 However, it is not approved by the USA and Europe. Ripasudil, a Rho-kinase inhibitor eye drop, is used for the treatment of glaucoma and ocular hypertension in Japan.26 However, the Rho-kinase inhibitors have not yet been approved by the USA and Europe. The Rho-kinase inhibitors have potential adverse effects such as systemic vasodilation or hypotension.27,28,29 Thus, selective inhibition of LIMK, a downstream effector of ROCK, would be better than targeting ROCK itself in terms of both efficacy and safety.14 LIMK is a serine protein kinase that plays a critical role in the regulation of the actin cytoskeleton by phosphorylating and deactivating Cofilin.30 The LX7101, a pyrrolopyrimidine-based inhibitor of LIMK2, has been recently developed.23 A randomized, double-blind, placebo-controlled Phase 1/2a trial of topically administered LX7101 for patients with open-angle glaucoma or ocular hypertension is ongoing.31
Interestingly, the LX7101 is reported to be a dual LIMK2 and ROCK inhibitor.23 According to the densitometry of our study, the administered dose of 10 mg LX-7101 in our study tended to suppress the increase of ROCK1 expression. However, there was no statistically significant suppression of the increased ROCK1 expression. Meanwhile, 10 mg LX7101 significantly improved the dysregulated LIMK2 phosphorylation. There are some plausible explanations for it. First, the LX7101 is thought to be a potent inhibitor of LIMK2, although we cannot rule out the possibility that some of the observed activities are due to weak inhibition of ROCK.23 This is supported by a recent finding showing that the LX7101 has proved significantly selective for LIMK2 (300-fold compared to ROCK1).23 Second, there is a possibility that the administered dose of 10 mg LX7101 in our study is not sufficient for significant suppression of ROCK1 expression.
According to our results, the inhibition of LIMK2 by administering LIMK2i improved erectile function, but could not completely restore it to normal values. In addition, the SM/collagen ratio was significantly corrected; however, it did not recover to the point of control values despite the normalization of fibroblasts' content positive for phosphorylated LIMK2 by administration of 10.0 mg kg−1 bwt LIMK2i. On the basis of the incomplete erectile functions' recovery observed in our study, other structural alterations including corporal apoptosis may also contribute to the development of post-RP ED. Moreover, a possible explanation for the SM/collagen ratio's incomplete recovery in the cavernosum is that other molecular pathways such as the Smad pathway might play a role in corporal fibrosis after CN injury.32 Thus, further research is needed to explore whether a combination of LIMK2i with anti-apoptotic agents or anti-fibrotic agents targeting other fibrotic pathways can restore erectile function to control values. Furthermore, additional time course studies with long-term follow-ups using dynamic infusion cavernosometry might be necessary to determine the effect of chronic treatment with LIMK2i on CVOD caused by CN injury.
A limitation of our study is that although both ROCK1 and ROCK2 are expressed in the penis, expression of cavernosal ROCK2 was not evaluated. Several studies have suggested a potential role for RhoA/ROCK1 signaling pathway in the development of fibrosis.33,34 We also noticed that the activation of RhoA is followed by the activation of downstream target kinases, such as ROCK1, and subsequently accompanied by phosphorylation of the downstream effectors LIMK2 and Cofilin, resulting in fibroblast-to-myofibroblast differentiation, a pathophysiologic feature of fibrosis.11,12 However, previous studies reported that ROCK2 was increased after CN injury.35,36 Therefore, expression of ROCK2 needs to be evaluated in future studies. Another limitation is that vimentin can be stained in macrophages and SM cells of mesenchymal origin as well as fibroblasts. Thus, although previous studies have used vimentin as a fibroblast marker, the subsequent studies need to use a more specific marker of fibroblasts or additional protocols such as flow cytometry for sorting vimentin-positive cells into fibroblasts. Finally, although the inhibition of LIMK2 could improve the maximal ICP/MAP in a similar level to S control, it did not normalize the AUC/MAP (maintenance of erection) to the level observed in S control. Thus, the maximal ICP/MAP was increased, but the animals were unable to maintain an erection. Nevertheless, using a rat model of CNCI that could approximate a clinical situation in human males undergoing nerve-sparing RP, this study's results suggest the inhibition of LIMK2 as an early treatment strategy, at least as a part of combination therapy, for improving post-RP ED through suppressing the corporal fibrosis caused by CN injury.
CONCLUSIONS
Our data suggest that the inhibition of LIMK2 beginning from the early postoperative period, particularly with administration of 10.0 mg kg−1 bwt LIMK2i, can improve corporal fibrosis and erectile functions by normalizing the LIMK2/Cofilin pathway in a rat model of CNCI, although the animals did not completely recover to normal values. Thus, an early therapeutic strategy targeting the LIMK2/Cofilin pathway may alleviate corporal fibrosis caused by CN injury, resulting in improvement of post-RP ED. Future time course studies with long-term follow-ups for determining treatment effects of LIMK2 inhibition combined with anti-apoptotic agents or anti-fibrotic agents targeting other fibrotic pathways are needed to render our results more clinically useful.
AUTHOR CONTRIBUTIONS
JP carried out substantial contributions to conception/design, animal experiments, data acquisition, data analysis, data interpretation, drafting the manuscript, and statistical analysis. SYC and KP carried out substantial contributions to conception/design and data interpretation. JSC helped in animal experiments and data acquisition. HS, SWK, and JSP carried out data interpretation and critical revision of the manuscript for scientific and factual content and helped to draft the manuscript. MCC carried out substantial contributions to conception/design, data interpretation, drafting the manuscript, supervision, and final approval of the version to be published. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (grant No.: NRF-2014R1A1A1006572).
REFERENCES
- 1.Tal R, Alphs HH, Krebs P, Nelson CJ, Mulhall JP. Erectile function recovery rate after radical prostatectomy: a meta-analysis. J Sex Med. 2009;6:2538–46. doi: 10.1111/j.1743-6109.2009.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallina A, Ferrari M, Suardi N, Capitanio U, Abdollah F, et al. Erectile function outcome after bilateral nerve sparing radical prostatectomy: which patients may be left untreated? J Sex Med. 2012;9:903–8. doi: 10.1111/j.1743-6109.2011.02622.x. [DOI] [PubMed] [Google Scholar]
- 3.Tavukçu HH, Aytac O, Atug F. Nerve-sparing techniques and results in robot-assisted radical prostatectomy. Investig Clin Urol. 2016;57(Suppl 2):S172–84. doi: 10.4111/icu.2016.57.S2.S172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capogrosso P, Salonia A, Briganti A, Montorsi F. Postprostatectomy erectile dysfunction: a review. World J Mens Health. 2016;34:73–88. doi: 10.5534/wjmh.2016.34.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullerad M, Donohue JF, Li PS, Scardino PT, Mulhall JP. Functional sequelae of cavernous nerve injury in the rat: is there model dependency. J Sex Med. 2006;3:77–83. doi: 10.1111/j.1743-6109.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- 6.Weyne E, Castiglione F, Van der Aa F, Bivalacqua TJ, Albersen M. Landmarks in erectile function recovery after radical prostatectomy. Nat Rev Urol. 2015;12:289–97. doi: 10.1038/nrurol.2015.72. [DOI] [PubMed] [Google Scholar]
- 7.Kovanecz I, Rambhatla A, Ferrini M, Vernet D, Sanchez S, et al. Long-term continuous sildenafil treatment ameliorates corporal veno-occlusive dysfunction (CVOD) induced by cavernosal nerve resection in rats. Int J Impot Res. 2008;20:202–12. doi: 10.1038/sj.ijir.3901612. [DOI] [PubMed] [Google Scholar]
- 8.Leungwattanakij S, Bivalacqua TJ, Usta MF, Yang DY, Hyun JS, et al. Cavernous neurotomy causes hypoxia and fibrosis in rat corpus cavernosum. J Androl. 2003;24:239–45. doi: 10.1002/j.1939-4640.2003.tb02668.x. [DOI] [PubMed] [Google Scholar]
- 9.Hu WL, Hu LQ, Song J, Li SW, Zheng XM, et al. Fibrosis of corpus cavernosum in animals following cavernous nerve ablation. Asian J Androl. 2004;6:111–6. [PubMed] [Google Scholar]
- 10.El-Sakka AI. Alleviation of post-radical prostatectomy cavernosal fibrosis: future directions and potential utility for PDE5 inhibitors. Expert Opin Investig Drugs. 2011;20:1305–9. doi: 10.1517/13543784.2011.609315. [DOI] [PubMed] [Google Scholar]
- 11.Cho MC, Park K, Chai JS, Lee SH, Kim SW, et al. Involvement of sphingosine-1-phosphate/RhoA/Rho-kinase signaling pathway in corporal fibrosis following cavernous nerve injury in male rats. J Sex Med. 2011;8:712–21. doi: 10.1111/j.1743-6109.2010.02147.x. [DOI] [PubMed] [Google Scholar]
- 12.Song SH, Park K, Kim SW, Paick JS, Cho MC. Involvement of Rho-Kinase/Lim Kinase/Cofilin signaling pathway in corporal fibrosis after cavernous nerve injury in male rats. J Sex Med. 2015;12:1522–32. doi: 10.1111/jsm.12903. [DOI] [PubMed] [Google Scholar]
- 13.Cho MC, Park K, Kim SW, Paick JS. Restoration of erectile function by suppression of corporal apoptosis, fibrosis and corporal veno-occlusive dysfunction with Rho-Kinase inhibitors in a rat model of cavernous nerve injury. J Urol. 2015;193:1716–23. doi: 10.1016/j.juro.2014.10.099. [DOI] [PubMed] [Google Scholar]
- 14.Morin P, Wickman G, Munro J, Inman GJ, Olson MF. Differing contributions of LIMK and ROCK to TGFβ-induced transcription, motility and invasion. Eur J Cell Biol. 2011;90:13–25. doi: 10.1016/j.ejcb.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Boland S, Bourin A, Alen J, Geraets J, Schroeders P, et al. Design, synthesis and biological characterization of selective LIMK inhibitors. Bioorg Med Chem Lett. 2015;25:4005–10. doi: 10.1016/j.bmcl.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Jung G, Kim BS, Song WH, Park J, Park K, et al. 886 Improvement of erectile function by suppression of corporal fibrosis with LIM-kinase2 inhibitors in a rat model of cavernous nerve injury. Eur Urol Suppl. 2016;15:e886. [Google Scholar]
- 17.Surma M, Wei L, Shi J. Rho Kinase as a therapeutic target in cardiovascular disease. Future Cardiol. 2011;7:657–71. doi: 10.2217/fca.11.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kardassis D, Murphy C, Fotsis T, Moustakas A, Stournaras C. Control of transforming growth factor beta signal transduction by small GTPases. FEBS J. 2009;276:2947–65. doi: 10.1111/j.1742-4658.2009.07031.x. [DOI] [PubMed] [Google Scholar]
- 19.Rockey DC, Bell PD, Hill JA. Fibrosis – A common pathway to organ injury and failure. N Engl J Med. 2015;373:96. doi: 10.1056/NEJMc1504848. [DOI] [PubMed] [Google Scholar]
- 20.Ferrini MG, Kovanecz I, Sanchez S, Umeh C, Rajfer J, et al. Fibrosis and loss of smooth muscle in the corpora cavernosa precede corporal veno-occlusive dysfunction (CVOD) induced by experimental cavernosal nerve damage in the rat. J Sex Med. 2009;6:415–28. doi: 10.1111/j.1743-6109.2008.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vignozzi L, Morelli A, Filippi S, Vannelli GB, Mungai S, et al. Effect of sildenafil administration on penile hypoxia induced by cavernous neurotomy in the rat. Int J Impot Res. 2008;20:60–7. doi: 10.1038/sj.ijir.3901596. [DOI] [PubMed] [Google Scholar]
- 22.Gorovoy M, Koga T, Shen X, Jia Z, Yue BY, et al. Downregulation of LIM kinase 1 suppresses ocular inflammation and fibrosis. Mol Vis. 2008;14:1951–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison BA, Almstead ZY, Burgoon H, Gardyan M, Goodwin NC, et al. Discovery and development of LX7101, a dual LIM-kinase and ROCK Inhibitor for the treatment of glaucoma. ACS Med Chem Lett. 2015;6:84–8. doi: 10.1021/ml500367g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15:2208–16. [PMC free article] [PubMed] [Google Scholar]
- 25.Satoh S, Ikegaki I, Kawasaki K, Asano T, Shibuya M. Pleiotropic effects of the Rho-Kinase inhibitor fasudil after subarachnoid hemorrhage: a review of preclinical and clinical studies. Curr Vasc Pharmacol. 2014;12:758–65. doi: 10.2174/1570161112666140613115813. [DOI] [PubMed] [Google Scholar]
- 26.Garnock-Jones KP. Ripasudil: first global approval. Drugs. 2014;74:2211–5. doi: 10.1007/s40265-014-0333-2. [DOI] [PubMed] [Google Scholar]
- 27.Shibuya M, Hirai S, Seto M, Satoh S, Ohtomo E. Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial. J Neurol Sci. 2005;238:31–9. doi: 10.1016/j.jns.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Shibuya M, Suzuki Y, Sugita K, Saito I, Sasaki T, et al. Effect of AT877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Results of a prospective placebo-controlled double-blind trial. J Neurosurg. 1992;76:571–7. doi: 10.3171/jns.1992.76.4.0571. [DOI] [PubMed] [Google Scholar]
- 29.Defert O, Boland S. Rho kinase inhibitors: a patent review (2014 – 2016) Expert Opin Ther Pat. 2017;27:507–15. doi: 10.1080/13543776.2017.1272579. [DOI] [PubMed] [Google Scholar]
- 30.Scott RW, Olson MF. LIM kinases: function, regulation and association with human disease. J Mol Med (Berl) 2007;85:555–68. doi: 10.1007/s00109-007-0165-6. [DOI] [PubMed] [Google Scholar]
- 31.Freiman J. Study to Evaluate the Safety, Tolerability, and Efficacy of LX7101 in Subjects With Primary Open-angle Glaucoma or Ocular Hypertension NLM Identifier: NCT01528111. [Last accessed on 2017 Oct 15]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01528111 .
- 32.Canguven O, Lagoda G, Sezen SF, Burnett AL. Losartan preserves erectile function after bilateral cavernous nerve injury via antifibrotic mechanisms in male rats. J Urol. 2009;181:2816–22. doi: 10.1016/j.juro.2009.01.097. [DOI] [PubMed] [Google Scholar]
- 33.Haudek SB, Gupta D, Dewald O, Schwartz RJ, Wei L, et al. Rho kinase-1 mediates cardiac fibrosis by regulating fibroblast precursor cell differentiation. Cardiovasc Res. 2009;83:511–8. doi: 10.1093/cvr/cvp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rikitake Y, Oyama N, Wang CY, Noma K, Satoh M, et al. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/- haploinsufficient mice. Circulation. 2005;112:2959–65. doi: 10.1161/CIRCULATIONAHA.105.584623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hannan JL, Kutlu O, Stopak BL, Liu X, Castiglione F, et al. Valproic acid prevents penile fibrosis and erectile dysfunction in cavernous nerve-injured rats. J Sex Med. 2014;11:1442–51. doi: 10.1111/jsm.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gratzke C, Strong TD, Gebska MA, Champion HC, Stief CG, et al. Activated RhoA/Rho kinase impairs erectile function after cavernous nerve injury in rats. J Urol. 2010;184:2197–204. doi: 10.1016/j.juro.2010.06.094. [DOI] [PubMed] [Google Scholar]