Abstract
This study was performed to investigate a potential marker for the presence of spermatozoa in the ejaculate following varicocelectomy in Chinese men with nonobstructive azoospermia and varicoceles. The micro-RNA (miR)-192a levels in seminal plasma and testicular tissue were evaluated by quantitative real-time polymerase chain reaction from 60 men with nonobstructive azoospermia and varicoceles (Group A: 27 men with spermatozoa found in the ejaculate after surgery; Group B: 33 men without spermatozoa found in the ejaculate after surgery) and 30 controls. The seminal plasma and testicular tissue miR-192a levels were higher in Group B than in Group A and the controls (P < 0.001), and there was no significant difference between Group A and the controls (P > 0.05). Apoptosis and proliferation assays with miR mimics and inhibitors showed that miR-192a induced GC-2 cell apoptosis through the activation of Caspase-3 protein. Thus, seminal plasma miR-192a appears to be a potential marker for successfully indicating spermatozoa in the ejaculate following microsurgical varicocelectomy in men with nonobstructive azoospermia and varicoceles. Seminal plasma miR-192a may be a useful clinical marker for prescreening to determine which patients with nonobstructive azoospermia and varicoceles would benefit from varicocelectomy.
Keywords: miR-192a, nonobstructive azoospermia, seminal plasma, varicocelectomy
INTRODUCTION
Clinical varicoceles have been reported in 4.3%–13.3% of infertile men with nonobstructive azoospermia (NOA).1 Previous studies have indicated that varicocelectomy may improve sperm production. Weedin et al.2 recently published a meta-analysis of 11 studies from the previous 20 years. This involved 233 patients with NOA and varicoceles who underwent varicocelectomy. Overall, 39.1% (91/233) of patients had motile spermatozoa in the ejaculate following varicocele repair. A total of 10.3% (24/233) pregnancies were reported, of which 6.0% (14/233) were spontaneous. A similar study by Esteves and Glina showed that 47.1% (8/17) of men with NOA had spermatozoa in the ejaculate after varicocelectomy.3 Kim et al.4 also found that 42.9% (12/28) of men with NOA had motile spermatozoa in the ejaculate after surgery. Furthermore, Pasqualotto et al.5 reported improved semen quality after varicocelectomy in men with NOA who exhibited germ cell aplasia in a single large testis biopsy. A recent meta-analysis showed that motile spermatozoa were detected in the ejaculate of 60.0% (18/30) of men with hypospermatogenesis, 46.2% (12/26) of men with maturation arrest, and 2.9% (1/34) of men with Sertoli cell-only syndrome who underwent microsurgical subinguinal varicocelectomy.6
Overall, about 10%–50% of patients with NOA may show motile spermatozoa in the ejaculate after varicocelectomy.7 Varicocelectomy is an invasive and expensive procedure; thus, a clinical marker that can indicate which patients are most likely to benefit from varicocelectomy would be useful. The surgical procedure would then only target those patients who have a high chance of obtaining ejaculated spermatozoa after varicocelectomy.
Micro-RNAs (miRs) are a naturally occurring class of noncoding regulatory RNAs that modulate protein expression by binding to the 3'-untranslated region of mRNA, inhibiting mRNA translation and affecting nearly 30% of all protein-coding genes.8 Some miRs previously identified in cells and tissues have also recently been found in extra-cellular fluids such as semen plasma, serum, saliva, and urine.9 One study has shown that reduced microRNA-188-3p expression contributes to apoptosis of spermatogenic cells in patients with azoospermia.10 Seminal plasma micro-RNAs (Let-7and miR-19b) are potential biomarkers for spermatogenic status.9 Another study has shown expression of miR-15a and its target gene heat shock protein family A (Hsp70) member 1B (Hspa1B) in the spermatozoa of infertile men with varicoceles exhibiting oxidative stress.11 Our previous study also revealed that seminal miR-192a expression was significantly higher in patients with idiopathic NOA; however, whether seminal miR-192a expression is associated with varicocele repair in men with NOA remains unknown.12
In the present retrospective study, we examined the expression of seminal miR-192a in men with NOA and clinical varicoceles following microsurgical subinguinal varicocelectomy who either did or did not achieve postoperation ejaculatory spermatozoa. We evaluated how the expression of seminal miR-192a affects apoptosis and proliferation of GC-2 germ cell lines. The overall aim of the study was to identify noninvasive predictors of achieving successful ejaculatory spermatozoa following varicocelectomy.
PATIENTS AND METHODS
Study design and patients
This study was approved by Shanghai General Hospital Research and Ethics Committee (license number of ethics statement: 2012-01). All patients provided written informed consent. Patients with clinically palpable varicoceles (grades 1–3) were confirmed to have NOA by Doppler ultrasonography and repeated semen analysis. NOA with clinical varicocele patients following microsurgical subinguinal varicocelectomy with successful (Group A, n = 27) and unsuccessful (Group B, n = 33) ejaculatory spermatozoa, and 30 fertile controls from January 2014 to December 2016. Semen samples were obtained by masturbation into a sterile wide-mouth container after 3–5 days of sexual abstinence, and testicular tissue specimens were obtained during varicocelectomy. Doppler ultrasonography of the scrotum was performed before and after surgery. Sex hormones were measured by chemiluminescence assay.
Patients were excluded from the study if they had an abnormal karyotype, Y chromosome micro-deletion, a history of alcohol or drug abuse, diabetes or hypertension, or a history of vasectomy or other surgery within the previous 3 months.
Surgical procedure
Microsurgical subinguinal varicocelectomy was performed as previously described.13 Briefly, the subcutaneous tissue and fascia of scarpa were divided by electrocautery. The testis was then externalized, and a testicular biopsy was obtained. The external spermatic veins and gubernacular veins were ligated. The testis was then returned to the scrotum. A rubber band was applied, below the cord and fix. Under 4–6 -fold magnification, the external and internal spermatic fasciae were opened. The vas deferens and vessels were separated with another rubber band to avoid injury. The magnification was increased to 10-fold. All veins were doubly ligated while preserving the testicular artery, cremasteric arteries, cremaster muscle fibers, vas deferens nerves, and lymph vessels. Finally, the wound was closed in layers after the procedure. All patients were followed for 6 months postoperatively.
Hematoxylin and eosin staining
Deparaffinized and rehydrated specimens were stained with hematoxylin for 15 min, followed by eosin for 10 min at room temperature. They were then mounted in neutral resin and visualized by light microscopy (940552, Nikon, Tokyo, Japan).
Sample collection and RNA isolation
The semen samples were first centrifuged for 5 min at 1600 g, and the supernatant centrifuged for 15 min at 16 000 g, and 1 ml of the resultant supernatant was used for total RNA isolation. The total RNA was isolated from the seminal plasma using an RNeasy Micro Kit (cat. No. 74004; Qiagen, New York, USA) according to the manufacturer's protocol. Eight rounds of phenol/chloroform purification were performed to eliminate proteins and ensure collection of pure seminal plasma total RNA. The concentration of the RNA samples was determined using a Nano-Drop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
Quantitative real-time polymerase chain reaction (PCR) of miR expression
According to the manufacturer's instructions (miScript PCR Starter Kit, cat. No. 218193; Qiagen), RNA was reverse transcribed in a final volume of 20 ml containing 4 μl 5 × miScript HiSpec Buffer, 2 μl 10 × miScript Nucleics Mix, 2 μl miScript Reverse Transcriptase Mix, 7 μl RNase-free water, and 5 μl template RNA. The mix was incubated at 37°C for 60 min and 95°C for 5 min, and then held at 4°C. Real-time quantification was then performed using an Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems, Waltham, MA, USA).
Each real-time PCR included 0.5 ml universal reverse primer, 0.5 ml sense primer, 1 ml EvaGreen (Biotium, Hayward, CA, USA), 1 U ml−1 TaqMan (Biotium), and 1 ml reverse transcription product. The reactions were incubated in a 96-well optical plate at 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. U6 snRNA was used as the endogenous control gene to normalize the miR-192a content among different samples. The expression of miR-192a relative to U6 snRNA was determined using the 2−ΔCt method. The tests were repeated three times per experiment.
Cell proliferation and apoptosis analysis
GC-2 cells were seeded at a density of 1000 cells per well in 96-well microplates in Dulbecco's Modified Eagle Media/Nutrient Mixture F-12 (DMEM/F12) supplemented with 2% (w/v) fetal bovine serum. They were then transfected with mimics control, miR-192a mimics, a inhibitor control, or an miR-192a inhibitor and maintained in a humid 5% (v/v) CO2 atmosphere at 37°C. After 5 days of culture, the proliferation potential of GC-2 cells was assessed by CCK-8 assay (cat. No. CK04; Dojin Laboratories, Tokyo, Japan) according to the manufacturer's instructions. To investigate the role of miR-19b in apoptosis, GC-2 cells were homogenized in immunoprecipitation assay lysis buffer (Solarbio, Shanghai, China) containing protease inhibitors. The lysate was centrifuged at 16 000 g for 20 min at 4°C. After removal of the supernatant, 1 × loading buffer was added to the sample. The protein concentration was measured with a protein assay kit (Qiagen) according to the manufacturer's instructions. Approximately 20 μg of protein was loaded on each gel and electrotransferred to polyvinylidene difluoride membranes by standard procedures. Caspase-3 was detected using a rabbit polyclonal antibody to Caspase-3 (1:300; Proteintech, Chicago, IL, USA), followed by a mouse anti-rabbit secondary antibody (1:2000; Multi Sciences Biotech, Chicago, IL, USA). The band intensity was quantified relative to that of actin beta (ACTB; Multi Sciences Biotech) using ImageQuant TL 7.0 software (GE Healthcare, Little Chalfont, UK).
Statistical analyses
Data analysis was performed with SPSS 19.0 (IBM Corp., Armonk, NY, USA). The differences in selected demographic variables between the cases and controls were evaluated by the Chi-square test. Statistical analyses for miR expression levels were performed by the Mann–Whitney U-test. P ≤ 0.001 was considered statistically significant.
RESULTS
Patients' clinical characteristics
The patients' clinical characteristics are summarized in Table 1. Age, body mass index, varicocele grades, and follicle-stimulating hormone (FSH) levels were similar between Group A and Group B (P > 0.05).
Table 1.
Baseline characteristics of nonobstructive azoospermia patients with varicocele
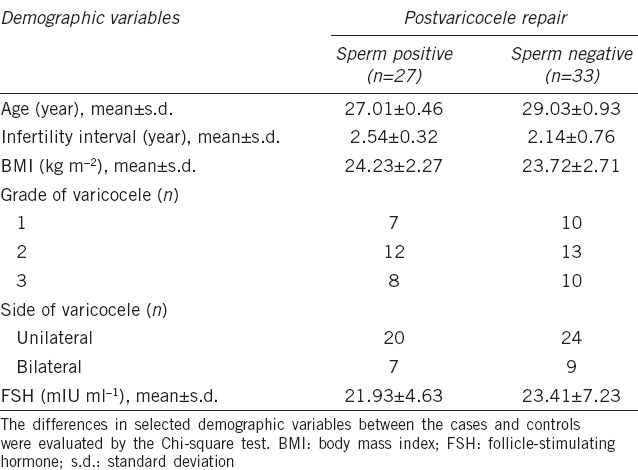
Levels and expression of miR-192a in seminal plasma and testicular tissue
To determine whether the level of miR-192a in seminal plasma or expression in testicular tissue is associated with sperm recovery in patients with NOA following varicocelectomy, the level of miR-192a in seminal plasma and testicular tissue was compared between patients in Group A, Group B and controls. Figure 1a shows that the level of seminal miR-192a in Group B was more than 2-fold higher than that in Group A and the controls. Similar results were obtained for testicular miR-192a expression between patients in Groups A and B (Figure 1b). However, the seminal miR-192a level was very similar between patients in Group A and controls (P = 0.17).
Figure 1.
Comparison of miR-192a expression in (a) seminal plasma and (b) testicular tissue. Panel a shows the expression levels of seminal miR-192a in Group B is over 2-fold higher than in Group A or the controls. (b) Similar results were obtained for testicular miR-192a expression between patients Group A and Group B. Group A: 27 men with spermatozoa in the ejaculate following surgery; Group B: 33 men without spermatozoa in the ejaculate follow surgery; Group C: 30 controls. **P < 0.001, the number is a relative level as relative to U6 snRNA, and the box is the 25th–75th centiles, the line is the median, the whiskers is the 10th and 90th centiles.
Induction of GC-2 cell apoptosis by miR-192a
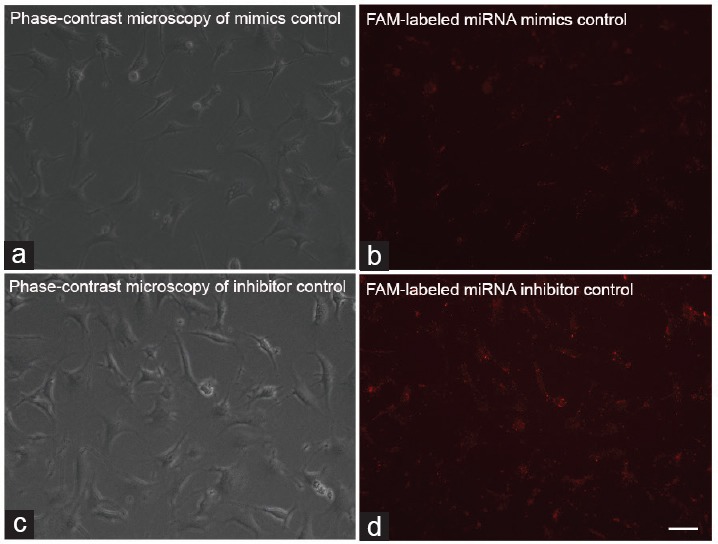
To investigate the role of miR-19b in cell proliferation, we transfected miR mimics control, miR-192a mimics, miR inhibitor control, and miR-192a inhibitor into GC-2 cells (Figure 2). A high level of miR-192a mimics induced apoptosis of GC-2 cells. Western blot was used to quantify the expression levels of Caspase-3 protein and showed an increase in Caspase-3 in the miR-192a mimics group compared with the other groups (Figure 3).
Figure 2.
Transfection efficiency of miR-192a mimics and inhibitor in GC-2 cells. (a) Phase-contrast microscopy of mimics control. (b) The transfection efficiency of miR-192a mimics using FAM-labeled miRNA mimic control oligonucleotides. (c) Phase-contrast microscopy of inhibitor control. (d) The transfection efficiency of miR-192a inhibitor using FAM-labeled miRNA inhibitor control oligonucleotides. Scale bar = 20 μm for all panels. FAM: fluorescein amide marker.
Figure 3.
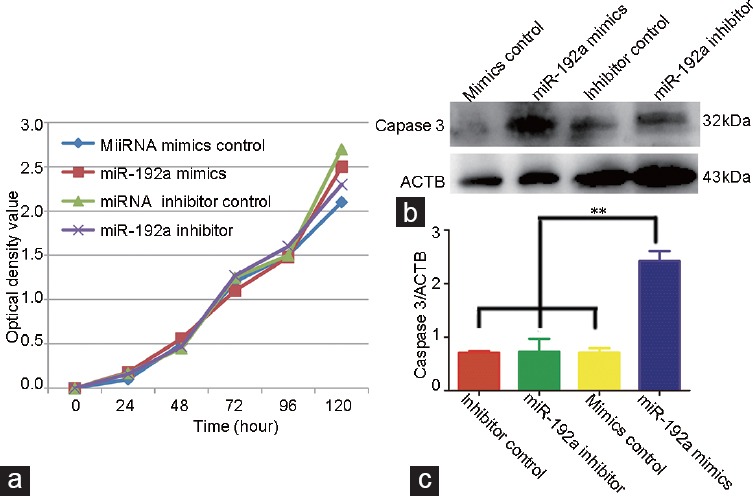
The effect of miR-192a on the proliferation and apoptosis of GC-2 cells. (a) Cell proliferation was measured using a 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay. (b) Western blot analysis of the protein Caspase-3 at 72 h after transfection. (c) Quantification of western analysis. Data are presented as the mean of three experiments. ACTB: actin beta; OD: optical density. **P<0.01.
DISCUSSION
The past decade has witnessed a growing interest in the management of clinical varicoceles in men with NOA.14 However, whether varicocelectomy is beneficial for men with NOA and varicoceles remains controversial, and many such patients exhibit no spermatozoa in the postoperative ejaculate, and varicocelectomy is an invasive and expensive procedure.7 Thus, if research reveals a predictive marker for successfully finding sperm in the ejaculate after varicocelectomy in patients with NOA, prescreening of men with NOA should be performed to determine which patients will benefit from varicocelectomy.
Seminal miRs may be a potential biomarker for the spermatogenesis status in men with NOA because they are released from living cells of the testis and their expression is very stable and resistant to degradation by ribonucleases.15 Our study clearly showed different expression levels of the seminal plasma miR-192a between Group A and Group B. The expression pattern in testicular tissue is the same as that in the seminal plasma; thus, we speculate that the higher expression level of miR-192a in the seminal plasma might be due to germ cell secretion. Its physiological functions can regulate the expression of target genes that are induced by varicoceles; such functions include testicular hypoxia, venous hypertension, elevated temperature, increased oxidative stress, and increased levels of catecholamines in the spermatic vein.7,16,17,18 Growing evidence has indicated that miRs have critical functions in regulating germ cell development in male rodents.
To identify these physiological functions, we transfected GC-2 cells with miR mimics controls, miR-192a mimics, miR inhibitor controls, and miR-192a inhibitors mimics. The miR-192a induced GC-2 cells apoptosis; however, no significant change was observed in the proliferation of cells. We also found that Caspase-3 protein increased in the miR-192a mimics group compared with the other groups. Caspase-3 (apoptosis-related cysteine peptidase) is a member of the cysteine-aspartic acid protease family, which plays a central role in the execution phase of cell apoptosis and cleaves and activates Caspases 6, 7, and 9. Thus, we suspect that miR-192a induces GC-2 cell apoptosis through Caspase-3 protein.
Our study has several limitations. First, it was a single-center retrospective study. Second, men with NOA and clinical varicoceles represent a small group of infertile men. Future studies with a larger sample size are needed to validate this association. Third, more seminal miRs expression profiling should be performed using micro-array assay, and the biological functions of miRs and their target genes should be identified.
CONCLUSIONS
The seminal plasma miR-192a expression level may be a useful clinical marker for prediction of successfully obtaining spermatozoa in the ejaculate after varicocelectomy in men with NOA and varicoceles. However, a prospective study with a large number of patients is required to confirm this finding.
AUTHOR CONTRIBUTIONS
ZL and PX conceived and designed the experiments. ELZ and GQL performed the experiments. GQL, PL, HXC, and RHT contributed to reagents, materials, and analysis tools. ELZ wrote the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This work was supported by grants from the Shanghai Shen Kang Medical Development Center (SHDC12015122. SHDC12014236), the National Nature Science Foundation of China (31230048/C12010), and the National Science Foundation of China (No. 31201109).
REFERENCES
- 1.Abdel-Meguid TA. Predictors of sperm recovery and azoospermia relapse in men with non-obstructive azoospermia after varicocele repair. J Urol. 2012;187:222–6. doi: 10.1016/j.juro.2011.09.047. [DOI] [PubMed] [Google Scholar]
- 2.Weedin JW, Khera M, Lipshultz LI. Varicocele repair in patients with nonobstructive azoospermia: a meta-analysis. J Urol. 2010;183:2309–15. doi: 10.1016/j.juro.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Esteves SC, Glina S. Recovery of spermatogenesis after microsurgical subinguinal varicocele repair in azoospermic men based on testicular histology. Int Braz J Urol. 2005;31:541–8. doi: 10.1590/s1677-55382005000600005. [DOI] [PubMed] [Google Scholar]
- 4.Kim ED, Leibman BB, Grinblat DM, Lipshultz LI. Varicocele repair improves semen parameters in azoospermic men with spermatogenic failure. J Urol. 1999;162:737–40. doi: 10.1097/00005392-199909010-00031. [DOI] [PubMed] [Google Scholar]
- 5.Pasqualotto FF, Sobreiro BP, Hallak J, Pasqualotto EB, Lucon AM. Induction of spermatogenesis in azoospermic men after varicocelectomy repair: an update. Fertil Steril. 2006;85:635–9. doi: 10.1016/j.fertnstert.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 6.Elzanaty S. Varicocele repair in non-obstructive azoospermic men: diagnostic value of testicular biopsy – a meta-analysis. Scand J Urol. 2014;48:494–8. doi: 10.3109/21681805.2014.932839. [DOI] [PubMed] [Google Scholar]
- 7.Ustuner M, Yilmaz H, Yavuz U, Ciftci S, Saribacak A, et al. Varicocele repair improves testicular histology in men with nonobstructive azoospermia. Biomed Res Int. 2015;2015:709452. doi: 10.1155/2015/709452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilz S, Modzelewski AJ, Cohen PE, Grimson A. The roles of microRNAs and siRNAs in mammalian spermatogenesis. Development. 2016;143:3061–73. doi: 10.1242/dev.136721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu W, Hu Z, Qin Y, Dong J, Dai J, et al. Seminal plasma microRNAs: potential biomarkers for spermatogenesis status. Mol Hum Reprod. 2012;18:489–97. doi: 10.1093/molehr/gas022. [DOI] [PubMed] [Google Scholar]
- 10.Song WY, Meng H, Wang XG, Jin HX, Yao GD, et al. Reduced microRNA-188-3p expression contributes to apoptosis of spermatogenic cells in patients with azoospermia. Cell Prolif. 2017;50:e12297. doi: 10.1111/cpr.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji Z, Lu R, Mou L, Duan YG, Zhang Q, et al. Expressions of miR-15a and its target gene HSPA1B in the spermatozoa of patients with varicocele. Reproduction. 2014;147:693–701. doi: 10.1530/REP-13-0656. [DOI] [PubMed] [Google Scholar]
- 12.Wu W, Qin Y, Li Z, Dong J, Dai J, et al. Genome-wide microRNA expression profiling in idiopathic non-obstructive azoospermia: significant up-regulation of miR-141, miR-429 and miR-7-1-3p. Hum Reprod. 2013;28:1827–36. doi: 10.1093/humrep/det099. [DOI] [PubMed] [Google Scholar]
- 13.Samoylov AS, Martov AG, Kyzlasov PS, Zabelin MV, Kazhera AA. [Comparative effectiveness of Marmar technique and laparoscopic clipping of testicular vein in surgical treatment of varicocele in athletes] Urologiia. 2016;6:44–6. [Article in Russian] [PubMed] [Google Scholar]
- 14.Mehta A, Goldstein M. Varicocele repair for nonobstructive azoospermia. Curr Opin Urol. 2012;22:507–12. doi: 10.1097/MOU.0b013e328358e27b. [DOI] [PubMed] [Google Scholar]
- 15.Johansen I, Andreassen R. Validation of miRNA genes suitable as reference genes in qPCR analyses of miRNA gene expression in Atlantic salmon (Salmo salar) BMC Res Notes. 2014;8:945. doi: 10.1186/1756-0500-7-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esteves SC, Miyaoka R, Roque M, Agarwal A. Outcome of varicocele repair in men with nonobstructive azoospermia: systematic review and meta-analysis. Asian J Androl. 2016;18:246–53. doi: 10.4103/1008-682X.169562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milone M, Musella M, Fernandez ME, Maietta P, Sasso A, et al. Varicocele repair in severe oligozoospermia: a case report of post-operative azoospermia. World J Clin Cases. 2014;2:94–6. doi: 10.12998/wjcc.v2.i4.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niederberger C. Re: Relationship between testicular sperm extraction and varicocelectomy in patients with varicocele and nonobstructive azoospermia. J Urol. 2014;191:1080. doi: 10.1016/j.juro.2014.01.064. [DOI] [PubMed] [Google Scholar]


