Abstract
This study aimed to gain insight into the underlying pathogenesis of erectile dysfunction in young men under the age of 40 years without widely-known risk factors. Compared with normal controls, patients with erectile dysfunction had increased carotid intima–media thickness, fasting levels of blood glucose and insulin, and homeostatic model assessment index, as well as lower flow-mediated vasodilation and testosterone levels (P < 0.05), though all of these values were within their respective normal range. Multivariate logistic regression analysis identified carotid intima–media thickness, flow-mediated vasodilation, insulin level, and homeostatic model assessment index as significant predictors of erectile dysfunction. Young men with flow-mediated vasodilation <10.65% were 11.645 times more likely to have erectile dysfunction, young men with carotid intima–media thickness >0.623 mm had a 4.16-fold, and young men with homeostatic model assessment index >1.614 had a 5.993-fold greater risk of having erectile dysfunction. In conclusions, in young men with normal results from general clinical screening, an increased carotid intima–media thickness and homeostatic model assessment index and reduced flow-mediated vasodilation were associated with a higher incidence of erectile dysfunction. Erectile dysfunction may appear before the detection of traditional cardiovascular risk factors and may be the earliest clinical sign of subclinical cardiovascular disease.
Keywords: endothelial dysfunction, erectile dysfunction, intima–media thickness, risk factors
INTRODUCTION
Cardiovascular disease is the major cause of morbidity and mortality worldwide. The treatment of end-stage atherosclerotic cardiovascular disease is extremely difficult, highlighting the importance of early diagnosis and prevention. Assessment of erectile dysfunction (ED) may help identify early atherosclerosis in men, because ED has been observed several years before coronary symptoms and assumed to be an independent marker of cardiovascular disease risk. ED is the consistent inability to achieve or maintain a penile erection adequate for satisfactory sexual performance. It is a common public health problem affecting 44% of men aged 60–69 years and up to 70% of men aged ≥70 years. In men <40 years old, about 5% are affected by ED.1,2 The etiology of ED is often multifactorial, with vascular, hormonal, lifestyle, aging, neurologic, and psychological factors playing roles. Vascular disease is by far the most common cause of ED.3 However, many young patients suffering from ED do not have clinical cardiovascular disease or abnormal laboratory findings, and their underlying pathogenesis of ED remains unknown.
The measurement of carotid intima–media thickness (CIMT) through duplex ultrasound is a common noninvasive tool used for the evaluation of early atherosclerosis and vascular remodeling. Epidemiologic evidence has proven that, similar to carotid plaques, an elevated CIMT is associated with increased atherosclerotic risk factors, prevalence and extent of coronary artery disease, and incidence of cardiovascular events.4,5,6 Endothelial dysfunction, characterized by reduced endothelium-dependent flow-mediated vasodilation (FMD), often precedes arterial structural alterations, such as increased intima–media thickness (IMT) or plaque presentation. Endothelial dysfunction is considered as an important biological mechanism linking ED and cardiovascular disease.7,8
This study was designed to gain insight into the underlying pathogenesis of ED without an established etiology. We aimed to determine the association between erectile function and markers of subclinical atherosclerosis in young men under the age of 40 years.
PATIENTS AND METHODS
Patients selection
This study was conducted on 261 patients aged 20–40 years and complaining of ED, who presented to the Urology and Andrology Outpatient Center of theFirst Affiliated Hospital, Sun Yat-sen University, during 2012–2015. Age-matched healthy controls were recruited from the Health Screening Center.
Erectile function was evaluated using the International Index of Erectile Function-5 (IIEF-5) questionnaire, where a score of 21 or less indicates ED. The degree of ED severity was classified by the score as mild (17–21), moderate (11–16), or severe (<10). Individuals were eligible for inclusion in the study if they had ED for more than 3 months, a stable monogamous relationship with a female partner, and at least one attempt of sexual intercourse over the last 4 weeks. Exclusion criteria were patients with systemic diseases such as coronary artery disease, congestive heart failure, hypertension or orthostatic hypotension, diabetes or abnormal fasting blood glucose, dyslipidemia, abnormal hormone level, significant renal or hepatic dysfunction and anemia, malignancies, smoking history (in the past 5 years), alcohol and substance abuse or addiction, reliance on long-term pharmacotherapy, and those who had pelvic surgery or trauma of the perineal region. Those with psychiatric disorders or signicant mental health problems requiring psychotropic drugs were assessed by clinical interviews with a psychologist and excluded from this study. Penile color duplex Doppler ultrasound (ACUSON S2000, Siemens Healthcare, Erlangen, Germany) was used to find and exclude ED caused by anatomical abnormalities. The study was approved by Institutional Research Ethics Committee, First Affiliated Hospital, Sun Yat-sen University, and all patients provided informed consent to participate in the study. This trial was registered at ClinicalTrials.gov as NCT01249703. Blood chemistry and endocrine assays were performed in the clinical testing center of our hospital.
Ultrasound examination
Patients and controls were examined by well-trained operators blinded to the participants' demographic data, according to a standard protocol.9 All individuals had fast for at least 8 h and stopped coffee drinking for at least 2 h before the ultrasound examination. After 15 min of resting in a quiet environment, FMD was evaluated in the right brachial artery using a high-resolution ultrasound machine equipped with a high-frequency and linear array ultrasound probe (5–12 MHz, VIVID7, GE Healthcare, Chicago, IL, USA). The probe was positioned approximately 2–5 cm proximal to the elbow. Diameter and blood flow velocity of brachial artery were recorded by R-wave gating of the synchronic electrocardiogram (ECG). Baseline parameters were obtained before reactive hyperemia, and then an arterial occlusion cuff was placed around the arm and inflated above 250 mmHg for 5 min before deflating. After cuff deflation, the greatest diameter and maximal velocity were recorded at end diastole (tip of ECG R-wave). FMD is expressed as the percentage change in diameter before and after cuff inflation, reflecting endothelium-dependent dilatation during reactive hyperemia in superficial arteries.
The same operator measured the combined thickness of the intima and media of common carotid arteries (CCA). CIMT, defined as the distance between two parallel lines: the lumen–intima and media–adventitia boundaries, was measured on the far wall of CCA. Longitudinal sonographic images of the right and the left CCA of each patient, at approximately 1 cm proximal to the carotid bulb, were obtained. A plaque was defined as a focal structure encroaching into the arterial lumen of at least 0.5 mm or 50% of the surrounding IMT value or demonstrated a thickness >1.5 mm measured from the media–adventitia interface to the intima–lumen interface.
Intra- and inter-operator coefficients of variation were 2.8% and 3.0%, respectively, and intra- and inter-operator intraclass correlations were both 0.96.
Laboratory assessment
Blood was collected from patients and controls after overnight fast and analyzed for low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglyceride (TG), uric acid (UA), fasting blood glucose (FBG), high-sensitive C-reactive protein (hs-CRP) (AU5800 automatic biochemical analyzer, Beckman Coulter, Fullerton, CA, USA), and insulin and testosterone levels (Architect i2000SR analyzer, Abbott Diagnostic, Chicago, IL, USA). The homeostatic model assessment (HOMA) index was calculated as fasting insulin concentration (μU ml−1) × fasting glucose concentration (mmol l−1)/22.5.
Statistical analyses
Data were expressed as mean ± s.d. or median (interquartile range), as appropriate. Kolmogorov–Smirnov criterion was applied to all variables to test for normal distribution. Differences were assessed by the Student's t-test in normal variables and by the Mann–Whitney U-test in nonnormal variables. Multivariate logistic regression analysis was performed to identify significant risk factors for ED. Correlation between these significant risk factors and ED severity was tested by Spearman's or Pearson's rank-order correlation, as appropriate. Multiple linear regression was also used to estimate their association. Receiver-operating characteristic (ROC) analysis was performed to evaluate these risk factors' ability to predict ED presence, with an area under the curve (AUC) value of 0.5 indicating no accuracy and a value of 1.00 indicating maximal accuracy. Optimal cutoff points were defined by maximizing the sum of sensitivity and specificity.
Statistical analysis was performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 (2-tailed) was considered statistically significant.
RESULTS
Clinical characteristics and laboratory parameters
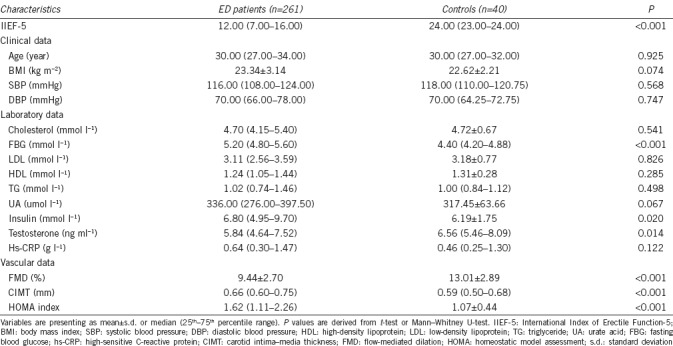
The clinical characteristics, laboratory, and vascular parameters of patients and controls are presented in Table 1. There was no difference with regard to the age, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), LDL, HDL, and TG levels in patients and controls. Compared with control individuals lacking ED, patients with ED had greater CIMT values, and higher FBG, HOMA index and insulin levels, but lower brachial artery FMD and lower testosterone levels (all P < 0.05), though all values were within their respective normal range. No carotid plaques were observed in both groups.
Table 1.
Characteristics of patients with erectile dysfunction and controls
Risk factors associated with ED
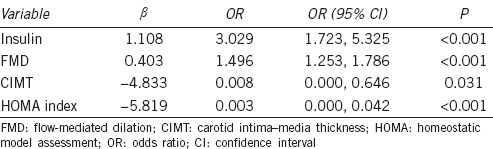
The six variables (CIMT, FMD, FBG, HOMA index, insulin level, and testosterone level) that showed significant differences in ED versus control patients were examined by logistic regression analysis to identify risk factors for ED. The FMD, CIMT, HOMA index, and insulin variables were predictors of ED (P < 0.05). Their odds ratios are shown in Table 2.
Table 2.
Logistic regression analysis for the presence of erectile dysfunction in young men
The Spearman's or Pearson's correlation was used to further investigate the relationship between these risk factors and the severity of ED (as measured by the IIEF-5 score). Throughout the entire study population, FMD values were positively correlated with the IIEF-5 scores (r = 0.216, P < 0.001), while CIMT (r = −0.143, P < 0.013), HOMA index (r = −0.219, P < 0.001), and insulin (r = −0.126, P < 0.029) levels were inversely correlated with the IIEF-5 scores.
The results of multiple linear regression analysis showed that one unit (mm) thicker CIMT was associated with a 6.432-fold lower IIEF-5 score (P < 0.05), while one unit (%) higher FMD was associated with a 0.475-fold higher IIEF-5 score adjusted for age (P < 0.001).
Diagnostic performance for ED identification in young men
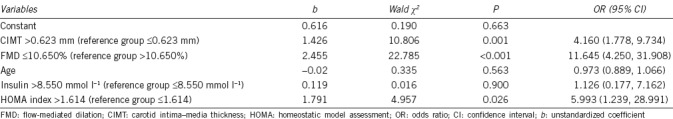
As shown in Figure 1 and Table 3, of the four predictors identified by logistic regression, FMD had the best diagnostic performance for the identification of ED in men under the age of 40 years. Young men with FMD <10.65% were 11.645 times more likely to have ED; this cutoff value had sensitivity of 68.6% and specificity of 87.5% for the diagnosis of ED. Young men with CIMT >0.623 mm had a 4.16 times greater risk of having ED; this cutoff value had sensitivity of 66.3% and specificity of 75.0% to predict ED. A HOMA index >1.614 was associated with a 5.993 times greater risk of having ED.
Figure 1.
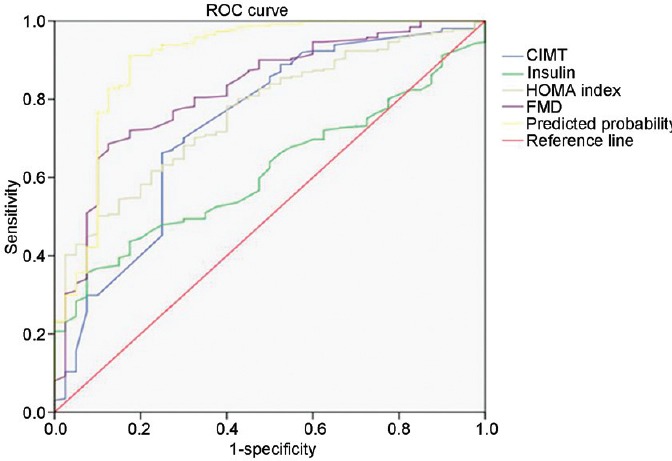
Receiver-operating characteristic (ROC) curves to identify ED in young men without abnormal laboratory findings. ED: erectile dysfunction; CIMT: carotid intima–media thickness; FMD: flow-mediated dilation; HOMA: homeostatic model assessment.
Table 3.
Association of cardiovascular risk factors with erectile dysfunction in young man
DISCUSSION
The incidence of ED increases with age. Although ED is not life-threatening, it affects the quality of life of the patient and his partner in a negative way, especially for young men, whose etiology of ED remains difficult to elucidate. In this study, we investigated the underlying pathogenesis of ED in young patients lacking obvious etiology after general assessment. The current results showed that young patients with ED had greater CIMT and lower FMD values than the control group, indicating subclinical atherosclerosis and endothelial dysfunction may be key pathological changes during the development of ED. Although all laboratory parameters were within respective normal ranges, the relatively high levels of FBG, insulin, and insulin resistance index and relatively low testosterone levels in young ED versus normal patients may be linked to impaired endothelial function and accelerated subclinical atherosclerosis, which may cause ED and subsequent cardiovascular disease.
The relationship between ED and cardiovascular disease is well established.10,11 They share common risk factors, such as diabetes, hypertension, hyperlipidemia, and smoking. Endothelial dysfunction may be the common link to both conditions. Impaired endothelial function and the subsequent reduced capacity of vascular smooth muscle relaxation are regarded as precursor conditions of atherosclerosis and impaired cavernosal relaxation.12,13 ED and cardiovascular disease often coexist in the same patients, and ED may precede symptoms of coronary heart disease (CHD) by 2–3 years and CHD events by 3–5 years.14,15
The development of ED before CHD may be explained by the artery size hypothesis, which proposed that a smaller penile artery may be more susceptible to systemic risk factors than larger vessels in the heart and brain, and the same level of plaque compromises blood flow more profoundly through the smaller arteries of the penis than through the larger vessels of the heart and limbs.16,17 Intima–media thickness is considered as a marker of early artery disease and an independent predictor of major cardiovascular events. The ARIC study showed that for every 0.19 mm increment in CIMT, the risk of death or myocardial infarction increased by 36% in middle-aged patients.18 However, although there were reference values, no standardized thresholds were determined for the general population. CIMT can vary with age, gender, and race/ethnicity, so these factors should be considered when comparing CIMT between study groups. As recommended in guidelines for the assessment of cardiovascular risk in asymptomatic adults, an elevated CIMT is commonly defined as a level that surpasses the population-based 75th percentile value.19 When cardiovascular risk is assessed using percentiles, a CIMT greater than the 75th percentile value or ≥1 mm is considered high risk, and values between the 25th and 75th percentiles or <1 mm are average or moderate.20,21 In our study of men under the age of 40 years, the mean CIMT of both groups was <0.70 mm, but greater CIMT values were observed in ED patients compared with healthy controls, suggesting more accelerated subclinical vascular disease in these ED patients. Statistical analysis showed that thicker CIMT was associated with a lower IIEF-5 score and greater risk of having ED.
The evaluation of brachial artery FMD using duplex ultrasound is now widely accepted as a noninvasive technique to assess endothelial function. Many studies have documented a relationship between FMD and traditional cardiovascular risk factors. The FMD values are lower (abnormal) in individuals with higher Framingham Risk Scores or more risk factors.9 Mutlu et al.22 reported that a FMD <8% and CIMT ≥0.65 mm could predict patients with coronary artery disease. The relationship between FMD and risk factors was most clear in the category with the lowest baseline risk, suggesting that FMD was an early marker of vascular dysfunction.23 Our previous study reported that FMD was impaired in young ED patients and correlated with the severity of ED.24 The present study further confirmed the relationship of FMD with ED by showing that young men with lower FMD values were more likely to have ED, whereas higher FMD was associated with higher IIEF-5 scores.
This report quantitatively investigated the association of CIMT, FMD, and the HOMA index with the prevalence of ED in young men lacking traditional risk factors. Most studies involved relatively senior patients with known vascular causes, whereas this study focused on patients under the age of 40 years and without an established etiology of ED.
Considering the link between ED and cardiovascular disease, the assessment and management of ED may help identify those who require early intervention to reduce their risk of future cardiovascular events.25 As the first point of medical contact for men with ED symptoms, the primary care physician or urologist has a unique opportunity to perform screening and initiate primary prevention. Young men should be informed about the association of ED with atherosclerosis and encouraged to make lifestyle modifications and seek medical advice to improve their general health, so as to limit cardiovascular risk as well as ED prevalence.26
Limitations
Limitations of this study include relying on FMD as the sole indicator of endothelial function, and CIMT as the sole indicator of subclinical atherosclerosis. The relatively small size of the control group may reduce the power of statistical analysis and cross-sectional design. The evaluation of disease was based on a self-reporting questionnaire, although previous work had demonstrated the validity of this measure.
CONCLUSIONS
In young men with normal clinical findings, a greater CIMT and lower FMD was associated with a higher incidence of ED. Our study highlights endothelial dysfunction, subclinical atherosclerosis, and insulin resistance as potential factors in the underlying pathogenesis of ED lacking an established etiology. The diagnosis and evaluation of ED in young men provides unique opportunities for cardiovascular risk prevention. Conversely, early detection of subclinical vascular disease may provide a warning sign for the subsequent onset of ED and help improve the quality of life of affected men.
AUTHOR CONTRIBUTIONS
FJY carried out substantial contributions to conception/design, data acquisition, data analysis, and drafting the manuscript. YDZ, ZW, WL, and HL carried out data acquisition and analysis. YZ and CHD conceived of the study, carried out data interpretation, critical revision of the manuscript, and supervision. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (81070488 and 30871047) and Science and Technology Program of Guangdong, China (2013B021800294, 2014A020212438).
REFERENCES
- 1.Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, et al. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol. 2000;163:460–3. [PubMed] [Google Scholar]
- 2.Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84:50–6. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 3.Feldman DI, Cainzos-Achirica M, Billups KL, DeFilippis AP, Chitaley K, et al. Subclinical vascular disease and subsequent erectile dysfunction: the multiethnic study of atherosclerosis (MESA) Clin Cardiol. 2016;39:291–8. doi: 10.1002/clc.22530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma RK, Donekal S, Rosen BD, Tattersall MC, Volpe GJ, et al. Association of subclinical atherosclerosis using carotid intima-media thickness, carotid plaque, and coronary calcium score with left ventricular dyssynchrony: the multiethnic study of atherosclerosis. Atherosclerosis. 2015;239:412–8. doi: 10.1016/j.atherosclerosis.2015.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xanthakis V, Enserro DM, Murabito JM, Polak JF, Wollert KC, et al. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham offspring study. Circulation. 2014;130:1676–83. doi: 10.1161/CIRCULATIONAHA.114.009273. [DOI] [PubMed] [Google Scholar]
- 6.Baldassarre D, Hamsten A, Veglia F, de Faire U, Humphries SE, et al. Measurements of carotid intima-media thickness and of interadventitia common carotid diameter improve prediction of cardiovascular events: results of the IMPROVE (Carotid Intima Media Thickness [IMT] and IMT-Progression as Predictors of Vascular Events in a High Risk European Population) study. J Am Coll Cardiol. 2012;60:1489–99. doi: 10.1016/j.jacc.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 7.Novo S, Iacona R, Bonomo V, Evola V, Corrado E, et al. Erectile dysfunction is associated with low total serum testosterone levels and impaired flow-mediated vasodilation in intermediate risk men according to the Framingham risk score. Atherosclerosis. 2015;238:415–9. doi: 10.1016/j.atherosclerosis.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Yao F, Huang Y, Zhang Y, Dong Y, Ma H, et al. Subclinical endothelial dysfunction and low-grade inflammation play roles in the development of erectile dysfunction in young men with low risk of coronary heart disease. Int J Androl. 2012;35:653–9. doi: 10.1111/j.1365-2605.2012.01273.x. [DOI] [PubMed] [Google Scholar]
- 9.Yeboah J, Folsom AR, Burke GL. Predictive value of brachial flow-mediated dilation for Incident cardiovascular events in a population-based study: the multiethnic study of atherosclerosis. Circulation. 2009;120:502–9. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandaglia G, Briganti A, Jackson G, Kloner RA, Montorsi F, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol. 2014;65:968–78. doi: 10.1016/j.eururo.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Dattatrya KY, Vedpalsingh TH, Gorakhnath WV, Kiran PS. Can erectile dysfunction in young patients serve as a surrogate marker for coronary artery disease? J Clin Diagn Res. 2015;9:PC01–3. doi: 10.7860/JCDR/2015/14207.6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reriani M, Flammer AJ, Li J, Prasad M, Rihal C, et al. Microvascular endothelial dysfunction predicts the development of erectile dysfunction in men with coronary atherosclerosis without critical stenoses. Coron Artery Dis. 2014;25:552–7. doi: 10.1097/MCA.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lane-Cordova AD, Kershaw K, Liu K, Herrington D, Lloyd-Jones DM. Association between cardiovascular health and endothelial function with future erectile dysfunction: the multi-ethnic study of atherosclerosis. Am J Hypertens. 2017;30:815–21. doi: 10.1093/ajh/hpx060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsiki N1, Wierzbicki AS, Mikhailidis DP. Erectile dysfunction and coronary heart disease. Curr Opin Cardiol. 2015;30:416–21. doi: 10.1097/HCO.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 15.Gandaglia G, Briganti A, Montorsi P, Mottrie A, Salonia A, et al. Diagnostic and therapeutic implications of erectile dysfunction in patients with cardiovascular disease. Eur Urol. 2016;70:219–22. doi: 10.1016/j.eururo.2016.01.054. [DOI] [PubMed] [Google Scholar]
- 16.Keenan HA. Do erectile dysfunction and cardiovascular disease have the same mechanism? Eur Urol. 2014;65:979–80. doi: 10.1016/j.eururo.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Montorsi P, Ravagnani PM, Galli S, Rotatori F, Briganti A, et al. The artery size hypothesis: a macrovascular link between erectile dysfunction and coronary artery disease. Am J Cardiol. 2005;96:19M–23M. doi: 10.1016/j.amjcard.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the atherosclerosis risk in communities (ARIC) study, 1987–1993. Am J Epidemiol. 1997;146:483–94. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 19.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:e584–636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 20.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Cobble M, Bale B. Carotid intima-media thickness: knowledge and application to everyday practice. Postgrad Med. 2010;122:10–8. doi: 10.3810/pgm.2010.01.2091. [DOI] [PubMed] [Google Scholar]
- 22.Mutlu B, Tigen K, Gurel E, Ozben B, Karaahmet T, et al. The predictive value of flow-mediated dilation and carotid artery intima-media thickness for occult coronary artery disease. Echocardiography. 2011;28:1141–7. doi: 10.1111/j.1540-8175.2011.01492.x. [DOI] [PubMed] [Google Scholar]
- 23.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2–12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao FJ, Liu LJ, Zhang Y, Huang Y, Liu D, et al. Erectile dysfunction may be the first clinical sign of insulin resistance and endothelial dysfunction in young man. Clin Res Cardiol. 2013;102:645–51. doi: 10.1007/s00392-013-0577-y. [DOI] [PubMed] [Google Scholar]
- 25.Vlachopoulos C, Ioakeimidis N, Stefanadis C. Biomarkers, erectile dysfunction, and cardiovascular risk prediction: the latest of an evolving concept. Asian J Androl. 2015;17:17–20. doi: 10.4103/1008-682X.143250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miner M, Nehra A, Jackson G, Bhasin S, Billups K, et al. All men with vasculogenic erectile dysfunction require a cardiovascular workup. Am J Med. 2014;127:174–82. doi: 10.1016/j.amjmed.2013.10.013. [DOI] [PubMed] [Google Scholar]


