Abstract
Differences in intravaginal ejaculation latency reflect normal biological variation, but the causes are poorly understood. Here, we investigated whether variation in ejaculation latency in an experimental rat model is related to altered sympathetic nervous system (SNS) activity and expression of N-methyl-D-aspartic acid (NMDA) receptors in the paraventricular nucleus of the hypothalamus (PVN). Male rats were classified as “sluggish,” “normal,” and “rapid” ejaculators on the basis of ejaculation frequency during copulatory behavioral testing. The lumbar splanchnic nerve activity baselines in these groups were not significantly different at 1460 ± 480 mV, 1660 ± 600 mV, and 1680 ± 490 mV, respectively (P = 0.71). However, SNS sensitivity was remarkably different between the groups (P < 0.01), being 28.9% ± 8.1% in “sluggish,” 48.4% ± 7.5% in “normal,” and 88.7% ± 7.4% in “rapid” groups. Compared with “normal” ejaculators, the percentage of neurons expressing NMDA receptors in the PVN of “rapid” ejaculators was significantly higher, whereas it was significantly lower in “sluggish” ejaculators (P = 0.01). In addition, there was a positive correlation between the expression of NMDA receptors in the PVN and SNS sensitivity (r = 0.876, P = 0.02). This study shows that intravaginal ejaculatory latency is associated with SNS activity and is mediated by NMDA receptors in the PVN.
Keywords: ejaculation, N-methyl-D-aspartic acid receptor, paraventricular nucleus, sympathetic nervous system
INTRODUCTION
Lifelong premature ejaculation (PE) is the most common male sexual dysfunction, which negatively impacts men and their partners and may prevent single men from forming new partner relationships.1 Lifelong PE has been defined as a neurobiological dysfunction, which is caused by somatic disorders and/or neurobiological imbalances.2 To further understand the pathophysiology of lifelong PE, animal studies in which specific brain areas have been manipulated have been used.3,4 Ejaculation is characterized by seminal fluid expulsing from the urethral meatus, accompanied by orgasm. It consists of two different but successive phases: emission and expulsion.5 In male rats, ejaculation occurs after a series of mounts and vaginal intromission with a receptive female.6
Different lines of data from rats indicate ejaculation to be predominantly mediated by a spinal control center, which is in turn influenced by the descending inhibitory and excitatory supraspinal sites in the brainstem, hypothalamus, and preoptic area.7 Many types of neurotransmitter with inhibitory or excitatory roles, such as serotonin, dopamine, adrenaline, acetylcholine, norepinephrine, oxytocin, gamma-aminobutyric acid, N-methyl-D-aspartic acid (NMDA), and nitric oxide, have been implicated in the central regulation of ejaculation reflex.8,9 More recently, we have demonstrated that NMDA receptors in the paraventricular nucleus of the hypothalamus (PVN) can facilitate ejaculation through enhancing the activity of the sympathetic nervous system (SNS).10
To further investigate the mechanism of lifelong PE, we examined whether differences in ejaculatory behavior of rats are related to different states of SNS activity and NMDA receptor expression in the PVN. Lifelong PE has been considered as normal biological variation of the intravaginal ejaculation latency time.11 Based on the ejaculation distribution theory, we and others have investigated “rapid,” “normal,” and “sluggish” ejaculator rats.6,12,13 Compared with “normal” ejaculator rats, “rapid” ejaculators show shorter ejaculation latencies, higher ejaculation frequencies, and lower number of mounts before ejaculation, while “sluggish” ejaculators display the opposite behaviors.6,12,13 To our knowledge, no previous studies have explored differences in SNS activity and NMDA receptor expression in the PVN of rats with differential ejaculatory behavior.
MATERIALS AND METHODS
Animals
Sprague–Dawley rats aged 3 months weighing 250–300 g (49 males and 32 females) were housed in a 12-h light-dark cycle and temperature-controlled room with free access to standard rat chow and tap water. All the experiments were approved by the Experimental Animal Care and Use Committee of Nanjing Medical University and carried out in accordance with the Guide for the Care and Use of Laboratory Animals.14
Selecting male rats with different ejaculatory behavior
Details of copulatory behavioral testing are described in our previous study.10 Briefly, ovariectomized female rats were induced to be receptive and perceptive by subcutaneous injection of 20 μg estradiol benzoate and 500 μg progesterone. The female rats were then paired with male rats for 30 min in a testing arena (60 cm × 30 cm × 35 cm steel cage) for copulation. To get a stable sexual performance, the males performed six copulation tests. Tests were conducted weekly (on the same day of the week and at about the same time) with six different females. The copulatory behaviors were recorded with a high-definition digital video processing system (IXUS 245 HS, Canon, Zhuhai, China). The following behavioral parameters were recorded and analyzed: mount latency (ML), mount frequency (MF), intromission latency (IL), ejaculation latency (EL), intromission frequency (IF), postejaculatory interval (PEI), ejaculation frequency (EF), and intromission ratio (IR) = IF/(MF + IF).10 When a male rat achieved ejaculation, it developed violent trembling throughout its body accompanied with deep penetration. At the same time, they held the females tightly with their forelimbs for a few seconds. After ejaculation, male rats experienced a refractory period for several minutes. EF of the male rats increased over the test sessions, as the animals acquired the expected sexual behavior. From the third session onward, EF remained mostly stable between test sessions.6,13 Therefore, we included rats with stable sexual activity in the last three sessions (T3–T6) with mean EF ≤2.
Lumbar splanchnic nerve activity (LSNA) recording
LSNA can indicate the status of sympathetic nerve activity.10,15 The lumbar splanchnic nerve was carefully exposed with a retroperitoneal approach, and a pair of silver electrodes was placed on the nerve with warm mineral oil. An AC/DC differential amplifier (DP-304, Warner Instruments, Hamden, CT, USA) recorded LSNA signals through a band-pass filter (10–3000 Hz). The amplified and filtered nerve activity signals were integrated and collected at a time constant of 100 ms. Ganglionic blockade was induced by 30 mg kg−1 hexamethonium hydrochloride (HEX) injection into the carotid vein. Then, the number of LSNA spikes was averaged using the LabChart/Spike histogram tool. At the end of each experiment, the background noise was determined after the central end of the nerve was severed and was subtracted from the integrated values of the LSNA. The sensitivity of sympathetic activity was calculated as a percentage change from baseline LSNA using the formula = (baseline LSNA − the LSNA after HEX)/baseline LSNA × 100%.16,17
Plasma norepinephrine (NE) measurement
The procedure was conducted under the instructions of the manufacturer. The standards or sample diluents were incubated in the appropriate well of a specific antibody precoated microtiter plate. Conjugate was added and incubated for 1 h at 37°C, and then wells were washed. The reactions were stopped with stop solution and read at 450 nm using a microtiter plate reader (ELX800, BioTek, Winooski, VT, USA).
Immunohistochemistry for NR1
The NMDA receptor is primarily composed of two NR1 subunits and two NR2A or NR2B subunits.18 The NR1 subunit determines the function of the NMDA receptor.19 Rats were anesthetized by intraperitoneal injection of sodium 100 mg kg−1 pentobarbitone and then perfused transcardially with 0.01 mol l−1 phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. Thirty-micron coronal brain sections were cut with a freezing microtome (CM 1900-1-1, Leica, Frankfurt, Germany) and then immersed in cryoprotectant solution (30% sucrose, 30% ethylene glycol in 0.1 mol l−1 phosphate buffer, and 0.01% sodium azide) and stored at −20°C until further processing. Sections were incubated with a rabbit anti-NR1 antibody (Sigma, USA, G0541) diluted in 0.01 mol l−1 PBS at 4°C overnight. Sections were further incubated with a biotinylated secondary antibody in incubation solution from the ABC staining system kit (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 30 min at 37°C according to the manufacturer's instructions. NR1-positive neurons in the PVN were observed with a conventional light microscope (DP70, Olympus, Tokyo, Japan). PVN sites were identified according to the rat brain atlas of Paxinos and Watson.20 We observed neurons with NR1-like immunoreactivity in four PVN sections and averaged the numbers as the final result for each animal.
Western blot analysis of NR1 protein
Brains were removed and immediately frozen on dry ice and stored at −70°C until sectioned. Coronal sections, 450 μm thick, were cut through the hypothalamus, which incorporated the PVN area. The PVN area was punched out with a 15-gauge needle, according to the coordinates in the rat brain atlas,20 and processed as described previously.21 Briefly, the punched tissue was homogenized in 20 mmol l−1 HEPES, pH 7.5, 1.5 mmol l−1 MgCl2, 0.2 mmol l−1 EDTA, 0.45 mol l−1 NaCl, 0.4 mmol l−1 phenylmethylsulfonyl fluoride, 0.5 mmol l−1 sodium orthovanadate, protease inhibitor cocktail, and 1 mmol l−1 CoCl2. Homogenates were centrifuged at 12 000 g at 4°C for 10 min. The bicinchoninic acid method was used to estimate the protein content of the PVN lysates using bovine serum albumin as a standard. The protein lysate was mixed with Laemmli sample buffer (250 mmol l−1 Tris–HCl, pH 6.8, 10% sodium dodecyl sulfate (SDS), 30% glycerol, 5% mercaptoethanol, and 0.02% bromophenol blue) in a 1:1 (v/v) ratio and boiled for 5 min. Protein lysates (35 μg) were fractionated on 8% SDS-polyacrylamide gels in Tris-glycine running buffer and electrophoretically transferred onto PVDF membranes at 300 mA for 90 min. Nonspecific binding sites were blocked by incubating the membrane with 5% nonfat dried milk (w/v) in TBST (10 mmol l−1 Tris, 150 mmol l−1 NaCl, 0.05% Tween-20) at ambient temperature for 1 h. The membrane was probed with a rabbit anti-rat NR1 antibody (Sigma, San Francisco, CA, USA, 1:1000) or a rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Sigma, 1:1000) diluted in 0.01 mol l−1 PBS at 4°C overnight. Membranes were washed with TBST for 5 min at room temperature three times and then incubated with secondary antibody (peroxidase-conjugated goat anti-rabbit IgG, 1:5000) for 1 h at room temperature. Enhanced chemiluminescence was used to develop the positive protein bands. The band intensities were quantified using ImageJ gel analysis software (National Institutes of Health, Bethesda, MD, USA).
Experimental design
To screen the male rats for differential ejaculatory behavior, we conducted the copulatory behavioral tests on 33 male rats for 6 weeks. After selecting male rats in accordance with the principle of the “10%” category,6,12,13,22 we analyzed 22 rats (six “rapid,” nine “normal,” and seven “sluggish”). In the week after the copulatory behavioral tests, the male rats were deeply anesthetized with intraperitioneal injection of urethane (800 mg kg−1) and α-chloralose (40 mg kg−1). LSNA recordings were then performed. Subsequently, 0.5 ml of the blood was collected from the carotid artery. Blood samples were centrifuged immediately, and a 100 μl aliquot of each plasma sample was stored at −70°C. A commercial ELISA kit (ALPCO Diagnostics, Windham, NH, USA) was used for NE measurement. At the end of experiment, rats were euthanized and brains removed to be used for immunohistochemistry (n = 10) and western blot analysis (n = 12).
Statistical analysis
Values are expressed as the mean ± standard error. The data for multiple comparisons were analyzed by one-way or two-way ANOVA. Post hoc comparisons were conducted using Newman–Keuls test, followed by Dunn's multiple post hoc test. A P < 0.05 was considered statistically significant.
RESULTS
Behavioral characteristics
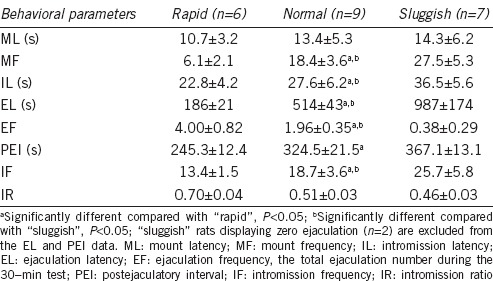
During the six copulatory behavioral test sessions, 10 of the 49 male rats were excluded from the analysis for no copulation with the female. EF of the remaining 39 male rats increased over the first three test sessions and then remained relatively stable in individual rats for the remaining three sessions. Based on the last three test sessions, we selected the stable sexual performers (EF value less than two between different mating sessions) and categorized them as “rapid,” “normal,” or “sluggish” ejaculators according to the mean EF value. Thirty-three male rats were included in the final analysis. The scattergram of mean EF during the last three training sessions showed approximately normal distribution (Figure 1), which is consistent with the previous studies.6,22 According to the principle of “10%” within each category, we classified rats with a mean EF <1 as “sluggish,” 1.5< EF <2.5 as “normal,” and EF >3 as “rapid.” Accordingly, six rats (two with EF = 1 and four with EF between 1 and 1.5) were eliminated from the analysis. Compared with “normal” ejaculators, EL of “rapid” rats was significantly shortened (P < 0.01), while that of “sluggish” rats was remarkably prolonged (P < 0.01), with two out of seven “sluggish” rats presenting no ejaculation. In addition, IL, IF, PEI, and MF also showed differences between the three groups. IR of the “rapid” ejaculators was the highest among the three groups, although the difference was not statistically significant (F = 7.2, P = 0.25). However, ML, a marker of sexual motivation, was not significantly different among groups. Table 1 presents the details of the parameters related to sexual behavior.
Figure 1.
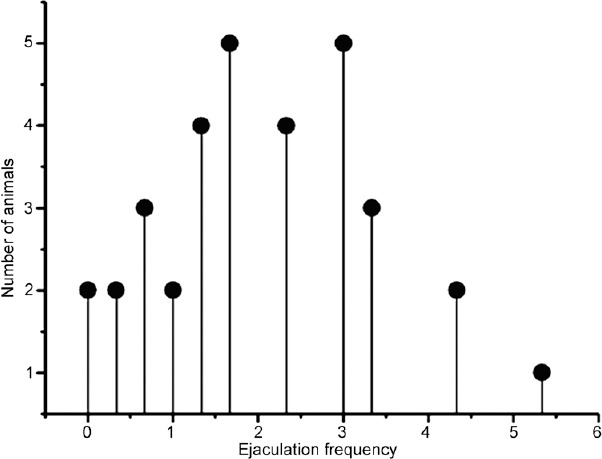
Mean ejaculation frequency (the total number of ejaculations during the 30 min test) (n = 33).
Table 1.
Behavioral parameters monitored during mating tests for “rapid”, “normal”, and “sluggish” ejaculating rats
Differences in sympathetic nervous system activity
The LSNA baselines were 1460 ± 480 mV, 1660 ± 600 mV, and 1680 ± 490 mV, in “sluggish,” “normal,” and “rapid” ejaculators, respectively; these values were not significantly different from each other (F = 0.4, P = 0.71). However, SNS sensitivity was remarkably different among groups (F = 63.1, P < 0.01, Figure 2) at 28.9% ± 8.1% in “sluggish,” 48.4% ± 7.5% in “normal,” and 88.7% ± 7.4% in “rapid” groups. Furthermore, serum NE levels were statistically different among the three groups at 758 ± 421 pg ml−1, 1429 ± 675 pg ml−1, and 1674 ± 651 pg ml−1 in “sluggish,” “normal,” and “rapid” ejaculators, respectively (F = 4.2, P = 0.03). The lowest NE level was in the “sluggish” ejaculators group.
Figure 2.
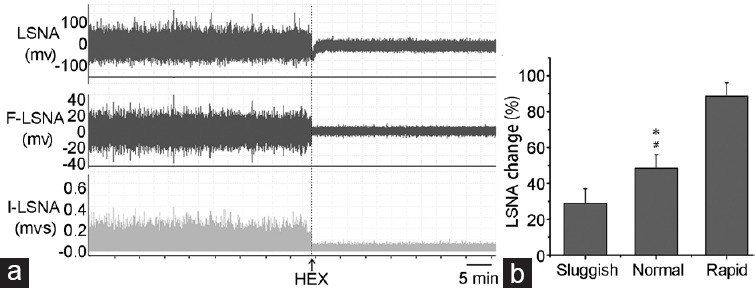
(a) Representative recordings showing LSNA changes from baseline after HEX treatment (30 mg kg−1 injected into the carotid vein). (b) LSNA sensitivity of “sluggish,” “normal,” and “rapid” ejaculating rats. *P < 0.01, “normal” rats compared with “sluggish” rats; #P < 0.01, “normal” rats compared with “rapid” rats. HEX: hexamethonium hydrochloride; LSNA: lumbar sympathetic nerve activity; R-LSNA: raw LSNA; I-LSNA: integrated LSNA.
Expression of NMDAR1 in the PVN of male rats
Finally, 10 rats (three “rapid,” four “normal,” and three “sluggish”) were used for immunohistochemistry, and the other 12 rats (three “rapid,” five “normal,” and four “sluggish”) were used for western blotting. Qualitative and quantitative analyses revealed that neurons in the PVN express the NMDA receptor. Compared with “normal” ejaculators, the percentage of neurons expressing NMDA receptors in the PVN of “rapid” ejaculators was significantly higher, whereas it was significantly lower in “sluggish” ejaculators (F = 15.1, P = 0.01; Figure 3 and 4). In addition, there was a positive correlation between the expression of NR1 in the PVN and SNS sensitivity (r = 0.876, P = 0.02, Figure 5).
Figure 3.
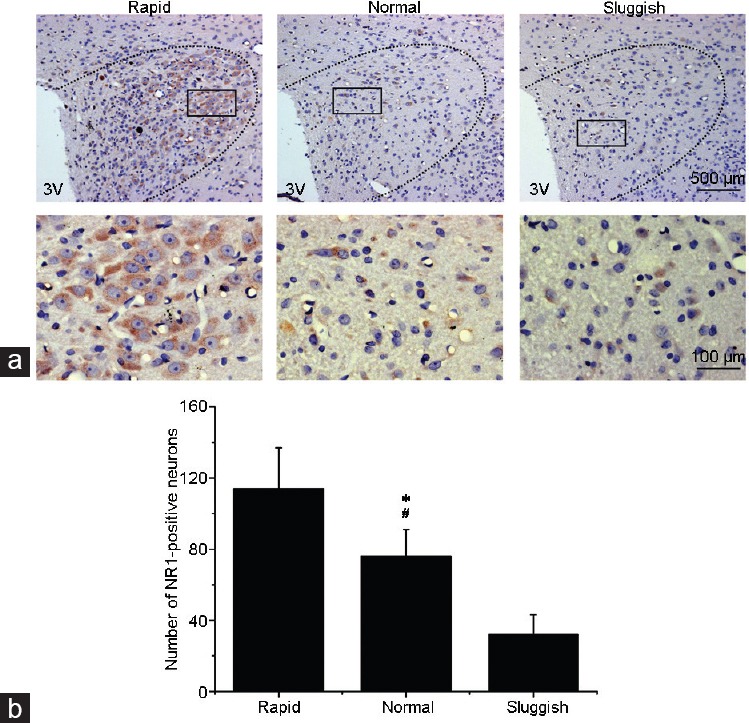
(a) Immunohistochemical analysis of NMDANR1 distribution (brown color) in the PVN in “rapid,” “normal,” and “sluggish” rats. (b) Bar graph showing the number of NMDANR1-positive cells in the PVN. Values are presented as mean ± s.e. *P < 0.01, “normal” group compared with the “rapid” group; #P < 0.01, “normal” group compared with the “sluggish” group. 3V: third ventricle; NMDANR1: N-methyl-D-aspartic acid-NR1; PVN: paraventricular nucleus of the hypothalamus; s.e.: standard error.
Figure 4.
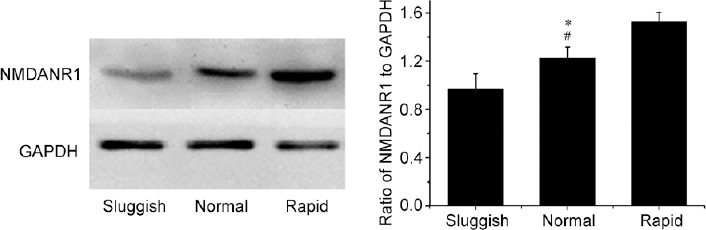
Western blot analysis of NMDANR1 normalized against GAPDH in the punched PVN samples of “sluggish,” “normal,” and “rapid” rats. *P < 0.01, “normal” group compared with the “sluggish” group; #P < 0.01, “normal” group compared with the “rapid” group. NMDANR1: N-methyl-D-aspartic acid-NR1; GAPHD: glyceraldehyde-3-phosphate dehydrogenase; PVN: paraventricular nucleus of the hypothalamus.
Figure 5.
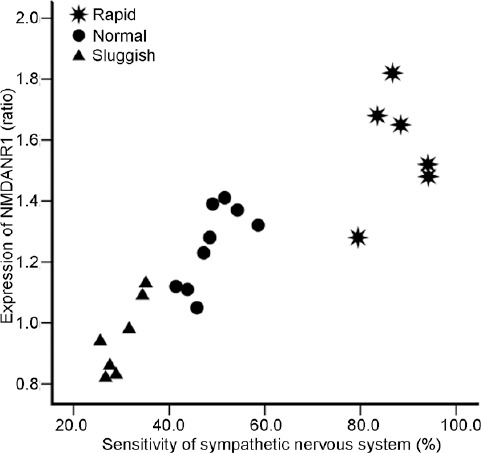
Positive correlation between the expression of NMDANR1 in the paraventricular nucleus of the hypothalamus and sympathetic nervous system sensitivity (r = 0.876, P = 0.02). NMDANR1: N-methyl-D-aspartic acid-NR1.
DISCUSSION
In the present study, we found that male rats with differential ejaculatory behavior had different SNS sensitivities, which correlated with NMDA receptor levels in the PVN. This result supports different levels of NMDA receptors in the PVN as contributing to changes in ejaculatory latency during sexual activity.
The broad EF distribution in the copulatory behavioral tests is similar to observations in the previous mating tests.6,22 The male rats were divided into “rapid,” “normal,” and “sluggish” groups based on their EF scores. Sluggish ejaculators did not ejaculate during the third session of the experiment onward, whereas rapid ejaculators achieved more than three ejaculations during the sessions. The mean EL was considerably different among the three groups, with the rapid ejaculators having the shortest EL and the sluggish ejaculators the longest. Correspondingly, IF, which the rats displayed before ejaculation, was lowest in the rapid ejaculators and highest in the sluggish ejaculators. Interestingly, ML is usually regarded as sexual motivation,23 but there were no differences in ML among the three groups. This indicates that the appetitive components of sexual behavior did not differ between the three groups. IR is an index of erectile function, and it differed between the three groups, being lowest in the sluggish ejaculators.24 This may account for the inhibited ejaculatory performance, which is in accordance with the clinical findings of retarded or anejaculation men suffering increased erectile dysfunction.25 As mentioned above, the behavioral differences of rapid and sluggish ejaculators show attributes common to human premature and retarded ejaculation, respectively.
The LSNA baselines of “rapid,” “normal,” and “sluggish” rats were not different. Although LSNA can represent the status of the SNS,15 its recording may be affected by variation in different preparations. We adopted a standard experimental procedure, which reduces potential variation. The SNS is responsible for regulating homeostatic mechanisms, such as the cardiovascular system.26 However, SNS sensitivity differed remarkably, being highest in the “rapid” ejaculators and the lowest in the “sluggish” ones. Similarly, serum NE levels were highest in the “rapid” ejaculators compared with the other two groups. Plasma NE is mainly derived from noradrenergic nerve endings modulated by the SNS, and NE levels are positively correlated with increased SNS activity.27 The elevation of plasma NE found in rapid ejaculators may be interpreted as a result of increased neurotransmitter release caused by sympathetic neuronal sensitivity. Clinically, we and others find that SNS activity is increased in men with lifelong PE, and overactivity of the SNS is regarded as the mechanism of PE.28,29 Therefore, differences in SNS sensitivity probably contribute to the different EL of each ejaculator group.
Recently, we explored the roles of NMDA receptors in the PVN and their association with SNS activity in male sexual behaviors and found that NMDA receptors in the PVN facilitate ejaculation by enhancing SNS activity.10 In the present study, we demonstrated that the density of NMDA receptors in the PVN distinguished between the three groups, with the density being the highest in “rapid” ejaculators. In addition, the density of NMDA receptors positively correlated with SNS sensitivity. Therefore, it can be speculated that differential expression of NMDA receptors in the PVN has a differential effect on SNS activity and, therefore, on differences in ejaculatory behavior. Interestingly, it has been hypothesized that an initial increase in NMDA receptor transmission is critical for short-term sexual experience and subsequent reward behavior.30 This hypothesis was based on the finding that sexual experience and subsequent reward abstinence in males caused immediate and long-lasting facilitation of sexual behavior with an upregulation of total NMDA receptor expression in the nucleus accumbens, which is consistent with an earlier study.31 Although not identical, this experience-induced neuroplasticity may have similarities in the PVN, which needs to be demonstrated in the future. Therefore, we speculate that changes in NMDA receptor levels may result from individual differences in sexual experience during maternal care. Some studies have indicated that variations in maternal behavior are related to differential expression of genes encoding NMDA receptors in certain brain areas.32,33
Obviously, multiple other neurotransmitters/modulators are involved in SNS and ejaculation function, including serotonin, oxytocin, and dopamine.9,34 The roles of other pathways in the observed variation in ejaculatory behavior remain to be explored. Furthermore, ejaculation is influenced by other brain areas, such as the medial preoptic area, the nucleus paragigantocellularis, and the bed nucleus of the stria terminalis.7 Thus, the interconnected network and the mechanism of ejaculation need further investigation.
CONCLUSION
This study shows that “rapid,” “normal,” and “sluggish” ejaculators have neurophysiological differences. We show that intravaginal ejaculatory latency is associated with SNS activity and is mediated by NMDA receptors in the PVN.
AUTHOR CONTRIBUTIONS
JDX, JY, and ZJW designed this experiment. JDX, JC, BBY, and HJS performed the research. Statistical analyses were conducted by JDX, JC, and JY. JDX wrote the first draft of the manuscript. JDX, YTD, ZJW, and GQZ revised the manuscript for intellectual content. All authors reviewed, edited, and approved the final manuscript.
COMPETING INTERESTS
The authors declare no competing interests.
ACKNOWLEDGMENTS
The study was supported by the National Natural Science Foundation of China (Grant No. 81501245).
REFERENCES
- 1.Althof SE, McMahon CG, Waldinger MD, Serefoglu EC, Shindel AW, et al. An update of the International Society of Sexual medicine's guidelines for the diagnosis and treatment of premature ejaculation (PE) Sex Med. 2014;2:60–90. doi: 10.1002/sm2.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buvat J. Pathophysiology of premature ejaculation. J Sex Med. 2011;8(Suppl 4):316–27. doi: 10.1111/j.1743-6109.2011.02384.x. [DOI] [PubMed] [Google Scholar]
- 3.Larsson K, Ahlenius S. Brain and sexual behavior. Ann N Y Acad Sci. 1999;877:292–308. doi: 10.1111/j.1749-6632.1999.tb09274.x. [DOI] [PubMed] [Google Scholar]
- 4.Pfaus JG. Neurobiology of sexual behavior. Curr Opin Neurobiol. 1999;9:751–8. doi: 10.1016/s0959-4388(99)00034-3. [DOI] [PubMed] [Google Scholar]
- 5.Giuliano F. Neurophysiology of erection and ejaculation. J Sex Med. 2011;8(Suppl 4):310–5. doi: 10.1111/j.1743-6109.2011.02450.x. [DOI] [PubMed] [Google Scholar]
- 6.Pattij T, Olivier B, Waldinger MD. Animal models of ejaculatory behavior. Curr Pharm Des. 2005;11:4069–77. doi: 10.2174/138161205774913363. [DOI] [PubMed] [Google Scholar]
- 7.Coolen LM, Allard J, Truitt WA, McKenna KE. Central regulation of ejaculation. Physiol Behav. 2004;83:203–15. doi: 10.1016/j.physbeh.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Argiolas A, Melis MR. Neuropeptides and central control of sexual behaviour from the past to the present: a review. Prog Neurobiol. 2013;108:80–107. doi: 10.1016/j.pneurobio.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Veening JG, Coolen LM. Neural mechanisms of sexual behavior in the male rat: emphasis on ejaculation-related circuits. Pharmacol Biochem Behav. 2014;121:170–83. doi: 10.1016/j.pbb.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Xia JD, Chen J, Sun HJ, Zhou LH, Zhu GQ, et al. Centrally mediated ejaculatory response via sympathetic outflow in rats: role of N-methyl-D-aspartic acid receptors in paraventricular nucleus. Andrology. 2017;5:153–9. doi: 10.1111/andr.12274. [DOI] [PubMed] [Google Scholar]
- 11.Waldinger MD, Olivier B. Selective serotonin reuptake inhibitor-induced sexual dysfunction: clinical and research considerations. Int Clin Psychopharmacol. 1998;13(Suppl 6):S27–33. doi: 10.1097/00004850-199807006-00006. [DOI] [PubMed] [Google Scholar]
- 12.Waldinger MD, Olivier B. Animal models of premature and retarded ejaculation. World J Urol. 2005;23:115–8. doi: 10.1007/s00345-004-0493-x. [DOI] [PubMed] [Google Scholar]
- 13.Xia JD, Zhou LH, Yang BB, Chen Y, Dai YT. Establishment of an animal model of primary ejaculation. Natl J Androl. 2016;22:579–83. [PubMed] [Google Scholar]
- 14.National Research Council. Washington (DC): National Academies Press; 2010. Guide for the Care and use of Laboratory Animals. [Google Scholar]
- 15.Cassaglia PA, Shi Z, Li B, Reis WL, Clute-Reinig NM, et al. Neuropeptide Y acts in the paraventricular nucleus to suppress sympathetic nerve activity and its baroreflex regulation. J Physiol. 2014;592:1655–75. doi: 10.1113/jphysiol.2013.268763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomes da Silva AQ, Xavier CH, Campagnole-Santos MJ, Caligiorne SM, Baltatu OC, et al. Cardiovascular responses evoked by activation or blockade of GABA(A) receptors in the hypothalamic PVN are attenuated in transgenic rats with low brain angiotensinogen. Brain Res. 2012;1448:101–10. doi: 10.1016/j.brainres.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 17.Zhu GQ, Xu Y, Zhou LM, Li YH, Fan LM, et al. Enhanced cardiac sympathetic afferent reflex involved in sympathetic overactivity in renovascular hypertensive rats. Exp Physiol. 2009;94:785–94. doi: 10.1113/expphysiol.2008.046565. [DOI] [PubMed] [Google Scholar]
- 18.Herman JP, Eyigor O, Ziegler DR, Jennes L. Expression of ionotropic glutamate receptor subunit mRNAs in the hypothalamic paraventricular nucleus of the rat. J Comp Neurol. 2000;422:352–62. [PubMed] [Google Scholar]
- 19.Kew JN, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl) 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson C. San Diego: Academic Press; 2007. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- 21.Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res. 2003;93:990–7. doi: 10.1161/01.RES.0000102865.60437.55. [DOI] [PubMed] [Google Scholar]
- 22.Borgdorff AJ, Rossler AS, Clement P, Bernabe J, Alexandre L, et al. Differences in the spinal command of ejaculation in rapid ejaculating rats. J Sex Med. 2009;6:2197–205. doi: 10.1111/j.1743-6109.2009.01308.x. [DOI] [PubMed] [Google Scholar]
- 23.Agmo A. Sexual motivation – An inquiry into events determining the occurrence of sexual behavior. Behav Brain Res. 1999;105:129–50. doi: 10.1016/s0166-4328(99)00088-1. [DOI] [PubMed] [Google Scholar]
- 24.Allouh MZ. Effects of swimming activity on the copulatory behavior of sexually active male rats. Int J Impot Res. 2015;27:113–7. doi: 10.1038/ijir.2014.42. [DOI] [PubMed] [Google Scholar]
- 25.Rowland DL, Keeney C, Slob AK. Sexual response in men with inhibited or retarded ejaculation. Int J Impot Res. 2004;16:270–4. doi: 10.1038/sj.ijir.3901156. [DOI] [PubMed] [Google Scholar]
- 26.Barrett CJ. Renal sympathetic nerves – what have they got to do with cardiovascular disease? Exp Physiol. 2015;100:359–65. doi: 10.1113/expphysiol.2014.080176. [DOI] [PubMed] [Google Scholar]
- 27.Katholi RE, Whitlow PL, Winternitz SR, Oparil S. Importance of the renal nerves in established two-kidney, one clip goldblatt hypertension. Hypertension. 1982;4(3 Pt 2):166–74. [PubMed] [Google Scholar]
- 28.Xia JD, Han YF, Zhou LH, Xu ZP, Chen Y, et al. Sympathetic skin response in patients with primary premature ejaculation. Int J Impot Res. 2014;26:31–4. doi: 10.1038/ijir.2013.23. [DOI] [PubMed] [Google Scholar]
- 29.Zorba OU, Cicek Y, Uzun H, Cetinkaya M, Onem K, et al. Autonomic nervous system dysfunction in lifelong premature ejaculation: analysis of heart rate variability. Urology. 2012;80:1283–6. doi: 10.1016/j.urology.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 30.Pitchers KK, Schmid S, Di Sebastiano AR, Wang X, Laviolette SR, et al. Natural reward experience alters AMPA and NMDA receptor distribution and function in the nucleus accumbens. PLoS One. 2012;7:e34700. doi: 10.1371/journal.pone.0034700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fleming AS, Kucera C. Sexual experience effects are blocked both by the protein-synthesis inhibitor, cycloheximide, and by the noncompetitive NMDA antagonist, MK-801. Behav Neural Biol. 1991;56:319–28. doi: 10.1016/0163-1047(91)90499-g. [DOI] [PubMed] [Google Scholar]
- 32.Bredy TW, Zhang TY, Grant RJ, Diorio J, Meaney MJ. Peripubertal environmental enrichment reverses the effects of maternal care on hippocampal development and glutamate receptor subunit expression. Eur J Neurosci. 2004;20:1355–62. doi: 10.1111/j.1460-9568.2004.03599.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 34.Pyner S. Neurochemistry of the paraventricular nucleus of the hypothalamus: implications for cardiovascular regulation. J Chem Neuroanat. 2009;38:197–208. doi: 10.1016/j.jchemneu.2009.03.005. [DOI] [PubMed] [Google Scholar]


