Abstract
Background
Mycoplasma hominis is a human urogenital pathogen involved in gynaecological, neonatal and extra-genital infections. However, no versatile genetic tools are currently available to study the pathogenicity of this bacterium. Targeting-Induced Local Lesions IN Genomes (TILLING) is a reverse-genetic method that combines point mutations induced by chemical mutagenesis with a DNA screening technique. We used ethyl methanesulfonate (EMS) that introduces C-G to T-A transition mutations to generate a library of M. hominis mutants. As a proof of concept, mutagenized organisms were screened for mutations in two target genes previously associated with the mycoplasma pathogenicity, the vaa gene encoding an adhesin lipoprotein and the oppA gene encoding the main ectoATPase of the bacterium. The resulting mutants were evaluated using functional assays, an adhesion to HeLa cell assay for vaa-mutants and an ATPase activity test for oppA-mutants.
Results
A 1200-clone library was generated by exposing M. hominis PG21 to 9 mg/mL EMS for 3 h. To identify mutants of interest, targeted gene fragments were amplified, heat-denatured, slowly reannealed and digested with the mismatch-specific endonuclease ENDO1. If multiple alleles were present in the PCR amplicons, these alleles formed heteroduplexes during reannealing that were specifically cleaved by ENDO1 at mismatching positions.
A total of four vaa-mutants and two oppA-mutants harbouring missense mutations were obtained and fully sequenced. Zero to eight additional mutations were identified in the genomes of each mutant. The vaa-mutants were tested for adhesion to immobilized HeLa cells but their adhesion was not significantly different from the adhesion of M. hominis PG21. One of the two oppA-mutants that were tested for ATPase activity presented a higher affinity for its ATP substrate than the parental strain.
Conclusion
For the first time, we demonstrated that M. hominis gene-targeted mutants could be successfully obtained using this TILLING strategy. In the absence of robust genetic tools for studying M. hominis, the TILLING strategy that can target any gene of the genome could help to elucidate gene functions and to better understand the pathogenesis of this human pathogenic species.
Keywords: Mycoplasma hominis, Chemical mutagenesis, Ethyl methanesulfonate, TILLING
Background
Mycoplasma hominis is a human urogenital pathogen involved in gynaecological, neonatal and extra-genital infections [1]. The genome of the reference strain, M. hominis PG21, is the second smallest genome among self-replicating free-living organisms [2]. Although a conjugal transfer of the transposon Tn916 from Streptococcus faecalis to M. hominis was reported 30 years ago [3], no versatile genetic tools are currently available for this species. Whereas Mycoplasma arthritidis, a phylogenetically closely related species belonging to the same Hominis group, has successfully been transformed using the PEG method [4], all attempts to transform M. hominis using either PEG or electroporation methods, as well as transposons, suicide plasmids or replicative plasmids have failed. Only one study reported the transformation of M. hominis by the plasmid pAM120 using electroporation in the 2000s [5], but the experiments could not be reproduced. A cutting-edge synthetic biology approach, which consists of cloning and subsequent engineering of M. hominis genome in yeast is currently under development but the back transplantation of the genome from yeast to a recipient bacterium has not been achieved yet [6]. The lack of genetic engineering tools for M. hominis has limited our capacity to modify its genome in order to elucidate gene functions and to understand its pathogenesis.
Chemical mutagenesis methods may offer an interesting alternative method to modify the genome of this intractable species. Targeting-Induced Local Lesions IN Genomes (TILLING) combines point mutations induced by standard chemical mutagenesis with a sensitive DNA screening technique that identifies single nucleotide polymorphisms (SNPs) in the targeted gene. This reverse-genetic strategy has mainly been used in plants such as Arabidopsis thaliana [7], tomato [8] and Cucurbita pepo [9] but also in parasites such as Toxoplasma gondii [10]. Only one publication reported its use in a bacterium, the intracellular Chlamydia trachomatis species [11].
In this study, we used ethyl methanesulfonate (EMS) that introduces C-G to T-A transition mutations to generate a library of M. hominis mutants. As a proof of principle, mutagenized organisms were screened for mutations in two target genes, the vaa gene encoding the variable adherence-associated (Vaa) adhesin lipoprotein [12] and the oppA gene encoding the substrate binding subunit of an oligopeptide permease and the main ectoATPase of the bacteria [13, 14]. The generated vaa- and oppA-mutants were fully sequenced and evaluated using two functional assays, an adhesion test to HeLa cells for vaa-mutants and an ATPase activity test for oppA-mutants.
Results
Generation of a M. hominis mutant library
The effect of EMS on M. hominis survival was assessed after contact times of 1.5 h and 3 h (Fig. 1). A positive correlation was observed between the concentration of EMS and the killing of the bacteria. The contact time with EMS also slightly affected the survival of M. hominis. For the generation of the mutant library, a concentration of 9 mg/ml EMS applied for 3 h that resulted in 75% killing was chosen according to the bibliography [11]. After culture filtration to avoid bacterial aggregates and plating on agar medium, 1200 individual colonies were picked, grown and frozen.
Fig. 1.
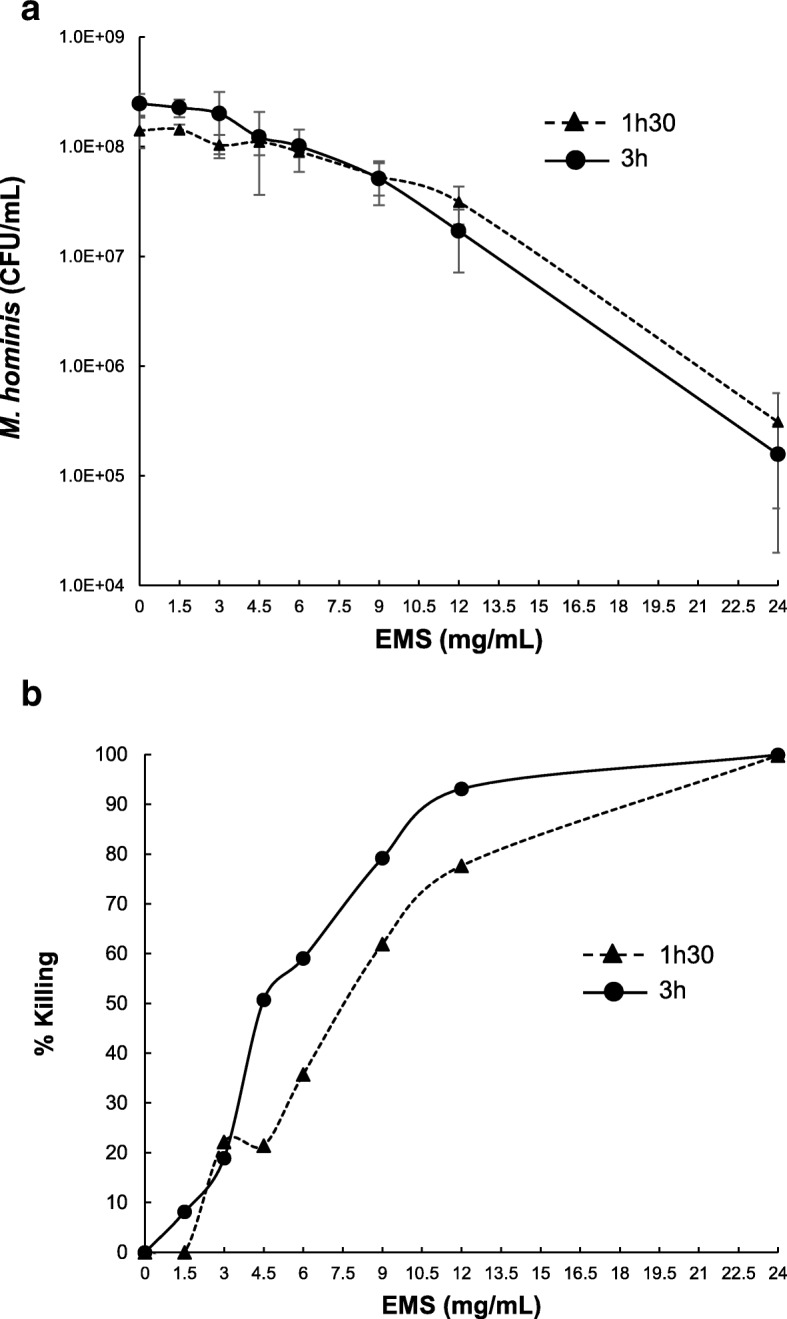
Effects of EMS mutagenesis on M. hominis survival. Concentrations of M. hominis PG21 were determined after treatment with various concentration of EMS for 1.5 h and 3 h (a). Three independent experiments were performed. The percentage of killing was calculated (b). In accordance with the literature, a 75% killing rate corresponding to exposure to approximately 9 mg/ml EMS for 3 h was chosen to generate the M. hominis PG21 mutant library
Screening of the M. hominis mutant library for vaa- and oppA-mutants
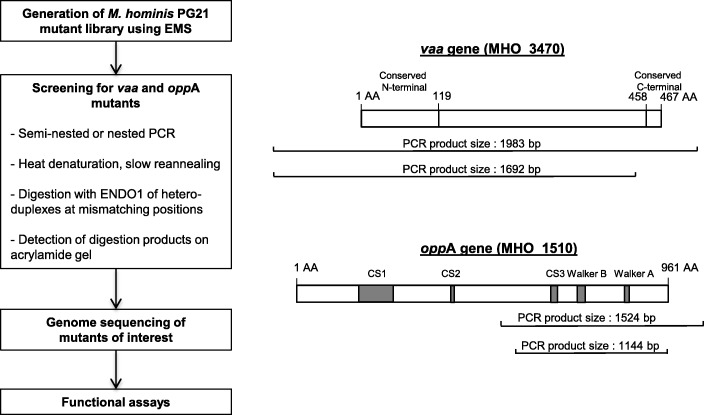
To screen the library for vaa-mutants, a 1692-bp PCR fragment of the M. hominis genome encompassing the 1295 nucleotides at the 5′-end of the vaa gene (MHO_3470, 1416 bp [2]) was targeted (Table 1, Fig. 2). To screen for oppA-mutants, a 1144-bp PCR fragment of the oppA gene encompassing the regions encoding the CS3, Walker B and Walker A domains involved in ATPase activity [14] of the OppA protein (MHO_1510, 2886 bp) was amplified (Table 1, Fig. 2).
Table 1.
Oligonucleotides used in this study
| Primer target and designation | Primer sequence | Product size (bp) |
|---|---|---|
| vaa genea | ||
| VaaPG21-FE | 5’-AATGGGATAGTTAGTAAAGTCGG-3’ | 1983 |
| VaaPG21-RE | 5’-AACCCTACCATTGTCGCTAAGA-3’ | |
| VaaPG21-FE-IRD700 | 5’-IRD700-AATGGGATAGTTAGTAAAGTCGG-3’ | 1692 |
| VaaPG21-RI-IRD800 | 5’-IRD800-GAAAAATCTCCTCGTGCTGATG-3’ | |
| oppA gene | ||
| OppAPG21-FE | 5’-GACAGTGGAAAAGCAGACAC-3’ | 1524 |
| OppAPG21-RE | 5’-AGGAACTAAAATGTCCGGGT-3’ | |
| OppAPG21-FI-IRD700 | 5’-IRDye700- CATCTTCTGGCCAAGCAACT − 3’ | 1144 |
| OppAPG21-RI-IRD800 | 5’-IRDye800-CAGTCTTGGTATGAATCAAC-3’ | |
aVaaPG21-FE and VaaPG21-RE primers are located upstream and downstream the vaa gene, respectively
Fig. 2.
Schematic representation of the TILLING strategy used in this study
After the screening procedure using ENDO1 nuclease, a total of six vaa and four oppA M. hominis mutants were obtained (Table 2). Four mutants were discarded: (i) two vaa-mutants that harboured a C to T transition located upstream of the vaa gene (these mutants were initially screened because the amplified DNA fragment encompassed 397 nucleotides upstream of the gene) and (ii) two oppA-mutants that harboured a G181068A and a G180439A silent substitution, respectively. Overall, four vaa-mutants (Mho66, Mho119, Mho787 and Mho1133) and two oppA-mutants (Mho369 and Mho940), each harbouring one amino-acid substitution in the targeted region of the vaa and oppA genes, respectively, were selected. The genomes of these six selected mutants were fully sequenced. The expected mutation was found in every mutant and zero to eight additional mutations were retrieved in the whole genome of each mutant (Table 3). Notably, mutant Mho66 harboured no additional mutation and mutant Mho369 harboured only one additional mutation in an intergenic region.
Table 2.
vaa- and oppA-mutants obtained by screening the 1200 EMS-generated M. hominis clone library
| Mutant designation | Targeted gene | Nucleotide substitutiona | Amino-acid substitutionb | Comments |
|---|---|---|---|---|
| Mho66 | vaa | G410726A | Glu54Lys | |
| Mho119 | vaa | C411332T | Leu328Phe | |
| Mho787 | vaa | C410526A | Pro59His | |
| Mho864 | vaa | C410197T | none | Mutation located upstream of the vaa gene (intergenic region) |
| Mho1055 | vaa | C410154T | none | Mutation located upstream of the vaa gene (intergenic region) |
| Mho1133 | vaa | G410726A | Gly126Ser | |
| Mho258 | oppA | G181068A | Leu813Leu | Silent mutation |
| Mho369 | oppA | G180439A | Asp604Asn | |
| Mho785 | oppA | C181239T | Asp870Asp | Silent mutation |
| Mho940 | oppA | G180634A | Glu669Lys |
aNucleotide substitutions are numbered according to the M. hominis PG21 genome (GenBank accession number FP236530)
bAmino acid substitutions are numbered from the N-terminal end of the targeted protein
Table 3.
Mutations harboured by EMS-generated M. hominis mutants in comparison to the sequence of the M. hominis PG21 strain not exposed to EMS
| Mutant designation | Expected mutationa (gene, position in the gene) | Other mutations in the genomea (Locus, gene) | Number of additional mutations |
|---|---|---|---|
| Mho66 | G410726A (vaa, 376) | none | 0 |
| Mho119 | C411332T (vaa, 982) | - C230929T (intergenic) - C316577T (MHO_2640, CHP) - G463593A (MHO_3770, licA, Protein LicA homolog) - G494526A (MHO_3990, nusG, Transcription antitermination protein) |
4 |
| Mho787 | C410526A (vaa, 176) | - C5000T (MHO_0050, dnaN, DNA polymerase III beta chain) - C44025T (MHO_0340, ktrA, Potassium uptake protein KtrA) - C172081T (MHO_1460, rpsG, 30S ribosomal protein S7) - C258548T (MHO_2150, rpoD, RNA polymerase sigma factor) - C283090T (MHO_2320, ABC transporter, ATP-binding protein) - G288972A (MHO_2370, tRNA/rRNA methyltransferase) - C441828T (MHO_3650, asnA, Aspartate-ammonia ligase) - C571686T (MHO_4600, pyrH, Uridylate kinase) |
8 |
| Mho1133 | G410726A (vaa, 376) | - G179389A (MHO_1510, oppA) - C206418T (MHO_1700, recU, Recombination protein U homolog) - C381191T (MHO_3200, CHP, Hypothetical lipoprotein) - G462316A (MHO_3760, gyrB, DNA gyrase subunit B) - G490546A (intergenic) - C644067T (MHO_5190, pip, Proline iminopeptidase) |
6 |
| Mho369 | G180439A (oppA, 1813) | - G230779A (intergenic) | 1 |
| Mho940 | G180634A (oppA, 2008) | - G136127A (MHO_1080, ATB-binding protein) - G138607A (MHO_1090, HP) - G203092A (MHO_1670, cmk, Cytidylate kinase) - G211597A (MHO_1740, oppB, Oligopeptide transport system permease protein) - A227310G (MHO_1880, aspS, Aspartyl-tRNA synthetase) - G357674A (MHO_3060, leuS, Leucyl-tRNA synthetase) - G371725A (MHO_3130, atpA, F1-likeX0) - G649646C (MHO_5220, hsdS, Type I restriction enzyme specificity protein) |
8 |
amutations are numbered according to the M. hominis PG21 genome (GenBank accession number FP236530)
CHP conserved hypothetical protein, HP hypothetical protein
Functional assays applied to vaa- and oppA-mutants
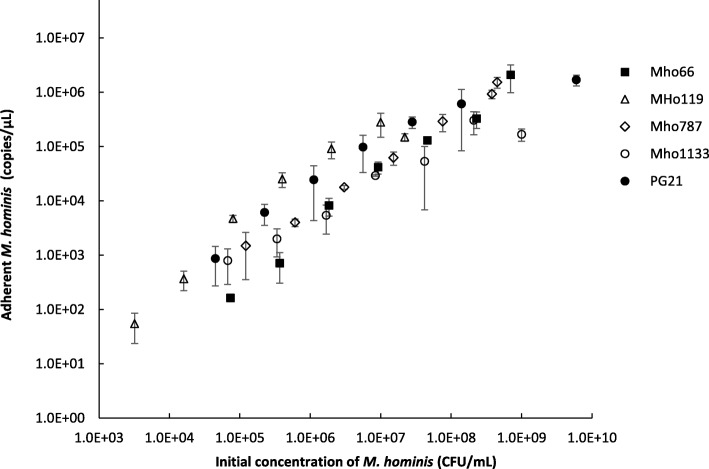
The four vaa-mutants, Mho66, Mho119, Mho787 and Mho1133, and the parental PG21 M. hominis strain, which has a functional in-frame vaa gene, were tested for adhesion to HeLa cells immobilized on microtiter plates to quantify bacterial adherence. In all cases, the number of adherent M. hominis cells increased with the amount of M. hominis inoculum used in the assay (Fig. 3). The adhesion of the four vaa-mutants and the non-mutated M. hominis PG21 strain was not significantly different (p = 0.73, Kruskal-Wallis test).
Fig. 3.
Adhesion to HeLa cells of EMS-generated M. hominis vaa-mutants. M. hominis cells were added to immobilized HeLa cells in triplicate and incubated 4 h at 37 °C. After removal of unbound M. hominis cells by washing, the adherent M. hominis cells were quantified by real-time PCR. One representative experiment of three is shown
The two oppA-mutants, Mho369 and Mho940, and the parental PG21 strain were tested for ATPase activity (Fig. 4) during which the kinetics of ATP hydrolysis were analysed by measuring the release of free phosphate [14]. Both mutants had a higher Vmax than M. hominis PG21 and the Mho369 mutant had the lowest Michaelis-Menten Km constant, although the 95% confidence intervals were overlapping.
Fig. 4.
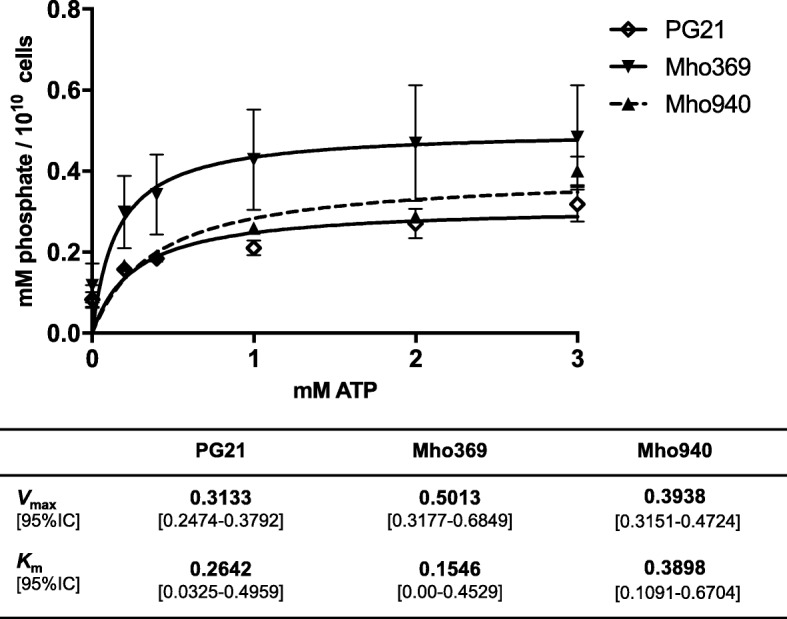
ectoATPase activity of EMS-generated M. hominis oppA-mutants. ectoATPase activity of EMS-generated Mho369 and Mho940 M. hominis mutants and of M. hominis PG21 parental strain was determined by measuring the release of free phosphate per 1010 cells as a function of the ATP concentration. The data represent the means and standard errors of five (Mho369) and four (Mho940 and PG21) independent experiments with triplicate samples in each experiment. Vmax, maximum rate, Km, Michaelis-Menten constant, 95% IC, 95% confidence interval
Discussion
Because the transformation of M. hominis PG21 has so far been elusive, chemical mutagenesis using EMS appears to be an alternative option to create gene-targeted mutants. In several organisms, a positive correlation was observed between the concentrations of EMS applied, the killing of the organism and the mutation frequency obtained [8, 11]. Only one study has reported the generation of EMS mutants and TILLING strategy in bacteria. Indeed, to generate C. trachomatis mutants using EMS, the concentration of EMS that generated a single C-G base pair mutation per genome corresponded to a 75% killing. Because we also observed a positive correlation between the concentration of EMS and the killing of M. hominis, we decided to choose, for the generation of the library, the concentration of EMS that killed approximately 75% of the M. hominis population, i.e. 9 mg/ml applied for 3 h.
Using these conditions, we demonstrated, after screening the 1200-clone library, that four and two M. hominis mutants were successfully generated in two targeted genes previously associated with the bacterium’s pathogenicity, the vaa gene involved in cytadherence [12] and the oppA gene involved in oligopeptide binding and the ectoATPase activity [13, 15], respectively. Because the ATPase activity of the OppA protein is due mainly to the CS3, Walker B and Walker A gene regions [14], a fragment of 1144 bp encompassing these regions was chosen. Regarding the vaa gene, a 1692-bp fragment encompassing the 5′-end conserved region of the gene was targeted for this proof of concept experiment because an early premature stop in protein synthesis would have had the greatest effect on loss of Vaa-mediated cytoadhesion. To our knowledge, the substitutions that were found in the mutated Vaa and OppA have never been reported in the alleles present in other M. hominis strains.
The four vaa-mutants were tested for adhesion to immobilized HeLa cells [14]. Unfortunately, the adhesion of the four mutants did not show any significant difference with the adhesion of the parental M. hominis PG21 strain. However, because M. hominis harbours several adhesin proteins such as the P120 lipoprotein and the Lmp-related proteins [16], this adhesion assay may not be sensitive enough to detect a potential adhesion decrease caused by the mutation of the Vaa protein. A control strain with an inactivated Vaa protein or a phase variable “OFF” variant would be necessary to determine whether the sensitivity of this assay was accurate. Analysis of the vaa gene sequence of the M. hominis PG21 genome showed that C-G to T-A EMS-generated transitions could theoretically create 23 stop codons, leading to truncated proteins. Thus, enlarging our EMS library may generate null mutants.
Regarding the two oppA-mutants, mutant Mho369 was of interest because (i) it harboured only one additional mutation in an intergenic region of its genome, suggesting that the observed phenotype could be attributed to the EMS-generated oppA mutation with a high degree of certainty and (ii) it had a higher Vmax and a lower Km than M. hominis PG21, although 95% confidence intervals were overlapping. This finding suggests that Mho369 may have the capacity to hydrolyse ATP with a higher turnover. This mutant harboured an aspartic acid-to-asparagine substitution located upstream of the CS3 motif, known to be involved in the ATPase activity of the protein [14]. Further studies such as cloning, expression and analysis of the mutated OppA protein could be informative in future works. Nevertheless, although recombinant OppA-mutant proteins have previously been generated [14], oppA-mutated M. hominis cells had never been obtained before. Since OppA is involved in M. hominis pathogenicity [13, 15], such mutated M. hominis cells will allow for the characterization of the effect of single amino-acid changes in OppA on the pathogenicity of the mycoplasma.
The TILLING strategy used in this study presents some advantages and some drawbacks. The principal advantage is its capacity to generate gene-targeted mutants in species such as M. hominis that are difficult to genetically manipulate. The ultimate goal of the method is to generate null mutants. All genes could be potentially targeted except those genes that are essential for growth in vitro, and thus cannot be targeted for null mutations. In addition, once a library has been generated, it can be reused to search for mutants in other genes of interest. However, this method is labour-intensive, both for the generation of the library and its handling. The 1200-clone library generated in this study was not representative of the whole M. hominis genome and the isolation of a null mutant of Vaa and OppA was not achieved. Sequencing the whole genome of the selected mutants is necessary to check for additional mutations that could have been created in other regions of the chromosome. Indeed, the occurrence of multiple mutations in the same clone may be disruptive for functional studies. However, in a future use of the TILLING strategy, whole genome sequencing will only have to be performed on mutants presenting interesting phenotypes upon functional assays. In the present study, only one of the six sequenced mutants had no other mutation in the genome. Because one to eight additional mutations were present in the five other mutants, it is likely that the concentration and/or the EMS exposure time with M. hominis should be decreased slightly in future experiments. Decreasing these parameters would require screening more than 1200 clones to select for a mutant, which would increase the workload of the method. However, the technical improvement of robotic colony pickers may unlock this limitation, making the TILLING strategy a broader avenue for the functional genomic of intractable bacteria.
Conclusion
Although genetic manipulations are still hardly achievable in M. hominis, targeted mutants were successfully generated using the TILLING strategy. Further experiments are needed to adjust the conditions of EMS mutagenesis to limit the mutation frequency to one mutation per genome, but this strategy will be helpful to elucidate the gene functions and to better understand the pathogenesis of this human urogenital species.
Methods
EMS mutagenesis of M. hominis PG21
The M. hominis PG21 reference strain (ATCC 23114) was grown in Hayflick broth supplemented with arginine (hereafter named Hayflick arginine medium) [17]. One millilitre of a culture of M. hominis PG21 ranging between 1 × 108 and 3 × 108 colony forming unit (CFU)/ml was exposed to concentrations of EMS ranging from 0 to 24 mg/ml for 1.5 h and 3 h at room temperature, including a 30-min centrifugation step at 15,000 g. Pellets were resuspended in 1 ml of Hayflick arginine medium, and M. hominis concentration was determined by plating on Hayflick agar plates to assess M. hominis survival.
To generate the mutant library, a 108 CFU/ml M. hominis culture was exposed to EMS, filtered on 0.45 μm sterile mixed cellulose ester membrane (Millex-HA, Merck Millipore, Cork, Ireland), and 10− 1 to 10− 4 serial dilutions in Hayflick arginine medium were plated on Hayflick agar plates. After 48 h at 37 °C under 5% CO2, individual colonies were picked and grown in 1 ml of Hayflick arginine medium until the colour changed. A total of 800 μl of the latter culture was frozen at − 80 °C, and 200 μl was used for DNA extraction.
Screening for vaa- and oppA-mutants in M. hominis
Two hundred microliters from four distinct clone cultures were pooled together before DNA extraction using the MagNA Pure 96 DNA and Viral NA Small Volume kit on the MagNA Pure 96 instrument (Roche Diagnostics, Meylan, France). DNA extraction was also performed on 200 μl of each of four single clone cultures when a mutated pool of clones was identified.
PCR fragments of 1692 bp and 1144 bp in regions of interest of the vaa and the oppA genes were amplified by semi-nested and nested PCR, respectively, using primers presented in Table 1. The first round of PCR was performed on a CFX96 real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA, USA) in a final volume of 25 μl using 2.5 μM of tartrazine 10X buffer (50 mM Tris HCl pH 8, 500 μg/ml bovine serum albumin, 0.5% Ficoll, 1% sucrose, 30 mM KCl, 3 mM MgCl2, 1 mM tartrazine), 0.1 μM of each primer, 120 μM dNTP, 0.0005 U of GoTaq® qPCR Master Mix (Promega, Madison, WI, USA), and 1 μl of DNA extract. The amplification conditions were 3 min at 95 °C followed by 35 cycles of 15 s at 95 °C, 20 s at 58–68 °C, 2 min at 72 °C then 10 min at 72 °C. The second round of PCR was performed using 0.5 μl from the first PCR and IRD700- and IRD800-labelled primers (Table 1). After checking accurate amplification by agarose gel electrophoresis, 5 μl of the PCR products was used to screen for vaa- and oppA-mutants. PCR amplicons were heat-denatured 3 min at 95 °C and slowly reannealed using a temperature gradient from 95 °C to 8 °C (0.1 °C/s) before a 4 °C final step. The reannealed amplicons were digested using ENDO1 nuclease at 0.33 U/μl, prepared as previously reported [8], for 20 min at 45 °C and cooled to 4 °C to stop the enzyme activity. At this step, if multiple alleles were present in the PCR amplicons, these alleles formed heteroduplexes during reannealing that were specifically cleaved by ENDO1 at mismatching positions. The digested amplicons were purified and dried as described previously [18].
SNP screening was performed using a LiCOR® DNA analyser as previously described [19]. Pools of clones containing vaa- and oppA-mutants were identified by the appearance of lower-mass DNA digestion products after electrophoresis on a 6.5% acrylamide gel. The PCR products of vaa or oppA genes from the four individual clones of the mutated pool were sequenced by Sanger method (Eurofins Genomics, Ebersberg, Germany) to identify the mutated clone and the mutation type.
M. hominis genome sequencing
The genomic DNA from four vaa-mutants, two oppA-mutants and from the M. hominis PG21 parental strain was extracted using NucleoBond® AXG100 columns (Macherey-Nagel, Düren, Germany) and the NucleoBond® buffer Set III (Macherey-Nagel). The genomes were sequenced using paired-end V2 2X250 bp sequencing on MiSeq Illumina apparatus (San Diego, CA, USA) after generating the genomic DNA libraries using the of Nextera XT DNA Library Preparation Kit (Illumina). About 160,000 to 200,000 paired reads were obtained for each genome. Data processing including quality check, trimming, alignment with BWA (Galaxy Version 1.2.3) and variant calling using Varscan (Galaxy Version 0.1) was performed using Galaxy at https://usegalaxy.org/ [20].
Adhesion to HeLa cell assay
M. hominis adhesion to immobilized HeLa cells was performed as previously reported with some modifications [14]. A calibrated culture of each M. hominis mutant, with concentrations ranging between 1 × 108 and 4 × 108 CFU/ml, was concentrated 10 times by centrifugation and serially diluted 1:5 in Hayflick arginine medium. Fifty microliters of each dilution was incubated in triplicate with HeLa cells that had been lysine-coated on 96-well microplates in 50 μl of Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 1% fetal bovine serum (DMEM-FBS) for 4 h at 37 °C. Unbound M. hominis cells were removed by three washings with DMEM-FBS. Adherent M. hominis cells were lysed using 20 μl of proteinase K 20 mg/ml from the NucleoSpin® Tissue kit (Macherey-Nagel) for 1 h at 56 °C and DNA was extracted using the MagNA Pure 96 DNA and Viral NA Small Volume kit on the MagNA Pure 96 instrument (Roche Diagnostics). A quantitative real-time PCR targeting the yidC gene of M. hominis was then performed as previously described [21]. The non-parametric Kruskal-Wallis test was used to compare the adhesion of mutants and the PG21 parental strain. A significant p-value was set at < 0.05.
ATPase activity test
The ATPase assay was conducted by incubating M. hominis cells with ATP and subsequently using an ammonium molybdate solution to quantify the emerging free phosphates [13]. M. hominis cells were collected from 10 ml of logarithmic growth culture by centrifugation (6700 g for 10 min at 6 °C), washed twice in buffer A (120 mM NaCl, 5 mM KCl, 20 mM Tris-HCl, pH 7.5) and resuspended in 1.2 ml of buffer A. M. hominis cells were quantified by Taqman PCR as published earlier [22]. Triplicates of 20 μl of mycoplasma cell suspension and 20 μl of buffer A (as negative control) were each adjusted to 0, 0.2, 0.4, 1, 2 or 3 mM ATP and immediately assayed for free phosphate (to estimate phosphate background of mycoplasma suspension) or incubated for 4 h at 37 °C.
Hydrolysis of ATP was terminated by adding 200 μl of malachite green reagent (5.72% [w/v] ammonium molybdate in 6 N HCl, 2.32% [w/v] polyvinyl alcohol, 0.0812% [w/v] malachite green, and distilled water at a ratio of 1:1:2:2). The relative absorbance of the samples was measured in relation to a blank at 620 nm (Tecan Rainbow, SLT Labinstruments, Crailsheim, Germany). Inorganic phosphate at concentrations ranging from 1 to 20 nmol was used as quantification standard. Values of ATPase activity of M. hominis expressed as the release of nmol phosphate were corrected for the phosphate background of ATP, adjusted to 1010 M. hominis cells and used in the Michaelis-Menten equation to calculate the values of Vmax and Km using the Graph Pad Prism 6.01 software.
Acknowledgements
The authors thank E. Chancerel for genome sequencing.
Funding
This study was mainly supported by internal funding. Genome sequencing was performed at the Genome Transcriptome Facility of Bordeaux (PGTB, https://pgtb.cgfb.u-bordeaux.fr/) that is supported by grants from the Conseil Régional d’Aquitaine n°20030304002FA and 20040305003FA, from the European Union FEDER n°2003227 and from Investissements d’Avenir ANR-10-EQPX-16-01. PGTB staff produced genome sequences of the M. hominis mutants and provided the corresponding FastQ files.
Availability of data and materials
The PG21 M. hominis reference strain and the EMS-generated M. hominis mutant sequence information were deposited in NCBI Sequence Read Archive (SRA) with the following accession numbers: PG21 (ATCC 23114) SRX4325267, Mho66 SRX4325266, Mho119 SRX4325269, Mho369 SRX4325268, Mho787 SRX4325264, Mho940 SRX4325263 and Mho1133 SRX4325265.
Abbreviations
- C. trachomatis
Chlamydia trachomatis
- CHP
Conserved hypothetical protein
- EMS
Ethyl methanesulfonate
- HP
Hypothetical protein
- M. hominis
Mycoplasma hominis
- TILLING
Targeting-Induced Local Lesions IN Genomes
Authors’ contributions
SP, PSP, BH and CBéb conceived and designed the study. SP, CBén, CBr, CLR, JPM, FR and BH conducted the experiments and performed data analysis. SP wrote the manuscript with contributions from CBr, PSP, BH and CBéb. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable. No human samples and no animals were used.
The M. hominis PG21 reference strain was from ATCC (ATCC 23114). The HeLa cells were Human cervical carcinoma cell line HeLa S3 (ATCC CCL2.2).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
B. Henrich and C. Bébéar contributed equally to this work.
Contributor Information
S. Pereyre, Phone: +33 5 57 57 16 25, Email: sabine.pereyre@u-bordeaux.fr
C. Bénard, Email: chloe.benard@live.fr
C. Brès, Email: cecile.bres@bordeaux.inra.fr
C. Le Roy, Email: chloe.le-roy@u-bordeaux.fr
J. P. Mauxion, Email: jpmauxio@bordeaux.inra.fr
F. Rideau, Email: fabien.rideau@u-bordeaux.fr
P. Sirand-Pugnet, Email: pascal.sirand-pugnet@inra.fr
B. Henrich, Email: birgit.henrich@hhu.de
C. Bébéar, Email: cecile.bebear@u-bordeaux.fr
References
- 1.Waites K, Talkington D. New developments in human diseases due to mycoplasmas. Mycoplasmas: Molecular Biology, Pathogenicity, and Strategies for Control. 2005. pp. 289–354. [Google Scholar]
- 2.Pereyre S, Sirand-Pugnet P, Beven L, Charron A, Renaudin H, Barré A, Avenaud P, Jacob D, Couloux A, Barbe V, et al. Life on arginine for Mycoplasma hominis: clues from its minimal genome and comparison with other human urogenital mycoplasmas. PLoS Genet. 2009;5(10):e1000677. doi: 10.1371/journal.pgen.1000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts MC, Kenny GE. Conjugal transfer of transposon Tn916 from Streptococcus faecalis to Mycoplasma hominis. J Bacteriol. 1987;169(8):3836–3839. doi: 10.1128/jb.169.8.3836-3839.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dybvig K, French CT, Voelker LL. Construction and use of derivatives of transposon Tn4001 that function in Mycoplasma pulmonis and Mycoplasma arthritidis. J Bacteriol. 2000;182(15):4343–4347. doi: 10.1128/JB.182.15.4343-4347.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aleksandrova NM, Bevova MR, Govorun VM. Transformation of Mycoplasma hominis with plasmid pAM120 through electroporation. Russ J Genet. 2000;36(3):237–240. [PubMed] [Google Scholar]
- 6.Rideau F, Le Roy C, Descamps ECT, Renaudin H, Lartigue C, Bébéar C. Cloning, stability, and modification of Mycoplasma hominis genome in yeast. ACS Synth Biol. 2017;6(5):891–901. doi: 10.1021/acssynbio.6b00379. [DOI] [PubMed] [Google Scholar]
- 7.McCallum CM, Comai L, Greene EA, Henikoff S. Targeted screening for induced mutations. Nat Biotechnol. 2000;18(4):455–457. doi: 10.1038/74542. [DOI] [PubMed] [Google Scholar]
- 8.Okabe Y, Asamizu E, Saito T, Matsukura C, Ariizumi T, Bres C, Rothan C, Mizoguchi T, Ezura H. Tomato TILLING technology: development of a reverse genetics tool for the efficient isolation of mutants from micro-tom mutant libraries. Plant Cell Physiol. 2011;52(11):1994–2005. doi: 10.1093/pcp/pcr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vicente-Dolera N, Troadec C, Moya M, del Rio-Celestino M, Pomares-Viciana T, Bendahmane A, Pico B, Roman B, Gomez P. First TILLING platform in Cucurbita pepo: a new mutant resource for gene function and crop improvement. PLoS One. 2014;9(11):e112743. doi: 10.1371/journal.pone.0112743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrell A, Coleman BI, Benenati B, Brown KM, Blader IJ, Marth GT, Gubbels MJ. Whole genome profiling of spontaneous and chemically induced mutations in Toxoplasma gondii. BMC Genomics. 2014;15:354. doi: 10.1186/1471-2164-15-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kari L, Goheen MM, Randall LB, Taylor LD, Carlson JH, Whitmire WM, Virok D, Rajaram K, Endresz V, McClarty G, et al. Generation of targeted Chlamydia trachomatis null mutants. Proc Natl Acad Sci U S A. 2011;108(17):7189–7193. doi: 10.1073/pnas.1102229108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boesen T, Emmersen J, Baczynska A, Birkelund S, Christiansen G. The vaa locus of Mycoplasma hominis contains a divergent genetic islet encoding a putative membrane protein. BMC Microbiol. 2004;4(1):37. doi: 10.1186/1471-2180-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hopfe M, Henrich B. OppA, the substrate-binding subunit of the oligopeptide permease, is the major Ecto-ATPase of Mycoplasma hominis. J Bacteriol. 2004;186(4):1021–1028. doi: 10.1128/JB.186.4.1021-1028.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopfe M, Dahlmanns T, Henrich B. In Mycoplasma hominis the OppA-mediated cytoadhesion depends on its ATPase activity. BMC Microbiol. 2011;11:185. doi: 10.1186/1471-2180-11-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopfe M, Henrich B. OppA, the ecto-ATPase of Mycoplasma hominis induces ATP release and cell death in HeLa cells. BMC Microbiol. 2008;8(1):55. doi: 10.1186/1471-2180-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ladefoged SA. Molecular dissection of Mycoplasma hominis. APMIS. 2000;108:5–45. doi: 10.1111/apm.2000.108.s97.5. [DOI] [PubMed] [Google Scholar]
- 17.Waites KB, Bébéar CM, Roberston JA, Talkington DF, Kenny GE. Cumitech 34, laboratory diagnosis of mycoplasmal infections. In: Nolte FS, editor. Cumitech. Washington D. C: American Society for Microbiology. p. 2001.
- 18.Till BJ, Zerr T, Comai L, Henikoff S. A protocol for TILLING and Ecotilling in plants and animals. Nat Protoc. 2006;1(5):2465–2477. doi: 10.1038/nprot.2006.329. [DOI] [PubMed] [Google Scholar]
- 19.Triques K, Piednoir E, Dalmais M, Schmidt J, Le Signor C, Sharkey M, Caboche M, Sturbois B, Bendahmane A. Mutation detection using ENDO1: application to disease diagnostics in humans and TILLING and eco-TILLING in plants. BMC Mol Biol. 2008;9:42. doi: 10.1186/1471-2199-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afgan E, Baker D, Batut B, van den Beek M, Bouvier D, Cech M, Chilton J, Clements D, Coraor N, Gruning BA, et al. The galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018 [DOI] [PMC free article] [PubMed]
- 21.Férandon C, Peuchant O, Janis C, Benard A, Renaudin H, Pereyre S, Bébéar C. Development of a real-time PCR targeting the yidC gene for the detection of Mycoplasma hominis and comparison with quantitative culture. Clin Microbiol Infect. 2011;17(2):155–159. doi: 10.1111/j.1469-0691.2010.03217.x. [DOI] [PubMed] [Google Scholar]
- 22.Mobius N, Brenneisen W, Schaeffer A, Henrich B. Protocol for the rapid detection of the urogenital tract mollicutes and Chlamydia with concomitant LGV-(sub)typing. Methods Mol Biol. 2012;903:235–253. doi: 10.1007/978-1-61779-937-2_15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The PG21 M. hominis reference strain and the EMS-generated M. hominis mutant sequence information were deposited in NCBI Sequence Read Archive (SRA) with the following accession numbers: PG21 (ATCC 23114) SRX4325267, Mho66 SRX4325266, Mho119 SRX4325269, Mho369 SRX4325268, Mho787 SRX4325264, Mho940 SRX4325263 and Mho1133 SRX4325265.