Abstract
Background
Definitions and practices regarding use of contact precautions and isolation to prevent the spread of gram-positive and gram-negative multidrug-resistant organisms (MDRO) are not uniform.
Methods
We conducted an on-site survey during the European Congress on Clinical Microbiology and Infectious Diseases 2014 to assess specific details on contact precaution and implementation barriers.
Results
Attendants from 32 European (EU) and 24 non-EU countries participated (n = 213). In EU-respondents adherence to contact precautions and isolation was high for Methicillin-resistant Staphylococcus aureus (MRSA), carbapenem-resistant Enterobacteriaceae, and MDR A. baumannii (84.7, 85.7, and 80%, respectively) whereas only 68% of EU-respondents considered any contact precaution measures for extended-spectrum-beta-lactamase (ESBL) producing non-E. coli. Between 30 and 45% of all EU and non-EU respondents did not require health-care workers (HCW) to wear gowns and gloves at all times when entering the room of a patient in contact isolation. Between 10 and 20% of respondents did not consider any rooming specifications or isolation for gram-positive MDRO and up to 30% of respondents abstain from such interventions in gram-negative MDRO, especially non-E. coli ESBL. Understaffing and lack of sufficient isolation rooms were the most commonly encountered barriers amongst EU and non-EU respondents.
Conclusion
The effectiveness of contact precautions and isolation is difficult to assess due to great variation in components of the specific measures and mixed levels of implementation. The lack of uniform positive effects of contact isolation to prevent transmission may be explained by the variability of interpretation of this term. Indications for contact isolation require a global definition and further sound studies.
Electronic supplementary material
The online version of this article (10.1186/s13756-018-0366-5) contains supplementary material, which is available to authorized users.
Keywords: Contact precaution, Isolation, Multi-drug resistant organisms, Implementation, Barriers
Background
The European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Healthcare Infection Control Practices Advisory Committee (HICPAC) have defined multidrug-resistant organisms (MDRO) that qualify for contact precautions and isolation [1]. According to HICPAC, contact precaution measures are indicated if transmission of an infectious agent is not interrupted by the use of standard precautions alone due to environmental contamination and, therefore, requires HCW to wear gloves and gowns upon room entry, not only if contact with blood or body fluid is anticipated. HICPAC also recommends that such patients should be placed preferably in a single room [2]. Similarly, the guidelines on prevention of transmission of gram-negative MDRO issued by ESCMID require contact precaution for patients colonized or infected with an epidemiologically targeted organism, that includes wearing gloves and gowns upon entry to the room and the use of patient-dedicated or single-use disposable non-critical equipment [3] (Table 1).
Table 1.
Core elements of contact precautions (CP) recommended by recent ESCMID and HICPAC/CDC guidelines
| ESCMID 2014 (3) | HICPAC/CDC 2007 (2) | |
|---|---|---|
| Indication for CP | Colonization or infection with MDRO | Colonization or infection with MDRO |
| Donning and wearing of gloves and gowns | Gown and gloves are donned upon entry to a room | Gown and gloves are donned upon room entry Gown and gloves are indicated for all interactions that may involve contact with the patient or potentially contaminated areas in the patient’s environment. |
| Disposal of gowns and gloves | Not stated | Gown and gloves are discarded before exiting the patient room |
| Additional requirements & recommendations | Use of disposable single-use or patient-dedicated non-critical care equipment (e.g. blood pressure cuffs and stethoscopes). | Use of patient-dedicated or single-use disposable noncritical equipment |
| Placement of patients | Special isolation wards Nursing cohort with separate rooms on general wards Single room or cohort in same room without dedicated personnel Placement in a room with patients unaffected by MDROs but maintaining CP by use of gowns and gloves based on the patient’s extent of MDRO carriage |
Single patient room preferred Cohort patients with same MDRO Multi-bed rooms with non-infected/non-colonized patients: at least 3 ft spatial separation between beds |
CDC centers for disease control and prevention, CP contact precaution, ESCMID european society of clinical microbiology and infectious diseases, HICPAC healthcare infection control practices advisory committee, MDRO multidrug resistant organism
However, there is no uniform definition of multidrug resistance in gram-negative bacteria and the indications to implement isolation precaution measures for MDRO vary substantially [4]. Reasons for this are not well understood. The variability in practices and the strictness of implementation (e.g. whether gowns and gloves are worn upon room entry or only if contact with blood or bodily fluid is anticipated, or whether implementation of contact precaution and isolation depends on the presence or absence of patient risk factors), has not been well studied amongst health-care professionals. This is of major relevance when examining the success of prevention and control of the spread of MDRO and when designing studies to look at the effectiveness of such interventions. Interestingly, comparison of national MRSA guidelines of 13 European (EU) countries has also shown divergent implementation regarding donning of gloves and gowns [5].
Methods
Our main survey aims were to explore the diversity in adopting contact precaution and isolation practices for gram-positive and gram-negative MDRO and to assess barriers to their implementation.
After an in-depth discussion amongst the ESCMID nosocomial infection study group (ESGNI) committee members we decided to focus on the indication, circumstances and implementation of contact precautions and isolation for MRSA, glycopeptide-resistant enterococci (GRE), extended-spectrum-beta-lactamase-producing Enterobacteriaceae (ESBL-E) and carbapenem-resistant Enterobacteriaceae (CRE), MDR P. aeruginosa, and MDR A. baumannii.
A questionnaire survey was developed by the authors and distributed amongst the ESGNI committee members for revision. Levels of agreement on barriers frequently encountered during implementation were measured on a 5-point Likert scale (3 being neutrality) [6]. The survey was then transferred onto Survey Monkey® [7] and pilot-tested among a broader group including five infection control nurses and five infectious diseases physicians from Switzerland, Germany and the USA.
The online survey was applied to attendees of the 2014 ECCMID in Barcelona, Spain. On-site participant recruitment was by study team members during the regular opening hours. Individuals were invited to complete the survey at a booth. Study team members addressed any issues of comprehension. As a recruitment incentive, there was a lottery with three prizes. In addition, the online survey was open to all ESCMID members for six weeks after the congress.
Statistical analyses
Numbers, percentages, median and interquartile range (IQR) were used for descriptive statistics. Countries were categorised into EU and non-EU in compliance with a reference classification system [8]. We regrouped the transcontinental Eurasian countries, e.g. Turkey, as belonging to the Southern EU Area rather than Western Asia to be consistent with other publications [9]. We compared differences in proportions among EU and non-EU responders using Chi-square or Fisher’s exact test. Missing answers were removed from the respective analysis on a case-by-case basis. In our primary analysis, we considered all non-missing responses equivalently without taking potential nesting into account. In order to evaluate a possible overestimation of effects due to nested data, we eliminated all duplicates that we defined as respondents from the same country and from the same hospital size. We then repeated the primary analysis with the de-duplicated dataset. All analyses were performed using SPSS statistical software version 23.0 [10].
Results
Overall, 213 individuals from 32 EU and 28 non-EU countries participated in the survey.The majority were European and had their workplace in either the Southern European Area (31%), in Western (22%), Northern (16%), or Eastern Europe (7%); about a quarter of the respondents came from countries out-side Europe (Asia and The Middle East 11%, South America 8%, Africa 5%). A total of 77 (36.1%) respondents were specialized in infection control and prevention and 108 respondents (50.7%) had either a background in microbiology and/or infectious diseases. The median experience in infection control was 8 (IQR: 3–15) years. There were 159 (74.6%) medical doctors and the majority (71.8%) worked in acute care. Details on the participants’ country of workplace and professional responsibilities are listed in the supplementary appendix. Numbers (%) of completely missing answers to questions concerning indications of contact precautions were: 14 (6.6) for MRSA, 38 (17.8) for GRE, 27 (12.7) for E. coli ESBL, 14 (6.6) for non-E. coli ESBL, 14 (6.6) for CR E. coli, 17 (8.0) for CRE (other than E. coli), 14 (6.6) for MDR P. aeruginosa, and 14 (6.6) for MDR A. baumannii. The proportion of EU-respondents reporting any form of contact precautions/isolation, irrespective whether a patient was colonized or infected, was high (≥ 80%) for MRSA, CRE (other than E. coli), and MDR A. baumannii (84.7, 85.7, and 80%, respectively) with lower, but still similar percentages among non-EU respondents (Table 2). The proportions amongst EU-respondents who would apply any form of contact precaution were markedly lower for ESBL-producing E. coli and non-E. coli ESBL (59.4 and 68%, respectively). Answers from EU and non-EU responders differed significantly regarding overall contact precaution indications for ESBL-E other than E. coli (p = 0.044) in that approximately one third of non-EU responders either did not consider any contact precaution measures or did not determine the presence of ESBL. Amongst those who implemented contact precautions more non-EU responders than EU-responders did so if the patient was only colonized (Table 2).
Table 2.
Indication and specification for contact precautions (CP) and isolationa
| MRSA | GRE | E. coli ESBL | Non-E. coli ESBL | |||||||||
| EU | Non EU | p-valueb | EU | Non EU | p-valueb | EU | Non EU | p-valueb | EU | Non EU | p-valueb | |
| No CP | 12.7 | 24.5 | 0.165 | 30.0 | 32.7 | 0.396 | 32.7 | 34.7 | 0.636 | 23.3 | 34.7 | 0.044 |
| CP only if infected | 16.7 | 10.2 | 54.7 | 44.9 | 14.7 | 10.2 | 17.3 | 6.1 | ||||
| CP if colonised and/or infected | 68.0 | 65.3 | 15.3 | 22.4 | 44.7 | 40.8 | 50.7 | 40.8 | ||||
| Unknown | 42.7 | 0 | 0 | 0 | 5.3 | 10.2 | 6.0 | 14.3 | ||||
| ESBL not determined | n.a. | n.a. | n.a. | n.a. | 2.7 | 4.1 | 2.7 | 33.3 | ||||
| Total no. responses (%) | 150 (75.4) | 49 (24.6) | 131 (74.9) | 44 (24.1) | 150 (75.4) | 47 (24.6) | 150 (75.4) | 49 (24.6) | ||||
| Gowns and gloves whenever entering the room | 57.3 | 62.9 | 0.398 | 59.0 | 56.7 | 0.623 | 44.9 | 59.1 | 0.234 | 47.3 | 71.4 | 0.046 |
| Gowns and gloves if direct contact is anticipated | 37.9 | 37.1 | 37.1 | 43.3 | 55.1 | 40.9 | 52.7 | 28.6 | ||||
| Other procedures (e.g. standard precautions only) | 4.8 | 0.0 | 3.8 | 0.0 | 0 | 0 | 0 | 0 | ||||
| Total no. responses (%) | 124 (78.0) | 35 (22.0) | 105 (77.8) | 30 (22.2) | 89 (80.2) | 22 (19.8) | 93 (81.6) | 21 (18.4) | ||||
| Single room | 62.5 | 63.8 | 59.4 | 56.3 | 0.703 | 31.4 | 31.3 | 0.960 | 36.4 | 27.1 | 0.494 | |
| Cohorting | 17.4 | 10.6 | 16.1 | 16.7 | 19.3 | 18. | 20.7 | 18.8 | ||||
| Spatial separationc | 10.4 | 6.4 | 0.235 | 9.8 | 6.3 | 13.6 | 16.7 | 13.6 | 20.8 | |||
| No specific measures | 9.7 | 19.1 | 14.7 | 20.8 | 35.7 | 33.3 | 29.3 | 33.3 | ||||
| Total no. responses (%) | 144 (75.4) | 47 (24.6) | 143 (74.9) | 48 (25.1) | 140 (74.5) | 48 (25.5) | 140 (74.5) | 48 (25.5) | ||||
| Carbapenem resistant E. coli | Carbapenem resistant non-E. coli | MDR P. aeruginosa | MDR A. baumannii | |||||||||
| EU | Non EU | p-valueb | EU | Non EU | p-valueb | EU | Non EU | p-valueb | EU | Non EU | p-valueb | |
| No CP | 11.3 | 16.3 | 0.745 | 8.2 | 18.4 | 0.246 | 13.3 | 20.4 | 0.597 | 9.3 | 20.4 | 0.171 |
| CP only if infected | 14.0 | 10.2 | 13.6 | 10.2 | 17.3 | 14.3 | 17.3 | 20.4 | ||||
| CP if colonised and/or infected | 65.3 | 63.3 | 72.1 | 65.3 | 60.0 | 59.2 | 62.7 | 51.0 | ||||
| Unknown | 9.3 | 10.2 | 6.1 | 6.1 | 9.3 | 6.1 | 10.7 | 8.2) | ||||
| Total no. responses (%) | 150 (75.4) | 49 (24.6) | 147 (75.0) | 49 (25.0) | 150 (75.4) | 49 (24.6) | 150 (75.4) | 49 (24.6) | ||||
| Gowns and gloves whenever entering the room | 61.3 | 68.8 | 0.440 | 63.5 | 60.6 | 0.763 | 56.3 | 64.7 | 0.389 | 58.7 | 61.3 | 0.797 |
| Gowns and gloves if direct contact is anticipated | 38.7 | 31.3 | 36.5 | 39.4 | 43.7 | 35.3 | 41.3 | 38.7 | ||||
| Total no. responses (%) | 111 (77.6) | 32 (22.4) | 115 (77.7) | 33 (22.3) | 103 (75.2) | 34 (24.8) | 109 (77.9) | 31 (22.1) | ||||
| Single room | 64.1 | 41.7 | 0.029 | 71.6 | 47.9 | 0.026 | 56.7 | 43.8 | 0.156 | 61.6 | 45.8 | 0.067 |
| Cohorting | 13.4) | 14.6 | 12.1 | 18.8 | 18.7 | 14.6 | 18.1 | 14.6 | ||||
| Spatial separationc | 9.9 | 20.8 | 7.1 | 14.6 | 12.7 | 18.8 | 8.7 | 18.8 | ||||
| No specific measures | 12.7 | 22.9 | 9.2 | 18.8 | 11.9 | 22.9 | 11.6 | 20.8 | ||||
| Total no. responses (%) | 142 (74.7) | 48 (25.3) | 141 (74.6) | 48 (25.4) | 134 (73.6) | 48 (26.4) | 138 (74.2) | 48 (25.8) | ||||
aValues are percentages (related to the corresponding total respondents) unless indicated otherwise
bA two-sided p-value of < 0.05 was considered statistically significant
cShared room with MDRO-negative patients but with optical barrier (e.g. red margin on the floor) or separated by screen/curtains
The majority (> 56%) of EU responders reported donning of gowns and gloves upon entry into the room at all times for all MDRO except ESBL-E. However, only non-EU responders followed this practice in ESBL-E in contrast to EU responders, where a majority (55 and 53%, for E. coli and non-E. coli, respectively) indicated that donning of gowns and gloves was required only when patient-care was likely. The differences in proportions of EU and non-EU responders were statistically significant for ESBL-E other than E. coli (p = 0.046) (Table 2). After removing potential duplicate answers, the discrepancy became even more evident with statistically significant lower proportions of responders from EU countries that had strict gowning and gloving at all times implemented for ESBL-producing E. coli (p = 0.017) and other Enterobacteriaceae (p = 0.005) (Additional file 1).
A majority of EU and non-EU country participants preferred single room placement for MRSA (62.5 and 63.8%) and GRE (59.4 and 56.3%) (Table 2). The answers, however, were less consistent for gram-negative MDRO. Only about one third of EU and non-EU responders advocated single room placement for ESBL-E. major differences between responses from participants from EU and non-EU countries were encountered for rooming specifications in CR E. coli and CRE (other than E. coli), where EU responders compared to non-EU responders favoured single room placement (64.1% vs. 41.7 and 71.6% vs. 47.9%, respectively) over cohorting or spatial separation, whereas responses from non-EU participants were more divergent among the different placement options. Differences in placements of patients with MDRO among EU and non-EU responders, however, were not statistically significant in the sensitivity analysis (see Additional file 1).
The answers were highly consistent among all participants and for any MDRO, except for MRSA, that pre-emptive contact precautions/isolation had a significant value, whereas only a minority considered limiting implementation of contact precautions to patients with certain risk factors (e.g. diarrhoea or urinary incontinence) in their local practice (Table 3). None of the differences between responses from EU and non-EU countries were significant after deduplication (Additional file 1). When comparing the responses between infection control practitioners (ICP) and non-ICPs, as well as the responses between clinicians and non-clinicians, we also detected significantly different approaches to infection control measures across different pathogens (Additional file 1: Tables S3-S10). However, the results also demonstrated large incongruities amongst ICPs as well as amongst clinicians as to what strictness level of contact precaution is pursued.
Table 3.
Other specific requirements and conditions for contact precaution (CP)
| MDRO | Origin of responses | Total responses | Additional pre-emptive CP based on patient’s historya | CP only required if specific risk factors presentb | Additional pre-emptive CP but only if specific risk factors | None applicable | p-valuec |
|---|---|---|---|---|---|---|---|
| MRSA | EU | 126 | 117 (92.9) | 1 (0.8) | 1 (0.8) | 7 (5.6) | 0.038 |
| Non EU | 38 | 30 (78.9) | 1 (2.6) | 2 (5.3) | 5 (13.2) | ||
| GRE | EU | 94 | 81 (86.2) | 3 (3.2) | 1 (1.1) | 9 (9.6) | 0.265 |
| Non EU | 30 | 24 (80.00) | 2 (6.7) | 2 (6.7) | 2 (6.7) | ||
| ESBL E. coli | EU | 91 | 74 (81.3) | 8 (8.8) | 4 (4.7) | 5 (4.7) | 0.812 |
| Non EU | 26 | 22 (84.6) | 1 (3.8) | 2 (7.7) | 1 (3.8) | ||
| ESBL non-E. coli | EU | 101 | 86 (85.1) | 5 (5.0) | 3 (3.0) | 7 (6.9) | 0.131 |
| Non EU | 23 | 18 (78.3) | 3 (13.0) | 2 (8.7) | 0 (0.0) | ||
| Carbapenem resistant E. coli | EU | 120 | 103 (85.8) | 4 (3.3) | 4 (3.3) | 9 (7.5) | 0.465 |
| Non EU | 36 | 33 (91.7) | 0 (0.0) | 2 (5.6) | 1 (2.8) | ||
| Carbapenem resistant Enterobacteriaceae (non-E. coli) | EU | 126 | 111 (88.1) | 2 (1.6) | 4 (3.2) | 9 (7.1) | 0.560 |
| Non EU | 37 | 33 (89.2) | 1 (2.7) | 2 (5.4) | 1 (2.7) | ||
| MDR P. aeruginosa | EU | 112 | 96 (85.7) | 3 (2.7) | 4 (3.6) | 9 (8.0) | 0.178 |
| Non EU | 35 | 31 (88.6) | 1 (2.9) | 3 (8.6) | 0 (0.0) | ||
| MDR A. baumannii | EU | 120 | 107 (89.2) | 1 (0.8) | 5 (4.2) | 7 (5.8) | 0.758 |
| Non EU | 33 | 31 (91.2) | 0 (0.0) | 2 (5.9) | 1 (2.9) |
aFormerly positive for respective MDRO or presumptive infection/colonization with respective MDRO
bCP only when certain risk factors present e.g. incontinence, diarrhoea, draining wounds
cA two-sided p-value of < 0.05 was considered statistically significant
Most respondents demonstrating poor knowledge were either no medical doctors, were not working in hospitals or had fewer years of experience in infection control (Additional file 1 Table S13).
Most commonly encountered barriers
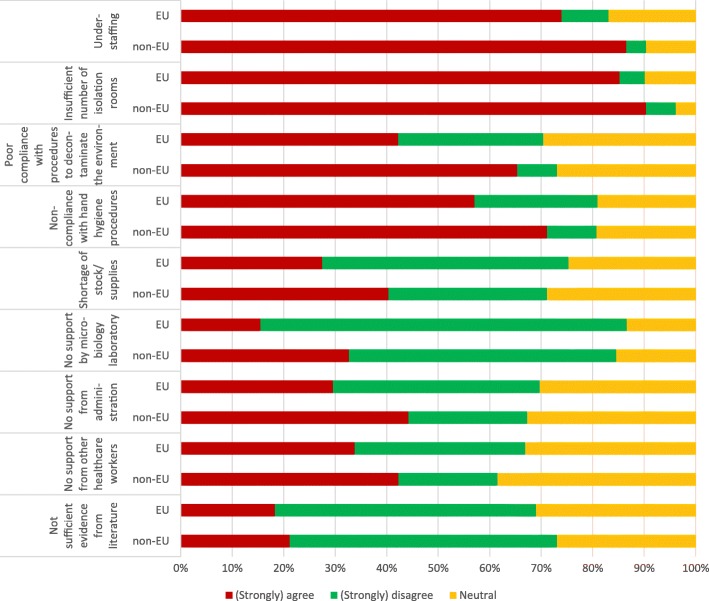
Of the 213 participants, 15 (10%) Europeans and 4 (7%) non-Europeans did not respond to these questions. Respondents from EU- and non-EU countries largely agreed that the major obstacles to implement appropriate contact precaution/isolation measures were shortage of personnel (EU-respondents: 67%; non-EU respondents: 80%) and lack of rooms for isolation (77 and 84%, respectively). The opinions were more divergent between EU- and non-EU-respondents regarding lack of environmental cleanliness (EU-respondents: 38%, non-EU respondents: 61%), support from administration (27 and 41%, respectively) or microbiology (14 and 30%, respectively), and provision of supplies (25 and 38%, respectively), where non-EU respondents perceived more frequent constraints than EU respondents (Fig. 1).
Fig. 1.
Most commonly encountered barriers when trying to implement contact precaution and isolation measures from the survey respondents’ perspective (n = 194, 4 missing from non-EU and 15 missing from EU countries)
Discussion
MDRO comprise a global threat [11] causing economic damage comparable to the 2008 financial crisis [12]. International experts rated their control the highest priority [13]. Surprisingly, to the best of our knowledge, this is the first multinational survey addressing specifically potential differences and major hindrances in practical implementation of contact precaution/isolation measures in MDROs. Representatives from most European countries and from a large number of non-EU countries across Africa, Asia, and South America participated. The results have confirmed our suspicions that indications and practical implementation of contact precautions including isolation measures vary considerably. This study also showed there were major inconsistencies particularly in the handling of ESBL-E, CR E. coli, and CREs.
Firstly, in contrast to ESCMID [3] recommendations, 23.3% of EU-respondents did not consider any contact precaution measures in non-E. coli ESBL; the proportion was even higher amongst non-EU respondents (34.7%). Secondly, we found between 30 and 45% of all respondents neither followed the HICPAC nor the ESCMID recommendations requiring HCW to wear gowns and gloves at all times when entering the room of a patient in contact isolation [14]. In clinical practice it seems sufficient not to don a gown (and gloves) if no contact with blood or bodily fluid is anticipated, rendering more time urgently needed for care and treatment. In any case, the emphasis has to be on thorough education and proper implementation of standard precautions and hand hygiene as their integral component because they constitute the mainstay of controlling the spread of all micro-organisms (including MDROs).
Thirdly, contrary to these recommendations, between 10 and 20% of respondents from all countries did not consider any rooming specifications, e.g. cohorting or isolation for gram-positive MDRO. Up to 30% of all respondents abstained from such interventions in gram-negative MDRO, especially non-E. coli ESBL. These deficits seem somewhat alarming, since omitting such control measures is likely to facilitate the nosocomial spread of these organisms [15].
Our survey found the inability to separate patients colonized or infected with MDRO was due to the lack of personnel and insufficient single rooms, rather than a consequence of guideline scepticism or evidence-base paucity. Isolation practices implementation barriers were similar to those found for MRSA interventions in USA HCW interviews [16]. These findings underpin the view that the greatest challenge to implement contact precautions/isolation is the need for more staffing and isolation facilities, reinforced by a strong infection prevention ethos amongst HCWs and supported by a skilled infection control team as outlined in a previous European project [17].
A more recent survey among members of the Society for Healthcare Epidemiology of America (SHEA) on contact precaution use for MRSA and GRE revealed that over 60% of respondents were interested in alternative approaches, such as enhanced standard precautions and environmental cleaning/disinfection or targeted contact precautions and isolation (e.g., in conditions enhancing horizontal spread, such as diarrhoea or urinary incontinence) [18]. Our survey underlines that risk-stratified precautions are implemented for ESBL-E in few institutions or countries, respectively.
However, whether limiting contact precaution to those who have diarrhoea or urinary incontinence is equally effective in reducing transmission than application of contact precautions irrespective of the presence of risk factors, and whether this newer approach may be considered for gram-positive as well as gram-negative MDROs, remains to be determined in future studies and are matters of some urgency.
The strengths of this survey were its comprehensiveness about use of personal protective equipment and augmenting the response with on-site recruitment using a booth at ECCMID. Compared to other surveys we explicitly differentiated between E. coli and other Enterobacteriaceae, since the transmission risk of ESBL E. coli is deemed to be lower compared to non-E. coli ESBL, at least in the acute care setting [3, 19, 20]. The survey encompassed a broad geographical area across the world, including 32 EU and 28 non-EU countries.
Our study has some limitations. The online survey was potentially available to approx. 7000 ESCMID members and the ECCMID attendance was 10,839. Thus, the response rate was very poor, but still of significant size to draw interesting conclusions. Also, ECCMID attendants may have differed from other infection control experts and 10% of participants, though mostly non-clinicians with less experience in infection control and infectious diseases, showed unexpectedly poor knowledge about their local practice.
We therefore would urge some caution in generalising from these results, but they are a worrying potential indicator of variability in recommended practices, and are surely causes for concern which cannot be ignored. Larger studies, perhaps by individual countries, are required and measures to relieve recognised hindrances to improvement reflected upon and implemented.
The need for more rigorous studies comparing standard precautions to contact precautions/isolation in reducing the spread of MDRO has been previously highlighted [18]. These are essential to informing the best prevention strategies to combat spread of MDRO. The lack of uniform positive effects of contact isolation to prevent transmission may be explained by the variability of interpretation of this term. Indications for contact isolation require a global definition and further sound studies. ESCMID, HICPAC and any other MDR guidelines could perhaps add a score to the current infection control guidelines that would allow estimation of the level of implementation of contact precautions.
Conclusion
We discovered great variation in components of the specific measures of contact precaution and isolation and mixed levels of implementation.
Our findings should inform the design of future trials ensuring that the methodology and different levels of contact precautions need to be described clearly to enhance comparability between studies.
Additional files
Table S1. Country of workplace of the 213 survey participants (number of respondents per country). Table S2. Survey respondents affiliations (n = 213). Table S3. MRSA contact precaution measures according to professional background. Table S4. GRE contact precaution measures according to professional background. Table S5. ESBL-E. coli contact precaution measures according to professional background. Table S6. ESBL-non-E. coli contact precaution measures according to professional background. Table S7. CR-E. coli contact precaution measures according to professional background. Table S8. CRE contact precaution measures according to professional background. Table S9. MRD P. aeruginosa contact precaution measures according to professional background. Table S10. MRD A. baumannii contact precaution measures according to professional background. Table S11. Indication and specification for contact precautions (CP) and isolation (cont. Next page) after deduplication*. Table S12. Other specific requirements for CP, results after deduplication*. Table S13. Characteristics of respondents that indicated “unknown” compared to respondents that provided any other answer. (DOCX 78 kb)
Acknowledgements
We would like to acknowledge the European Study Group for Nosocomial Infections and its Executive Committee members for their commitment.
Funding
The study has not been funded.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Preliminary results have been presented as a poster (P0847) at the ECCMID 2015 in Copenhagen, Denmark.
Abbreviations
- CRE
Carbapenem-resistant enterobacteriaceae
- ECCMID
European congress on clinical microbiology and infectious diseases
- ESBL
Extended-spectrum-beta-lactamase
- ESCMID
European society of clinical microbiology and infectious diseases
- ESGNI
ESCMID nosocomial infection study group
- EU
European
- GRE
Glycopeptide-resistant enterococci
- HCW
Health-care workers
- HICPAC
Healthcare infection control practices advisory committee
- ICP
Infection control practitioner
- IQR
Interquartile range
- MDRO
Multidrug resistant organism
- MRSA
Methicillin resistant Staphylococcus aureus
Authors’ contributions
DVG developed the survey, collected and analysed the data and drafted the manuscript. MD, HS, and BC offered essential expertise during the survey development and critically revised the manuscript, HS further helped with technical and logistical issues regarding survey deployment. AFW conceived the idea of the survey, gave important inputs and critically revised the content of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This was an online survey open to all attendants of the European Congress of Microbiology and Infectious diseases. The survey was considered non-interventional research, posing the participants at minimal physical, psychological, social, economic, legal, or dignitary risk. The survey was completely anonymous and participation was voluntary. Participants’ identity was kept separately from their responses. The authors, therefore, deemed this study to be exempted from ethical approval.
Competing interests
All authors declare to have no conflict of interests with regard to this manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13756-018-0366-5) contains supplementary material, which is available to authorized users.
Contributor Information
Danielle Vuichard Gysin, Email: danielle.vuichard-gysin@stgag.ch.
Barry Cookson, Email: bsajcookson@btinternet.com.
Henri Saenz, Email: henri.saenz@escmid.org.
Markus Dettenkofer, Email: Markus.Dettenkofer@glkn.de.
Andreas F. Widmer, Email: andreas.widmer@usb.ch
References
- 1.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 2.Siegel JD, Rhinehart E, Jackson M, et al. 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35:S65–164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tacconelli E, Cataldo MA, Dancer SJ, et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014;20(Suppl 1):1–55. doi: 10.1111/1469-0691.12427. [DOI] [PubMed] [Google Scholar]
- 4.Drees M, Pineles L, Harris AD, Morgan DJ. Variation in definitions and isolation procedures for multidrug-resistant gram-negative bacteria: a survey of the Society for Healthcare Epidemiology of America research network. Infect Control Hosp Epidemiol. 2014;35:362–366. doi: 10.1086/675600. [DOI] [PubMed] [Google Scholar]
- 5.Kalenic S, Cookson B, Gh R, et al. Comparison of recommendations in national/regional guidelines for prevention and control of MRSA in thirteen European countries. International Journal of Infection Control. 2010;6:1–10. doi: 10.3396/ijic.V6i2.016.10. [DOI] [Google Scholar]
- 6.Likert R. A technique for the measurement of attitudes. Arch Psychol. 1932;20(140):5–55. [Google Scholar]
- 7.Survey Monkey Europe UC. Dublin. Theatr Irel. www.surveymonkey.com. Accessed 20 Aug 2014.
- 8.Population Reference Bureau. World Population Data Sheet. Washington, DC; 2016. p. 10–4. https://www.prb.org/2016-world-population-data-sheet. Accessed 05 Nov 2017.
- 9.MacKenzie FM, Bruce J, Van Looveren M, et al. Antimicrobial susceptibility testing in European hospitals: report from the ARPAC study. Clin Microbiol Infect. 2006;12:1185–1192. doi: 10.1111/j.1469-0691.2006.01549.x. [DOI] [PubMed] [Google Scholar]
- 10.IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk: IBM Corp; 2015.
- 11.WHO. Antimicrobial resistance: global report on surveillance. 2014. http://www.who.int/drugresistance/documents/surveillancereport/en/. Accessed 11 June 2018.
- 12.The World Bank. Drug-resistant infections: A Threat to Our Economic Future. Washington, DC; 2017. http://documents.worldbank.org/curated/en/323311493396993758/final-report. Accessed 05 Nov 2017.
- 13.Dettenkofer M, Humphreys H, Saenz H, et al. Key priorities in the prevention and control of healthcare-associated infection: a survey of European and other international infection prevention experts. Infection. 2016;44:719–724. doi: 10.1007/s15010-016-0904-0. [DOI] [PubMed] [Google Scholar]
- 14.Clock SA, Cohen B, Behta M, et al. Contact precautions for multidrug-resistant organisms: current recommendations and actual practice. Am J Infect Control. 2010;38:105–111. doi: 10.1016/j.ajic.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial infections. Ann Intern Med. 2004;140:26–32. doi: 10.7326/0003-4819-140-1-200401060-00008. [DOI] [PubMed] [Google Scholar]
- 16.Seibert DJ, Speroni KG, Oh KM, et al. Knowledge, perceptions, and practices of methicillin-resistant Staphylococcus aureus transmission prevention among health care workers in acute-care settings. Am J Infect Control. 2014;42:254–259. doi: 10.1016/j.ajic.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Brusaferro S, Cookson B, Kalenic S, et al. Training infection control and hospital hygiene professionals in Europe, 2010: agreed core competencies among 33 European countries. Euro Surveill. 2014;19:45-54. [DOI] [PubMed]
- 18.Morgan DJ, Murthy R, Munoz-Price LS, et al. Reconsidering contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus. Infect Control Hosp Epidemiol. 2015;36:1163–1172. doi: 10.1017/ice.2015.156. [DOI] [PubMed] [Google Scholar]
- 19.Tschudin-Sutter S, Frei R, Dangel M, et al. Rate of transmission of extended-spectrum beta-lactamase-producing enterobacteriaceae without contact isolation. Clin Infect Dis. 2012;55:1505–1511. doi: 10.1093/cid/cis770. [DOI] [PubMed] [Google Scholar]
- 20.Tschudin-Sutter S, Frei R, Schwahn F, et al. Prospective validation of cessation of contact precautions for extended-Spectrum beta-lactamase-producing Escherichia coli. Emerg Infect Dis. 2016;22:1094–1097. doi: 10.3201/eid2206.150554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Country of workplace of the 213 survey participants (number of respondents per country). Table S2. Survey respondents affiliations (n = 213). Table S3. MRSA contact precaution measures according to professional background. Table S4. GRE contact precaution measures according to professional background. Table S5. ESBL-E. coli contact precaution measures according to professional background. Table S6. ESBL-non-E. coli contact precaution measures according to professional background. Table S7. CR-E. coli contact precaution measures according to professional background. Table S8. CRE contact precaution measures according to professional background. Table S9. MRD P. aeruginosa contact precaution measures according to professional background. Table S10. MRD A. baumannii contact precaution measures according to professional background. Table S11. Indication and specification for contact precautions (CP) and isolation (cont. Next page) after deduplication*. Table S12. Other specific requirements for CP, results after deduplication*. Table S13. Characteristics of respondents that indicated “unknown” compared to respondents that provided any other answer. (DOCX 78 kb)
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.