Abstract
Mycoplasma canis is an opportunistic bacterial pathogen that may colonize dogs and cattle. In the present case, we report for the first time the isolation of M. canis from a wound tissue specimen of a 62-year-old woman after a dog bite.
Keywords: Dog bite, MALDI, Microaerophilic cultivation, Mycoplasma canis, Wound infection
Mycoplasma canis has been isolated from mucosal surfaces of dogs or cattle [1], [2], [3]. It has also been associated with respiratory or urinary tract infections in dogs [2], [4], [5], [6] and has recently been identified in brain tissue samples of dogs presenting with meningoencephalitis [7]. Because it is well established that dogs may harbour M. canis in the mucosal flora, it is likely that transmission to humans may occur, especially in situations with close contact to body liquids like saliva. Yet it is unknown if M. canis may also cause infections in humans. Only one case of respiratory tract infection in an immunocompromised patient with close canine contact has been described in the literature so far [8]. To date, M. canis has not been isolated from human tissue samples.
In the present case, a 62-year-old woman presented with an amputation of the distal phalanx of D3 of the left hand after a dog bite. A first surgical debridement was done (Fig. 1(A)), and intravenous antimicrobial therapy (ampicillin/sulbactam) was applied immediately after her arrival at the emergency room. A second-look surgery and amputation of the distal phalanx was performed on the next day. During the second surgery, two tissue samples (one of the distal phalanx and one of the proximal stump) were obtained for microbiologic analysis.
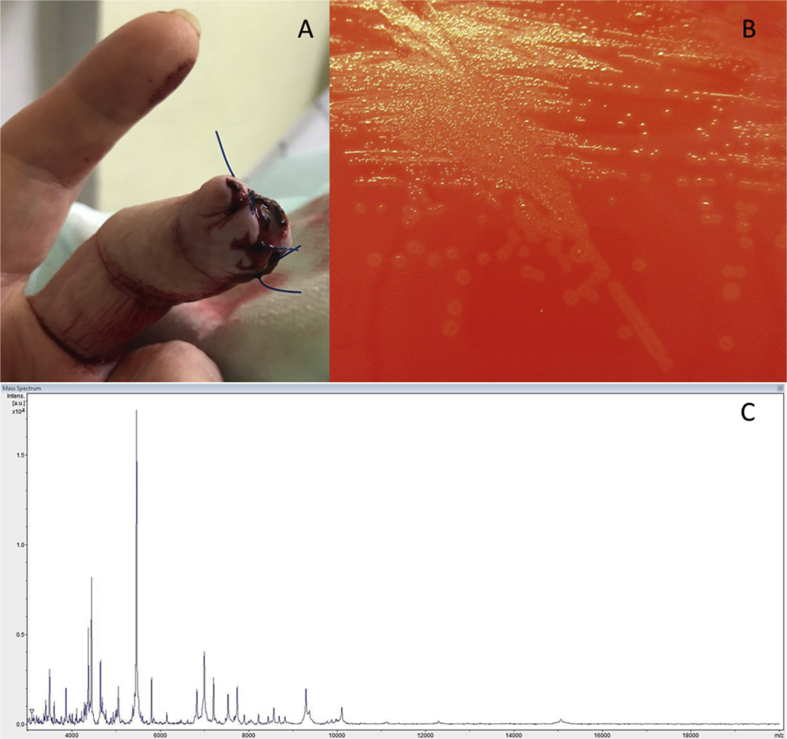
Fig. 1.
(A) Finger wound before second-look surgery and amputation of distal phalanx. (B) Colonies of Mycoplasma canis on Columbia sheep's blood agar after 48 hours of microaerophilic incubation. (C) Matrix-assisted desorption ionization–time of flight mass spectrometry spectrum of M. canis.
After 4 days' incubation, very small greenish colonies were grown on Columbia blood's sheep agar in 5% CO2 after enrichment in thioglycollate broth for 24 hours from both tissue samples. Bacterial growth in subsequent subcultures was greatly enhanced under microaerophilic conditions (CampyPak; BD Diagnostics, Heidelberg, Germany). The colony morphology was similar to α-haemolysing streptococci (Fig. 1(B)). Identification by matrix-assisted desorption ionization–time of flight mass spectrometry (Bruker Diagnostics, Billerica, MA, USA) revealed M. canis (Fig. 1(C)), which was confirmed by 16S ribosomal DNA sequencing [9] (GenBank accession no. MH169225). No other bacterial pathogen was detected. Antimicrobial susceptibility testing was performed with Etest (Liofilchem, Roseto degli Abruzzi, Italy) on Mueller-Hinton agar with 5% defibrinated horse blood (BD, Heidelberg, Germany) with McFarland 0.5 in microaerophilic conditions. Ciprofloxacin had a MIC of 0.094 mg/L, doxycycline 0.023 mg/L and erythromycin >256 mg/L.
The patient was discharged 2 days after the second surgery. The further clinical course was without complication, and follow-up on day 30 after discharge showed complete wound healing.
M. canis is known as a veterinary pathogen but has rarely been isolated from human samples. Here, it was grown after 4 days' incubation in 5% CO2, but sufficient growth may require broth enrichment and microaerophilic conditions. It may therefore not be detected in routine microbiology diagnostics. Although the patient did not present with a purulent infection, M. canis may still be of relevance in the current case. In addition, other infections with Mycoplasma spp. like pneumonia can be nonpurulent as well. Although the patient did not receive antimicrobial therapy efficient against M. canis, the infection might have been treated sufficiently with the second wound debridement.
In case of human tissue samples from dog bites, broth enrichment and microaerophilic incubation may be necessary to cultivate M. canis.
Conflict of interest
None declared.
Acknowledgements
We acknowledge financial support by Deutsche Forschungsgemeinschaft within the funding programme Open Access Publishing, by the Baden-Württemberg Ministry of Science, Research and the Arts and by Ruprecht-Karls-Universität Heidelberg.
References
- 1.ter Laak E.A., Tully J.G., Noordergraaf H.H., Rose D.L., Carle P., Bove J.M. Recognition of Mycoplasma canis as part of the mycoplasmal flora of the bovine respiratory tract. Vet Microbiol. 1993;34:175–189. doi: 10.1016/0378-1135(93)90171-3. [DOI] [PubMed] [Google Scholar]
- 2.Chalker V.J. Canine mycoplasmas. Res Vet Sci. 2005;79:1–8. doi: 10.1016/j.rvsc.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Thomas A., Ball H., Dizier I., Trolin A., Bell C., Mainil J. Isolation of mycoplasma species from the lower respiratory tract of healthy cattle and cattle with respiratory disease in Belgium. Vet Rec. 2002;151:472–476. doi: 10.1136/vr.151.16.472. [DOI] [PubMed] [Google Scholar]
- 4.Chalker V.J., Owen W.M., Paterson C., Barker E., Brooks H., Rycroft A.N. Mycoplasmas associated with canine infectious respiratory disease. Microbiology. 2004;150(Pt 10):3491–3497. doi: 10.1099/mic.0.26848-0. [DOI] [PubMed] [Google Scholar]
- 5.L'Abee-Lund T.M., Heiene R., Friis N.F., Ahrens P., Sorum H. Mycoplasma canis and urogenital disease in dogs in Norway. Vet Rec. 2003;153:231–235. doi: 10.1136/vr.153.8.231. [DOI] [PubMed] [Google Scholar]
- 6.Ulgen M., Cetin C., Senturk S., Ozel A.E., Ozdemir U. Urinary tract infections due to Mycoplasma canis in dogs. J Vet Med A Physiol Pathol Clin Med. 2006;53:379–382. doi: 10.1111/j.1439-0442.2006.00858.x. [DOI] [PubMed] [Google Scholar]
- 7.Michaels D.L., Leibowitz J.A., Azaiza M.T., Shil P.K., Shama S.M., Kutish G.F. Cellular microbiology of Mycoplasma canis. Infect Immun. 2016;84:1785–1795. doi: 10.1128/IAI.01440-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong D., Yu B.H., Yagoda A., Kagnoff M.F. Colonization of humans by Mycoplasma canis. J Infect Dis. 1971;124:607–609. doi: 10.1093/infdis/124.6.607. [DOI] [PubMed] [Google Scholar]
- 9.Lane D.J. 16S/23S rRNA sequencing. In: Stackebrandt G.M., Goodfellow M., editors. Nucleic acid techniques in bacterial systematics. Wiley; Chichester, UK: 1991. pp. 115–175. [Google Scholar]