Abstract
Background
Anti-Mullerian hormone (AMH) in women is secreted by granulosa cells of antral follicles. AMH appears to be a very stable marker for ovarian function. It may be used to diagnosis cases of premature ovarian failure, polycystic ovary syndrome (PCOS), and ovarian tumors. It has been suggested that cadmium exposure can reduce female fecundity. The purpose of this study was to investigate whether environmental exposure to cadmium was associated with alterations in AMH with regards to age.
Methods
In a cross-sectional study, the data of premenopausal women living in Seoul, ranging from 30 to 45 of age was collected. The study included a total of 283 women who completed serum AMH and whole blood cadmium assessments. Linear regression analyses were used in order to examine the association between cadmium and AMH. Given that age was the strongest confounder in both cadmium and AMH concentrations, we stratified subjects by 5 years old and analyzed their data.
Results
Geometric mean concentrations of blood cadmium and AMH were 0.97 μg/L and 3.02 ng/ml, respectively. Total association between cadmium and AMH was statistically significant (adjusted coefficient = − 0.34 (0.15), p = 0.02). After stratification, the only age group with a negative association between cadmium and AMH were the women raging between 30 and 35 years (adjusted coefficient = − 0.43 (0.18), p = 0.01).
Conclusions
The results of this study suggest that environmental exposure to cadmium may alter the AMH level of premenopausal women, depending on their age group.
Keywords: Anti-Mullerian hormone, Cadmium, Premenopausal women, Ages
Background
Anti-Müllerian hormone (AMH) in women is secreted by granulosa cells of antral follicles [1, 2]. AMH is a member of transforming growth factor-beta (TGF-β) superfamily. It appears to be a very stable marker for ovarian function. It might be useful for diagnosing cases of premature ovarian failure, polycystic ovary syndrome (PCOS), and ovarian tumors [1, 3, 4]. It has been reported that the number of primordial follicles is related to their activation rate. This is reflected by the number of growing follicles. This type of relationship might be useful as an indicator of ovarian reserve. Cadmium is known to be an endocrine disruptor. It has been suggested that exposure to cadmium reduces fecundity [5–7]. The reproductive toxicity of cadmium is mediated through various pathways, such as structural damage to testicular vascular structure and blood-testis barrier, cytotoxicity to Sertoli and Leydig cells, oxidative stress, apoptosis, and disruption of the hypothalamus-pituitary gonadal axis in males [8]. However, recent studies have solely reported on the effects of cadmium exposure with regards to the reproductive capacity of males. Few studies have reported the health effects of cadmium has on women. In addition, studies have shown that the exposure to cadmium in humans increases with age, while the concentration of AMH in women decreases with age. Therefore, the purpose of this study was to investigate whether cadmium exposure was related to changes in AMH in premenopausal women according to age.
Methods
Study participants
We conducted a cross-sectional survey of premenopausal women in Seoul, Republic of Korea. The women volunteered after seeing ads on bulletin boards in various places, including the Ewha Womans University Medical Center, Healthy Families Support Center, Community Health Center, Community Service Center, and Community Blog for Mothers. In order to investigate the effects of environmental exposure on premenopausal women’s health, the 308 participants, ranging in age from 30 to 49 years old, were recruited from September to November 2014. To exclude women who might have already undergone menopause, subjects 45 years of age and older were excluded (n = 13) [9]. It has been determined that PCOS (polycystic ovarian syndrome) patients are associated with high AMH levels [2]. Therefore, PCOS patients were excluded from the study (n = 1). In the process of measuring AMH and cadmium, it was not possible measure below or above the limit of detection (LOD) in some cases. These cases were also excluded (n = 11). Therefore, a total of 283 women were included in this study.
Data concerning covariates was extracted from questionnaires, physical examinations and pelvic ultrasonography by trained researchers and nurses. We examined the following potential confounders: premenopausal women’s age (30–35, 35–40, 40–45 years), body mass index (BMI), parity (nulliparous, 1, 2, 3, 4), smoking status (current smoker, past smoker, never smoker), and fish intake (less than half a fish, half a fish, or more than one fish per one meal). This study protocol was approved by the Institutional Review Board of the Ewha Womans University Medical Center. Written informed consent was obtained from each participant.
Cadmium assessment
Venous blood was collected from premenopausal women using EDTA treated tubes. The blood samples were frozen and stored at 4–8 °C throughout the entire analysis period. All analyses were conducted within three days of storage at the Seoul Medical Science Institute.
Analysis of the cadmium content in blood was performed using a Zeeman Atomic Absorption Spectrometer (AAS 280Z, Agilent, USA). The Spectrometer was verified using certified standard and quality control materials. In short, 100 μl of 1% nitric acid and 1.5 ml of diluent were added to 100 μl of the blood sample and vortexed. In order to prepare the diluent, Triton X-100 5 ml, 20% NH4H2PO4 10 ml, and HNO3 1 ml were added to 1 l of water (HPLC grade). The detection limit of cadmium (three times the standard deviation of the SD; SD * 3) was 0.015 μg/L, and the limit of cadmium quantification (10 times standard deviation of the SD; SD * 10) was 0.05 μg/L. ClinChek Whole Blood Controls level I and level II (RECIPE, GERMANY) were measured before, during, and after the analysis. The value of the Coefficient of Variation(CV) (%) of the quality control substances for level I and level II were precisely 6.1 and 5.7%, respectively. The analysis accuracy of the blood cadmium analysis was verified by the cadmium survey (five times a year) conducted by the College of American Pathologists(CAP) and Lead and Multielement Proficiency(LAMP) Survey (three times a year) by the Centers for Disease Control and Prevention(CDC).
AMH assessment
The concentrations of AMH were assessed with V MAX (microplate reader, Molecular Devices, USA). It was verified using certified standard and quality control materials. The detection limit of AMH (SD * 3) ranged from 0.08 ng/mL to 21.00 ng/mL. Its limit of detection (SD * 10) was 0.17 μg/L. AMH Gen II enzyme-linked immunosorbent assay (Beckman Coulter, USA) was used for the AMH measurement before, during, and after the assay. The CV (%) values of the level control materials were precisely 7.7 and 5.8% for level I and level II, respectively.
Statistical analysis
Descriptive statistics for the participants characteristics were expressed as the number (%) or geometrical mean and standard deviation. Geometric means for cadmium and AMH concentrations were calculated and analyzed using the Wilcoxon rank sum test or Kruskal–Wallis rank sum test in order to determine the significant difference between the following covariate groups. After descriptive analyses, cadmium and AMH concentrations were transformed logarithmically due to their skewed distribution.
We used regression analysis in order to examine the effect of cadmium levels on the AMH level in premenopausal women within each age group. In an effort to select covariates for inclusion in multivariate models, we performed a literature review in order to identify the risk factors associated with cadmium exposure or AMH. The following key covariates were used in this study: Body Mass Index (BMI), parity, smoking status, and fish intake. It has been reported that the level of AMH varies according to age, and the level of AMH is changing in diseases such as PCOS and early ovarian failure [1]. Thus, AMH levels have been reported to be significantly lower in obese women (high BMI) [10, 11]. Smoking is a major source of cadmium exposure [12]. Cadmium exposure occurs mainly through diet, such as grains, fish, and more. In addition, heavy metals such as cadmium are increased in aquatic environments through industrial processes and fertilizers and can be introduced to humans through fish [13]. Moreover, fish is also a source of other compounds, such as vitamin D, which is associated with serum AMH [14]. Some participants had missing data for a given variable. They were excluded from the multivariate analysis. In order to examine the interactive effects of the age of premenopausal women and cadmium levels on AMH, we included an interaction term between cadmium level and age in the multivariate analysis. After identifying the interaction effects, stratified analysis was performed for each age group (in five years interval). All statistical analyses were carried out using SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Participant characteristics were described in Table 1.
Table 1.
Characteristics of participants
| N(%) or mean ± standard deviation | p-value* | |
|---|---|---|
| Total | 283 | |
| Age (year) | 36.22 ± 3.86 | <.0001 |
| 30 < ≤35 | 135 (47.70) | |
| 35 < ≤40 | 108 (38.16) | |
| 40 < ≤45 | 40 (14.13) | |
| Missing | 0 | |
| BMI (kg/m2) | 22.26 ± 3.12 | |
| Missing | 1 | |
| Parity (NO.) | <.0001 | |
| 0 | 9 (3.19) | |
| 1 | 94 (33.33) | |
| 2 | 150 (53.19) | |
| 3 | 27 (9.57) | |
| 4 | 2 (0.71) | |
| Missing | 1 | |
| Smoking status | <.0001 | |
| Current smoker | 5 (1.79) | |
| Past smoker | 27 (9.64) | |
| Never smoker | 248 (88.57) | |
| Missing | 3 | |
| Fish intake per serving | <.0001 | |
| Less than half a fish | 146 (52.33) | |
| Half a fish | 99 (35.48) | |
| More than one fish | 34 (12.19) | |
| Missing | 4 |
Data are presented as number (%), mean ± SD
BMI body mass index
*P-value for Chi square
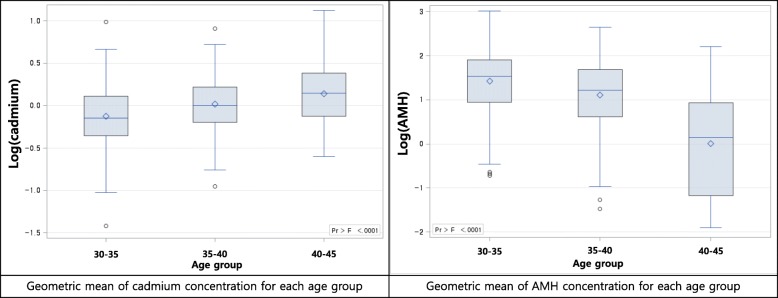
We identified 283 individuals who were 30–45 years old after excluding those who were diagnosed with PCOS and those whose cadmium and AMH levels were beyond LOD. After descriptive analyses, cadmium and AMH concentrations were then logarithmically transformed because of their skewed distribution. Geometric mean levels of cadmium and AMH concentrations were 0.97 μg/L and 3.02 ng/ml, respectively (Table 2).
Table 2.
Distribution of cadmium and anti mullerian hormone
| N(%) | Cadmium (ug/L) | Anti-Müllerian hormone (ng/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Median [1Q, 3Q] | GM | GSD | P-value | Median [1Q, 3Q] | GM | GSD | P-value | ||
| Total | 283 | 0.96 [0.77, 1.22] | 0.97 | 1.44 | 3.62 [1.88, 6.01] | 3.02 | 2.61 | ||
| Age (year) | <.0001 | <.0001 | |||||||
| 30 < ≤35 | 135 (47.70) | 0.86 [0.70, 1.12] | 0.88 | 1.44 | 4.64 [2.58, 6.75] | 4.19 | 2.07 | ||
| 35 < ≤40 | 108 (38.16) | 1.00 [0.82, 1.25] | 1.02 | 1.40 | 3.41 [1.86, 5.39] | 3.01 | 2.27 | ||
| 40 < ≤45 | 40 (14.13) | 1.16 [0.88, 1.47] | 1.15 | 1.46 | 1.17 [0.31, 2.54] | 1.00 | 3.20 | ||
Data are presented as number (%), Median [1Q, 3Q]
GM geometric mean, GSD Geometric standard deviation. 1Q = 1st quartile. 3Q = 3rdquartile
Tested by p-value calculated by the Wilcoxon rank sum test or Kruskal–Wallis rank sum test because of the skewness of cadmium and anti-mullerian hormone
The differences in cadmium and AMH concentrations between age groups were calculated using the Wilcoxon rank sum test or Kruskal–Wallis rank sum test (Fig. 1).
Fig. 1.
Distribution of cadmium and anti-mullerian hormone concentration with subgroup. AMH: Anti-Mullerian hormone
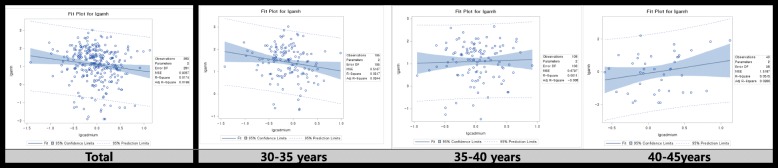
We then tested whether or not cadmium level was associated with the AMH level by using adjusted linear regression models (Fig. 2 and Table 3). Cadmium exposure decreased AMH level in the total group (β = − 0.34193 (0.14990), p-value = 0.0233). After stratification of the patients at 5-year intervals, the cadmium level decreased AMH levels in the age group of 30–35-year age group (β = − 0.43462 (0.17599), p-value = 0.0149).
Fig. 2.
Graphs of simple regression serum cadmium concentration and anti-mullerian hormone concentration with subgroup
Table 3.
Linear regression with serum cadmium concentration and anti-mullerian hormone concentration
| Anti mullerian Hormone | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Crude | 95% CI | P-value | N | Adjusteda | 95% CI | P-value | |||
| Total | 283 | −0.34385 (0.15428) | −0.64754 | − 0.04015 | 0.0266 | 276 | −0.34193 (0.14990) | − 0.63706 | − 0.04679 | 0.0233 |
| BY age | ||||||||||
| 30 < ≤35 | 135 | −0.35569 (0.17052) | −0.69296 | − 0.01841 | 0.0389 | 131 | −0.43462 (0.17599) | − 0.78298 | −0.08625 | 0.0149 |
| 35 < ≤40 | 108 | 0.08280 (0.23812) | −0.38929 | 0.55488 | 0.7287 | 106 | 0.14879 (0.23103) | −0.30968 | 0.60726 | 0.5211 |
| 40 < ≤45 | 40 | 0.69231 (0.48192) | −0.28328 | 1.66791 | 0.1590 | 39 | 0.94249 (0.48586) | −0.04717 | 1.93216 | 0.0613 |
aAdjusted for BMI, parity, smoking status, and fish intake
Discussion
In this study, we determined the effect exposure to cadmium has on AMH levels in premenopausal women. Our results suggest that cadmium exposure affects AMH concentration depending on the specific age group. When cadmium concentration is increased, the concentration of AMH is decreased in the age group of 30–35-year age group.
Very few previous epidemiologic studies have been conducted on the correlation between cadmium exposure and AMH concentrations in women. An earlier study, which examined the effect of cadmium exposure on AMH concentrations among 117 women, found that cadmium exposure had a positive association with AMH concentration in borderline statistically significant [15]. However, our findings differ from the findings of that earlier study because the characteristics of the subjects in both studies were very heterogeneous. There are many differences between the two studies, including the distribution of the subjects’ age, cadmium concentration, and AMH concentration. In the earlier study, the mean age of the subjects was 26.5 ± 5.6, the median AMH concentration was 11.71 (1.00–123.29), and the median cadmium concentration was 0.46 (0.17–2.98). However, in our study, the mean age of subjects was 36.22 ± 3.86, the median AMH concentration of 3.62 (1.88–6.01), and the median cadmium concentration was 0.96 (0.77–1.22). In particular, age has been known to be a crucial determinant of cadmium exposure and AMH concentration, respectively. As age increases, exposure to cadmium increases and the concentration of AMH decreases [1, 16]. Because the age range in the earlier study is about 10 years lower than the age range in our research; the cadmium concentrations were lower and AMH concentrations were higher in their study than in our research. In addition, given that the concentrations of cadmium and AMH vary with age, it is more reasonable to analyze the relationship between cadmium and AMH at similar ages after stratification. On the other hand, several studies have determined the effect of cadmium exposure on the reproductive system in men. A current review has outlined epidemiological observational findings for environmental and occupational exposure in humans through experimental studies in humans and animals [8]. These reports suggested that cadmium causes structural damage to testis vascular endothelium, which ultimately results in necrosis of the testis as well as affecting the blood-follicle-barrier (BFB) integrity, possibly leading to the development of autoimmunity against germ cells. Furthermore, cadmium might affect both spermatogenesis and testis by directly affecting inflammation mediators as well as pro-apoptotic and anti-apoptotic factors. In an in vitro study on pre-pubertal pig testis, Sertoli cell culture has shown that CdCl2 can adversely affect cell viability in a dose-dependent and time-dependent manner. The results showed that CdCl2 treatment can induce impaired function of the Sertoli cells as demonstrated by the reduction in INH-B and AMH secretion. CdCl2 also disrupts FSH receptor responsiveness (measured by a reduction in E2 production) and induces cell apoptosis. [17]. The molecular weight of AMH is 140 kDa. It is semi-restricted by normal functioning BFB. The disruption of the BFB could potentially cause an increase in the release of AMH from the follicles to circulation, thus theoretically explaining the increase in serum AMH in males [18]. The present study confirmed that cadmium exposure could affect AMH levels in women, similar to in men. The effect of cadmium exposure on the AMH concentration was also affected based on the age of the subjects. It is known that cadmium can alter the cycle pattern of oxidoreductase gene expression. Cadmium can also alter the clock gene in the neuroendocrine axis because it induces oxidative stress and regulates daily changes in HHG axis activity [19]. A separate review has outlined cadmium-induced neuroendocrine disruption of the hypothalamus-pituitary-gonad (HHG) axis [3]. Cadmium interferes with the regulatory mechanisms of this physiological axis by altering the neurotransmitters involved at the hypothalamic level, changing gonadotropin hormone secretion, and affecting testicular or ovarian structure and activity. These effects can be related to the periodic rhythm of the physiological axis associated with aging. Thus, there was also a study about cadmium toxicity, depending on age, in male rats. According to this study, the accumulation of cadmium in the hypothalamus, pituitary gland, and testis varied according to age. Cadmium accumulated in the three organs of young adult male rats but not in the hypothalamus and pituitary glands of older male rats. When they were exposed to cadmium, norepinephrine, dopamine and serotonin metabolism in the hypothalamus. Also levels of plasma LH and testosterone were different in young and old male rats. Thus, the study reported that the neurochemical levels of male rats and daily toxicity of cadmium in the reproductive axis were different in young and old rats. Since our study involved only women, it is difficult to interpret it with regard to previous studies. However, the results showed statistically different significance by age group. This suggests that there may be an association between AMH and cadmium only at a particular age, or there may be a difference in the mechanism depending on age, as a reference.
In this study, we surveyed the subject’s history of disease through questionnaires. Pelvic ultrasonography was used in order to determine any gynecologic diseases. In addition, environmental exposures were determined for subjects. These were the key strengths of this study. However, this study has limitations. First, it was a cross-sectional study that could not take time into consideration. In addition, all participants were healthy volunteers which might create bias research results. Although we have adjusted for the possible confounding effects of a number of covariates, there is always the possibility of remaining residual confounders, such as unmeasured grain intake in this study. It is also important to consider endocrine disruptors (ECDs), such as BPA and phthalates which are known to affect vital hormones, in examining the effects of heavy metals and hormones. Bias, such as co-exposure or collinearity, are well-known problems in epidemiological analysis. They occur because of a very high correlation due to common causes, such as shared cause, metabolic pathway, and other factors [20]. Therefore, the present study first identifies the single effects of cadmium and AMH; it then confirms the subsequent effects of the multi-pollutant approach (such as lasso, PCA, and factor analysis) on the combined effect of cadmium and ECDs on AMH [21].
Conclusions
This study provides evidence suggesting that environmental exposure to cadmium may alter the level of AMH in premenopausal women, depending on their age.
Acknowledgements
HW Chung, KA Jeong, SY Park, YA Sung, HJ Lee, EH Ha, and SH Ye received funding from ‘Research on Health Effects of Exposure to Environmental Risk Factors in Reproductive Age Women’, a project of the Ministry of Environment, Republic of Korea.
HJ Kim and YM Lee did not receive funding.
Availability of data and materials
Due to data protection laws, data is only available to the participating scientists, involved in ‘Research on Health Effects of Exposure to Environmental Risk Factors in Reproductive Age Women’.
Abbreviations
- AMH
Anti-Mullerian hormone
- BMI
Body mass index
- CAP
College of American Pathologists
- CDC
By Centers for Disease Control and Prevention
- LAMP
Lead and Multielement Proficiency
- LOD
Limit of detection
- PCOS
Polycystic ovary syndrome
- TGF-β
Transforming growth factor-beta
Authors’ contributions
SHY and EHH conceptualized and designed the study, collected the data. YM Lee conducted analysis, and drafted and finalized the manuscript. HWC, KAJ, YAS, HJL, and SYP conceptualized and designed the study, collected the data, and reviewed the draft manuscript. HJK reviewed the draft manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study protocol was approved by the institutional review board at Ewha Womans University Hospital, and written informed consent was obtained from all of the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yu min Lee, Email: yumin.lee.oem@gmail.com.
Hye Won Chung, Email: hyewon@ewha.ac.kr.
Kyungah Jeong, Email: ogjeong@ewha.ac.kr.
Yeon-Ah Sung, Email: yasung@ewha.ac.kr.
Hyejin Lee, Email: hyejinlee@ewha.ac.kr.
Shinhee Ye, Email: shinheeye@naver.com.
Eun-Hee Ha, Phone: 82-2-2650-5757, Email: eunheeha@naver.com.
References
- 1.Kruszynska A, Slowinska-Srzednicka J. Anti-Mullerian hormone (AMH) as a good predictor of time of menopause. Prz Menopauzalny. 2017;16(2):47–50. doi: 10.5114/pm.2017.68591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bungum L, Franssohn F, Bungum M, Humaidan P, Giwercman A. The circadian variation in anti-Mullerian hormone in patients with polycystic ovary syndrome differs significantly from normally ovulating women. PLoS One. 2013;8(9):e68223. doi: 10.1371/journal.pone.0068223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lafuente A. The hypothalamic–pituitary–gonadal axis is target of cadmium toxicity. An update of recent studies and potential therapeutic approaches. Food Chem Toxicol. 2013;59:395–404. doi: 10.1016/j.fct.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Sahmay S, Aydin Y, Oncul M, Senturk LM. Diagnosis of polycystic ovary syndrome: AMH in combination with clinical symptoms. J Assist Reprod Genet. 2014;31(2):213–220. doi: 10.1007/s10815-013-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lafuente A, Esquifino AI. Effects of oral cadmium exposure through puberty on plasma prolactin and gonadotropin levels and amino acid contents in various brain areas in pubertal male rats. Neurotoxicology. 2002;23(2):207–213. doi: 10.1016/S0161-813X(02)00020-7. [DOI] [PubMed] [Google Scholar]
- 6.Hachfi L, Sakly R. Effect of cd transferred via food product on spermatogenesis in the rat. Andrologia. 2010;42(1):62–64. doi: 10.1111/j.1439-0272.2009.00965.x. [DOI] [PubMed] [Google Scholar]
- 7.Mendiola J, Moreno JM, Roca M, Vergara-Juarez N, Martinez-Garcia MJ, Garcia-Sanchez A, Elvira-Rendueles B, Moreno-Grau S, Lopez-Espin JJ, Ten J, et al. Relationships between heavy metal concentrations in three different body fluids and male reproductive parameters: a pilot study. Environ Health. 2011;10(1):6. doi: 10.1186/1476-069X-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Angelis C, Galdiero M, Pivonello C, Salzano C, Gianfrilli D, Piscitelli P, Lenzi A, Colao A, Pivonello R. The environment and male reproduction: the effect of cadmium exposure on reproductive system and semen quality and its implication in fertility. Reprod Toxicol. 2017;73:105–127. doi: 10.1016/j.reprotox.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65(2):161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steiner AZ, Stanczyk FZ, Patel S, Edelman A. Antimullerian hormone and obesity: insights in oral contraceptive users. Contraception. 2010;81(3):245–248. doi: 10.1016/j.contraception.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moslehi N, Shab-Bidar S, Ramezani Tehrani F, Mirmiran P, Azizi F. Is ovarian reserve associated with body mass index and obesity in reproductive aged women? A meta-analysis. Menopause. 2018; 10.1097/GME.0000000000001116. [DOI] [PubMed]
- 12.Mezynska M, Brzoska MM. Environmental exposure to cadmium-a risk for health of the general population in industrialized countries and preventive strategies. Environ Sci Pollut Res Int. 2018;25(4):3211–3232. doi: 10.1007/s11356-017-0827-z. [DOI] [PubMed] [Google Scholar]
- 13.Baki MA, Hossain MM, Akter J, Quraishi SB, Haque Shojib MF, Atique Ullah AKM, Khan MF. Concentration of heavy metals in seafood (fishes, shrimp, lobster and crabs) and human health assessment in Saint Martin island, Bangladesh. Ecotoxicol Environ Saf. 2018;159:153–163. doi: 10.1016/j.ecoenv.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 14.Naderi Z, Kashanian M, Chenari L, Sheikhansari N. Evaluating the effects of administration of 25-hydroxyvitamin D supplement on serum anti-mullerian hormone (AMH) levels in infertile women. Gynecol Endocrinol. 2018;34(5):409–412. doi: 10.1080/09513590.2017.1410785. [DOI] [PubMed] [Google Scholar]
- 15.Christensen PS, Bonde JP, Bungum L, Giwercman A, Toft G, Jonsson BA, Specht IO. Environmental cadmium and lead exposure and anti-Mullerian hormone in pregnant women. Reprod Toxicol. 2016;61:114–119. doi: 10.1016/j.reprotox.2016.03.047. [DOI] [PubMed] [Google Scholar]
- 16.Jarup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238(3):201–208. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Luca G, Lilli C, Bellucci C, Mancuso F, Calvitti M, Arato I, Falabella G, Giovagnoli S, Aglietti MC, Lumare A, et al. Toxicity of cadmium on Sertoli cell functional competence: an in vitro study. J Biol Regul Homeost Agents. 2013;27(3):805–816. [PubMed] [Google Scholar]
- 18.Siu MK, Cheng CY. The blood-follicle barrier (BFB) in disease and in ovarian function. Adv Exp Med Biol. 2012;763:186–192. doi: 10.1007/978-1-4614-4711-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jimenez-Ortega V, Cardinali DP, Fernandez-Mateos MP, Rios-Lugo MJ, Scacchi PA, Esquifino AI. Effect of cadmium on 24-hour pattern in expression of redox enzyme and clock genes in rat medial basal hypothalamus. Biometals. 2010;23(2):327–337. doi: 10.1007/s10534-010-9292-6. [DOI] [PubMed] [Google Scholar]
- 20.Weisskopf MG, Seals RM, Webster TF. Bias amplification in epidemiologic analysis of exposure to mixtures. Environ Health Perspect. 2018;126(4):047003. doi: 10.1289/EHP2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim KN, Hong YC. The exposome and the future of epidemiology: a vision and prospect. Environ Health Toxicol. 2017;32:e2017009. doi: 10.5620/eht.e2017009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to data protection laws, data is only available to the participating scientists, involved in ‘Research on Health Effects of Exposure to Environmental Risk Factors in Reproductive Age Women’.