The crystallization and structural analysis of the various players in the ataRT toxin–antitoxin module from enteropathogenic Escherichia coli are reported: the toxin AtaT, the intrinsically disordered antitoxin AtaR and its complex with a 22 bp fragment of the operator DNA.
Keywords: toxin–antitoxin, acetyltransferase, protein–DNA complexes, AtaR, AtaT, Escherichia coli
Abstract
The ataRT operon from enteropathogenic Escherichia coli encodes a toxin–antitoxin (TA) module with a recently discovered novel toxin activity. This new type II TA module targets translation initiation for cell-growth arrest. Virtually nothing is known regarding the molecular mechanisms of neutralization, toxin catalytic action or translation autoregulation. Here, the production, biochemical analysis and crystallization of the intrinsically disordered antitoxin AtaR, the toxin AtaT, the AtaR–AtaT complex and the complex of AtaR–AtaT with a double-stranded DNA fragment of the operator region of the promoter are reported. Because they contain large regions that are intrinsically disordered, TA antitoxins are notoriously difficult to crystallize. AtaR forms a homodimer in solution and crystallizes in space group P6122, with unit-cell parameters a = b = 56.3, c = 160.8 Å. The crystals are likely to contain an AtaR monomer in the asymmetric unit and diffracted to 3.8 Å resolution. The Y144F catalytic mutant of AtaT (AtaTY144F) bound to the cofactor acetyl coenzyme A (AcCoA) and the C-terminal neutralization domain of AtaR (AtaR44–86) were also crystallized. The crystals of the AtaTY144F–AcCoA complex diffracted to 2.5 Å resolution and the crystals of AtaR44–86 diffracted to 2.2 Å resolution. Analysis of these structures should reveal the full scope of the neutralization of the toxin AtaT by AtaR. The crystals belonged to space groups P6522 and P3121, with unit-cell parameters a = b = 58.1, c = 216.7 Å and a = b = 87.6, c = 125.5 Å, respectively. The AtaR–AtaT–DNA complex contains a 22 bp DNA duplex that was optimized to obtain high-resolution data based on the sequence of two inverted repeats detected in the operator region. It crystallizes in space group C2221, with unit-cell parameters a = 75.6, b = 87.9, c = 190.5 Å. These crystals diffracted to 3.5 Å resolution.
1. Introduction
Bacteria have evolved multiple mechanisms to adapt and survive in an ever-changing environment that is often dominated by strong competition from other microorganisms (Koonin et al., 2017 ▸; Zhang et al., 2012 ▸). Toxin–antitoxin (TA) modules are ubiquitous operons found across bacterial genomes that are involved in the stabilization of genomic material, defence mechanisms and post-segregational killing in the case of plasmids and phages (Hayes & van Melderen, 2011 ▸). There are several types of TA module, but they all represent a minimal system involving a toxic element and its antidote which prevents the activity of the toxin (Hayes & van Melderen, 2011 ▸; Loris & Garcia-Pino, 2014 ▸). Type II TA modules, the most prevalent TA modules observed in bacteria, involve proteins functioning as a toxin and an antitoxin, with some type II TA modules also having an additional regulatory element (Goeders & Melderen, 2014 ▸). Both proteins bind and form a tight nontoxic complex that is often involved in autoregulation of the transcription of the operon (Garcia-Pino, Balasubramanian et al., 2010 ▸; Loris & Garcia-Pino, 2014 ▸; Monti et al., 2007 ▸; De Jonge et al., 2009 ▸; Hadži et al., 2017 ▸; Jurėnas, Chatterjee et al., 2017 ▸; Afif et al., 2001 ▸). It has been shown that TA modules that are carried by mobile genetic elements such as bacteriophages, integrons and plasmids contribute to the mechanisms of genetic maintenance and are lethal to cells that are ‘cured’ from infection (Van Melderen & Bast, 2009 ▸). In general, the labile antitoxins have a rapid turnover compared with the more stable and long-lived toxins (Buts et al., 2005 ▸; Dao-Thi et al., 2000 ▸; De Jonge et al., 2010 ▸; Van Melderen et al., 1994 ▸, 1996 ▸). Thus, upon plasmid loss the TA-free progeny will be poisoned and only the population that retains the plasmid can divide and multiply, in a phenomenon known as post-segregational killing (Gerdes et al., 1986 ▸; Jaffé et al., 1985 ▸). The role of chromosomally encoded systems is still highly debated in the field (Van Melderen, 2010 ▸). However, some TA operons encoded in bacterial chromosomes have been associated with growth regulation in eukaryotic host cells and further linked to pathogenesis and intracellular persistence (Norton & Mulvey, 2012 ▸; Helaine et al., 2014 ▸).
Translation is one of the preferred targets of type II toxins (Jurėnas, Garcia-Pino et al., 2017 ▸; Guglielmini & Van Melderen, 2011 ▸). Among the group of toxins that target translation, toxins with the GCN5-related N-acetyltransferase (GNAT) protein fold have recently been identified (Cheverton et al., 2016 ▸; Jurėnas, Chatterjee et al., 2017 ▸; Van Melderen et al., 2018 ▸). GNAT-fold enzymes use acetyl coenzyme A (AcCoA) as a donor to acetylate the free amine groups located on the amino acid charged onto a tRNA in the case of TA GNATs. This modification has been observed in Met-tRNAfMet and other elongation aa-tRNAs, in both cases leading to strong inhibition of translation (Cheverton et al., 2016 ▸; Jurėnas, Chatterjee et al., 2017 ▸). However, the molecular bases of the action of the enzyme as well as the way that the antitoxin neutralizes its toxic action remain unknown.
The AtaR antitoxin counteracts this activity by forming a strong multimeric complex with AtaT (Jurėnas, Chatterjee et al., 2017 ▸); however, this toxin–antitoxin interaction has not yet been studied for this or other TA systems with GNAT-domain toxins. Based on protein-sequence analysis, the AtaR antitoxin was proposed to have a ribbon–helix–helix (RHH) DNA-binding domain (Schreiter & Drennan, 2007 ▸) in the N-terminal region (Jurėnas, Chatterjee et al., 2017 ▸; Jurėnas, Garcia-Pino et al., 2017 ▸), suggesting a possible role in transcriptional autoregulation, as observed in other type II antitoxins (Goeders & Van Melderen, 2014 ▸). The functional unit of this RHH domain is a dimer, in which two RHH motifs are intertwined to form a stable domain with twofold symmetry that binds DNA. The twofold-symmetric domain is usually formed by homodimerization, which pairs two short β-strands from each RHH monomer to form an antiparallel β-sheet. The binding of the RHH dimer to DNA positions this β-sheet in the DNA major groove (Schreiter & Drennan, 2007 ▸).
In this paper, we describe the overexpression, purification, preliminary characterization and crystallization of all of the players in self-regulation of the AtaR–AtaT system from Escherichia coli (strain O157:H7 EDL933): the AtaR antitoxin, the AtaT toxin bound to AcCoA, a complex between AtaT and the C-terminus of AtaR, and the full AtaR–AtaT complex bound to the operator region of the ataRT operon.
2. Materials and methods
2.1. Cloning
The ataR and ataT genes (accession Nos. AAG58568 and AAG58567) described in Jurėnas, Chatterjee et al. (2017 ▸) were amplified from the E. coli O157:H7 EDL933 genome with Q5 polymerase using the oligonucleotides (Sigma–Aldrich) listed in Table 1 ▸. All cloning enzymes were obtained from NEB. The pET-28b-HisTEV-AtaT Y144F plasmid was generated by the following steps. Firstly, the Y144F mutation was introduced into the pBAD33-AtaT plasmid (Jurėnas, Chatterjee et al., 2017 ▸) by amplifying the entire plasmid with the primers F-ataT-Y144F and R-ataT-Y144F that introduce the mutation. The PCR product was treated with DpnI to remove the template plasmid, purified on a PCR purification column (Sigma), phosphorylated and ligated. The ligation mixture was transformed into DJ624 Δara strain and the resulting plasmid pBAD33-AtaTY144F was sequenced. The ataTY144F gene was then amplified using the primers F-ataT-Bmt and R-ataT-Xho, and the pET-28b plasmid was amplified with R-pET-hisTEV-Bmt and F-pET-synth to replace the thrombin site with a Tobacco etch virus (TEV) protease site. Both amplicons were digested with BmtI and XhoI, and ligated. Plasmid sequences were confirmed by sequencing (Eurofins Genomics).
Table 1. Oligonucleotides used in the cloning and construction of the different AtaR and AtaT variants.
Restriction sites are underlined, His6 tags are in italics and TEV sites are in bold.
| F-ataT-Y144F | AAATCGCTGGGCTTTATCCCTTT |
| R-ataT-Y144F | GAAAAACGTATGGGCTTTTTCATTC |
| F-ataT-Bmt | GATCGCTAGCGATGATCTGACGATAGAGATTCTGAC |
| R-ataT-Xho | GATCCTCGAGTTAATCGCTCTGTGTAAAAAGCA |
| R-pET-hisTEV-Bmt | GATCGCTAGC GCCCTGGAAGTACAGGTTTTCGCCGCTGCTGTGATGATGAT |
| F-pET-synth | GGATCCGAATTCGAGCTCCG |
| F-ataR-Eco | GATCGAATTCATGTCTGCTGTTAAAAAGCAG |
| R-ataT-his-Pst | GATCCTGCAGTTAGTGGTGGTGGTGGTGGTGATCGCTCTGTGTAAAAAGCAGTTC |
| F-ataR44-Eco | GATCGAATTCATGGCTGCGGAAGTGATTGAA |
| F-ataR-hisTEV-Eco | CTAGGAATTCATGCACCACCACCACCACCAC GAAAACCTGTACTTCCAGTCTATGTCTGCTGTTAAAAAGCAGCG |
| R-ataT-Pst | GACTCTGCAGTTAATCGCTCTGTGTAAAAAGC |
For ataR-ataT operons cloned in the pKK223.3 vector under the pTac promotor, cloning was performed as follows: ataR-ataT-his was amplified using the F-ataR-Eco and R-ataT-his-Pst oligomers, ataR-cter-ataT-his was amplified using the F-ataR44-Eco and R-ataT-his-Pst oligomers, and hisTEV-ataR-ataT was amplified using the F-ataR-hisTEV-Eco and R-ataT-Pst oligomers using E. coli O157:H7 EDL933 genomic DNA as a template. The PCR products were cloned into the pKK223.3 vector opened with EcoRI and PstI. Ligation mixtures were transformed into the DJ624Δara strain using electroporation and plasmid sequences were confirmed by sequencing (Eurofins Genomics). Expression of the pKK223.3 plasmids was performed in the DJ624 Δara strain, while the pET-28b-HisTEV-AtaTY144F plasmid was further transformed into the BL21(DE3) strain for expression.
2.2. Protein expression and purification
Cultures were grown in LB medium supplemented with kanamycin (50 mg l−1) for pET-28b derivatives or ampicillin (100 mg l−1) for pKK223.3 derivatives at 310 K with aeration. Expression was induced by adding 0.5 mM IPTG to the culture at an OD600 nm of ∼0.8. After adding the IPTG, the culture was continued for ∼12 h at a temperature of 301 K. The cells were harvested by centrifugation and resuspended in lysis buffer (50 mM Tris–HCl pH 8.5, 500 mM NaCl, 2 mM imidazole, 1 mM TCEP) supplied with cOmplete protease-inhibitor cocktail (Roche). The cells were then lysed using a cell cracker and the lysate was centrifuged to remove cell debris for 30 min at 40 000g. The proteins were purified using an ÄKTAexplorer FPLC purifier. The supernatant was loaded onto a HiTrap TALON column (GE Healthcare) equilibrated with buffer A (25 mM Tris–HCl pH 8.5, 250 mM NaCl). The column was then washed with buffer A and the proteins were eluted with a linear gradient of buffer B (25 mM Tris–HCl pH 8.5, 250 mM NaCl, 500 mM imidazole). The fractions containing the protein of interest were concentrated using spin filters (Amicon) and loaded onto a Superdex 200 HR size-exclusion column equilibrated with buffer A or specific crystallization buffers. The purity of the proteins was analysed by SDS–PAGE.
All of the versions of AtaR or AtaT used in this work were tagged at the N-terminus with a His6 tag followed by the cleavage sequence for TEV protease (see Table 2 ▸ for the sequences of all of the proteins used in this work). We used recombinant His-TEV protease to digest the different proteins at 283 K for 12 h. The protein and protease were mixed in a 100:1 molar ratio and cleavage of the His tag was confirmed by SDS–PAGE and Western blotting (using an antipolyhistidine antibody; Sigma, catalogue No. H1029). Excess TEV protease was removed by reapplying the sample onto a HiTrap TALON crude column. The protein of interest (found in the flowthrough fractions) was concentrated and further purified by gel filtration using a Superdex 200 HR column.
Table 2. Sequences of the recombinant proteins used in this work.
| AtaR | MSAVKKQRIDLRLTDDDKSMIEEAAAISNQSVSQFMLNSASQRAAEVIEQHRRVILNEESWTRVMDALSNPPSPGEKLKRAAKRLQGM |
| HisTEV-AtaTY144F | MGSSHHHHHHSSGENLYFQGASDDLTIEILTDDADYDLQRFDCGEEALNLFLTTHLVRQHRNKILRAYILCRNTPERQVLGYYTLCGSCFERAALPSKSKQKKIPYKNIPSVTLGRLAIDRSLQGQGWGATLVAHAMNVVWSASLAVGIHGLFVEALNEKAHTFFKSLGFIPLVGENENALFFPTKSIELLFTQSD |
| AtaTY144F | MGASDDLTIEILTDDADYDLQRFDCGEEALNLFLTTHLVRQHRNKILRAYILCRNTPERQVLGYYTLCGSCFERAALPSKSKQKKIPYKNIPSVTLGRLAIDRSLQGQGWGATLVAHAMNVVWSASLAVGIHGLFVEALNEKAHTFFKSLGFIPLVGENENALFFPTKSIELLFTQSD |
| AtaR44–88 | MAAEVIEQHRRVILNEESWTRVMDALSNPPSPGEKLKRAAKRLQGM |
| AtaT | MDDLTIEILTDDADYDLQRFDCGEEALNLFLTTHLVRQHRNKILRAYILCRNTPERQVLGYYTLCGSCFERAALPSKSKQKKIPYKNIPSVTLGRLAIDRSLQGQGWGATLVAHAMKVVWSASLAVGIHGLFVEALNEKAHTFYKSLGFIPLVGENENALFFPTKSIELLFTQSD |
2.3. Production of selenomethionine-modified proteins
The pKK223.3-AtaR-AtaT-His plasmid was transformed into the methionine auxotroph E. coli strain B834(DE3) (Studier et al., 1990 ▸; Doherty et al., 1995 ▸) by electroporation. A single colony was tested for auxotrophy on minimal medium supplemented with 100 mg l−1 ampicillin, 0.2% glucose with or without 50 mg l−1 l-methionine. For protein expression, cells from an overnight pre-culture grown in SeMet medium (Molecular Dimensions) supplemented with 100 mg l−1 ampicillin, 0.2% glucose and 50 mg l−1 l-methionine were washed three times in sterile water and inoculated into fresh SeMet medium supplemented with 100 mg l−1 ampicillin, 0.2% glucose and 50 mg l−1 l-selenomethionine. The culture was grown at 310 K with aeration until the OD600 nm reached 0.8, when expression of the genes was induced by the addition of 0.5 mM IPTG and continued at 301 K overnight. The cells were then harvested and the proteins were purified as described above.
2.4. Transcription start site mapping with 5′ RACE
The E. coli O157:H7 EDL933 culture was grown to an OD of 1.5 and total RNA was extracted with 338 K phenol, followed by chloroform extraction and ethanol precipitation. 30 µg of RNA was treated with 25 units of tobacco acid pyrophosphatase (Westburg) for 2 h at 310 K. The RNA was then re-extracted with phenol and chloroform, precipitated with ethanol and resuspended in water, and the RNA adapter (ACUCUCUACUGUUUCUCCAU) was ligated using T4 RNA ligase (NEB) overnight at 289 K. After reaction and additional re-extraction, 3 µg of the resulting RNA was used in a reverse transcription reaction with Superscript II Reverse Transcriptase (Thermo Fisher) with primer RT1-AtaRT (CGCTTCCTCGCCGCAGTCGA). 4 µl of the RT reaction mixture was then used for a PCR reaction using a primer against the RNA adapter and a nested specific primer RT2-AtaRT (CCCTGAAGACGTTTTGCCGCAC). 4 µl of the PCR product was then used for TOPO-TA cloning (Invitrogen) and the reaction was then transformed into TOP10 electrocompetent cells. The resulting clones were analysed by PCR with M13 forward/reverse primers and sequenced to map the transcription start site.
2.5. Crystallization
Crystallization conditions were screened by sitting-drop vapour diffusion at 277 and 293 K for all protein samples (toxin, toxin–antitoxin, toxin–antitoxic peptide, toxin–antitoxin and DNA complexes). Sitting drops were set up in Swissci (MRC) 96-well 2-drop UVP sitting-drop plates using a Mosquito robotic system (TTP Labtech). Drops consisting of 100 nl protein solution and 100 nl precipitant solution were equilibrated against 80 µl precipitant solution in the reservoir. Crystallization conditions were screened using commercially available screens: Crystal Screen and Crystal Screen 2 from Hampton Research and JCSG-plus, ProPlex, PACT premier, Helix, LMB and Morpheus II from Molecular Dimensions. The concentrations of protein solutions and buffers are given in Table 3 ▸. The concentrations of the protein solutions were determined spectrophotometrically from the absorbance at 280 nm corrected by extinction coefficients calculated using the ProtParam tool (Gasteiger et al., 2003 ▸). For the AtaR–AtaT complex we estimated the extinction coefficient assuming a 2:4 toxin:antitoxin ratio. These estimates are based on molecular weight measurements derived from mass spectrometry (Jurėnas, Chatterjee et al., 2017 ▸) and analytical size-exclusion chromatography (SEC). In order to reconstitute the AtaR–AtaT–DNA complex the double-stranded DNA operator fragment was added to the complex in a 1.2-fold molar excess over the AtaR–AtaT complex. All DNA oligomers were purchased from Sigma and resuspended in 50 mM Tris–HCl pH 8.5, 100 mM NaCl, and strands were hybridized by heating at 368 K for 5 min and letting the sample cool at room temperature.
Table 3. Crystallization conditions observed for the different macromolecules involved in the autoregulation of the ataRT toxin–antitoxin system.
Conditions shown in bold yielded the crystals that were used to solve the structures.
| Protein solution | Reservoir solution | Temperature (K) | Cryoprotection (collected data sets) | |
|---|---|---|---|---|
| AtaR | 15 mg ml−1 AtaR–AtaT-His protein in 50 mM Tris–HCl pH 8.5, 500 mM NaCl | 0.2 M magnesium chloride hexahydrate, 0.1 M Tris–HCl pH 8.5, 30%(w/v) PEG 4000 | 293 | |
| 0.2 M calcium chloride dihydrate, 0.1 M sodium HEPES pH 7.5, 28%(v/v) PEG 400 | ||||
| 0.5 M NaCl, 0.01 M magnesium chloride hexahydrate, 0.01 M CTAB | ||||
| 35%(v/v) 1,4-dioxane | 25%(v/v) glycerol | |||
| 0.2 M magnesium chloride hexahydrate, 0.1 M Tris pH 7.0, 10%(w/v) PEG 8000 | ||||
| 0.1 M Tris pH 8.0, 20%(v/v) MPD | ||||
| 0.1 M succinic acid, 15%(w/v) PEG 3350 | ||||
| HisTEV-AtaTY144F | 15 mg ml−1 in 50 mM Tris–HCl pH 8.5, 500 mM NaCl, 5 mM AcCoA | 0.1 M calcium acetate hydrate, 0.1 M sodium acetate pH 4.5, 10%(w/v) PEG 4000 | 293 | 20%(w/v) PEG 400 |
| AtaTY144F | 12 mg ml−1 in 50 mM MES pH 6.5, 200 mM NaCl, 5 mM CoA | 0.01 M spermine tetrahydrochloride, 0.01 M spermidine trihydrochloride, 0.01 M 1,4-diaminobutane dihydrochloride, 0.01 M DL-ornithine monohydrochloride, 0.1 M MOPSO, bis-tris pH 6.5, 10%(w/v) PEG 8000, 20%(v/v) 1,5-pentanediol | 277 | |
| 0.01 M spermine tetrahydrochloride, 0.01 M spermidine trihydrochloride, 0.01 M 1,4-diaminobutane dihydrochloride, 0.01 M DL-ornithine monohydrochloride, 0.1 M BES, triethanolamine pH 7.5, 10%(w/v) PEG 8000, 20%(v/v) 1,5-pentanediol | ||||
| 0.01 M spermine tetrahydrochloride, 0.01 M spermidine trihydrochloride, 0.01 M 1,4-diaminobutane dihydrochloride, 0.01 MDL-ornithine monohydrochloride, 0.1 M Gly-Gly, AMPD pH 8.5, 10%(w/v) PEG 8000, 20%(v/v) 1,5-pentanediol | None | |||
| 0.05 M potassium chloride, 0.1 M lithium chloride, 0.012 M spermine tetrahydrochloride, 0.05 M MES pH 6.5, 25%(v/v) PEG 400 | ||||
| AtaR44–88–AtaT | 15 mg ml−1 in 50 mM MES pH 6.5, 200 mM NaCl | 0.1 M citrate pH 5.0, 20%(w/v) PEG 6000 | 277 | 20%(w/v) PEG 400 |
| 0.15 M NaCl, 0.1 M MES pH 6.0, 21%(w/v) PEG 3350 | ||||
| 0.1 M MES pH 6.2, 15%(w/v) PEG 3350 | ||||
| 0.2 M ammonium acetate, 0.1 M sodium citrate pH 5.2, 28%(w/v) PEG 4000 | ||||
| 0.1 M sodium citrate pH 5.5, 20%(v/v) 2-propanol, 20%(w/v) PEG 4000 | ||||
| AtaR–AtaT–DNA Rep1g | 10 mg ml−1 in 50 mM Tris–HCl pH 8.5, 100 mM NaCl | 0.1 M sodium HEPES pH 7.0, 10%(w/v) PEG 4000 | 277 | |
| 0.1 M sodium phosphate pH 6.5, 12%(w/v) PEG 8000 | ||||
| AtaR–AtaT–DNA Rep2g | 10 mg ml−1 in 50 mM Tris–HCl pH 8.5, 100 mM NaCl | 0.1 M Bicine pH 9.0, 10%(v/v) MPD | 277 | |
| 0.2 M NaCl, 0.05 M HEPES pH 6.5, 25%(v/v) MPD | ||||
| 0.05 M lithium sulfate, 0.05 M HEPES pH 6.5, 15%(v/v) MPD | ||||
| AtaR–AtaT–DNA Rep3g | 10 mg ml−1 in 50 mM Tris–HCl pH 8.5, 100 mM NaCl | 0.01 M iron(III) chloride hexahydrate, 0.1 M sodium citrate tribasic dihydrate pH 5.6, 10%(v/v) Jeffamine M-600 | 277 | |
| AtaR–AtaT–DNA Pal-fix-perfect | 0.1 M HEPES pH 7.5, 8%(v/v) ethylene glycol, 10%(w/v) polyethylene glycol 8000 | 30%(v/v) ethylene glycol | ||
| 0.1 M potassium chloride, 0.05 M bis-tris pH 7.0, 22%(w/v) PEG 2000 MME | ||||
| 0.05 M bis-tris pH 7.0, 14%(w/v) PEG 2000 MME | ||||
| 0.1 M Bicine pH 9.0, 10%(w/v) PEG 20 000 | 20%(w/v) PEG 200 | |||
| 0.1 M Bicine pH 9.0, 10%(w/v) PEG 6000 | ||||
| 0.1 M HEPES pH 7.5, 9%(v/v) ethylene glycol, 10%(w/v) PEG 8000 | ||||
| 0.2 M potassium chloride, 0.005 M hexammine cobalt(III) chloride, 0.05 M MES pH 6.5, 25%(v/v) PEG 400 |
2.6. Data collection and processing
Data were collected on the PROXIMA-1 (PX1) and PROXIMA-2A (PX2A) beamlines at the SOLEIL synchrotron, Gif-sur-Yvette, Paris, France. All crystals were vitrified in liquid nitrogen and stored in liquid nitrogen for transport and data collection. Data sets were collected from the AtaR crystals and the AtaR–AtaT–DNA crystals on PX1 using a PILATUS 6M detector and data sets were collected from the AtaT crystals and the AtaTY144F–AtaR44–88 crystals on PX2A using an EIGER detector. All data were indexed, integrated with XDS (Kabsch, 2010 ▸) and scaled with XSCALE (Kabsch, 2010 ▸) or AIMLESS (Evans, 2006 ▸). Data quality and twinning were assessed with phenix.xtriage (Afonine et al., 2012 ▸) and POINTLESS (Evans, 2006 ▸).
2.7. Structure solution
Data were collected from crystals of selenomethionine-modified AtaR (SeMetAtaR) at the Se K edge for experimental phasing. The program MATTHEWS_COEF was used to calculated Matthews coefficients for cell-content analysis (Winn et al., 2011 ▸). The heavy-atom substructure was determined with SHELXD as implemented in the HKL2MAP suite (Pape & Schneider, 2004 ▸; Sheldrick, 2010 ▸). Initial phasing, density modification and automatic model building were performed with SHELXE also from the HKL2MAP suite (Pape & Schneider, 2004 ▸; Sheldrick, 2010 ▸). In the cases of AtaTY144F and AtaTY144F–AtaR44–88, the structure of TacT, a homologous N-acetyltransferase from Salmonella (PDB entry 5fvj; Cheverton et al., 2016 ▸), was used as initial search model for molecular replacement with Phaser (McCoy et al., 2007 ▸).
3. Results and discussion
3.1. Crystallization of AtaTY144F, a nontoxic mutant of AtaT
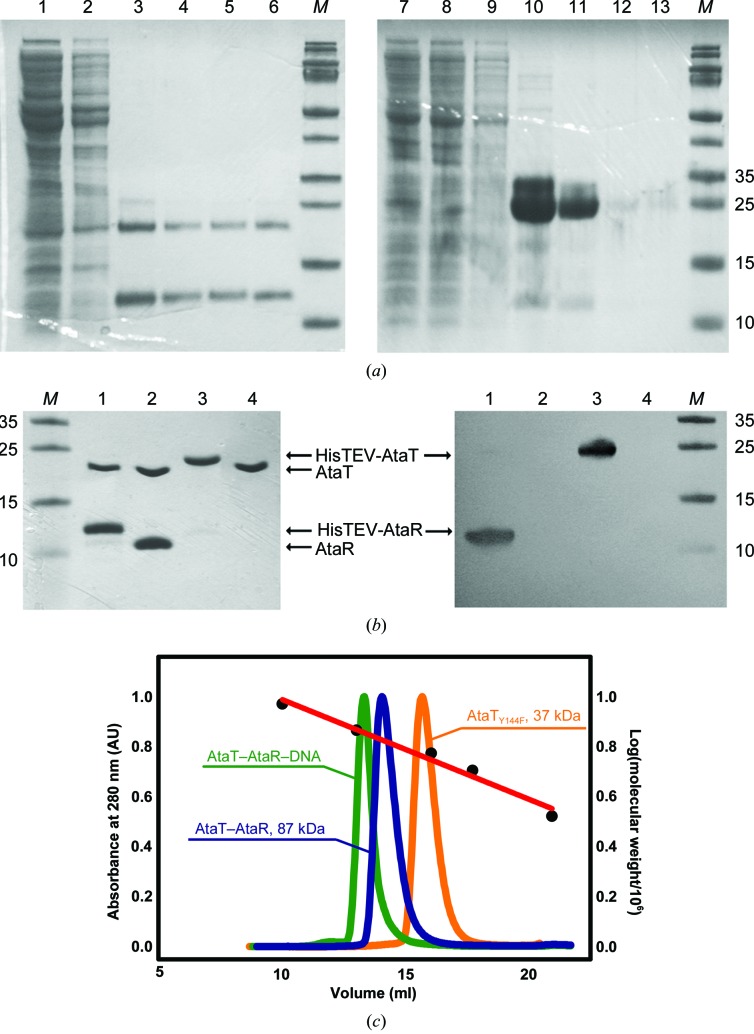
Because of the very high toxicity of AtaT, we could not express the free recombinant toxin. In previous work, it has been demonstrated that a tyrosine corresponding to Tyr144 in AtaT is a crucial catalytic residue (Cheverton et al., 2016 ▸). The introduction of the Y144F mutation renders the toxin inactive; however, it can still bind to AcCoA and to AtaR, and thus we used this mutant as a proxy for the native protein for structural studies. The genes encoding the ataRT operon and the ataTY144F toxin were cloned fused with a HisTEV tag to facilitate purification of the protein and subsequent removal of the His tag by treatment with TEV protease. The AtaR–AtaT complex and AtaTY144F were purified by Ni–NTA followed by size-exclusion chromatography (Fig. 1 ▸ a). After removal of the His tag, the proteins were repurified (Fig. 1 ▸ b) as described in §2. Based on analytical SEC, AtaTY144F was estimated to have a molecular mass of 37 kDa, which indicates that the toxin is in a dimeric state (Fig. 1 ▸ c). It has previously been reported that acetyltransferases from the GNAT family may function as dimers (Salah Ud-Din et al., 2016 ▸).
Figure 1.
Purification of AtaR–AtaT and AtaTY144F. (a) Coomassie-stained SDS–PAGE gel of protein purification on Ni Sepharose. Lanes 1–6, purification of HisTEV-AtaR–AtaT; lanes 7–13, purification of HisTEV-AtaTY144F. Lanes 1 and 7, protein extract; lanes 2 and 8, flowthrough; lane 9, wash; lanes 3–6 and 10–13, elution fractions. (b) SDS gel (16%) and Coomassie staining (left) or Western blotting with anti-His antibody (right) of HisTEV-AtaR–AtaT before and after TEV cleavage (lanes 1 and 2), HisTEV-AtaTY144F (lanes 3 and 4). Lane M, molecular-weight marker (labelled in kDa). (c) Analytical SEC on a Superdex 200 Increase SEC column; the measurements were performed in 50 mM Tris–HCl pH 8.5, 100 mM NaCl.
Free AtaTY144F toxin was screened for crystallization in the presence or absence of AcCoA at 277 and 293 K. Crystals were only obtained when AcCoA was added to the crystallization mother liquor. Crystals of the tagged version of AtaTY144F (HisTEV-AtaTY144F) were obtained at 293 K after several days of incubation in 10% PEG 4000 (see Fig. 2 ▸ a and Table 3 ▸ for details of the crystallization conditions and cryoprotection of all crystals prior to diffraction experiments). These crystals diffracted to 2.6 Å resolution. After treating the recombinant toxin mutant with TEV protease and repurifying the tag-free AtaTY144F, the enzyme crystallized at 277 K in 20% PEG 8000 and the crystals diffracted to 2.7 Å resolution (Fig. 2 ▸ b).
Figure 2.
Crystals of AtaR and AtaT. (a) Crystals of HisTEV-AtaTY144F. (b) Crystals of tag-free AtaTY144F. (c) Crystals of AtaR. (d) SDS–PAGE (16%) analysis of the AtaR crystals; only one band is observed in lane 1 next to the 10 kDa band from the molecular-weight markers (lane 2; labelled in kDa). This suggests that only the antitoxin AtaR is present in the crystal.
3.2. Crystallization of the AtaR antitoxin
As mentioned above, wild-type AtaT toxin cannot be expressed and purified alone as its expression causes inhibition of growth; however, co-expression of the AtaT toxin with the AtaR antitoxin is possible. We used this strategy to produce and purify the AtaT–AtaR complex (Jurėnas, Chatterjee et al., 2017 ▸) as well as a selenomethionine-modified (SeMet) version of the complex. We screened for crystals of the purified AtaR–AtaT complex with and without affinity tags. Crystals were obtained at 293 K after 2–3 weeks of incubation of SeMet-AtaT–AtaR and diffracted to 3.8 Å resolution (Fig. 2 ▸ c and Table 3 ▸). We harvested some of these crystals to analyse the content of the crystals by SDS–PAGE. For this, we transferred the crystals to a different drop containing only the mother liquor, and after several of these washing steps the crystals were dissolved in the SDS–PAGE loading dye. Fig. 2 ▸(d) shows the result of the SDS–PAGE. Interestingly, we could only observe a band corresponding to the expected molecular weight of AtaR, suggesting that these crystals only contained the antitoxin, and they are further referred to as AtaR crystals in this article.
3.3. Crystallization of the AtaR44–88–AtaTY144F complex
Like many type II antitoxins, AtaR is a modular protein (Fig. 3 ▸ a) with an N-terminal RHH domain and a highly charged C-terminus that is likely to be disordered in solution. The RHH domains observed in other TA antitoxins are usually involved in DNA binding and dimerization. They are notoriously flexible and only marginally stable (Madl et al., 2006 ▸). The strategy of simplifying type II antitoxins for structural biology studies has been very successful for other TA modules (De Gieter et al., 2014 ▸; De Jonge et al., 2009 ▸; Garcia-Pino et al., 2008 ▸) since it reduces the conformational heterogeneity of TA complexes associated with the disordered nature of TA antitoxins. Thus, after extensive screening for crystallization conditions of the full AtaR–AtaT complex that included the use of different protein constructs and sample conditions, we opted to crystallize a smaller, less flexible version of the AtaR–AtaT complex that lacked the N-terminal RHH domain of AtaR.
Figure 3.
(a) Sequence of the AtaR antitoxin; the functional modules of the antitoxin (N-terminal DNA-binding domain and C-terminal neutralization domain) are labelled in the figure. The arrow marks the start of the C-terminal domain of AtaR used in this study. (b) Bacterial growth assay on inducers indicating that the C-terminal domain of AtaR is sufficient to neutralize AtaT-His expression. Overnight cell cultures were serially diluted and spotted on LB-agar plates without (left) or with inducer (right). (c) AtaT-His expression was confirmed by assaying protein extracts 3 h after induction with 0.5 mM IPTG by SDS–PAGE (16%) and Coomassie staining (left) or Western blotting (right). Lane 1, AtaR–AtaT-His; lane 2, AtaRCter–AtaT-His; 3, control (empty expression vector). (d) Typical crystals of AtaTY144F in complex with the synthetic AtaR44–88 peptide. (e) Schematic representation of the ataRT promoter region. The transcription start site is marked +1 and shown in bold in the sequence. The −10 and −35 promoter elements and the start of the open reading frame of the ataR gene are indicated at the top of the sequence and the ATG start codon is shown in bold. The red arrows indicate the inverted repeat regions of the ataRT operator. (f) Sequences of the fragments used for crystallization. The sequence of the optimized DNA fragment that led to high-resolution diffraction is shown in bold. (g) Crystals of AtaR–AtaT in complex with double-stranded DNA. (h) 16% SDS–PAGE (left) and 2% agarose gel (right) of the dissolved AtaT–AtaR–DNA crystals, showing the presence of both proteins and of DNA.
We detected in vivo that the removal of the DNA-binding domain of the antitoxin did not affect the neutralization of AtaT (Figs. 3 ▸ a and 3 ▸ b). As shown in Fig. 3 ▸(c), using this construct AtaT could be co-expressed together with a truncated version of AtaR consisting of residues 44–88 (AtaR44–88). We used AtaTY144F and reconstituted the complex using a synthetic version of AtaR44–88 to obtain a more homogeneous preparation of the complex. For crystallization, AtaR44–88 (ChinaPeptides) was mixed with AtaTY144F in a 2:1 molar excess. Using this preparation, we obtained long needle-shaped crystals of the AtaR44–88–AtaTY144F complex in 20% PEG 6000 (Table 3 ▸) after a week of incubation at 277 K and these crystals diffracted to 2.5 Å resolution (Fig. 3 ▸ d).
3.4. Purification and characterization of the AtaR–AtaT–DNA complex
The AtaR antitoxin and the AtaT toxin form a very tight complex of around 87 kDa (as estimated by SEC). This complex can be directly purified when both proteins are co-expressed in E. coli. Fig. 1 ▸(c) shows that the complex is very stable and does not dissociate during SEC. Given the molecular weight of AtaR and AtaT, this complex is likely to be composed of two toxins and four antitoxins in a 1:2 ratio, resembling the repressing complexes of other TA modules (De Jonge et al., 2009 ▸; Loris & Garcia-Pino, 2014 ▸; Garcia-Pino, Balasubramanian et al., 2010 ▸).
A prevalent feature of type II TAs is the autoregulation of transcription of the operon (Hayes & Kędzierska, 2014 ▸). This autoregulation is characterized by the interaction of the DNA-binding domain of the antitoxin with the promoter region of the operon. In addition, in many cases it involves the toxin–antitoxin complex as a full repressor element and the toxin could act as a co-repressor or de-repressor. Therefore, we analysed the promoter region of the ataRT operon and found an inverted repeat sequence overlapping the −10 region of the putative operator sequence of the operon that could potentially contain a regulatory site (Fig. 3 ▸ e). The actual transcription start site was validated by 5′ RACE and the binding of the complex to this region was confirmed by SEC (Fig. 1 ▸ c). The 5′ RACE revealed the transcription start site to be located 43 bp upstream of ataR gene and the corresponding −10 and −35 elements to have sequences close to the consensus: TATgcT and TaGAaA, respectively (Fig. 3 ▸ e).
The inverted repeat region was reconstituted from synthetic DNA (HPLC-purified by Sigma–Aldrich) after the annealing of forward and reverse fragments and was further purified by SEC. The addition of an excess of duplex DNA to the AtaT–AtaR complex resulted in a left shift in the analytical SEC that indicates that the AtaR–AtaT complex binds tightly to the DNA (Fig. 1 ▸ c, green curve). Based on this result, we used SEC to prepare different variants of the AtaR–AtaT–DNA complex to screen for crystallization conditions (the different DNA fragments that were used in the crystallization screens are shown in Fig. 3 ▸(f).
3.5. Crystallization of the AtaR–AtaT–DNA complex
All of our attempts to produce crystals of the AtaR–AtaT complex failed, most likely owing to regions of high flexibility that still remained in the complex. In order to stabilize this complex, we decided to use the operator DNA region of the ataRT operon as an additional part that would not only stabilize the complex overall but would also stabilize local regions of the N-terminal DNA-binding domain of the antitoxin.
AtaR–AtaT forms a stable complex with DNA as seen by gel filtration (Fig. 1 ▸ c). We used different versions of dsDNA oligonucleotides of different lengths and overhangs to aid crystallization (Fig. 3 ▸ f). We obtained several crystals with different sizes and morphologies at 277 K. These crystals however, diffracted poorly to between 7 and 20 Å resolution. To further optimize the diffraction, we modified the DNA sequence to convert it to a perfect palindrome when reading the sequence from 5′ to 3′ on both strands (Fig. 3 ▸ f). The crystals obtained with the modified palindromic DNA diffracted to 3.5 Å resolution (Fig. 3 ▸ g, Tables 3 ▸ and 4 ▸).
Table 4. Data collection and processing.
The CC1/2 criterion was used to determine the resolution range. Values in parentheses are for the outer shell.
| SeMetAtaR | AtaT–AtaR44–88 | AtaTY144F | AtaR–AtaT–DNA | |
|---|---|---|---|---|
| Sample | AtaR | AtaTY144F–AtaR44–86 | AtaTY144F | AtaT–AtaR–DNA complex |
| Diffraction source | PX1, SOLEIL | PX2A, SOLEIL | PX2A, SOLEIL | PX1, SOLEIL |
| Wavelength (Å) | 0.9793 | 1.008 | 0.9801 | 0.9919 |
| Temperature (K) | 100 | 100 | 100 | 100 |
| Detector | PILATUS 6M | EIGER | EIGER | PILATUS 6M |
| Crystal-to-detector distance (mm) | 737.25 | 448.20 | 280.09 | 681.25 |
| Rotation range per image (°) | 0.1 | 0.1 | 0.1 | 0.1 |
| Total rotation range (°) | 360 | 200 | 120 | 200 |
| Exposure time per image (s) | 0.1 | 0.1 | 0.1 | 0.1 |
| Space group | P6122 | P3121 | P6522 | C2221 |
| a, b, c (Å) | 56.3, 56,3 160.8 | 87.6, 87.6, 125.5 | 58.1, 58.1, 216.7 | 75.6, 87.9, 190.5 |
| α, β, γ (°) | 90.0, 90.0, 120.0 | 90.0, 90.0, 120.0 | 90.0, 90.0, 120.0 | 90.0, 90.0, 90.0 |
| Mosaicity (°) | 0.25 | 0.17 | 0.19 | 0.18 |
| Resolution range (Å) | 46.64–3.78 (3.92–3.78) | 50.00–2.29 (2.35–2.29) | 45.54–2.51 (2.55–2.51) | 95.24–3.58 (3.64–3.58) |
| Total No. of reflections | 61729 (6402) | 276917 (16236) | 100100 (4851) | 51993 (2345) |
| No. of unique reflections | 1776 (197) | 25564 (1688) | 8089 (376) | 7075 (354) |
| Completeness (%) | 99.4 (95.2) | 99.3 (90.6) | 100 (100) | 91.2 (93.9) |
| Multiplicity | 34.8 (32.5) | 10.8 (9.6) | 12.2 (13.1) | 7.3 (6.6) |
| 〈I/σ(I)〉 | 9.2 (1.6) | 12.1 (0.8) | 8.6 (0.6) | 8.9 (0.8) |
| CC1/2 | 0.999 (0.552) | 0.998 (0.324) | 0.998 (0.448) | 0.998 (0.352) |
| R r.i.m. | 0.148 (1.423) | 0.065 (1.321) | 0.056 (1.520) | 0.040 (0.863) |
| Overall B factor from Wilson plot (Å2) | 146.5 | 72.62 | 62.0 | 170.6 |
| Resolution cutoff for SAD phasing (Å) | 6.00 | — | — | — |
| 〈d′′/σ〉 | 1.33 | — | — | — |
| CCall | 61.0 | — | — | — |
| CCweak | 34.7 | — | — | — |
| No. of Se atoms found with occupancy higher than 0.9 | 2 | — | — | — |
3.6. Preliminary X-ray analysis of the SeMetAtaR, AtaTY144F and AtaTY144F–AtaR44–88 crystals
The SeMetAtaR crystals diffracted to ∼3.8 Å resolution on average (Table 4 ▸). We performed a Se K-edge scan that showed a maximum at 0.9793 Å and we used this wavelength to collect data for single anomalous diffraction (SAD) phasing. Analysis of the data with SHELXC (Sheldrick, 2010 ▸) showed that there is strong anomalous signal to around ∼6.0 Å resolution (Table 4 ▸). SHELXD (Sheldrick, 2010 ▸) detected two heavy atoms with high occupancy, which was consistent with the presence of one AtaR chain in the asymmetric unit. This corresponds to a solvent content of ∼60% as estimated by MATTHEWS_COEFF (Winn et al., 2011 ▸). We used this solvent content for phasing and density modification with SHELXE (Sheldrick, 2010 ▸). The initial map calculated with SHELXE using a solvent content of 60% was of high quality and allowed the automatic tracing at 3.8 Å resolution of approximately 48 residues from the N-terminal DNA-binding domain of AtaR. This corresponds to most of the structured part of AtaR (the C-terminal part of the protein is predicted to be disordered) and confirms that the asymmetric unit contains a single AtaR monomer that forms a dimer when crystallographic symmetry is applied (Fig. 4 ▸). Although type II TA antitoxins are generally very difficult to crystallize because they contain intrinsically disordered regions, other antitoxins have also been crystallized in their unbound state (Arbing et al., 2010 ▸; Garcia-Pino, Sterckx et al., 2010 ▸). Using the PISA server (Krissinel & Henrick, 2007 ▸) we estimated that the AtaR dimer interface encompasses 920 Å2, which is of the order of the average interface area (1100 Å2) of other RHH antitoxin dimers (Francuski & Saenger, 2009 ▸; Madl et al., 2006 ▸; Ruangprasert et al., 2014 ▸). Further refinement and manual building to complete the structure is ongoing.
Figure 4.
Initial 2mF o − DF c map (σ = 1) for AtaR after density modification and automated tracing with SHELXE. The RHH dimer backbone autotraced with SHELXE and generated by crystallographic symmetry is shown as a ribbon.
In the case of AtaTY144F, the crystals of the native protein diffracted to approximately 2.5 Å resolution at a wavelength of 0.9801 Å (Table 4 ▸). However, we could not grow diffracting SeMetAtaTY144F crystals and the crystals were destroyed after soaking in solutions containing heavy atoms. Therefore, we used the coordinates of TacT (Cheverton et al., 2016 ▸) as a search model for molecular replacement for the native data set (PDB entry 5fvj). Although TacT is a remote homologue of AtaTY144F (29% sequence identity) we were able to find a solution in space group P6522 using Phaser, with a final TFZ of 40.7, PAK = 0 and LLG = 1640. The solution contains one AtaTY144F molecule in the asymmetric unit that forms a homodimer after applying crystallographic symmetry. The crystals of the AtaTY144F–AtaR44–88 complex diffracted to ∼2.3 Å resolution. As in the case of AtaTY144F, we used TacT as a search model and found a solution in space group P3121 with two AtaTY144F molecules in the asymmetric unit using Phaser, with a final TFZ of 20.2, PAK = 0 and LLG = 352. Structure refinement and completion is ongoing in all cases.
3.7. Preliminary X-ray analysis of the AtaR–AtaT–DNA crystals
To characterize the content of the AtaR–AtaT–DNA crystals, we treated them as described above followed by analysis with SDS–PAGE and agarose gels. The results shown in Fig. 3 ▸(h) confirmed that the crystals contained the AtaR–AtaT complex. In addition, the agarose gels of the redissolved crystals strongly absorbed UV light when incubated with ethidium bromide, suggesting that the crystals also contained the double-stranded DNA fragment (Fig. 3 ▸ h).
Since the analysis of the content of the AtaR–AtaT–DNA crystals performed by SDS–PAGE and agarose gel electrophoresis shows that both the AtaR–AtaT complex and DNA are present in the crystals, we generated a model of the DNA duplex of the modified DNA with the Make-NA server (http://structure.usc.edu/make-na/), a web-based utility to create ideal DNA and RNA models using Nucleic Acid Builder, and subsequently used this model for molecular replacement. Using this model and the coordinates of TacT, we were able to find a molecular-replacement solution at 3.5 Å resolution with TFZ = 11.9 and LLG = 166 in space group C2221with Phaser. Refinement of this model at 3.5 Å resolution is currently ongoing.
4. Conclusions
The role of TA modules in bacterial physiology has been extensively debated (Magnuson, 2007 ▸). Nevertheless, their role remains a thorny issue in modern microbiology. Type II toxins are by far the best-studied group. These studies involved various enzyme families such as RNAses (Christensen & Gerdes, 2003 ▸; Kamada et al., 2003 ▸; Kamada & Hanaoka, 2005 ▸; Winther & Gerdes, 2011 ▸), kinases (Castro-Roa et al., 2013 ▸; Kaspy et al., 2013 ▸; Mutschler et al., 2011 ▸), ADP-ribosyl transferases (Jankevicius et al., 2016 ▸), acetyltransferases (Cheverton et al., 2016 ▸; Jurėnas, Chatterjee et al., 2017 ▸) and adenylyltransferases (Engel et al., 2012 ▸). In addition, the different mechanisms of toxin neutralization and transcription autoregulation show that the intrinsically disordered regions present in TA antitoxins constitute an important feature of type II TA modules (Bordes et al., 2016 ▸; De Gieter et al., 2014; Garcia-Pino, Balasubramanian et al., 2010 ▸; Garcia-Pino et al., 2016 ▸; Loris & Garcia-Pino, 2014 ▸) beyond providing a target for protease degradation.
The toxin AtaT from the ataRT TA module found in enteropathogenic E. coli, Enterobacter, Citrobacter and other bacteria (Jurėnas, Garcia-Pino et al., 2017 ▸) was recently described as a member of the GNAT family of N-acetyltransferases. It triggers cell-growth arrest by acetylating the initiator Met-tRNAfMet at the amine group of the amino acid (Jurėnas, Chatterjee et al., 2017 ▸). Other toxins of this family also target amino-acylated tRNAs (aa-tRNAs) and have been linked to persister cell formation in Salmonella (Cheverton et al., 2016 ▸). Although there is structural information on other GNAT enzymes, very little is known regarding the molecular basis of the catalysis by GNAT N-acetyltransferases that target aa-tRNAs and the regulation of this type of TA operon. We have expressed, produced and crystallized AtaTY144F, the AtaTY144F–AtaR44–88 complex and an AtaT–AtaR–operator DNA complex. The corresponding crystal structures will reveal the basis of the regulation of the ataRT toxin–antitoxin module.
Acknowledgments
We thank the staff of the various beamlines at SOLEIL (PROXIMA-1 and PROXIMA-2A) and DLS (I24).
Funding Statement
This work was funded by Fonds De La Recherche Scientifique - FNRS grants F.4505.16, U.N043.17F, CR-2017S-03, T.0147.15F PDR, and J.0061.16F CDR. Université Libre de Bruxelles grant . Federaal Wetenschapsbeleid grant . Fonds Jean Brachet grant . Fonds Alice et David van Buuren grant .
References
- Afif, H., Allali, N., Couturier, M. & Van Melderen, L. (2001). Mol. Microbiol. 41, 73–82. [DOI] [PubMed]
- Afonine, P. V., Grosse-Kunstleve, R. W., Echols, N., Headd, J. J., Moriarty, N. W., Mustyakimov, M., Terwilliger, T. C., Urzhumtsev, A., Zwart, P. H. & Adams, P. D. (2012). Acta Cryst. D68, 352–367. [DOI] [PMC free article] [PubMed]
- Arbing, M. A. et al. (2010). Structure, 18, 996–1010. [DOI] [PMC free article] [PubMed]
- Bordes, P., Sala, A. J., Ayala, S., Texier, P., Slama, N., Cirinesi, A. M., Guillet, V., Mourey, L. & Genevaux, P. (2016). Nature Commun. 7, 13339. [DOI] [PMC free article] [PubMed]
- Buts, L., Lah, J., Dao-Thi, M.-H., Wyns, L. & Loris, R. (2005). Trends Biochem. Sci. 30, 672–679. [DOI] [PubMed]
- Castro-Roa, D., Garcia-Pino, A., De Gieter, S., van Nuland, N. A., Loris, R. & Zenkin, N. (2013). Nature Chem. Biol. 9, 811–817. [DOI] [PMC free article] [PubMed]
- Cheverton, A. M., Gollan, B., Przydacz, M., Wong, C. T., Mylona, A., Hare, S. A. & Helaine, S. (2016). Mol. Cell, 63, 86–96. [DOI] [PMC free article] [PubMed]
- Christensen, S. K. & Gerdes, K. (2003). Mol. Microbiol. 48, 1389–1400. [DOI] [PubMed]
- Dao-Thi, M.-H., Messens, J., Wyns, L. & Backmann, J. (2000). J. Mol. Biol. 299, 1373–1386. [DOI] [PubMed]
- De Gieter, S., Konijnenberg, A., Talavera, A., Butterer, A., Haesaerts, S., De Greve, H., Sobott, F., Loris, R. & Garcia-Pino, A. (2014). J. Biol. Chem. 289, 34013–34023. [DOI] [PMC free article] [PubMed]
- De Jonge, N., Garcia-Pino, A., Buts, L., Haesaerts, S., Charlier, D., Zangger, K., Wyns, L., De Greve, H. & Loris, R. (2009). Mol. Cell, 35, 154–163. [DOI] [PubMed]
- De Jonge, N., Hohlweg, W., Garcia-Pino, A., Respondek, M., Buts, L., Haesaerts, S., Lah, J., Zangger, K. & Loris, R. (2010). J. Biol. Chem. 285, 5606–5613. [DOI] [PMC free article] [PubMed]
- Doherty, A. J., Ashford, S. R., Brannigan, J. A. & Wigley, D. B. (1995). Nucleic Acids Res. 23, 2074–2075. [DOI] [PMC free article] [PubMed]
- Engel, P., Goepfert, A., Stanger, F. V., Harms, A., Schmidt, A., Schirmer, T. & Dehio, C. (2012). Nature (London), 482, 107–110. [DOI] [PubMed]
- Evans, P. (2006). Acta Cryst. D62, 72–82. [DOI] [PubMed]
- Francuski, D. & Saenger, W. (2009). J. Mol. Biol. 393, 898–908. [DOI] [PubMed]
- Garcia-Pino, A., Balasubramanian, S., Wyns, L., Gazit, E., De Greve, H., Magnuson, R. D., Charlier, D., van Nuland, N. A. & Loris, R. (2010). Cell, 142, 101–111. [DOI] [PubMed]
- Garcia-Pino, A., Christensen-Dalsgaard, M., Wyns, L., Yarmolinsky, M., Magnuson, R. D., Gerdes, K. & Loris, R. (2008). J. Biol. Chem. 283, 30821–30827. [DOI] [PMC free article] [PubMed]
- Garcia-Pino, A., De Gieter, S., Talavera, A., De Greve, H., Efremov, R. G. & Loris, R. (2016). Nature Chem. Biol. 12, 490–496. [DOI] [PubMed]
- Garcia-Pino, A., Sterckx, Y., Vandenbussche, G. & Loris, R. (2010). Acta Cryst. F66, 167–171. [DOI] [PMC free article] [PubMed]
- Gasteiger, E., Gattiker, A., Hoogland, C., Ivanyi, I., Appel, R. D. & Bairoch, A. (2003). Nucleic Acids Res. 31, 3784–3788. [DOI] [PMC free article] [PubMed]
- Gerdes, K., Rasmussen, P. B. & Molin, S. (1986). Proc. Natl Acad. Sci. USA, 83, 3116–3120. [DOI] [PMC free article] [PubMed]
- Goeders, N. & Van Melderen, L. (2014). Toxins, 6, 304–324. [DOI] [PMC free article] [PubMed]
- Guglielmini, J. & Van Melderen, L. (2011). Mob. Genet. Elements, 1, 283–290. [DOI] [PMC free article] [PubMed]
- Hadži, S., Garcia-Pino, A., Haesaerts, S., Jurėnas, D., Gerdes, K., Lah, J. & Loris, R. (2017). Nucleic Acids Res. 45, 4972–4983. [DOI] [PMC free article] [PubMed]
- Hayes, F. & Kędzierska, B. (2014). Toxins, 6, 337–358. [DOI] [PMC free article] [PubMed]
- Hayes, F. & Van Melderen, L. (2011). Crit. Rev. Biochem. Mol. Biol. 46, 386–408. [DOI] [PubMed]
- Helaine, S., Cheverton, A. M., Watson, K. G., Faure, L. M., Matthews, S. A. & Holden, D. W. (2014). Science, 343, 204–208. [DOI] [PMC free article] [PubMed]
- Jaffé, A., Ogura, T. & Hiraga, S. (1985). J. Bacteriol. 163, 841–849. [DOI] [PMC free article] [PubMed]
- Jankevicius, G., Ariza, A., Ahel, M. & Ahel, I. (2016). Mol. Cell, 64, 1109–1116. [DOI] [PMC free article] [PubMed]
- Jurėnas, D., Chatterjee, S., Konijnenberg, A., Sobott, F., Droogmans, L., Garcia-Pino, A. & Van Melderen, L. (2017). Nature Chem. Biol. 13, 640–646. [DOI] [PubMed]
- Jurėnas, D., Garcia-Pino, A. & Van Melderen, L. (2017). Plasmid, 93, 30–35. [DOI] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Kamada, K. & Hanaoka, F. (2005). Mol. Cell, 19, 497–509. [DOI] [PubMed]
- Kamada, K., Hanaoka, F. & Burley, S. K. (2003). Mol. Cell, 11, 875–884. [DOI] [PubMed]
- Kaspy, I., Rotem, E., Weiss, N., Ronin, I., Balaban, N. Q. & Glaser, G. (2013). Nature Commun. 4, 3001. [DOI] [PubMed]
- Koonin, E. V., Makarova, K. S. & Wolf, Y. I. (2017). Annu. Rev. Microbiol. 71, 233–261. [DOI] [PMC free article] [PubMed]
- Krissinel, E. & Henrick, K. (2007). J. Mol. Biol. 372, 774–797. [DOI] [PubMed]
- Loris, R. & Garcia-Pino, A. (2014). Chem. Rev. 114, 6933–6947. [DOI] [PubMed]
- Madl, T., Van Melderen, L., Mine, N., Respondek, M., Oberer, M., Keller, W., Khatai, L. & Zangger, K. (2006). J. Mol. Biol. 364, 170–185. [DOI] [PubMed]
- Magnuson, R. D. (2007). J. Bacteriol. 189, 6089–6092. [DOI] [PMC free article] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst. 40, 658–674. [DOI] [PMC free article] [PubMed]
- Monti, M. C., Hernández-Arriaga, A. M., Kamphuis, M. B., López-Villarejo, J., Heck, A. J., Boelens, R., Díaz-Orejas, R. & van den Heuvel, R. H. (2007). Nucleic Acids Res. 35, 1737–1749. [DOI] [PMC free article] [PubMed]
- Mutschler, H., Gebhardt, M., Shoeman, R. L. & Meinhart, A. (2011). PLoS Biol. 9, e1001033. [DOI] [PMC free article] [PubMed]
- Norton, J. P. & Mulvey, M. A. (2012). PLoS Pathog. 8, e1002954. [DOI] [PMC free article] [PubMed]
- Pape, T. & Schneider, T. R. (2004). J. Appl. Cryst. 37, 843–844.
- Ruangprasert, A., Maehigashi, T., Miles, S. J., Giridharan, N., Liu, J. X. & Dunham, C. M. (2014). J. Biol. Chem. 289, 20559–20569. [DOI] [PMC free article] [PubMed]
- Salah Ud-Din, A. I., Tikhomirova, A. & Roujeinikova, A. (2016). Int. J. Mol. Sci. 17, 1018. [DOI] [PMC free article] [PubMed]
- Schreiter, E. R. & Drennan, C. L. (2007). Nature Rev. Microbiol. 5, 710–720. [DOI] [PubMed]
- Sheldrick, G. M. (2010). Acta Cryst. D66, 479–485. [DOI] [PMC free article] [PubMed]
- Studier, F. W., Rosenberg, A. H., Dunn, J. J. & Dubendorff, J. W. (1990). Methods Enzymol. 185, 60–89. [DOI] [PubMed]
- Van Melderen, L. (2010). Curr. Opin. Microbiol. 13, 781–785. [DOI] [PubMed]
- Van Melderen, L., Bernard, P. & Couturier, M. (1994). Mol. Microbiol. 11, 1151–1157. [DOI] [PubMed]
- Van Melderen, L., Jurėnas, D. & Garcia-Pino, A. (2018). RNA Biol. 15, 303–307. [DOI] [PMC free article] [PubMed]
- Van Melderen, L. & Saavedra De Bast, M. (2009). PLoS Genet. 5, e1000437. [DOI] [PMC free article] [PubMed]
- Van Melderen, L., Dao Thi, M. H., Lecchi, P., Gottesman, S., Couturier, M. & Maurizi, M. R. (1996). J. Biol. Chem. 271, 27730–27738. [DOI] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- Winther, K. S. & Gerdes, K. (2011). Proc. Natl Acad. Sci. USA, 108, 7403–7407. [DOI] [PMC free article] [PubMed]
- Zhang, D., de Souza, R. F., Anantharaman, V., Iyer, L. M. & Aravind, L. (2012). Biol. Direct, 7, 18. [DOI] [PMC free article] [PubMed]