In the crystal structure of the title compound the molecules are coplanar and are linked into helical chains via N—H⋯N hydrogen bonding between one of the amino H atoms and the pyridine N atoms.
Keywords: crystal structure, hydrogen bonding, azopyridine
Abstract
The crystal structure of the title compound, C11H10N4, comprises molecules in a trans conformation for which all the atoms are located in general positions. The six-membered rings are coplanar and this arrangement might be stabilized by intramolecular N—H⋯N hydrogen bonding. In the crystal, the molecules are linked into helical chains parallel to the b axis via N—H⋯N hydrogen bonding. The molecular packing shows a herringbone-like pattern along the a axis. Comparison of the X-ray powder diffraction with that calculated from single crystal data proves that a pure crystalline phase was obtained and UV–Vis measurements reveal that only the trans isomer is present.
Chemical context
Azobenzenes are among the most frequently used photochromic compounds with numerous applications in different fields being reported (Szymański et al., 2013 ▸; Merino & Ribagorda, 2012 ▸; Kay et al., 2007 ▸). Moreover, azobenzenes are easily accessible, and their photochromic functions are quite reliable. The stretched trans isomer is usually the thermodynamically stable conformation. Upon irradiation with UV light, the bent cis isomer is formed. This cis conformation switches back to the trans isomer either upon irradiation with visible light or thermochemically (Hartley, 1937 ▸). A highly important variation of azobenzenes are azopyridines, as pyridines coordinate to various metals, e.g. nickel (Thies et al., 2010 ▸; Dommaschk et al., 2015c
▸). Thus, azopyridines can be used as switchable ligands. In this context, we have reported an approach to switch the spin state of azopyridine-functionalized Ni-porphyrins (Thies et al., 2011 ▸, 2012 ▸; Venkataramani et al., 2011 ▸; Dommaschk et al., 2015a
▸,b
▸). Aiming at further functionalization of azopyridines and in view of applications as molecular spin switches, we have synthesized 2-[2-(pyridin-3-yl)diazen-1-yl]aniline and report here its molecular and crystal structure.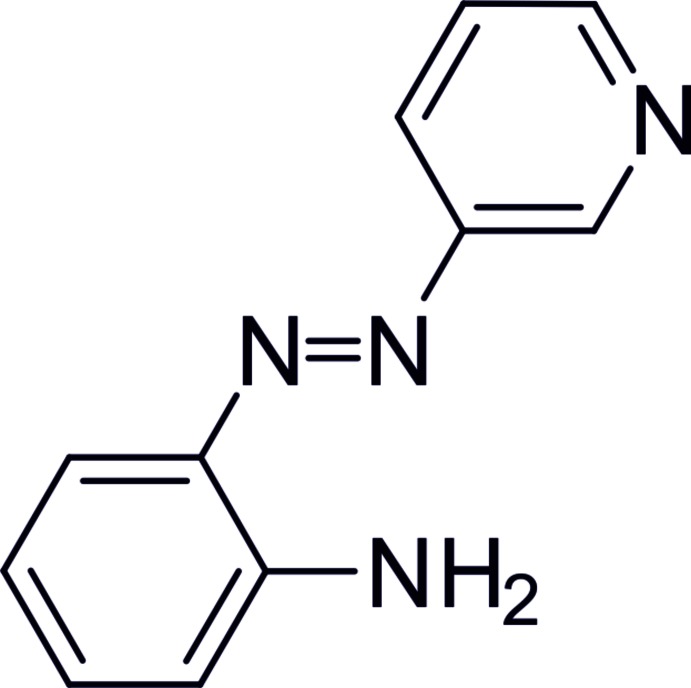
Structural commentary
The crystal structure of the title compound comprises 2-[2-(pyridin-3-yl)diazen-1-yl]aniline molecules, located on general positions, adopting a trans-conformation with a C1—N2—N3—C6 torsion angle of −179.80 (8)°, which corresponds to the energetically favored arrangement (Fig. 1 ▸). The two six-membered rings are coplanar [the maximum deviation from the least-squares plane for all non-H atoms is 0.0569 (9) Å for N4] and the dihedral angle between the ring planes is 0.11 (8)°. The amino H atoms are also in the plane of the adjacent benzene ring. There is an intramolecular N—H⋯N hydrogen bond between one of the amino H atoms and one nitrogen atom of the azo group with an N⋯H distance of 2.066 (15) Å and an N—H⋯N angle of 127.30 (12)° (Table 1 ▸). Even if this corresponds to a weak interaction, it might stabilize the planar arrangement.
Figure 1.
Molecular structure of the title compound with labeling and displacement elliposids drawn at the 50% probability level. The intramolecular N—H⋯N hydrogen bond is shown with dashed lines.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N4—H1N4⋯N2 | 0.881 (16) | 2.066 (15) | 2.6922 (14) | 127.3 (12) |
| N4—H2N4⋯N1i | 0.904 (16) | 2.163 (16) | 3.0274 (14) | 159.7 (14) |
Symmetry code: (i)  .
.
Supramolecular features
In the crystal structure of the title compound, the molecules are linked into chains along the b-axis direction via N—H⋯N hydrogen bonds between the amino hydrogen atom that is not involved in intramolecular hydrogen bonding and one of the nitrogen atoms of the azo group (Fig. 2 ▸, top). The N⋯H distance amounts to 2.163 (16) Å and the N—H⋯N angle of 159.7.14 (12)° is slightly bent, indicating that this is a relatively strong interaction (Table 1 ▸). The dihedral angle between the pyridine ring that carries the acceptor N atom and the aminophenyl moiety of a neighbouring molecule that carries the donor group is 66.12 (8)°. Therefore, the molecules exhibit a helical arrangement along the chain (Fig. 2 ▸, top). The chains are closely packed in such a way that each chain is surrounded by eight neighboring chains (Fig. 2 ▸, bottom). The molecules exhibit a herringbone-like pattern along the a axis (Fig. 3 ▸) in which the pyridine and benzene rings of adjacent molecules are perfectly coplanar. The distance between the ring planes is 3.462 Å and the centroid–centroid distance is 3.8040 (7) Å, indicating π–π interactions between the chains.
Figure 2.
Crystal structure of the title compound showing a chain (top) and a view along the b axis (bottom). Intra- and intermolecular hydrogen bonds are indicated by dashed lines.
Figure 3.
Crystal structure of the title compound in a view along the a axis.
Database survey
According to a search of the Cambridge Structural Database (CSD; Groom et al., 2016 ▸), 3-azopyridine molecules substituted with an amino group in the ortho-position are unknown. However, one ortho-substituted phenyl-azopyridine was reported, viz. 2,6-diamino-3-[(2-carboxymethyl)phenylazo]pyridine (Tan et al., 2010 ▸). 4-Aminophenyl-azopyridines such as N,N-diethyl-4-[(E)-(pyridine-3-yl)diazenyl]aniline (Draguta et al., 2015 ▸) and N,N-dimethyl-4-(pyridine-3-yldiazenyl)aniline (Draguta et al., 2013 ▸) are also known. Furthermore, structure reports on 2-azopyridine molecules that are substituted in the ortho-position, such as 5-[(5-bromo-2-pyridyl)azo]-2,4-toluenediamine (Jinzi et al., 1984 ▸) and 5-[(3,5-dibromo-2-pyridyl)azo]-2,4-diaminotoluene (Kailiang et al., 1985 ▸), have been published. Other azo compounds, substituted similarly to the title molecule, are highly important for coordination chemistry, which is shown in their crystal structures (Maiti et al., 2001 ▸, 2003 ▸; Pratihar et al., 2005 ▸, 2007 ▸).
Synthesis and crystallization
The synthesis of the title compound can be performed in two steps.
(i) Synthesis of 3-(2-acetanilide)azopyridine: 2-nitroacetanilide (4.00 g, 29.0 mmol) was dissolved in ethanol (150 ml). An aqueous solution of ammonium chloride (2.01 g, 37.7 mmol in 15 ml) was added. The mixture was warmed up to 313 K until the dispersion changed into a clear solution. After cooling to room temperature, zinc dust (4.93 g, 75.3 mmol) was added and the mixture was stirred for 1 h at 333 K. After filtration, the filtrate was poured into an aqueous ice-cooled iron(III) chloride solution (hexaaqua complex, 5.40 g, 20.3 mmol in 150 ml) whereby a green solid precipitated. After 15 min of stirring, the solid was filtered off and washed with water. The crude product was a mixture of 2-nitrosoacetanilide and starting material, which was used for azo condensation without further purification. 3-Aminopyridine (800 mg, 8.51 mmol) was dissolved in a mixture of pyridine (25 ml) and aqueous sodium hydroxide (5 ml, 25%). The crude product of 2-nitrosoacetanilide dissolved in pyridine (30 ml) was added to the solution containing the 3-aminopyridine. The reaction mixture was stirred for 1 h at 353 K and overnight at room temperature. After addition of dichloromethane (200 ml) the phases were separated. The organic layer was washed with water twice and dried over sodium sulfate. The solvent was removed under reduced pressure. The crude product was purified by column chromatography (ethyl ester/n-hexane, R f = 0.16). The product was obtained as an orange solid. Yield: 200 mg (0.83 mmol, 10%).
1H NMR (500 MHz, 300 K, CDCl3): δ = 9.88 (s, br, 1H, N-H), 9.11 (d, 4 J = 2.4 Hz, 1H), 8.71 (dd, 3 J = 4.7 Hz, 4 J = 1.5 Hz), 8.66 (d, br, 3 J = 8.3 Hz, 1H), 8.07 (ddd, 3 J = 8.2 Hz, 4 J = 2.4 Hz, 4 J = 1.5 Hz, 1H), 7.83 (dd, 3 J = 8.1 Hz, 4 J = 1.4 Hz 1H), 7.51–7.44 (m, 2H), 7.16 (td, 3 J = 8.1 Hz, 4 J = 1.3 Hz 1H), 2.26 (s, 3H) ppm. 13C NMR (150 MHz, 300 K, CDCl3): δ = 168.3, 151.9, 147.7, 146.3, 138.9, 136.4, 133.8, 127.3, 124.1, 123.4, 120.6, 120.4, 25.2 ppm. HRMS (EI): m/z [M]+ calculated for C13H12N4O: 240.10111, found: 240.10136.
(ii) Synthesis of 2-[2-(pyridin-3-yl)diazen-1-yl]aniline: 3-(2-acetanilide)azopyridine (640 mg, 2.66 mmol) was dissolved in methanol (50 ml). A sodium hydroxide solution (5 ml, 30%) was added and stirred for 6 h at 343 K. 2-[2-(Pyridin-3-yl)diazen-1-yl]aniline precipitated and was filtered off. The solid was washed with water. Recrystallization from acetone gave orange single crystals. Yield: 520 mg (2.63 mmol, 99%).
1H NMR (500 MHz, 300 K, CDCl3): δ = 9.08 (dd, 4 J = 2.4 Hz, 5 J = 0.8Hz, 1H), 8.63 (dd, 3 J = 4.7 Hz, 4 J = 1.6 Hz, 1H), 8.08 (ddd, 3 J = 8.2 Hz, 4 J = 2.4 Hz, 4 J = 1.6 Hz, 1H), 7.84 (dd, 3 J = 8.1 Hz, 4 J =1.4 Hz, 1H), 7.41 (ddd, 3 J =8.2 Hz, 3 J = 4.7 Hz, 5 J = 0.8 Hz, 1H),7.24 (ddd, 3 J = 8.3 Hz, 3 J = 7.1 Hz, 4 J = 1.4 Hz, 1H), 6.83 (ddd, 3 J = 8.1 Hz, 3 J = 7.1 Hz, 4 J = 1.3 Hz, 1H), 6.77 (dd, 3 J = 8.3 Hz, 4 J = 1.3 Hz, 1H), 6.01 (s, br, 2H, N—H) ppm. 13C NMR (150 MHz, 300 K, CDCl3): δ = 150.6, 148.3, 146.51, 143.0, 137.1, 133.1, 128.5, 126.4, 123.9, 117.5, 117.1 ppm. MS (EI, TOF): m/z (%) = 199 [M]+, 198 [M], 120 [M − C5H4N]+, 92 [M − C6H6N2]+.
Comparison of the experimental X-ray powder diffraction pattern with that calculated from single crystal data proves that the title compound was obtained as a pure phase (see Fig. S1 in the supporting information). The UV–Vis spectrum shows the strong π→π* band of the trans conformation (Fig. S2 in the supporting information). If the sample is exposed to light of 365 nm, no isomerization into the cis conformer is observed, and the sample starts to decompose. However, conversion of the amino to an amide group will probably restore the photochromic properties.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. The C—H hydrogen atoms were located in a difference map but were positioned with idealized geometry and refined with U iso(H) = 1.2U eq(C,N) using a riding model with Caromatic—H = 0.95 Å. The N—H hydrogen atoms were located in a difference map and were freely refined.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C11H10N4 |
| M r | 198.23 |
| Crystal system, space group | Monoclinic, P2/n |
| Temperature (K) | 200 |
| a, b, c (Å) | 13.2798 (8), 5.9792 (3), 13.4130 (9) |
| β (°) | 113.046 (7) |
| V (Å3) | 980.03 (11) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.09 |
| Crystal size (mm) | 0.20 × 0.15 × 0.15 |
| Data collection | |
| Diffractometer | Stoe IPDS1 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 8233, 2137, 1731 |
| R int | 0.073 |
| (sin θ/λ)max (Å−1) | 0.639 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.039, 0.113, 1.04 |
| No. of reflections | 2137 |
| No. of parameters | 145 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.21, −0.18 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989018008605/wm5450sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989018008605/wm5450Isup2.hkl
Fig. S1. Experimental (top) and calculated (bottom) X-ray powder diffraction patterns for the title compound, measured with copper radiation.. DOI: 10.1107/S2056989018008605/wm5450sup3.tif
Fig. S2. UV-Vis spectra for the title compound.. DOI: 10.1107/S2056989018008605/wm5450sup4.tif
Supporting information file. DOI: 10.1107/S2056989018008605/wm5450Isup5.cml
CCDC reference: 1848833
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
We thank Professor Dr Wolfgang Bensch for access to his experimental facilities.
supplementary crystallographic information
Crystal data
| C11H10N4 | F(000) = 416 |
| Mr = 198.23 | Dx = 1.344 Mg m−3 |
| Monoclinic, P2/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 13.2798 (8) Å | Cell parameters from 8233 reflections |
| b = 5.9792 (3) Å | θ = 3.3–27.0° |
| c = 13.4130 (9) Å | µ = 0.09 mm−1 |
| β = 113.046 (7)° | T = 200 K |
| V = 980.03 (11) Å3 | Block, orange |
| Z = 4 | 0.20 × 0.15 × 0.15 mm |
Data collection
| Stoe IPDS-1 diffractometer | Rint = 0.073 |
| Phi scans | θmax = 27.0°, θmin = 3.3° |
| 8233 measured reflections | h = −16→16 |
| 2137 independent reflections | k = −7→7 |
| 1731 reflections with I > 2σ(I) | l = −17→17 |
Refinement
| Refinement on F2 | Hydrogen site location: mixed |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.039 | w = 1/[σ2(Fo2) + (0.0675P)2 + 0.0778P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.113 | (Δ/σ)max < 0.001 |
| S = 1.04 | Δρmax = 0.21 e Å−3 |
| 2137 reflections | Δρmin = −0.18 e Å−3 |
| 145 parameters | Extinction correction: SHELXL2014 (Sheldrick, 2015), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 restraints | Extinction coefficient: 0.054 (14) |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.42109 (8) | 0.49909 (17) | 0.67373 (8) | 0.0197 (3) | |
| C2 | 0.40530 (8) | 0.65338 (19) | 0.74372 (9) | 0.0240 (3) | |
| H2 | 0.3449 | 0.6313 | 0.7638 | 0.029* | |
| N1 | 0.46934 (7) | 0.83068 (16) | 0.78456 (8) | 0.0264 (3) | |
| C3 | 0.55368 (9) | 0.85966 (19) | 0.75476 (9) | 0.0252 (3) | |
| H3 | 0.5997 | 0.9861 | 0.7817 | 0.030* | |
| C4 | 0.57689 (9) | 0.71309 (19) | 0.68624 (9) | 0.0261 (3) | |
| H4 | 0.6382 | 0.7387 | 0.6680 | 0.031* | |
| C5 | 0.51034 (9) | 0.53033 (18) | 0.64487 (9) | 0.0236 (3) | |
| H5 | 0.5248 | 0.4281 | 0.5979 | 0.028* | |
| N2 | 0.34292 (7) | 0.32432 (14) | 0.63669 (7) | 0.0218 (2) | |
| N3 | 0.35904 (7) | 0.19490 (15) | 0.56895 (7) | 0.0216 (2) | |
| C6 | 0.28594 (8) | 0.01793 (17) | 0.52757 (8) | 0.0205 (3) | |
| C7 | 0.19205 (8) | −0.03083 (18) | 0.55077 (8) | 0.0221 (3) | |
| C8 | 0.13128 (9) | −0.22461 (19) | 0.50278 (9) | 0.0272 (3) | |
| H8 | 0.0687 | −0.2617 | 0.5171 | 0.033* | |
| C9 | 0.16052 (9) | −0.3602 (2) | 0.43605 (9) | 0.0294 (3) | |
| H9 | 0.1186 | −0.4903 | 0.4059 | 0.035* | |
| C10 | 0.25156 (10) | −0.30942 (19) | 0.41168 (9) | 0.0291 (3) | |
| H10 | 0.2705 | −0.4022 | 0.3642 | 0.035* | |
| C11 | 0.31265 (9) | −0.12381 (19) | 0.45757 (9) | 0.0247 (3) | |
| H11 | 0.3747 | −0.0897 | 0.4418 | 0.030* | |
| N4 | 0.15968 (9) | 0.09982 (18) | 0.61468 (9) | 0.0308 (3) | |
| H1N4 | 0.2024 (12) | 0.210 (2) | 0.6510 (12) | 0.034 (4)* | |
| H2N4 | 0.1081 (13) | 0.045 (3) | 0.6365 (13) | 0.045 (4)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0178 (5) | 0.0203 (5) | 0.0213 (5) | 0.0009 (4) | 0.0080 (4) | 0.0027 (4) |
| C2 | 0.0186 (5) | 0.0268 (6) | 0.0293 (5) | 0.0009 (4) | 0.0125 (4) | −0.0011 (4) |
| N1 | 0.0232 (5) | 0.0264 (5) | 0.0313 (5) | 0.0005 (4) | 0.0125 (4) | −0.0045 (4) |
| C3 | 0.0219 (5) | 0.0222 (5) | 0.0309 (6) | −0.0024 (4) | 0.0098 (4) | −0.0007 (4) |
| C4 | 0.0221 (5) | 0.0284 (6) | 0.0321 (6) | −0.0021 (4) | 0.0152 (4) | 0.0021 (5) |
| C5 | 0.0242 (5) | 0.0251 (6) | 0.0262 (6) | 0.0005 (4) | 0.0150 (4) | 0.0000 (4) |
| N2 | 0.0211 (4) | 0.0221 (5) | 0.0240 (5) | −0.0010 (3) | 0.0106 (4) | 0.0000 (4) |
| N3 | 0.0220 (4) | 0.0196 (5) | 0.0238 (5) | −0.0001 (3) | 0.0097 (4) | 0.0019 (4) |
| C6 | 0.0203 (5) | 0.0198 (5) | 0.0211 (5) | 0.0008 (4) | 0.0078 (4) | 0.0032 (4) |
| C7 | 0.0208 (5) | 0.0240 (6) | 0.0208 (5) | −0.0005 (4) | 0.0073 (4) | 0.0037 (4) |
| C8 | 0.0226 (5) | 0.0300 (6) | 0.0274 (6) | −0.0050 (4) | 0.0080 (4) | 0.0028 (5) |
| C9 | 0.0279 (6) | 0.0238 (6) | 0.0301 (6) | −0.0046 (4) | 0.0045 (5) | −0.0016 (5) |
| C10 | 0.0304 (6) | 0.0255 (6) | 0.0296 (6) | 0.0034 (5) | 0.0099 (5) | −0.0029 (5) |
| C11 | 0.0236 (5) | 0.0249 (6) | 0.0267 (6) | 0.0025 (4) | 0.0110 (4) | 0.0023 (4) |
| N4 | 0.0291 (5) | 0.0351 (6) | 0.0362 (6) | −0.0100 (4) | 0.0214 (4) | −0.0073 (5) |
Geometric parameters (Å, º)
| C1—C2 | 1.3895 (15) | C6—C11 | 1.4094 (15) |
| C1—C5 | 1.3959 (14) | C6—C7 | 1.4283 (14) |
| C1—N2 | 1.4186 (13) | C7—N4 | 1.3484 (15) |
| C2—N1 | 1.3350 (14) | C7—C8 | 1.4148 (15) |
| C2—H2 | 0.9500 | C8—C9 | 1.3716 (17) |
| N1—C3 | 1.3397 (13) | C8—H8 | 0.9500 |
| C3—C4 | 1.3892 (15) | C9—C10 | 1.4035 (17) |
| C3—H3 | 0.9500 | C9—H9 | 0.9500 |
| C4—C5 | 1.3781 (15) | C10—C11 | 1.3703 (16) |
| C4—H4 | 0.9500 | C10—H10 | 0.9500 |
| C5—H5 | 0.9500 | C11—H11 | 0.9500 |
| N2—N3 | 1.2738 (12) | N4—H1N4 | 0.881 (16) |
| N3—C6 | 1.3958 (13) | N4—H2N4 | 0.904 (16) |
| C2—C1—C5 | 117.88 (10) | C11—C6—C7 | 119.42 (10) |
| C2—C1—N2 | 116.26 (9) | N4—C7—C8 | 119.78 (10) |
| C5—C1—N2 | 125.86 (10) | N4—C7—C6 | 122.88 (10) |
| N1—C2—C1 | 124.34 (10) | C8—C7—C6 | 117.34 (10) |
| N1—C2—H2 | 117.8 | C9—C8—C7 | 121.61 (10) |
| C1—C2—H2 | 117.8 | C9—C8—H8 | 119.2 |
| C2—N1—C3 | 116.94 (9) | C7—C8—H8 | 119.2 |
| N1—C3—C4 | 122.94 (10) | C8—C9—C10 | 120.92 (10) |
| N1—C3—H3 | 118.5 | C8—C9—H9 | 119.5 |
| C4—C3—H3 | 118.5 | C10—C9—H9 | 119.5 |
| C5—C4—C3 | 119.55 (10) | C11—C10—C9 | 118.89 (10) |
| C5—C4—H4 | 120.2 | C11—C10—H10 | 120.6 |
| C3—C4—H4 | 120.2 | C9—C10—H10 | 120.6 |
| C4—C5—C1 | 118.35 (10) | C10—C11—C6 | 121.81 (10) |
| C4—C5—H5 | 120.8 | C10—C11—H11 | 119.1 |
| C1—C5—H5 | 120.8 | C6—C11—H11 | 119.1 |
| N3—N2—C1 | 113.14 (9) | C7—N4—H1N4 | 119.2 (9) |
| N2—N3—C6 | 117.34 (9) | C7—N4—H2N4 | 117.8 (10) |
| N3—C6—C11 | 113.80 (9) | H1N4—N4—H2N4 | 119.8 (14) |
| N3—C6—C7 | 126.78 (10) | ||
| C5—C1—C2—N1 | 0.57 (16) | N3—C6—C7—N4 | 2.90 (17) |
| N2—C1—C2—N1 | −178.53 (10) | C11—C6—C7—N4 | −178.27 (10) |
| C1—C2—N1—C3 | 0.34 (16) | N3—C6—C7—C8 | −177.68 (9) |
| C2—N1—C3—C4 | −1.12 (16) | C11—C6—C7—C8 | 1.15 (15) |
| N1—C3—C4—C5 | 0.96 (17) | N4—C7—C8—C9 | 179.05 (10) |
| C3—C4—C5—C1 | 0.01 (16) | C6—C7—C8—C9 | −0.39 (15) |
| C2—C1—C5—C4 | −0.72 (15) | C7—C8—C9—C10 | −0.88 (17) |
| N2—C1—C5—C4 | 178.28 (10) | C8—C9—C10—C11 | 1.36 (17) |
| C2—C1—N2—N3 | 176.88 (9) | C9—C10—C11—C6 | −0.56 (17) |
| C5—C1—N2—N3 | −2.14 (15) | N3—C6—C11—C10 | 178.29 (9) |
| C1—N2—N3—C6 | −179.80 (8) | C7—C6—C11—C10 | −0.69 (16) |
| N2—N3—C6—C11 | −176.92 (9) | C1—N2—N3—C6 | −179.80 (8) |
| N2—N3—C6—C7 | 1.96 (15) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N4—H1N4···N2 | 0.881 (16) | 2.066 (15) | 2.6922 (14) | 127.3 (12) |
| N4—H2N4···N1i | 0.904 (16) | 2.163 (16) | 3.0274 (14) | 159.7 (14) |
Symmetry code: (i) −x+1/2, y−1, −z+3/2.
Funding Statement
This work was funded by Deutsche Forschungsgemeinschaft grant .
References
- Brandenburg, K. (2014). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Dommaschk, M., Näther, C. & Herges, R. (2015a). J. Org. Chem. 80, 8496–8500. [DOI] [PubMed]
- Dommaschk, M., Peters, M., Gutzeit, F., Schütt, C., Näther, C., Sönnichsen, F. D., Tiwari, S., Riedel, C., Boretius, S. & Herges, R. (2015b). J. Am. Chem. Soc. 137, 7552–7555. [DOI] [PubMed]
- Dommaschk, M., Thoms, V., Schütt, C., Näther, C., Puttreddy, R., Rissanen, K. & Herges, R. (2015c). Inorg. Chem. 54, 9390–9392. [DOI] [PubMed]
- Draguta, S., Fonari, M. S., Leonova, E. & Timofeeva, T. (2015). J. Mol. Struct. 1098, 206–215.
- Draguta, S., Leonova, E., Fokina, M., Denisyuk, I. & Timofeeva, T. V. (2013). Acta Cryst. E69, o1280. [DOI] [PMC free article] [PubMed]
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Hartley, G. S. (1937). Nature, 140, 281–281.
- Jinzi, Q., Jiaxing, Y., Haifu, F., Kailiang, S. & Shenghua, H. (1984). Acta Scient. Nat. Univ. Sunyatseni, 17, 2.
- Kailiang, S., Shenghua, H., Jinzi, Q., Jiaxing, Y. & Haifu, F. (1985). Chem. J. Chin. Univ. 6, 573.
- Kay, E. R., Leigh, D. A. & Zerbetto, F. (2007). Angew. Chem. Int. Ed. 46, 72–191. [DOI] [PubMed]
- Maiti, N., Dirghangi, B. K. & Chattopadhyay, S. (2003). Polyhedron, 22, 3109–3113.
- Maiti, N., Pal, S. & Chattopadhyay, S. (2001). Inorg. Chem. 40, 2204–2205. [DOI] [PubMed]
- Merino, E. & Ribagorda, M. (2012). Beilstein J. Org. Chem. 8, 1071–1090. [DOI] [PMC free article] [PubMed]
- Pratihar, J. L., Maiti, N. & Chattopadhyay, S. (2005). Inorg. Chem. 44, 6111–6114. [DOI] [PubMed]
- Pratihar, J. L., Shee, B., Pattanayak, P., Patra, D., Bhattacharyya, A., Puranik, V. G., Hung, C. H. & Chattopadhyay, S. (2007). Eur. J. Inorg. Chem. pp. 4272–4281.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. A71, 3–8.
- Stoe (2008). X-AREA. Stoe & Cie, Darmstadt, Germany.
- Szymański, W., Beierle, J. M., Kistemaker, H. A. V., Velema, W. A. & Feringa, B. L. (2013). Chem. Rev. 113, 6114–6178. [DOI] [PubMed]
- Tan, X., Xie, X., Chen, J. & Zhan, S. (2010). Inorg. Chem. Commun. 13, 1455–1458.
- Thies, S., Bornholdt, C., Köhler, F., Sönnichsen, F. D., Näther, C., Tuczek, F. & Herges, R. (2010). Chem. Eur. J. 16, 10074–10083. [DOI] [PubMed]
- Thies, S., Sell, H., Bornholdt, C., Schütt, C., Köhler, F., Tuczek, F. & Herges, R. (2012). Chem. Eur. J. 18, 16358–16368. [DOI] [PubMed]
- Thies, S., Sell, H., Schütt, C., Bornholdt, C., Näther, C., Tuczek, F. & Herges, R. (2011). J. Am. Chem. Soc. 133, 16243–16250. [DOI] [PubMed]
- Venkataramani, S., Jana, U., Dommaschk, M., Sönnichsen, F. D., Tuczek, F. & Herges, R. (2011). Science, 331, 445–448. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989018008605/wm5450sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989018008605/wm5450Isup2.hkl
Fig. S1. Experimental (top) and calculated (bottom) X-ray powder diffraction patterns for the title compound, measured with copper radiation.. DOI: 10.1107/S2056989018008605/wm5450sup3.tif
Fig. S2. UV-Vis spectra for the title compound.. DOI: 10.1107/S2056989018008605/wm5450sup4.tif
Supporting information file. DOI: 10.1107/S2056989018008605/wm5450Isup5.cml
CCDC reference: 1848833
Additional supporting information: crystallographic information; 3D view; checkCIF report





