The synthesis and crystal structure of a new N-substituted hydrazide are reported. In the crystal packing, O—H⋯O and N—H⋯O hydrogen bonds predominate together with π–π stacking interactions.
Keywords: crystal structure, N-substituted hydrazide, salicylic acid, Hirshfeld surface
Abstract
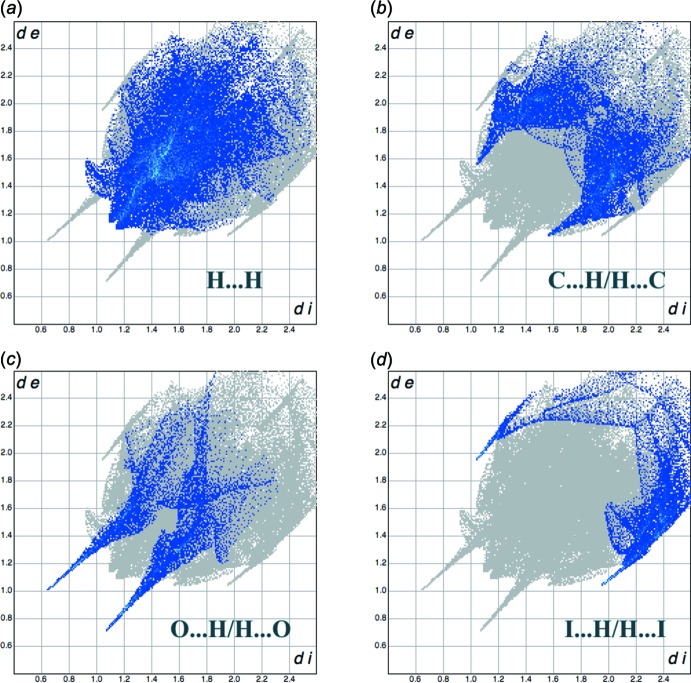
In the title compound, C15H14IN3O2·CH3OH, two aromatic rings are linked by an N-substituted hydrazide function. The dihedral angle between the aromatic rings is 10.53 (8)°. The stereochemistry about the imine function is E. The methanol molecule forms an O—H⋯O hydrogen bond to the hydrazide O atom. In the crystal, chains of molecules running along the c-axis direction are formed by O—H⋯O hydrogen bonds. Adjacent chains are linked through N—H⋯O hydrogen bonds and π–π stacking interactions. The intermolecular interactions in the crystal packing were investigated using Hirshfeld surface analysis, which indicated that the most significant contacts are H⋯H (38.2%), followed by C⋯H/H⋯C (20.6%), O⋯H/H⋯O (11.1%) and I⋯H/H⋯I (9.7%).
Chemical context
N-substituted hydrazides have been attracted much attention for their structures, coordination ability, biological activities and transformations to heterocyclic compounds (Majumdar et al., 2014 ▸; Asif & Husain, 2013 ▸; Khan et al., 2017 ▸). Derivatives of salicylic acid act as antibacterial (Kumar et al., 2012 ▸; Cui et al., 2014 ▸; Sarshira et al., 2016 ▸), antifungal (Wodnicka et al., 2017 ▸; Abbas et al., 2017 ▸) and antitumor (Murty et al., 2014 ▸) agents. In addition, some salicylhydrazones exhibit significant antitrypanosomal activity with IC50 ranging from 1 to 34 µM. N-substituted hydrazides containing the typical –C(O)—NH—N=C< functional group can be prepared by a condensation reaction between a hydrazide and a carbonyl compound (an aldehyde or a ketone).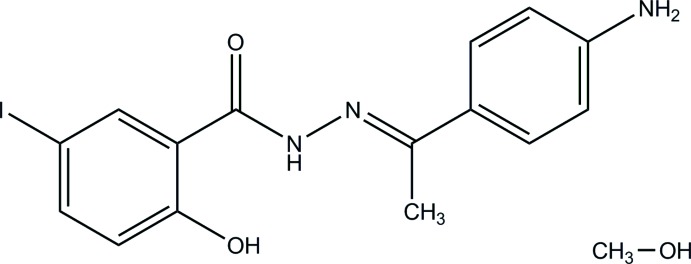
As a continuation of our research work to synthesize derivatives of 5-iodosalicylohydrazide (Nguyen et al., 2012 ▸), the new compound (E)-N’-[1-(4-aminophenyl)ethylidene]-2-hydroxy-5-iodobenzohydrazide methanol monosolvate was synthesized. The structure of the compound was determined by IR, 1H NMR, 13C NMR and HR–MS spectroscopy as well as X-ray diffraction and the crystal structure is reported herein.
Structural commentary
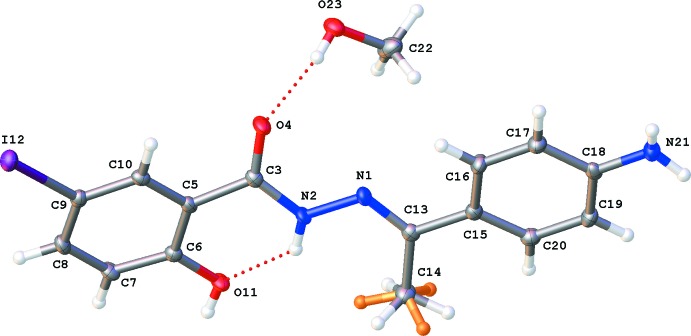
The title compound (Fig. 1 ▸) crystallizes as a methanol monosolvate in the monoclinic space group P21/c with one hydrazide molecule and a methanol solvate molecule in the asymmetric unit. The OH group of methanol is hydrogen bonded to the hydrazide oxygen atom O4 (Fig. 1 ▸, Table 1 ▸). The dihedral angle between the aromatic rings is 10.53 (8)°. This relatively planar character of the molecule is caused by an intramolecular hydrogen bond, N2—H2⋯O11 (Table 1 ▸), and the presence of the hydrazide functional group and the C13=N1 double bond. The r.m.s. deviation from a plane through all 21 non-Hatoms is 0.291 Å [with a maximum deviation of 0.838 (1) Å observed for atom O4]. The torsion angles about the bonds of the hydrazide link between the two aromatic rings are: C15—C13=N1—N2 = −175.48 (15)°, C13=N1—N2—C3 = 178.71 (16)° and N1—N2—C3—C5 = −172.18 (15)°. The stereochemistry about the imine function C13=N1 is E. The planar character causes short contacts for the H atoms of methyl group C14 with the H atoms on atoms N2 and C20. As a consequence, this methyl group displays rotational disorder with occupancies of 0.66 (2) and 0.34 (2).
Figure 1.
View of the asymmetric unit of the title compound, showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 50% probability level. H atoms are shown as small circles of arbitrary radii. Intra- and intermolecular hydrogen bonds are shown as dashed lines.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O23—H23⋯O4 | 0.80 (2) | 1.97 (2) | 2.7561 (18) | 170 (3) |
| N2—H2⋯O11 | 0.82 (3) | 2.02 (2) | 2.665 (2) | 134.4 (19) |
| O11—H11⋯O23i | 0.76 (3) | 1.88 (3) | 2.6323 (18) | 172 (2) |
| N21—H21A⋯O4ii | 0.85 (2) | 2.14 (2) | 2.961 (2) | 164 (2) |
Symmetry codes: (i) 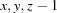 ; (ii)
; (ii) 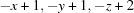 .
.
Supramolecular features
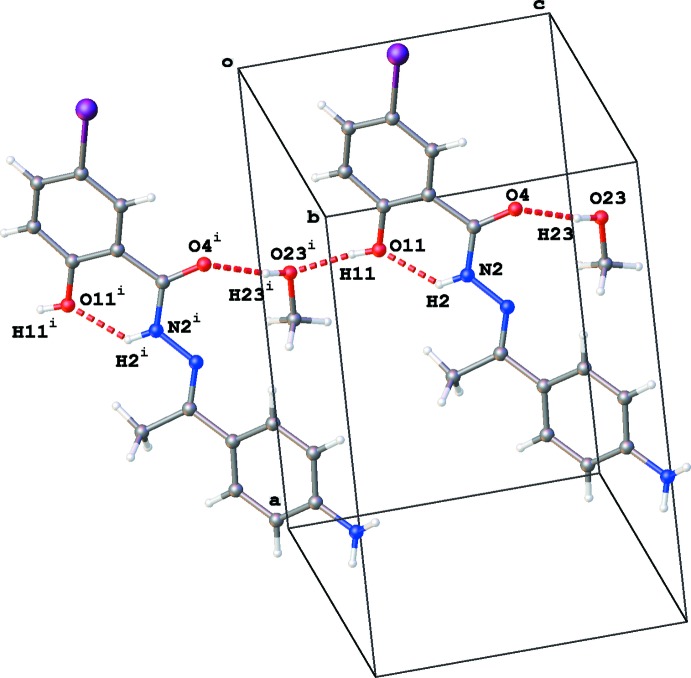
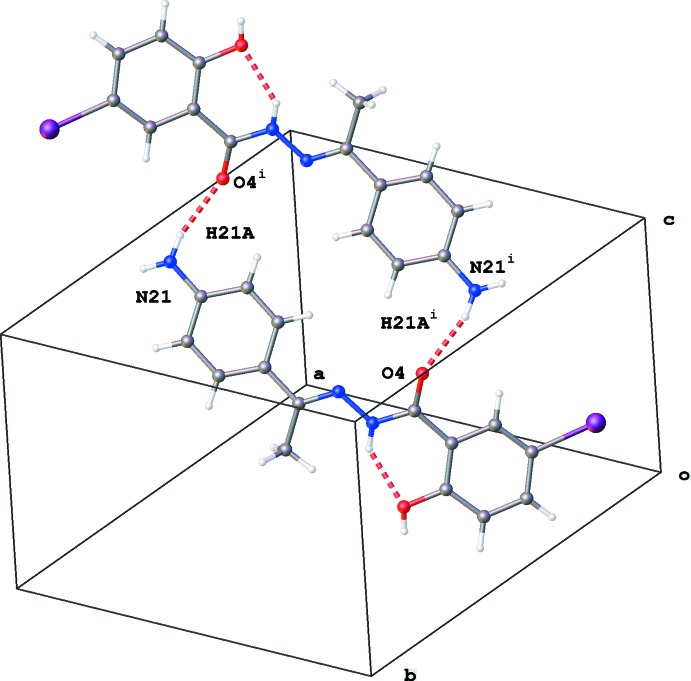
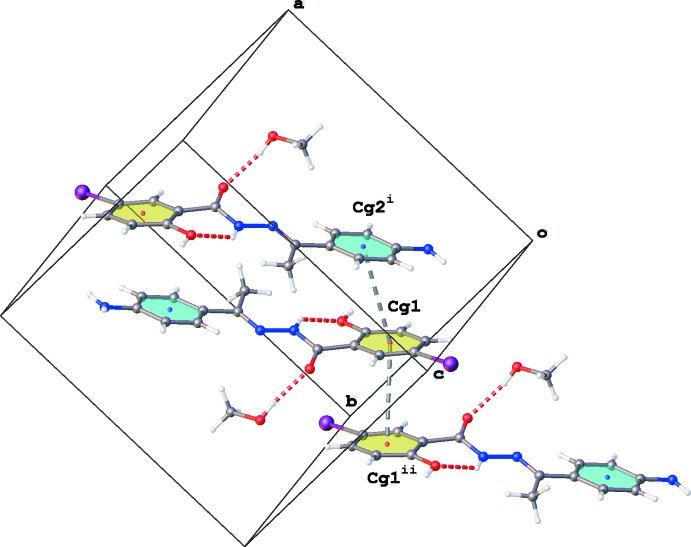
In the crystal, chains of molecules are formed along the c-axis direction by alternating O11—H11⋯O23i and O23—H23⋯O4 hydrogen bonds (Table 1 ▸ and Fig. 2 ▸). The interaction of adjacent chains through N21—H21A⋯O4ii hydrogen bonds results in the formation of dimers with graph set 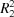 (22) (Table 1 ▸ and Fig. 3 ▸). Both aromatic rings are involved in π–π stacking interactions [Cg1⋯Cg1i = 3.9769 (10) Å, slippage 2.042 Å and Cg1⋯Cg2ii = 3.8635 (11) Å, slippage 1.596 Å; Cg1 and Cg2 are the centroids of rings C5–C10 and C15–C20, respectively; Fig. 4 ▸]. The crystal packing contains no voids.
(22) (Table 1 ▸ and Fig. 3 ▸). Both aromatic rings are involved in π–π stacking interactions [Cg1⋯Cg1i = 3.9769 (10) Å, slippage 2.042 Å and Cg1⋯Cg2ii = 3.8635 (11) Å, slippage 1.596 Å; Cg1 and Cg2 are the centroids of rings C5–C10 and C15–C20, respectively; Fig. 4 ▸]. The crystal packing contains no voids.
Figure 2.
Part of the crystal packing of the title compound, showing the chain along the c-axis direction formed by O—H⋯O hydrogen-bonding interactions [see Table 1 ▸; symmetry code: (i) x, y, z − 1]. Only the major component of the disordered methyl group C14 is shown.
Figure 3.
Ring of graph-set motif 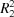 (22) formed by N—H⋯O hydrogen-bonding interactions [see Table 1 ▸; symmetry code: (i) x − 1, y − 1, z − 2].
(22) formed by N—H⋯O hydrogen-bonding interactions [see Table 1 ▸; symmetry code: (i) x − 1, y − 1, z − 2].
Figure 4.
Part of the crystal packing of the title compound, showing the π–π stacking interactions between the aminophenyl (blue) and iodophenyl (yellow) rings [symmetry codes: (i) −x + 1, −y + 1, −z + 1; (ii) −x, −y + 1, −z + 1].
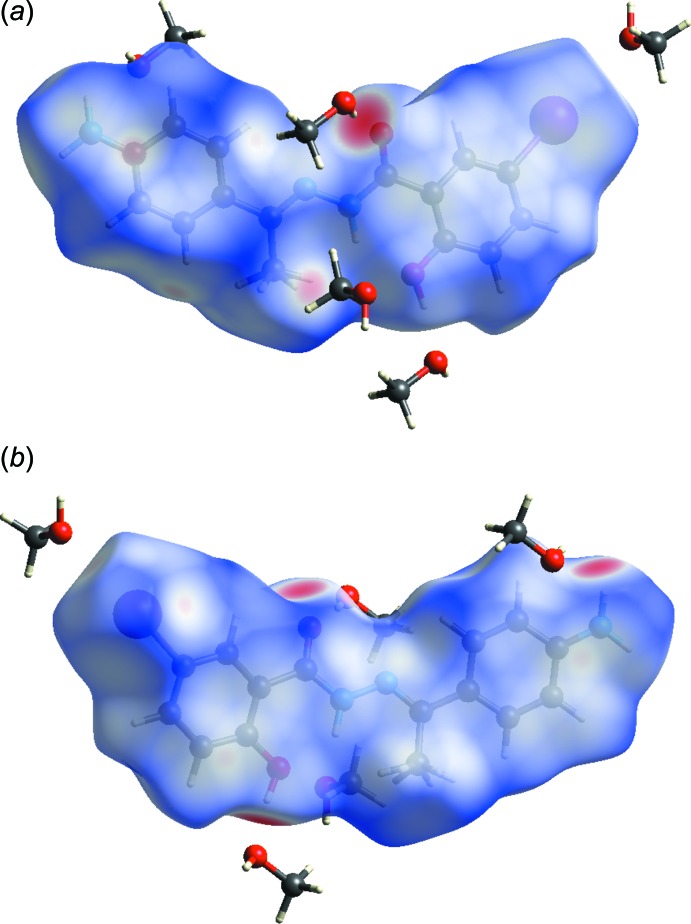
Additional insight into the crystal packing forces was obtained from a Hirshfeld surface analysis using CrystalExplorer (McKinnon et al., 2007 ▸; Spackman & Jayatilaka, 2009 ▸). The largest bright-red spots on the Hirshfeld surface mapped over d norm correspond to the (N,O)—H⋯O hydrogen-bonding contacts (Fig. 5 ▸). The pale-red spots are the weaker C⋯H (C18⋯H20), H⋯H (H14F⋯H22B), I⋯H (I12⋯H21B) and I⋯O (I12⋯O23) interactions. The most important 2D fingerprint plots, decomposed to highlight particular close contacts of atom pairs and their contribution, are given in Fig. 6 ▸. The relative contributions of the different intermolecular interactions to the Hirshfeld surface area in descending order are: H⋯H (38.2%), C⋯H/H⋯C (20.6%), O⋯H/H⋯O (11.1%), I⋯H/H⋯I (9.7%), N⋯H/H⋯N (7.2%) and C⋯C (5.7%). Contributions from the intermolecular non- or low-polar interactions are much greater than the contributions from the O⋯H contacts. The weak I⋯H interactions contribute significantly to the crystal packing.
Figure 5.
Views of the Hirshfeld surface for the title compound mapped over d norm over the range −0.740 to 1.296 a.u. showing the closest methanol molecules.
Figure 6.
Two-dimensional fingerprint plots delineated into different contact types (a)–(d) for the title compound. Each blue dot represents a 0.01 Å bin of points on the Hirshfeld surface, with coordinates corresponding to distances (Å) from the points to the nearest interior (d i) and exterior (d e) nuclei. Increasing intensity of overlapping points is shown by a colour coding from blue to cyan. The grey background contours correspond to the plot integrated for all contact types.
Database survey
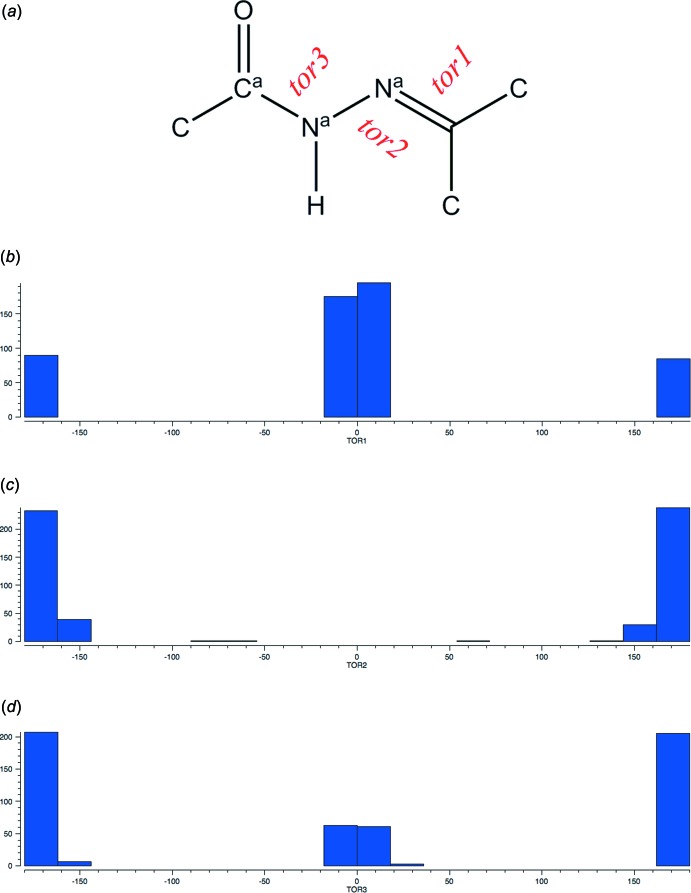
A search of the Cambridge Structural Database (CSD, Version 5.39, last update November 2017; Groom et al., 2016 ▸) for the central N-substituted hydrazide moiety (Fig. 7 ▸ a) resulted in 461 hits. The histograms of the torsion angles show the distribution for torsion angles tor1 (Fig. 7 ▸ b) and tor3 (Fig. 7 ▸ d) as expected for a planar conjugated system. However, the histogram of torsion angle tor2 (Fig. 7 ▸ c) shows the presence of three non-planar entries with torsion angle values of −72.1 (refcode XIJTAN; Buzykin et al., 2012 ▸), −67.9 (refcode NIZTUM; Muniz-Miranda et al., 2008 ▸) and +68.6° (XIJTAN; Buzykin et al., 2012 ▸).
Figure 7.
(a) The N-substituted hydrazide fragment used for a search in the CSD (a refers to acyclic). (b)–(d) Histograms of torsion angles tor1, tor2 and tor3, respectively.
Synthesis and crystallization
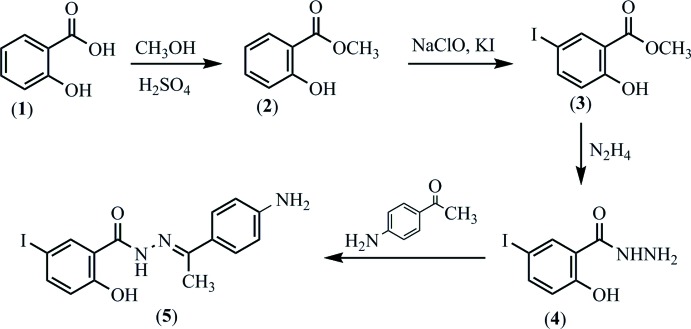
The reaction scheme used to synthesize the title compound, 5, is shown in Fig. 8 ▸. Methyl salicylate, methyl 2-hydroxy-5-iodobenzoate and 2-hydroxy-5-iodobenzohydrazide were prepared from salicylic acid according to the method described in our earlier work (Nguyen et al., 2012 ▸).
Figure 8.
Reaction scheme for the title compound.
Methyl salicylate, 2: liquid; b.p. 494-495 K, yield 73%.
Methyl 2-hydroxy-5-iodobenzoate (methyl 5-iodosalicylate), 3: white needles, m.p. 347–348 K, yield 85%; IR (ν, cm−1): 3156, 3080, 2949, 1676, 1604, 527.
2-Hydroxy-5-iodobenzohydrazide, 4: white needles, m.p. 451 K, yield 79%; IR (ν, cm−1): 3405, 3322, 1626, 1574, 529; 1H NMR (δ, ppm): 12.41 (1H, br, OH), 10.12 (1H, br, NH), 8.12 (1H, d, 4 J = 2.0, ArH), 7.65 (1H, dd, 3 J = 9.0 Hz, 4 J = 2.0 Hz, ArH), 6.75 (1H, d, 3 J = 9.0 Hz, ArH), 4.80 (2H, br, NH2); 13C NMR: 166.1 (CO), 158.9, 141.3, 135.5, 119.9, 117.4, 80.5.
(E)-N’-[1-(4-aminophenyl)ethylidene]-2-hydroxy-5-iodobenzohydrazide, 5: A solution of 2-hydroxy-5-iodobenzohydrazide 4 and 4′-aminoacetophenone was refluxed for 2 h. The reaction mixture was cooled down to room temperature and the precipitate obtained was filtered off and crystallized from methanol to give 5 as yellow crystals in 78% yield. M.p. 515–516 K. IR (ν, cm−1): 3440, 3298, 3201 (OH, N—H), 2932 (Csp 3—H), 1634, 1577 (C=O, C=N); 1H NMR (δ, ppm and J, Hz): 11.11 (1H, s, NH), 8.23 (1H, s, ArH), 7.70 (1H, d, 3 J = 8.5, ArH), 7.59 (2H, d, 3 J = 8.5, ArH), 6.86 (1H, d, 3 J = 8.5, ArH), 6.59 (2H, d, 3 J = 8.5, ArH), 5.55 (2H, br, NH2), 2.22 (3H, s, –CH3); 13C NMR (δ, ppm): 161.1 (C=O), 157.0, 154.8, 150.9, 141.6, 138.7, 128.3, 125.2, 121.0, 120.1, 113.7, 82.0, 14.1; MS: m/z 396.0069 (M+H)+, calculated for C15H15IN3O2: 396.0209.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. The H atoms attached to atoms N2, N21, O11 and O23 were found in a difference-Fourier map and refined freely. The other H atoms were placed at calculated positions and refined in riding mode, with C—H distances of 0.95 (aromatic) and 0.98 Å (CH3), and isotropic displacement parameters equal to 1.2U eq of the parent atoms (1.5U eq for CH3). The difference-Fourier map indicated disorder for the H atoms of methyl group C14. The final occupancy factors for the two sets of H atoms are 0.66 (2) and 0.34 (2). In the final cycles of refinement, two reflections showing very poor agreement were omitted as outliers.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C15H14IN3O2·CH4O |
| M r | 427.23 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 100 |
| a, b, c (Å) | 12.9877 (10), 14.8982 (10), 8.5593 (6) |
| β (°) | 91.806 (2) |
| V (Å3) | 1655.3 (2) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 1.95 |
| Crystal size (mm) | 0.41 × 0.27 × 0.22 |
| Data collection | |
| Diffractometer | Bruker D8 Quest CMOS |
| Absorption correction | Multi-scan (SADABS; Bruker, 2014 ▸) |
| T min, T max | 0.613, 0.746 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 45415, 3394, 3086 |
| R int | 0.044 |
| (sin θ/λ)max (Å−1) | 0.625 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.017, 0.041, 1.06 |
| No. of reflections | 3394 |
| No. of parameters | 231 |
| No. of restraints | 1 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.61, −0.24 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989018008204/sj5557sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989018008204/sj5557Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989018008204/sj5557Isup3.cml
CCDC reference: 1846971
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Crystal data
| C15H14IN3O2·CH4O | F(000) = 848 |
| Mr = 427.23 | Dx = 1.714 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 12.9877 (10) Å | Cell parameters from 9842 reflections |
| b = 14.8982 (10) Å | θ = 3.1–30.5° |
| c = 8.5593 (6) Å | µ = 1.95 mm−1 |
| β = 91.806 (2)° | T = 100 K |
| V = 1655.3 (2) Å3 | Block, yellow |
| Z = 4 | 0.41 × 0.27 × 0.22 mm |
Data collection
| Bruker D8 Quest CMOS diffractometer | 3086 reflections with I > 2σ(I) |
| φ and ω scans | Rint = 0.044 |
| Absorption correction: multi-scan (SADABS; Bruker, 2014) | θmax = 26.4°, θmin = 3.1° |
| Tmin = 0.613, Tmax = 0.746 | h = −16→16 |
| 45415 measured reflections | k = −18→18 |
| 3394 independent reflections | l = −10→10 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.017 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.041 | w = 1/[σ2(Fo2) + (0.0145P)2 + 1.4435P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.06 | (Δ/σ)max = 0.001 |
| 3394 reflections | Δρmax = 0.61 e Å−3 |
| 231 parameters | Δρmin = −0.24 e Å−3 |
| 1 restraint |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| N1 | 0.40946 (11) | 0.57600 (10) | 0.63743 (17) | 0.0163 (3) | |
| N2 | 0.33272 (11) | 0.55807 (10) | 0.52645 (18) | 0.0161 (3) | |
| H2 | 0.3379 (16) | 0.5699 (15) | 0.433 (3) | 0.023 (6)* | |
| C3 | 0.24587 (13) | 0.51777 (11) | 0.5728 (2) | 0.0148 (3) | |
| O4 | 0.22915 (10) | 0.50292 (9) | 0.71249 (14) | 0.0199 (3) | |
| C5 | 0.17050 (13) | 0.48847 (12) | 0.4474 (2) | 0.0140 (3) | |
| C6 | 0.16570 (13) | 0.52242 (11) | 0.2943 (2) | 0.0145 (3) | |
| C7 | 0.08776 (14) | 0.49318 (12) | 0.1909 (2) | 0.0170 (4) | |
| H7 | 0.081025 | 0.519641 | 0.090110 | 0.020* | |
| C8 | 0.01991 (14) | 0.42619 (12) | 0.2326 (2) | 0.0172 (4) | |
| H8 | −0.031928 | 0.405794 | 0.160228 | 0.021* | |
| C9 | 0.02861 (13) | 0.38912 (12) | 0.3817 (2) | 0.0150 (3) | |
| C10 | 0.10096 (13) | 0.42151 (12) | 0.48894 (19) | 0.0147 (3) | |
| H10 | 0.103575 | 0.398069 | 0.592210 | 0.018* | |
| O11 | 0.23620 (10) | 0.58419 (9) | 0.25082 (15) | 0.0182 (3) | |
| H11 | 0.233 (2) | 0.5920 (18) | 0.163 (3) | 0.042 (8)* | |
| I12 | −0.06829 (2) | 0.28322 (2) | 0.44470 (2) | 0.01876 (5) | |
| C13 | 0.49324 (13) | 0.61305 (12) | 0.5921 (2) | 0.0151 (3) | |
| C14 | 0.51380 (15) | 0.64301 (14) | 0.4276 (2) | 0.0217 (4) | |
| H14A | 0.454135 | 0.628694 | 0.359179 | 0.033* | 0.66 (2) |
| H14B | 0.574751 | 0.611872 | 0.390192 | 0.033* | 0.66 (2) |
| H14C | 0.525871 | 0.707940 | 0.426604 | 0.033* | 0.66 (2) |
| H14D | 0.506928 | 0.591666 | 0.356473 | 0.033* | 0.34 (2) |
| H14E | 0.583785 | 0.667320 | 0.423635 | 0.033* | 0.34 (2) |
| H14F | 0.464045 | 0.689520 | 0.395866 | 0.033* | 0.34 (2) |
| C15 | 0.57516 (13) | 0.62386 (12) | 0.7151 (2) | 0.0151 (3) | |
| C16 | 0.56846 (14) | 0.57802 (13) | 0.8582 (2) | 0.0187 (4) | |
| H16 | 0.510394 | 0.540934 | 0.875386 | 0.022* | |
| C17 | 0.64415 (14) | 0.58565 (12) | 0.9740 (2) | 0.0183 (4) | |
| H17 | 0.637198 | 0.554354 | 1.069845 | 0.022* | |
| C18 | 0.73134 (13) | 0.63918 (12) | 0.9518 (2) | 0.0156 (3) | |
| C19 | 0.73773 (14) | 0.68645 (12) | 0.8117 (2) | 0.0176 (4) | |
| H19 | 0.795006 | 0.724630 | 0.795497 | 0.021* | |
| C20 | 0.66115 (14) | 0.67820 (12) | 0.6957 (2) | 0.0175 (4) | |
| H20 | 0.667530 | 0.710410 | 0.600736 | 0.021* | |
| N21 | 0.80719 (13) | 0.64660 (12) | 1.06816 (19) | 0.0195 (3) | |
| H21A | 0.8080 (17) | 0.6075 (16) | 1.140 (3) | 0.025 (6)* | |
| H21B | 0.8644 (19) | 0.6701 (16) | 1.040 (3) | 0.028 (6)* | |
| C22 | 0.31350 (17) | 0.69396 (14) | 0.9344 (3) | 0.0285 (5) | |
| H22A | 0.294430 | 0.732318 | 0.845110 | 0.043* | |
| H22B | 0.380013 | 0.665329 | 0.916393 | 0.043* | |
| H22C | 0.318802 | 0.730503 | 1.029528 | 0.043* | |
| O23 | 0.23658 (11) | 0.62647 (9) | 0.95224 (15) | 0.0213 (3) | |
| H23 | 0.242 (2) | 0.5898 (15) | 0.885 (3) | 0.040 (8)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0147 (7) | 0.0195 (8) | 0.0143 (7) | −0.0010 (6) | −0.0037 (6) | −0.0009 (6) |
| N2 | 0.0166 (8) | 0.0217 (8) | 0.0096 (7) | −0.0024 (6) | −0.0033 (6) | 0.0005 (6) |
| C3 | 0.0170 (9) | 0.0125 (8) | 0.0147 (8) | 0.0020 (7) | −0.0012 (7) | −0.0008 (7) |
| O4 | 0.0229 (7) | 0.0257 (7) | 0.0109 (6) | −0.0068 (5) | −0.0023 (5) | 0.0014 (5) |
| C5 | 0.0140 (8) | 0.0150 (8) | 0.0128 (8) | 0.0023 (7) | −0.0015 (6) | −0.0025 (7) |
| C6 | 0.0163 (8) | 0.0131 (8) | 0.0142 (8) | 0.0010 (7) | 0.0009 (7) | −0.0009 (7) |
| C7 | 0.0216 (9) | 0.0175 (9) | 0.0117 (8) | 0.0021 (7) | −0.0031 (7) | 0.0013 (7) |
| C8 | 0.0166 (9) | 0.0193 (9) | 0.0154 (8) | 0.0010 (7) | −0.0048 (7) | −0.0025 (7) |
| C9 | 0.0135 (8) | 0.0141 (8) | 0.0175 (9) | 0.0002 (7) | 0.0009 (7) | −0.0018 (7) |
| C10 | 0.0166 (8) | 0.0155 (9) | 0.0118 (8) | 0.0031 (7) | −0.0001 (7) | 0.0004 (7) |
| O11 | 0.0225 (7) | 0.0215 (7) | 0.0104 (6) | −0.0055 (5) | −0.0012 (5) | 0.0029 (5) |
| I12 | 0.01767 (7) | 0.01657 (7) | 0.02193 (7) | −0.00289 (4) | −0.00078 (4) | 0.00057 (5) |
| C13 | 0.0166 (9) | 0.0131 (8) | 0.0155 (8) | 0.0020 (7) | 0.0000 (7) | −0.0006 (7) |
| C14 | 0.0206 (9) | 0.0279 (10) | 0.0166 (9) | −0.0017 (8) | −0.0009 (7) | 0.0053 (8) |
| C15 | 0.0143 (8) | 0.0154 (8) | 0.0155 (8) | 0.0018 (7) | 0.0000 (7) | −0.0009 (7) |
| C16 | 0.0154 (9) | 0.0216 (9) | 0.0190 (9) | −0.0051 (7) | 0.0002 (7) | 0.0021 (7) |
| C17 | 0.0180 (9) | 0.0210 (9) | 0.0160 (8) | −0.0022 (7) | 0.0009 (7) | 0.0026 (7) |
| C18 | 0.0149 (8) | 0.0157 (9) | 0.0162 (8) | 0.0027 (7) | −0.0006 (7) | −0.0050 (7) |
| C19 | 0.0150 (9) | 0.0169 (9) | 0.0210 (9) | −0.0027 (7) | 0.0012 (7) | 0.0005 (7) |
| C20 | 0.0187 (9) | 0.0172 (9) | 0.0168 (9) | 0.0007 (7) | 0.0022 (7) | 0.0017 (7) |
| N21 | 0.0170 (8) | 0.0230 (9) | 0.0182 (8) | −0.0038 (7) | −0.0019 (6) | 0.0006 (7) |
| C22 | 0.0291 (11) | 0.0251 (10) | 0.0318 (11) | −0.0050 (9) | 0.0107 (9) | −0.0034 (9) |
| O23 | 0.0277 (7) | 0.0223 (7) | 0.0140 (6) | −0.0043 (6) | 0.0014 (5) | −0.0005 (6) |
Geometric parameters (Å, º)
| N1—N2 | 1.381 (2) | C14—H14C | 0.9800 |
| N1—C13 | 1.291 (2) | C14—H14D | 0.9800 |
| N2—H2 | 0.82 (2) | C14—H14E | 0.9800 |
| N2—C3 | 1.349 (2) | C14—H14F | 0.9800 |
| C3—O4 | 1.242 (2) | C15—C16 | 1.407 (2) |
| C3—C5 | 1.495 (2) | C15—C20 | 1.394 (3) |
| C5—C6 | 1.404 (2) | C16—H16 | 0.9500 |
| C5—C10 | 1.399 (2) | C16—C17 | 1.379 (3) |
| C6—C7 | 1.394 (2) | C17—H17 | 0.9500 |
| C6—O11 | 1.359 (2) | C17—C18 | 1.403 (3) |
| C7—H7 | 0.9500 | C18—C19 | 1.396 (3) |
| C7—C8 | 1.386 (3) | C18—N21 | 1.383 (2) |
| C8—H8 | 0.9500 | C19—H19 | 0.9500 |
| C8—C9 | 1.392 (2) | C19—C20 | 1.388 (3) |
| C9—C10 | 1.380 (2) | C20—H20 | 0.9500 |
| C9—I12 | 2.0993 (17) | N21—H21A | 0.85 (2) |
| C10—H10 | 0.9500 | N21—H21B | 0.86 (2) |
| O11—H11 | 0.76 (3) | C22—H22A | 0.9800 |
| C13—C14 | 1.509 (2) | C22—H22B | 0.9800 |
| C13—C15 | 1.482 (2) | C22—H22C | 0.9800 |
| C14—H14A | 0.9800 | C22—O23 | 1.429 (2) |
| C14—H14B | 0.9800 | O23—H23 | 0.800 (16) |
| C13—N1—N2 | 118.21 (15) | C13—C14—H14A | 109.5 |
| N1—N2—H2 | 123.2 (15) | C13—C14—H14B | 109.5 |
| C3—N2—N1 | 118.42 (15) | C13—C14—H14C | 109.5 |
| C3—N2—H2 | 118.4 (15) | C13—C14—H14D | 109.5 |
| N2—C3—C5 | 116.98 (15) | C13—C14—H14E | 109.5 |
| O4—C3—N2 | 122.40 (16) | C13—C14—H14F | 109.5 |
| O4—C3—C5 | 120.59 (16) | C16—C15—C13 | 120.20 (16) |
| C6—C5—C3 | 125.00 (16) | C20—C15—C13 | 122.59 (16) |
| C10—C5—C3 | 116.05 (15) | C20—C15—C16 | 117.21 (16) |
| C10—C5—C6 | 118.94 (16) | C15—C16—H16 | 119.2 |
| H14Aa—C14—H14B | 109.5 | C17—C16—C15 | 121.57 (17) |
| H14Ba—C14—H14C | 109.5 | C17—C16—H16 | 119.2 |
| H14Aa—C14—H14C | 109.5 | C16—C17—H17 | 119.7 |
| H14Db—C14—H14E | 109.5 | C16—C17—C18 | 120.63 (17) |
| H14Eb—C14—H14F | 109.5 | C18—C17—H17 | 119.7 |
| H14Db—C14—H14F | 109.5 | C19—C18—C17 | 118.27 (16) |
| C7—C6—C5 | 119.36 (16) | N21—C18—C17 | 120.49 (17) |
| O11—C6—C5 | 119.30 (15) | N21—C18—C19 | 121.22 (17) |
| O11—C6—C7 | 121.33 (16) | C18—C19—H19 | 119.7 |
| C6—C7—H7 | 119.4 | C20—C19—C18 | 120.60 (17) |
| C8—C7—C6 | 121.12 (16) | C20—C19—H19 | 119.7 |
| C8—C7—H7 | 119.4 | C15—C20—H20 | 119.2 |
| C7—C8—H8 | 120.4 | C19—C20—C15 | 121.68 (17) |
| C7—C8—C9 | 119.19 (16) | C19—C20—H20 | 119.2 |
| C9—C8—H8 | 120.4 | C18—N21—H21A | 117.5 (16) |
| C8—C9—I12 | 120.11 (13) | C18—N21—H21B | 115.7 (15) |
| C10—C9—C8 | 120.35 (16) | H21A—N21—H21B | 119 (2) |
| C10—C9—I12 | 119.54 (13) | H22A—C22—H22B | 109.5 |
| C5—C10—H10 | 119.6 | H22A—C22—H22C | 109.5 |
| C9—C10—C5 | 120.78 (16) | H22B—C22—H22C | 109.5 |
| C9—C10—H10 | 119.6 | O23—C22—H22A | 109.5 |
| C6—O11—H11 | 111 (2) | O23—C22—H22B | 109.5 |
| N1—C13—C14 | 125.66 (16) | O23—C22—H22C | 109.5 |
| N1—C13—C15 | 115.19 (15) | C22—O23—H23 | 109.2 (19) |
| C15—C13—C14 | 119.13 (15) | ||
| N1—N2—C3—O4 | 5.9 (3) | C8—C9—C10—C5 | −3.6 (3) |
| N1—N2—C3—C5 | −172.18 (15) | C10—C5—C6—C7 | 4.3 (3) |
| N1—C13—C15—C16 | 13.9 (2) | C10—C5—C6—O11 | −176.74 (15) |
| N1—C13—C15—C20 | −166.49 (17) | O11—C6—C7—C8 | 175.98 (16) |
| N2—N1—C13—C14 | 3.0 (3) | I12—C9—C10—C5 | 176.29 (13) |
| N2—N1—C13—C15 | −175.48 (15) | C13—N1—N2—C3 | 178.71 (16) |
| N2—C3—C5—C6 | −20.8 (3) | C13—C15—C16—C17 | 179.02 (17) |
| N2—C3—C5—C10 | 158.64 (16) | C13—C15—C20—C19 | −179.11 (17) |
| C3—C5—C6—C7 | −176.25 (16) | C14—C13—C15—C16 | −164.75 (17) |
| C3—C5—C6—O11 | 2.7 (3) | C14—C13—C15—C20 | 14.9 (3) |
| C3—C5—C10—C9 | −179.52 (15) | C15—C16—C17—C18 | −0.6 (3) |
| O4—C3—C5—C6 | 161.03 (17) | C16—C15—C20—C19 | 0.6 (3) |
| O4—C3—C5—C10 | −19.5 (2) | C16—C17—C18—C19 | 1.9 (3) |
| C5—C6—C7—C8 | −5.1 (3) | C16—C17—C18—N21 | −179.92 (17) |
| C6—C5—C10—C9 | 0.0 (3) | C17—C18—C19—C20 | −2.0 (3) |
| C6—C7—C8—C9 | 1.5 (3) | C18—C19—C20—C15 | 0.8 (3) |
| C7—C8—C9—C10 | 2.9 (3) | C20—C15—C16—C17 | −0.6 (3) |
| C7—C8—C9—I12 | −177.01 (13) | N21—C18—C19—C20 | 179.84 (17) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O23—H23···O4 | 0.80 (2) | 1.97 (2) | 2.7561 (18) | 170 (3) |
| N2—H2···O11 | 0.82 (3) | 2.02 (2) | 2.665 (2) | 134.4 (19) |
| O11—H11···O23i | 0.76 (3) | 1.88 (3) | 2.6323 (18) | 172 (2) |
| N21—H21A···O4ii | 0.85 (2) | 2.14 (2) | 2.961 (2) | 164 (2) |
Symmetry codes: (i) x, y, z−1; (ii) −x+1, −y+1, −z+2.
Funding Statement
This work was funded by VLIR-UOS grant ZEIN2014Z182 to L. Van Meervelt.
References
- Abbas, D., Matter, A.-M., Sanaa, Q. B., Sattar, J. A. A.-S. & Ihsan, A. M. A.-A. (2017). World J. Pharm. Sci. 5, 25–28.
- Asif, M. & Husain, A. (2013). J. Appl. Chem. Article ID, 247203.
- Bruker (2013). SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2014). APEX2 and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Buzykin, B. I., Nabiullin, V. N., Mironova, E. V., Kostin, A. A., Tatarinov, D. A., Mironov, V. F. & Litvinov, I. A. (2012). Russ. J. Gen. Chem. 82, 1629–1645.
- Cui, Z., Ito, J., Dohi, H., Amemiya, Y. & Nishida, Y. (2014). PLoS One, 9, e108338. [DOI] [PMC free article] [PubMed]
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Khan, M. S., Siddiqui, S. P. & Tarannum, N. (2017). Hygeia. J. D. Med. 9, 61–79.
- Kumar, N. S., Amandoron, E. A., Cherkasov, A., Finlay, B. B., Gong, H., Jackson, L., Kaur, S., Lian, T., Moreau, A., Labrière, C., Reiner, N. E., See, R. H., Strynadka, N. C., Thorson, L., Wong, E. W., Worrall, L., Zoraghi, R. & Young, R. N. (2012). Bioorg. Med. Chem. 20, 7069–7082. [DOI] [PubMed]
- Majumdar, P., Pati, A., Patra, M., Behera, R. K. & Behera, A. K. (2014). Chem. Rev. 114, 2942–2977. [DOI] [PubMed]
- McKinnon, J. J., Jayatilaka, D. & Spackman, M. A. (2007). Chem. Commun. pp. 3814–3816. [DOI] [PubMed]
- Muniz-Miranda, M., Pagliai, M., Cardini, G., Messori, L., Bruni, B., Casini, A., Di Vaira, M. & Schettino, V. (2008). CrystEngComm, 10, 416–422.
- Murty, M. S. R., Penthala, R., Nath, L. R. & Anto, R. J. (2014). Lett. Drug. Des. Discov. 11, 1133–1142.
- Nguyen, T. C., Nguyen, Q. T., Nguyen, T. M. N. & Nguyen, T. C. (2012). Vietnam J. Chem. 50, 12–15.
- Sarshira, E. M., Hamada, N. M., Moghazi, Y. M. & Abdelrahman, M. M. (2016). J. Heterocycl. Chem. 53, 1970–1982.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Spackman, M. A. & Jayatilaka, D. (2009). CrystEngComm, 11, 19–32.
- Wodnicka, A., Huzar, E., Krawczyk, M. & Kwiecień, H. (2017). Pol. J. Chem. Technol. 19, 143–148.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989018008204/sj5557sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989018008204/sj5557Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989018008204/sj5557Isup3.cml
CCDC reference: 1846971
Additional supporting information: crystallographic information; 3D view; checkCIF report