Abstract
Calcitonin gene-related peptide (CGRP) has a potent protective action on the cardiovascular system; however, little is known about the role of CGRP in angiotensin II- (Ang II-) induced inflammation of vascular smooth muscle cells (VSMCs). This study is aimed at determining the anti-inflammatory effect of CGRP in Ang II-treated VSMCs and whether a disintegrin and metalloproteinase 17 (ADAM17) modulates this protective action. Small interference RNA (siRNA) and inhibitors of CGRP, epidermal growth factor receptor (EGFR), and extracellular signal-regulated kinase 1/2 (ERK1/2) were adopted to investigate their effect on Ang II-induced inflammation in VSMCs. Here, we found that CGRP could inhibit inflammation and decrease ADAM17 expression and activation of EGFR and ERK1/2 in VSMCs stimulated with Ang II. Results of siRNA demonstrated that ADAM17 siRNA attenuated Ang II-induced inflammation and up-regulation of activities of EGFR and ERK1/2 in VSMCs. Furthermore, the EGFR-ERK1/2 pathway promoted Ang II-induced VSMC inflammation. In summary, these findings identify the anti-inflammatory effect of CGRP in VSMCs stimulated by Ang II and suggest that ADAM17 is involved in the protective effect of CGRP against Ang II-induced inflammation via the EGFR-ERK1/2 pathway in VSMCs.
1. Introduction
Chronic vascular inflammation contributes to the initiation, development, and progression of a series of cardiovascular diseases including hypertension and atherosclerosis. Proinflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), and c-reactive protein (CRP) have been recognized as the markers of inflammation [1]. In addition to leukocytes, vascular smooth muscle cells (VSMCs) could be another crucial source of proinflammatory cytokines in the vessel wall [2, 3]. In vivo and in vitro studies show that angiotensin II (Ang II) induces the expression of proinflammatory cytokines in the vasculature such as IL-6, vascular cell adhesion molecule-1 (VCAM-1), and monocyte chemoattractant protein-1 (MCP-1) [2–4]. Thus, Ang II-mediated VSMC inflammation plays a key role in the development of cardiovascular diseases; however, its mechanisms remain to be incompletely elucidated.
Calcitonin gene-related peptide (CGRP), a 37-amino acid neuropeptide, is the most potent vasodilated vasodilator secreted by the sensory nerve terminal. Previous studies have demonstrated that CGRP could protect against cardiovascular diseases such as hypertension and heart failure [5–8]. Recently, CGRP is also considered as a critical proinflammatory neuropeptide in the pathophysiology of migraine [9]. Furthermore, macrophage infiltration and TNF-α production were elevated within the laser-induced lesions of choroidal neovascularization in CGRP (−/−) mice [10]. These results suggest the possibility that the anti-inflammatory effect of CGRP might play an important role in the development of cardiovascular diseases; nevertheless, little is known about the relation between CGRP and inflammation in VSMCs.
A disintegrin and metalloproteinase 17 (ADAM17), also known as tumor necrosis factor-α-converting enzyme (TACE), promotes cardiovascular remodeling that plays a crucial role in cardiovascular diseases [11–15]. This enzyme has essential functions in cell-cell interactions, in signaling, and in proteolysis of cytokines, cytokine receptors, and other targets. Activated ADAM17 could induce the release of TNF-α and soluble IL-6 receptor (IL-6R) that forms a complex by binding with IL-6 [1, 15, 16]. However, the role of ADAM17 in VSMC inflammation has not been determined, nor has it been elucidated whether ADAM17 regulates the protective effect of CGRP against Ang II-induced VSMC inflammation.
In this study, we establish that CGRP attenuates inflammation by decreasing the expression of IL-1β, IL-6, and TNF-ɑ in VSMCs treated with Ang II, and ADAM17 mediates this anti-inflammatory effect of CGRP through the EGFR-ERK1/2 pathway.
2. Methods and Materials
2.1. Cell Culture
Cell lines of VSMCs, derived from thoracic artery in rats, were originated from the ATCC cell bank (Manassas, VA, America). These cells were placed and cultured in normal condition (37°C, 5% carbon dioxide) with Dulbecco modified Eagle's medium (DMEM, Thermo Fisher, America) containing 10% fetal bovine serum (FBS). When VSMCs reach at about 80% confluence, these cells were needed to incubate for 24 hours in DMEM with 0.1% FBS before stimulation.
2.2. RNA Interference
Small interference RNA (siRNA) sequences against rat ADAM17 were synthesized by Shanghai GenePharma. The ADAM17 antisense sequence is 5′-ACUUCACACUGUA CUCGCUTT-3′, while the scrambled sequence was used as a negative control (NC). Small interference RNA (100 nmol/l) was transiently transfected into the cells via adding it to 5 μl Lipofectamine 2000 (Invitrogen, America) per 20 mm dish according to the manufacturer's instruction.
2.3. Western Blotting
Protocols of Western blotting were referred by our previous reference [17]. Antibodies were described as follows: ADAM17 antibody (Abcam, Britain), phosphor-EGFR (p-EGFR) (Abcam, Britain), EGFR (Abcam, America), phosphor-ERK1/2 (p-ERK1/2) (Abcam, Britain), ERK1/2 (Abcam, America), and Goat Anti-Rabbit IgG HRP (Affinity, America). Relative levels of immunoreactive proteins were detected by chemiluminescence and quantified with ImageJ software.
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
After cells were stimulated with Ang II for 24 hours, the conditioned medium was collected to detect the mature IL-1β, IL-6, and TNF-α released from VSMCs. ELISA kits (Cusabio, China) was used to determine the protein levels of IL-1β, IL-6, and TNF-α in culture media according to the manufacturer's instructions, briefly described as follows: First, the standard was reconstituted with 1.0 ml of sample diluent to produce a stock solution, used to produce a 2-fold dilution series, and the sample diluent served as the zero standard (0 pg/ml). After treating with the corresponding biotin antibody, HRP Avidin, and TMB substrate, these 2-fold dilution series were adopted to determine the optical density of each well within 5 minutes under 450 nm using an automatic enzyme-linked immunoadsorbent assay system. Second, the concentration of 2-fold dilution series were plotted against the corresponding optical density resulting in a standard curve used to determine the protein level of IL-1β, or IL-6, or TNF-α in the conditioned medium. Third, the same procedures were adopted to determine the optical density in the conditioned medium, and then the concentration of IL-1β, or IL-6, or TNF-α was calculated.
2.5. Real-Time Quantitative PCR
Total cellular RNA was prepared using TRIzol purchased from Takara according to the instruction. The reverse transcription system (Takara, Japan) was used to synthesize the first-strand complementary DNA. Real-time PCR used primers for ADAM17, IL-1β, IL-6, and TNF-α in accordance with the manufacturer's instruction (Takara, Japan), and the gene specific for GAPDH was chosen as an inner control. Primers for ADAM17 are 5′-GTGAGCAGTTTCTCGAACGC-3′ (forward primer) and 5′-AGCTTCTC AAGTCGCAGGTG-3′ (reverse primer); primer for IL-1β are 5-TCCTCTGTGACTCG TGGGAT-3 (forward primer) and 5′-TCAGACAGCACGAGGCATTT-3′ (reverse primer); primers for IL-6 are 5′-TCCTACCCCAACTTCCAATGCTC-3′ (forward primer) and 5′-TTG GATGGTCTTGGTCCTTA GCC-3′ (reverse primer); primers for TNF-α are 5′-TGGCGT GTTCATCCGTTCTC-3′ (forward primer) and 5′-CCCAGAGCCACAATTCCCTT-3′ (reverse primer); and primers for GADPH are 5′-ATCAAGAAGGTGGTGAAGCA-3′ (forward primer) and 5′-AAGGTGGAAGAATGG GAGTTG-3′ (reverse primer).
2.6. Statistical Analysis
Quantitative variables are presented as mean ± standard deviation (SD). Statistical significance between 2 means was performed by unpaired t-test, whereas those among more than 2 means with two independent variables were by two-way ANOVA with Bonferroni posttest. P values of <0.05 were considered to have statistical significance.
3. Results
3.1. CGRP Protected against Ang II-Induced Inflammation in VSMCs
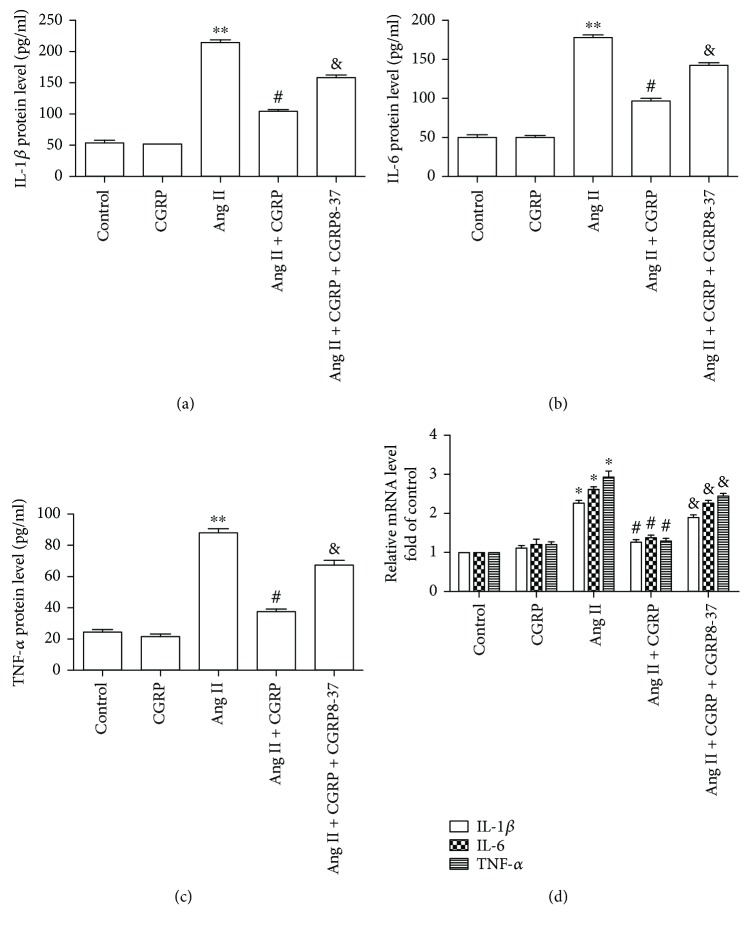
As shown in Figure 1, Ang II induced the release of IL-1β, IL-6, and TNF-α from VSMCs and the increase in mRNA levels of IL-1β, IL-6, and TNF-α compared with the control group. Pretreatment with CGRP inhibited Ang II-induced upregulation of protein release and mRNA levels of IL-1β, IL-6, and TNF-α, whereas these effects were canceled by CGRP antagonist CGRP 8-37. These data suggest that CGRP could attenuate Ang II-induced inflammation in VSMCs.
Figure 1.
CGRP dampened Ang II-induced inflammation in vascular smooth muscle cells. After pretreating with 10 nmol/l CGRP or/and 50 nmol/l CGRP8-37 for 30 minutes, vascular smooth muscle cells were stimulated with 100 nmol/l Ang II for 24 hours, then the medium was collected to assay for the concentration of IL-1β, IL-6, and TNF-α by ELISA, while the cells were adopted to measure the mRNA levels of IL-1β, IL-6, and TNF-α. (a–c) the protein levels of IL-1β (a), IL-6 (b), and TNF-α (c), n = 3–5 independent experiments. (d) The mRNA levels of IL-1β, IL-6, and TNF-α, n = 3 − 4 independent experiments. Ang II represents angiotensin II. ∗ P < 0.05 versus control, ∗∗ P < 0.01 versus control, # P < 0.05 versus Ang II, and & P < 0.05 versus Ang II + CGRP.
3.2. ADAM17 Mediated the Anti-Inflammatory Effect of CGRP
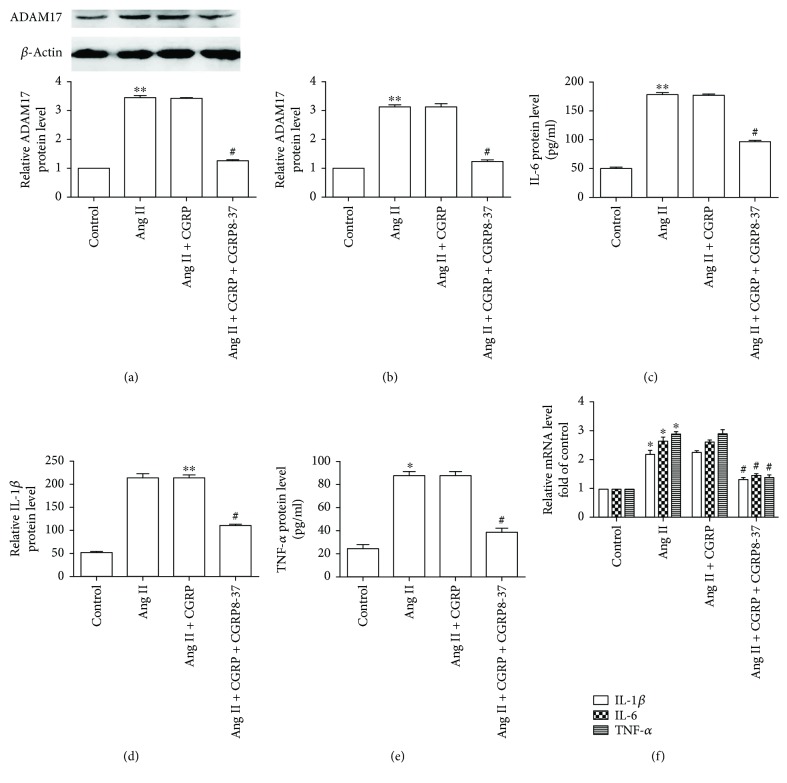
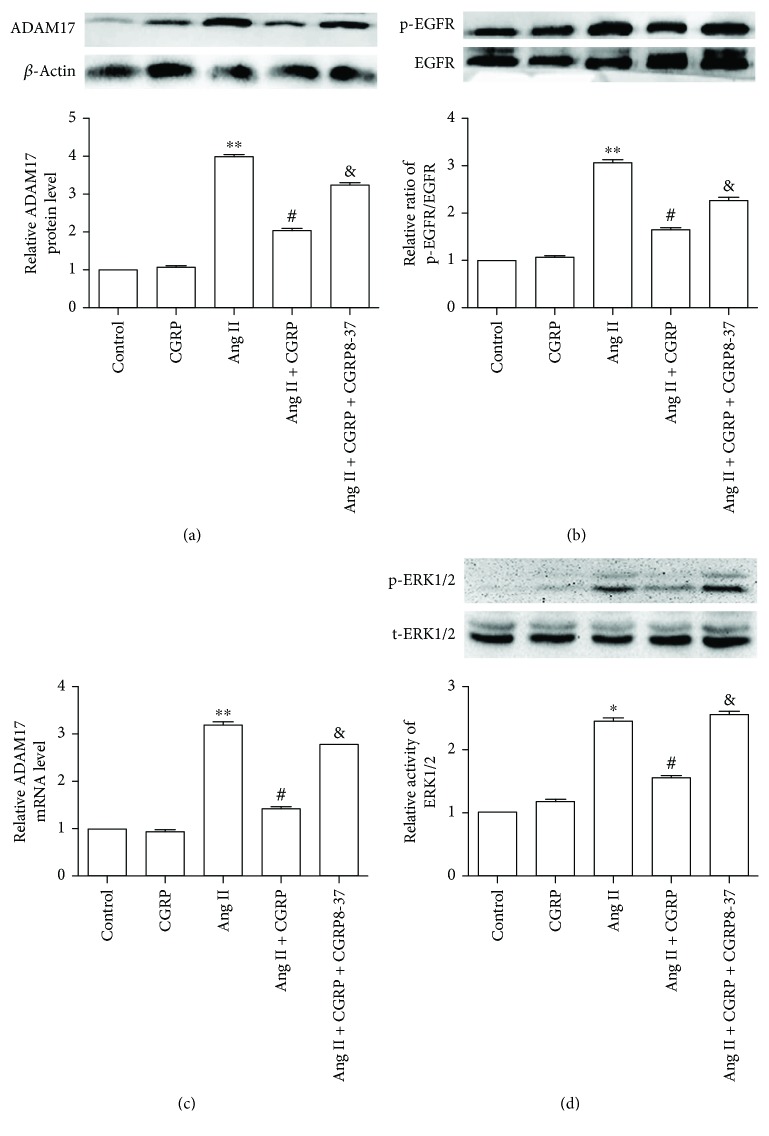
Technology of RNA interference was adopted to observe the effect of ADAM17 on Ang II-induced inflammation. The representative photos of VSMCs transfected with siRNA marked with green fluorescent protein are shown in Figure S1. As displayed in Figures 2(a) and 2(b), ADAM17 siRNA significantly reduced ADAM17 mRNA and protein levels in VSMCs, demonstrating the validity of the siRNA. Compared with the Ang II group, ADAM17 siRNA remarkably diminished the protein release and mRNA expression of IL-1β, IL-6, and TNF-α in VSMCs stimulated by Ang II (Figures 2(c)–2(f)). These results showed that ADAM17 promoted Ang II-induced VSMC inflammation. Moreover, CGRP could significantly decrease the ADAM17 protein and mRNA levels in VSMCs treated with Ang II for 24 hours (Figures 3(a) and 3(b)). Overall, ADAM17 modulated the protective effect of CGRP on Ang II-induced inflammation in VSMCs.
Figure 2.
ADAM17 siRNA attenuated Ang II-induced inflammation in vascular smooth muscle cells. After being transfected with 100 nmol/l ADAM17 siRNA for 30 hours, vascular smooth muscle cells were then stimulated with 100 nmol/l Ang II for 24 hours. (a) ADAM17 protein level, n = 4 independent experiments. (b) ADAM 17 mRNA level, n = 3 independent experiments. (c–e) At the end of the experiment, the medium was collected to detect the concentration of IL-1β, IL-6, and TNF-α by ELISA, n = 3–5 independent experiments. (c) IL-1β protein level. (d) IL-6 protein level. (e) TNF-α protein level. (f) The mRNA levels of IL-1β, IL-6, and TNF-α, n = 3 − 4 independent experiments. Ang II: angiotensin II; NC: negative control. ∗ P < 0.05 versus control, ∗∗ P < 0.01 versus control, and # P < 0.05 versus Ang II.
Figure 3.
CGRP reduced ADAM17 expression and activation of EGFR and ERK1/2 in vascular smooth muscle cells treated with Ang II. After being pretreated with 10 nmol/l CGRP or/and 10 nmol/l CGRP 8–37 for 30 minutes, vascular smooth muscle cells were treated with 100 nmol/l Ang II for 24 hours. (a) ADAM17 protein level, n = 4 independent experiments. (b) ADAM17 mRNA level, n = 3 independent experiments. (c) EGFR activity, n = 4 independent experiments. (d) ERK1/2 activity, n = 3 independent experiments. Ang II represents angiotensin II. ∗ P < 0.05 versus control, ∗∗ P < 0.01 versus control, # P < 0.05 versus Ang II, and & P < 0.05 versus Ang II + CGRP.
3.3. EGFR-ERK1/2 Signaling Pathway Modulated the Protective Action of CGRP against Ang II-Induced Inflammation in VSMCs
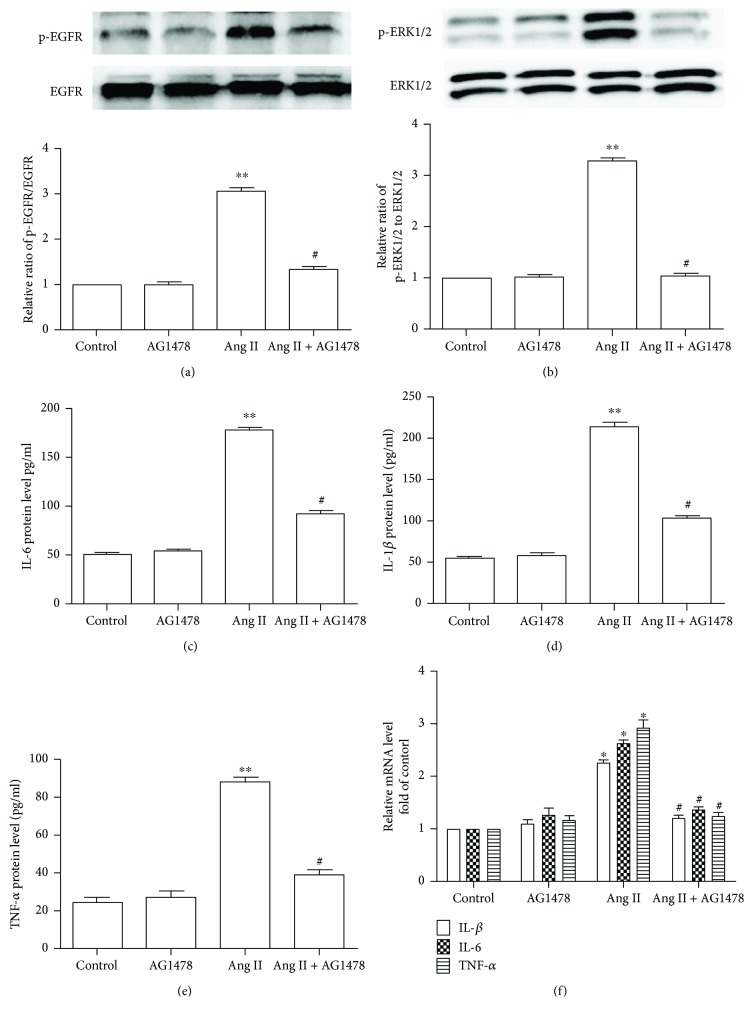
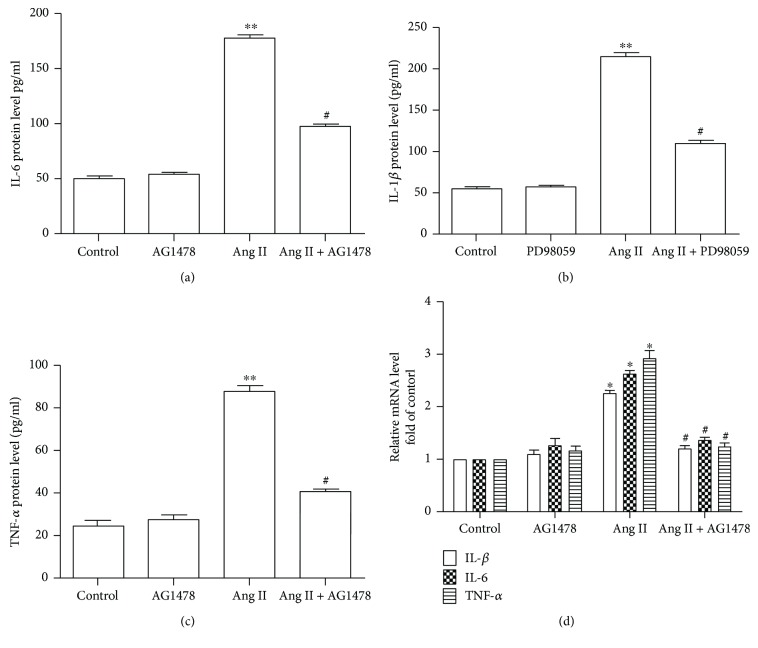
Pretreatment with CGRP for 30 minutes markedly decreased the phosphorylation levels of EGFR and ERK1/2 in VSMCs treated with Ang II for 24 hours, whereas CGRP8-37 canceled these effects (Figures 3(c) and 3(d)). Subsequently, EGFR inhibitor AG1478 and ERK1/2 antagonist PD98059 were used to test whether the EGFR-ERK1/2 pathway advanced Ang II-induced inflammation. Figure 4 shows that Ang II significantly elevated the protein release and mRNA levels of IL-1β, IL-6, and TNF-α and the ERK1/2 phosphorylation level compared with the control group, whereas pretreating with a selective EGFR inhibitor AG1478 for 30 minutes reversed these effects. Next, the results of ERK1/2 antagonist PD98059 intervention indicated that ERK1/2 activation promoted Ang II-induced elevation of protein release and mRNA levels of IL-1β, IL-6, and TNF-α in VSMCs (Figure 5). Collectively, CGRP alleviated Ang II-induced inflammation via inhibiting the EGFR-ERK1/2 pathway in VSMCs.
Figure 4.
EGFR activation is required in Ang II-induced inflammation in vascular smooth muscle cells. After being pretreated with selective EGFR inhibitor AG1478 (5 μmol/l) for 30 minutes, vascular smooth muscle cells were then stimulated with 100 nmol/l Ang II for 24 hours. (a) EGFR activity, n = 4 independent experiments. (b) ERK1/2 activity, n = 3 independent experiments. (c–e) At the end of the experiment, the medium was collected to detect the concentration of IL-1β, IL-6, and TNF-α by ELISA, n = 3–5 independent experiments. (c) IL-1β protein level. (d) IL-6 protein level. (e) TNF-α protein level. (f) The mRNA levels of IL-1β, IL-6, and TNF-α, n = 3 − 4 independent experiments. Ang II represents angiotensin II. ∗ P < 0.05 versus control, ∗∗ P < 0.01 versus control, and # P < 0.05 versus Ang II.
Figure 5.
ERK1/2 activation mediates Ang II-induced inflammation in vascular smooth muscle cells. After being pretreated with ERK1/2 inhibitor PD98059 (10 μmol/l) for 30 minutes, vascular smooth muscle cells were stimulated with 100 nmol/l Ang II for 24 hours. (a–c) At the end of the experiment, the medium was collected to detect the concentration of IL-1β, IL-6, and TNF-α by ELISA. (a–c) the protein levels of IL-1β (a), IL-6 (b), and TNF-α (c), n = 3–5 independent experiments. (d) The mRNA levels of IL-1β, IL-6, and TNF-α, n = 3-4 independent experiments. Ang II represents angiotensin II. ∗ P < 0.05 versus control, ∗∗ P < 0.01 versus control, and # P < 0.05 versus Ang II.
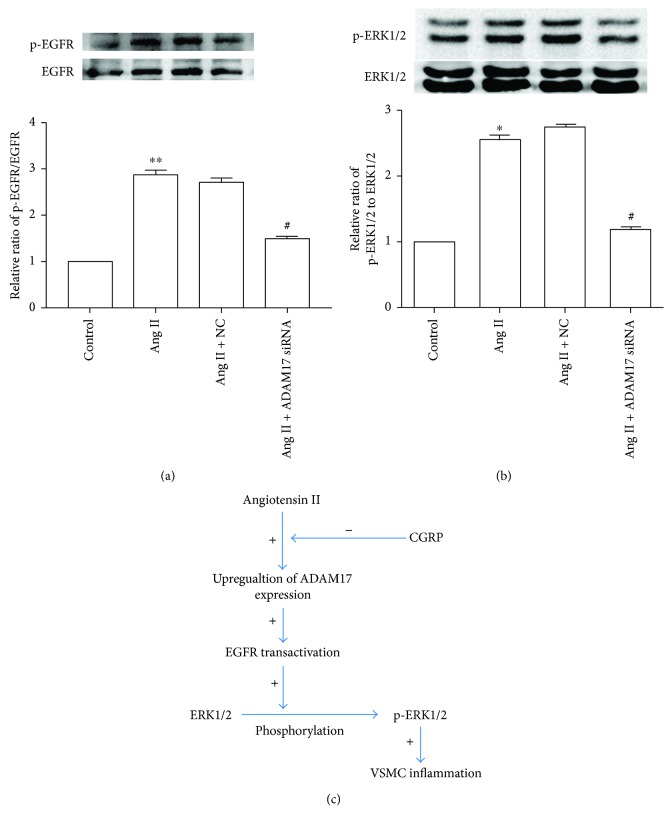
3.4. ADAM17 Is Required in the Activation of the EGFR-ERK1/2 Pathway Induced by Ang II
To determine the relationship of ADAM17 and activation of EGFR and ERK1/2, ADAM17 siRNA was used in the present study. As shown in Figure 6, Ang II-induced EGFR and ERK1/2 activation was attenuated by treatment of ADAM17 siRNA in VSMCs, suggesting that the activation of the EGFR-ERK1/2 pathway induced by Ang II is mediated by ADAM17.
Figure 6.
ADAM17 siRNA decreased Ang II-induced activation of EGFR and ERK1/2 in vascular smooth muscle cells. After being transfected with 100 nmol/l ADAM17 siRNA for 30 hours, vascular smooth muscle cells were then stimulated with 100 nmol/l Ang II for 24 hours. (a) EGFR activity, n = 4 independent experiments; (b) ERK1/2 activity, n = 3 independent experiments; (c) proposed model of ADAM17 in regulating the inhibitory effect of CGRP on Ang II-induced inflammation through the EGFR-ERK1/2 pathway. Ang II: angiotensin II; NC: negative control; +: positive effect; −: negative effect; VSMCs: vascular smooth muscle cells. ∗ P < 0.05 versus control, ∗∗ P < 0.01 versus control, and # P < 0.05 versus Ang II.
4. Discussion
The mechanisms of Ang II-mediated VSMC inflammation includes NF-κB activation and toll-like receptor 4 (TLR4) expression. NF-κB activation plays a central role in Ang II-induced IL-6 expression in VSMCs [18, 19]. In additional to NF-κB, Ang II-induced upregulation of IL-6 expression still requires cAMP response element-binding protein (CREB) and ERK-dependent histone acetylation mediated by p300 and steroid receptor coactivator-1 (SRC-1) [20]. Pretreatment with anti-TLR4 antibody, TLR4 inhibitor, or TLR4 siRNA prior to Ang II stimulation significantly diminished VSMC inflammation [21, 22]. In the present study, results show that Ang II induces inflammation of VSMCs by increasing ADAM17 expression and EGFR activation, presenting a new sight for understanding Ang II-induced VSMC inflammation.
EGFR transactivation plays a key role in Ang II-induced VSMC inflammation associated with cardiovascular diseases. EGFR transactivation has been implied in several cardiovascular conditions, including hypertension, heart failure, and cardiac and vascular hypertrophy [23–26]. It is reported that loss of vascular smooth muscle cell-EGFR increases mRNA levels of proinflammatory cytokines (such as MCP-1 and TNF-α) in the aorta, indicating that maintaining a certain activity of EGFR in vasculature is required in physiological conditions [27]. Our results showed that inhibition of EGFR could attenuate inflammation in VSMCs treated by Ang II. These results indicated that activation of EGFR might be a two-edged sword; excessive EGFR activation might produce harmful effects to cardiovasculature. EGFR deletion significantly decreases ERK1/2 phosphorylation level induced by endothelin 1 or α1-adrenoceptor or oxidative stress in VSMCs [28], which is inconsistent with our finding that EGFR activation could promote Ang II-induced ERK 1/2 activation. Moreover, our data also showed that inhibition of ERK1/2 inhibited Ang II-induced inflammation in VSMCs. Overall, the EGFR-ERK1/2 pathway plays an important role in Ang II-induced inflammation.
ADAM17 liberates and activates EGFR ligands from their membrane anchor, such as transforming growth factor-α (TGF-α), amphiregulin, and heparin-binding EGF-like growth factor (HB-EGF). A growing number of studies reported that ADAM17 induces EGFR transactivation in different types of cells such as vascular smooth muscle cell [29], cardiac cell [17], hepatic stellate cell [30], and endothelial cell [31], and the same effect was also observed in our results as well. Further, our findings suggested that ADAM17 siRNA decreased Ang II-induced inflammation and ERK1/2 activation in VSMCs. Therefore, ADAM17 induces Ang II-induced inflammation through the EGFR-ERK1/2 pathway in VSMCs.
CGRP, including α-CGRP and β-CGRP, has a potent protection for the cardiovascular system. The results of α-CGRP knockout and analogue demonstrate the protective action of α-CGRP against vascular remodeling in Ang II-induced hypertension [5, 8]. Our previous results showed that endogenous CGRP involves in the depressor effect and regression of vascular remodeling of losartan or perindopril in 2-kidney, 1-clip hypertensive rats [32]. The inhibitory effect of CGRP on VSMC proliferation constitutes the basis of the protective action of CGRP against vascular remodeling [33, 34], and the mechanisms include ERK1/2 activation, P53, and cAMP/protein kinase A (PKA) [33, 35] Our findings showed that CGRP inhibited Ang II-induced inflammation in VSMCs, providing a new insight in understanding the protective action of CGRP against Ang II-induced vascular remodeling. Further research, however, is still needed to elucidate about the mechanisms of anti-inflammatory action of CGRP in VSMCs stimulated with Ang II. In the present study, indirect evidences showed that ADAM17 negatively modulated the protective effect of CGRP against Ang II-induced inflammation through the EGFR-ERK1/2 pathway.
5. Conclusions
In summary, we identify the anti-inflammatory effect of CGRP on Ang II-induced VSMC inflammation, and indirect evidences indicate that ADAM17 mediates the protective effect of CGRP against Ang II-induced inflammation through the EGFR-ERK1/2 pathway in VSMCs (Figure 6(c)). This will help us better understand the mechanisms of VSMCs inflammation and how CGRP protects against cardiovascular disease resulting from the overactivation of the rennin-angiotensin system, providing further basis for ADAM17 and CGRP as potential targets against vascular inflammation and cardiovascular diseases. Nevertheless, further research is still needed to demonstrate these arguments in CGRP−/− animals and elucidate how CGRP mediates ADAM17 expression.
Acknowledgments
This work was supported by grants from Natural Science Foundation of Guangdong Province (no. 2015A030310076), Science and Technology Planning Project of Guangdong Province (no. 2016A020226005), Medical Scientific Research Foundation of Guangdong Province (no. A2014159), and Youth Foundation of the Guangdong Second Provincial General Hospital (no. YZ2015-008).
Contributor Information
Si-yu Zeng, Email: cosmo81@qq.com.
Xu-ping Qin, Email: qinxp333@hotmail.com.
Data Availability
All data used to support the findings of this study are available from the corresponding authors upon request.
Conflicts of Interest
The authors have no conflict of interests to disclose.
Supplementary Materials
Figure S1: representative microphotographs of vascular smooth muscle cells transfected with siRNA after being transfected with 100 nmol/l siRNA marked with green fluorescent protein for 12 hours. The photos of these cells were taken using a fluorescence microscope under 40× amplification.
References
- 1.Pacurari M., Kafoury R., Tchounwou P. B., Ndebele K. The renin-angiotensin-aldosterone system in vascular inflammation and remodeling. International Journal of Inflammation. 2014;2014:13. doi: 10.1155/2014/689360.689360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X. L., Tummala P. E., Olbrych M. T., Alexander R. W., Medford R. M. Angiotensin II induces monocyte chemoattractant protein-1 gene expression in rat vascular smooth muscle cells. Circulation Research. 1998;83(9):952–959. doi: 10.1161/01.RES.83.9.952. [DOI] [PubMed] [Google Scholar]
- 3.Kranzhofer R., Schmidt J., Pfeiffer C. A. H., Hagl S., Libby P., Kubler W. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19(7):1623–1629. doi: 10.1161/01.atv.19.7.1623. [DOI] [PubMed] [Google Scholar]
- 4.Tummala P. E., Chen X. L., Sundell C. L., et al. Angiotensin II induces vascular cell adhesion molecule-1 expression in rat vasculature: a potential link between the renin-angiotensin system and atherosclerosis. Circulation. 1999;100(11):1223–1229. doi: 10.1161/01.CIR.100.11.1223. [DOI] [PubMed] [Google Scholar]
- 5.Smillie S. J., King R., Kodji X., et al. An ongoing role of α-calcitonin gene-related peptide as part of a protective network against hypertension, vascular hypertrophy, and oxidative stress. Hypertension. 2014;63(5):1056–1062. doi: 10.1161/HYPERTENSIONAHA.113.02517. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z., Martorell B. C., Wälchli T., et al. Calcitonin gene-related peptide (CGRP) receptors are important to maintain cerebrovascular reactivity in chronic hypertension. PLoS One. 2015;10(4, article e0123697) doi: 10.1371/journal.pone.0123697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smillie S. J., Brain S. D. Calcitonin gene-related peptide (CGRP) and its role in hypertension. Neuropeptides. 2011;45(2):93–104. doi: 10.1016/j.npep.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Aubdool A. A., Thakore P., Argunhan F., et al. A novel α-calcitonin gene-related peptide analogue protects against end-organ damage in experimental hypertension, cardiac hypertrophy, and heart failure clinical perspective. Circulation. 2017;136(4):367–383. doi: 10.1161/CIRCULATIONAHA.117.028388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lukács M., Tajti J., Fülöp F., Toldi J., Edvinsson L., Vécsei L. Migraine, neurogenic inflammation, drug development - pharmacochemical aspects. Current Medicinal Chemistry. 2017;24(33):3649–3665. doi: 10.2174/0929867324666170712163437. [DOI] [PubMed] [Google Scholar]
- 10.Toriyama Y., Iesato Y., Imai A., et al. Pathophysiological function of endogenous calcitonin gene-related peptide in ocular vascular diseases. American Journal of Pathology. 2015;185(6):1783–1794. doi: 10.1016/j.ajpath.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Takayanagi T., Forrester S. J., Kawai T., et al. Vascular ADAM17 as a novel therapeutic target in mediating cardiovascular hypertrophy and perivascular fibrosis induced by angiotensin II novelty and significance. Hypertension. 2016;68(4):949–955. doi: 10.1161/HYPERTENSIONAHA.116.07620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan D., Takawale A., Shen M., et al. A disintegrin and metalloprotease-17 regulates pressure overload-induced myocardial hypertrophy and dysfunction through proteolytic processing of integrin β1. Hypertension. 2016;68(4):937–948. doi: 10.1161/HYPERTENSIONAHA.116.07566. [DOI] [PubMed] [Google Scholar]
- 13.Takaguri A., Kimura K., Hinoki A., Bourne A. M., Autieri M. V., Eguchi S. A disintegrin and metalloprotease 17 mediates neointimal hyperplasia in vasculature. Hypertension. 2011;57(4):841–845. doi: 10.1161/HYPERTENSIONAHA.110.166892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen M., Morton J., Davidge S. T., Kassiri Z. Loss of smooth muscle cell disintegrin and metalloproteinase 17 transiently suppresses angiotensin II-induced hypertension and end-organ damage. Journal of Molecular and Cellular Cardiology. 2017;103:11–21. doi: 10.1016/j.yjmcc.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Wang X., Oka T., Chow F. L., et al. Tumor necrosis factor-α-converting enzyme is a key regulator of agonist-induced cardiac hypertrophy and fibrosis. Hypertension. 2009;54(3):575–582. doi: 10.1161/HYPERTENSIONAHA.108.127670. [DOI] [PubMed] [Google Scholar]
- 16.Rose-John S. The soluble interleukin 6 receptor: advanced therapeutic options in inflammation. Clinical Pharmacology & Therapeutics. 2017;102(4):591–598. doi: 10.1002/cpt.782. [DOI] [PubMed] [Google Scholar]
- 17.Zeng S. Y., Chen X., Chen S. R., et al. Upregulation of Nox4 promotes angiotensin II-induced epidermal growth factor receptor activation and subsequent cardiac hypertrophy by increasing ADAM17 expression. Canadian Journal of Cardiology. 2013;29(10):1310–1319. doi: 10.1016/j.cjca.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 18.Funakoshi Y., Ichiki T., Ito K., Takeshita A. Induction of interleukin-6 expression by angiotensin II in rat vascular smooth muscle cells. Hypertension. 1999;34(1):118–125. doi: 10.1161/01.HYP.34.1.118. [DOI] [PubMed] [Google Scholar]
- 19.Han Y., Runge M. S., Brasier A. R. Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-κb transcription factors. Circulation Research. 1999;84(6):695–703. doi: 10.1161/01.RES.84.6.695. [DOI] [PubMed] [Google Scholar]
- 20.Sahar S., Reddy M. A., Wong C., Meng L., Wang M., Natarajan R. Cooperation of SRC-1 and p300 with NF-κB and CREB in angiotensin II-induced IL-6 expression in vascular smooth muscle cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(7):1528–1534. doi: 10.1161/ATVBAHA.107.145862. [DOI] [PubMed] [Google Scholar]
- 21.Ji Y., Liu J., Wang Z., Liu N. Angiotensin II induces inflammatory response partly via toll-like receptor 4-dependent signaling pathway in vascular smooth muscle cells. Cellular Physiology and Biochemistry. 2009;23(4–6):265–276. doi: 10.1159/000218173. [DOI] [PubMed] [Google Scholar]
- 22.Ji Y. Y., Liu J. T., Liu N., Wang Z. D., Liu C. H. PPARα activator fenofibrate modulates angiotensin II-induced inflammatory responses in vascular smooth muscle cells via the TLR4-dependent signaling pathway. Biochemical Pharmacology. 2009;78(9):1186–1197. doi: 10.1016/j.bcp.2009.06.095. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Patron C. Therapeutic potential of the epidermal growth factor receptor transactivation in hypertension: a convergent signaling pathway of vascular tone, oxidative stress, and hypertrophic growth downstream of vasoactive G-protein- coupled receptors? Canadian Journal of Physiology and Pharmacology. 2007;85(1):97–104. doi: 10.1139/y06-097. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y., Henning R. H., Sandovici M., van der Want J. J., van Gilst W. H., Buikema H. Enhanced myogenic constriction of mesenteric artery in heart failure relates to decreased smooth muscle cell caveolae numbers and altered AT1- and epidermal growth factor-receptor function. European Journal of Heart Failure. 2009;11(3):246–255. doi: 10.1093/eurjhf/hfn027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kagiyama S., Eguchi S., Frank G. D., Inagami T., Zhang Y. C., Phillips M. I. Angiotensin II-induced cardiac hypertrophy and hypertension are attenuated by epidermal growth factor receptor antisense. Circulation. 2002;106:909–912. doi: 10.1161/01.cir.0000030181.63741.56. [DOI] [PubMed] [Google Scholar]
- 26.Takayanagi T., Kawai T., Forrester S. J., et al. Role of epidermal growth factor receptor and endoplasmic reticulum stress in vascular remodeling induced by angiotensin II. Hypertension. 2015;65(6):1349–1355. doi: 10.1161/HYPERTENSIONAHA.115.05344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schreier B., Rabe S., Schneider B., et al. Loss of epidermal growth factor receptor in vascular smooth muscle cells and cardiomyocytes causes arterial hypotension and cardiac hypertrophy. Hypertension. 2013;61(2):333–340. doi: 10.1161/HYPERTENSIONAHA.112.196543. [DOI] [PubMed] [Google Scholar]
- 28.Schreier B., Dohler M., Rabe S., et al. Consequences of epidermal growth factor receptor (ErbB1) loss for vascular smooth muscle cells from mice with targeted deletion of ErbB1. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(7):1643–1652. doi: 10.1161/ATVBAHA.111.223537. [DOI] [PubMed] [Google Scholar]
- 29.Ohtsu H., Dempsey P. J., Frank G. D., et al. ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26(10):2208–2137. doi: 10.1161/01.ATV.0000236203.90331.d0. [DOI] [PubMed] [Google Scholar]
- 30.Oikawa H., Maesawa C., Tatemichi Y., et al. A disintegrin and metalloproteinase 17 (ADAM17) mediates epidermal growth factor receptor transactivation by angiotensin II on hepatic stellate cells. Life Sciences. 2014;97(2):137–144. doi: 10.1016/j.lfs.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 31.Maretzky T., Evers A., Zhou W., et al. Migration of growth factor-stimulated epithelial and endothelial cells depends on EGFR transactivation by ADAM17. Nature Communications. 2011;2, article 229 doi: 10.1038/ncomms1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin X. P., Ye F., Liao D. F., Li Y. J. Involvement of calcitonin gene-related peptide in the depressor effects of losartan and perindopril in rats. European Journal of Pharmacology. 2003;464(1):63–67. doi: 10.1016/S0014-2999(03)01370-0. [DOI] [PubMed] [Google Scholar]
- 33.QIN X., YE F., HU C., LIAO D., DENG H., LI Y. Effect of calcitonin gene-related peptide on angiotensin II-induced proliferation of rat vascular smooth muscle cells. European Journal of Pharmacology. 2004;488(1–3):45–49. doi: 10.1016/j.ejphar.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Fang L., Chen M. F., Xiao Z. L., et al. Calcitonin gene-related peptide released from endothelial progenitor cells inhibits the proliferation of rat vascular smooth muscle cells induced by angiotensin II. Molecular and Cellular Biochemistry. 2011;355(1-2):99–108. doi: 10.1007/s11010-011-0843-0. [DOI] [PubMed] [Google Scholar]
- 35.Chattergoon N. N., D'Souza F. M., Deng W., et al. Antiproliferative effects of calcitonin gene-related peptide in aortic and pulmonary artery smooth muscle cells. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2005;288(1):L202–L211. doi: 10.1152/ajplung.00064.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: representative microphotographs of vascular smooth muscle cells transfected with siRNA after being transfected with 100 nmol/l siRNA marked with green fluorescent protein for 12 hours. The photos of these cells were taken using a fluorescence microscope under 40× amplification.
Data Availability Statement
All data used to support the findings of this study are available from the corresponding authors upon request.