Abstract
In a prospective cohort study, we tested the hypothesis that children with sickle cell anemia (SCA) with normal transcranial Doppler ultrasound (TCD) velocities and without silent cerebral infarcts (SCIs) would have a lower incidence rate of new neurological events (strokes, seizures or transient ischemic attacks) compared to children with normal TCD measurements and SCIs, not receiving regular blood transfusions.
Non-randomized participants from the Silent Cerebral Infarct Transfusion (SIT) Trial who had screening magnetic resonance imaging (MRI) of the brain and normal TCD measurements were included. Follow-up ended at the time of first neurological event, start of regular blood transfusion, or loss to follow-up, whichever came first. The primary endpoint was a new neurological event.
Of 421 participants included, 68 had suspected SCIs. Mean follow-up was 3.6 years. Incidence rates of new neurological events in non-transfused participants with normal TCD values with SCIs and without SCIs were 1.71 and 0.47 neurological events per 100 patient-years, respectively, p=0.065. The absence of SCI(s) at baseline was associated with a decreased risk of a new neurological event (hazard ratio 0.231, 95% CI 0.062 – 0.858; p=0.029). Local pediatric neurologists examined 67 of 68 participants with suspected SCIs and identified 2 with overt strokes classified as SCIs by local hematologists; subsequently one had a seizure and the other an ischemic stroke. Children with SCA, without SCIs, and normal TCD measurements have a significantly lower rate of new neurological events when compared to those with SCIs and normal TCD measurements. Pediatric neurology assessment may assist risk stratification.
Keywords: sickle cell disease, stroke, silent cerebral infarct, transcranial Doppler ultrasound
Introduction
Strokes and other neurological complications in sickle cell anemia (SCA) are a major cause of morbidity in children living in high and low resource settings.1 Strokes are still a major risk factor for death in children living in low resource settings, where primary and secondary stroke prevention strategies are not part of routine care.2,3 Completion of six clinical trials in children with SCA has clearly identified the group of children with the highest risk of initial and recurrent strokes.4–8 A pooled analysis of stroke recurrence in children with initial overt strokes not receiving any treatment for secondary prevention showed that they have the highest rate of future strokes, 29 events per 100 patient-years.9 Children with elevated transcranial Doppler ultrasound (TCD) velocities who do not receive regular long-term blood transfusions have the highest incidence of initial strokes, 10.7 per 100 patient-years.10 No cohort of children with SCA has been identified with the lowest stroke incidence rate. Specifically, no multi-center prospective cohort study of children has determined whether children with a normal TCD measurement (time averaged mean maximum velocity of < 200 cm/second, non-imaging or <185 cm/second, imaging technique) and absence of silent cerebral infarcts (SCIs), have a low rate of strokes or other neurological events.
To address the absence of data estimating the stroke incidence rate in children with SCA and children with normal TCD measurements and without SCIs, we designed an ancillary prospective cohort study. The original informed consent of the Silent Cerebral Infarct Transfusion (SIT) Trial included the ability to follow prospectively all participants screened and not randomly allocated to blood transfusion or observation. The National Institute of Neurological Diseases and Stroke funded a SIT Trial ancillary cohort study with prospective longitudinal follow-up, permitting our team to test the hypothesis that SCA children with normal TCD measurements and without SCIs would have a lower incidence of new neurologic events than children with SCIs and normal TCD measurements.
Methods
Study Design
The SIT Trial was a multi-center clinical trial in which children with SCA and SCIs were randomized to either blood transfusion therapy or observation.8 The trial was approved by Institutional Review Boards (IRB) at all 26 participating institutions and registered at www.clinicaltrials.gov (NCT00072761) and www.ISRCTN.org (ISRCTN52713285). Parents provided written informed consent and participants provided assent in accordance with local IRB procedures. This planned ancillary study was approved by the SIT Trial Executive Committee and reviewed by the Data and Safety Monitoring Board. Twenty-three sites chose to participate in this prospective longitudinal study of all screened participants. As part of routine care, participants were followed from the time of the initial MRI of the brain until a site visit was performed to specifically collect designated data from the medical records. To ensure uniform collection of information, data were retrieved from the medical records after a visit from Clinical Coordinating Center staff (B. Covert and D. Roberts Williams) that were educated about SCA and served as research managers for the primary trial. Data were recorded on case report forms and later were entered into a REDCap database.11 When available, source documents for brain MRIs were obtained, along with source documents for any clinical events described in the medical records as a possible stroke, transient ischemic attack (TIA) or seizure.
The SIT Trial was a two-stage randomized controlled trial. During the first stage, eligible participants were screened for lesions present on brain MRI consistent with SCIs.8 Study screening visits to determine eligibility for the trial included informed consent, a comprehensive medical history, a site hematologist’s normal neurological examination, a screening brain MRI without sedation, a TCD assessment, and completion of the relevant screening case report forms. The site neurologist subsequently evaluated a subgroup of participants with infarct-like lesions to assess whether the lesions were without a corresponding neurological deficit. The site neurologists were unaware of the MRI findings, but were aware that participants would be referred if an infarct-like lesion was identified on MRI. During the second stage of the trial, participants with documented, non-progressive SCIs and a normal neurological examination as adjudicated by the trial neurology committee were randomly allocated to the blood transfusion arm or the observation arm of the trial. For participants that were not randomly allocated, with or without SCIs, all subsequent data collected were part of routine care. The cohort did not include children receiving regular blood transfusion. The follow up events were adjudicated by LCJ, a pediatric neurologist, and MRD, a pediatric hematologist with expertise in the neurological complication of SCA, who both reviewed all of the source documents from the clinical files and made a joint decision on whether an event had occurred.
Participants
Inclusion criteria for this analysis were: a confirmed diagnosis of hemoglobin SS or hemoglobin Sβ° thalassemia; 5 to 15 years of age at study entrance; informed consent and assent in accordance with local IRBs; neuroradiology committee assessment of screening brain MRI; and an initial normal TCD measurement (time averaged mean maximum velocity of <200 cm/second, non-imaging or <185 cm/second imaging technique). Exclusion criteria for this analysis included an incomplete or no screening MRI for the SIT Trial; history of a focal neurologic event lasting more than 24 hours with medical documentation or history of prior overt stroke; epilepsy; less than one year of follow-up at the local center after obtaining the initial brain MRI (screening MRI for SCI) unless a qualifying neurological event occurred; random allocation to transfusion or observation for the planned 36 months of the SIT Trial (N=196).
Primary Outcome
The primary outcome was the incidence of new neurologic events defined as stroke, seizure or TIA. Seizure was included as a new neurological event because seizures may reflect new cerebral ischemia in children with SCA.12–15
Definitions of cerebrovascular events:
Overt Stroke
Neurological deficit consistent with a stroke and new ischemic or hemorrhagic lesion on MRI that could explain the deficit. Overt strokes were sub-categorized as ischemic or hemorrhagic.
MRI-Negative Stroke
The SIT Trial neurology committee determined that an event was an MRI-negative stroke based on presentation consistent with stroke, neurological deficits lasting for > 24 hours, and negative brain MRI at the time of evaluation.
Silent Cerebral Infarct (SCI)
The presence of SCI was determined by the neurology committee based on: 1) An infarct-like lesion on screening MRI at least 3 mm in one dimension and visible in two planes on FLAIR T2-weighted images, determined by a consensus of at least two of the three study neuroradiologists and 2) a local site neurologic examination by a hematologist and when possible, a second examination by a neurologist, that was normal or an abnormality on neurologic examination that could not be explained based on location of the infarct-like lesion. A subgroup of participants had subtle neurological findings adjudicated as overt strokes based on the SIT Trial neurology committee evaluation of MRI of the brain and the local pediatric neurologist’s examination. However, these participants were not deemed to have a stroke locally based on the hematologist’s decision not to recommend regular blood transfusion therapy, a standard care intervention for secondary prevention of strokes in SCA, and were thus included in this analysis.16
Transient Ischemic Attack (TIA)
Clinical picture consistent with stroke and neurological deficit for < 24 hours and negative brain MRI.
New Seizure
Focal or generalized seizure occurring in a child without a history of seizure at the time of study entry.
Neurology examination
A pediatric neurologist completed the standardized neurological examination at each study site to determine eligibility for the SIT Trial using the Pediatric Stroke Outcome Measure (PSOM). The PSOM is a validated, reliable outcome measure17 that consists of standard neurological examination items (mental status, cranial nerves, motor, sensory, cerebellar and gait). At the conclusion of the PSOM, the clinician completes a summary of impressions that measures neurological function across five subscales: sensorimotor (right and left), language comprehension, language production and cognitive or behavioral deficit. The total PSOM is summed from the individual five sub-scales ranging from 0 (no deficits) to a maximum score of 10.
Study Procedures
Study managers visited each site and collected follow-up data including but not limited to clinic, hospital and radiology records on all participants. Site visits were conducted from February 2010 to August 2011. The study neurologist (LCJ) and the trial principal investigator (MRD) independently reviewed all source documents and reports in participants with suspected neurological events. Disagreements regarding the presence or absence of a neurological event were resolved by consensus. The final diagnosis for each clinical neurological event was based on information in the medical chart as well as the MRI of the brain. Head CT was not considered adequate to distinguish an ischemic stroke from a TIA.
Sample Characteristics
The sampling frame for this study was the population of children that completed a screening brain MRI for the SIT Trial, had at least one TCD measurement, and were followed until they developed a neurological event or for at least a year after signing the informed consent. Follow-up time was based on date of initial MRI of the brain until first neurological event, start of blood transfusion, or last day of follow-up, whichever came first. Participants with initial or subsequent abnormal TCD measurements (time averaged mean of maximum velocity of ≥ 200 cm/second non-imaging TCD or ≥185 cm/second imaging technique) were excluded.
Statistical Methods
Data analysis was performed using SPSS, version 24.0 (IBM, Armonk, NY). The relationship between the demographic and pre-specified clinical covariates (age, gender, systolic blood pressure, steady state hemoglobin, reticulocyte count, presence or absence of infarct-like lesion at baseline) for those with and without a new neurological event was assessed by chi-square test or Fisher’s exact test for categorical covariates, and a t-test for continuous covariates. For the incidence calculation, only the first neurological event was utilized.
Assessment of the association between those with or without SCI at baseline and a new event was investigated using Kaplan-Meier product-limit estimation and then Cox regression model. The incidence rate of new events was compared with a mid-P exact test. Due to the small number of new neurological events, which rules out the inclusion of the full set of variables directly in the Cox regression model,18 a propensity score was first calculated with logistic regression for the presence or absence of SCI at baseline, using the other covariates. The score was the predicted probability of SCI. A Cox regression model was then estimated with only the propensity score and the SCI covariate, comparing children with SCA, normal TCD velocities and SCIs (reference population) to children with normal TCD velocities and no SCIs. This approach effectively allowed the inclusion of all covariates previously identified as risk factors for SCIs (age, sex, baseline hemoglobin level, systolic blood pressure, and reticulocyte count),8, 18 even though this was not a direct measure of their association with a new neurological event. An analysis of various residual statistics identified no cases with a large influence on the model results. A hazard ratio < 1.0 that also excludes 1.0 in the 95% confidence interval indicates a statistically significant decreased risk of future neurological events when compared to the reference category (children with SCA, normal TCD, and SCIs). Analyses were performed to determine if any significant differences existed between those children included in the analysis and those excluded because of one of the following: absence of TCD value, or no follow up after initial evaluation. No systematic or clinically important differences were observed in the following parameters: age, sex, systolic blood pressure, white blood cell count or steady state hemoglobin (Supplementary Table 1).
Results
Demographics
In the SIT Trial, 1176 children consented for screening, 1063 completed the screening brain MRI and 196 were randomized between 04/2005 and 05/2010. Of the 867 children that had a screening brain MRI and were not randomly allocated to blood transfusion or observation, 135 were at sites that elected not to participate in this ancillary cohort study and 95 had no follow-up available. Thus, of 637 eligible children (73.4%), 421 had a normal TCD value and follow-up of at least one year until a site visit or start of chronic transfusion therapy (Supplementary Figure, study flow diagram). Demographic features of children with and without SCI at baseline are presented in Table 1. Of the 421 children, 68 (16.2%) had a baseline screening brain MRI that demonstrated ischemic lesions8,19 and were determined locally as having SCI(s). However, further review by the SIT trial neurology committee indicated that two participants should be considered as meeting the definition of stroke, although neurological findings were subtle (n=2), both children had very mild hemiparesis and one had cognitive difficulties. The local site hematologists did not consider these participants as having a stroke, but rather having SCI, and elected not to offer regular blood transfusion therapy, as would be standard care for an overt stroke.
Table 1.
Demographics of the cohort of children with normal transcranial Doppler (TCD) measurements (time averaged mean maximum velocity of < 200 cm/second, non-imaging or < 185 cm/second imaging technique) stratified by negative or positive MRI (n=421)
| Variable* | Normal TCD measurements, no evidence of silent cerebral infarct MRI (n=353) | Normal TCD measurements, evidence of silent cerebral infarct (n=68) | P Value* |
|---|---|---|---|
| Age at registration | 8.9 (2.4) | 9.1 (2.6) | 0.437 |
| Sex, Male (%) | 49.6 | 54.4 | 0.465 |
| Systolic blood pressure (n=416) | 107.5 (11.8) | 110.6 (10.5) | 0.044 |
| Steady state hemoglobin | 8.3 (1.2) | 7.9 (1.0) | 0.006 |
| Reticulocytes (%) (n=418) | 11.6 (5.6) | 14.3 (6.3) | <0.001 |
| Hydroxyurea use before end of follow-up (%) | 25.2 | 23.5 | 0.769 |
| Chronic transfusion before end of follow-up (%) | 4.8 | 7.4 | 0.375# |
| Follow-up time until event, end-of study, or chronic transfusion, years | 3.6 (1.3) | 3.4 (1.5) | 0.270 |
| New neurological event (stroke, seizure or TIA) (%) | 1.7 | 5.9 | 0.061# |
| Incidence rate, new neurological events (events/100 patient-years), 95% confidence interval | 0.47 (0.17 – 1.02) | 1.71 (0.46 – 4.37) | 0.065§ |
Mean and standard deviation or chi-square test unless otherwise noted
Fisher’s exact test
Mid-p exact test
Presence of silent cerebral infarct was associated with a higher incidence of future neurological events
Of the 10 children with new neurological events, 5.9% (4 of 68) were in the group with SCIs at baseline, compared to 1.7% (6 of 353) in the group with a negative brain MRI at baseline (p=0.061). Details of each of the 10 children with a new neurological event at follow-up are shown in Table 2, while further detail for those with seizures is given in Supplementary Table 2. The incidence rate of new events for those with and without SCIs at baseline, Table 1, was 1.71 (4 events in 234.2 patient-years) and 0.47 (6 events in 1286.2 patient-years) per 100 patient-years, respectively (p=0.065). The proportion of children that remained neurological event-free over time is shown in Figure 1, with a difference between the two groups (log rank test, p=0.032). A Cox regression model, including a propensity score for SCI at baseline, Supplementary Table 3, found that absence of SCI at baseline was associated with a decreased risk of a new neurological event (hazard ratio 0.231, 95% CI 0.062–0.858; p=0.029).
Table 2.
Participants with normal TCD measurements (time averaged mean maximum velocity of < 200 cm/second, non-imaging or < 185 cm/second imaging technique) and study screening magnetic resonance image (MRI)of the brain that subsequently developed a new stroke, seizure or transient ischemic attack.
| Age (years) | SCI at screening | Subtle Stroke at screening | Baseline SBP | Steady State Hb | Baseline TCD*, Follow Up TCD (cm/s) | Time from Baseline TCD to follow-up TCD (years)** | Time from screening MRI to endpoint (years) | Type of Neurological Event | Neurological Exam and MRI Findings | Medical events proximate to Neuro Event | On hydroxyurea (age started, after screening, before neurological event) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5.99 | Yes | No | 97 | 9.2 | 184, 186 | 0.01 | 0.04 | Ischemic Stroke | Right hemiparesis; punctate subacute infarcts left >right, normal MRA, | None | No |
| 10.68 | Yes | Yes | 109 | 7.7 | 118, 140 | 0.25 | 0.50 | Ischemic Stroke | Right hemiparesis, new ischemia, left and right centrum semi-ovale, MRA normal. | Pain crisis | No |
| 12.88 | Yes | No | 115 | 8.5 | 122, 97 | 1.0 | 2.88 | TIA (2)╪ | Right hemiparesis (transient), normal MRI, MRA, MRV. | None | No |
| 11.01 | Yes | Yes | 110 | 8.6 | 158, 96 | 2.08 | 0.30 | Seizure | Tonic clonic seizure, MRI unchanged SCI, MRA normal | None | No |
| 5.15 | No | No | 95 | 9.1 | 105, NA | NA | 2.55 | Ischemic Stroke | Right hemiparesis, aphasia, left frontoparietal acute stroke, left ICA high grade stenosis. | ACS, new intracranial stenosis | No |
| 6.97 | No | No | 108 | 6.7 | 163, NA | NA | 3.32 | Ischemic Stroke | Right leg weakness, acute left frontal stroke., No MRA. | None | No |
| 7.21 | No | No | 107 | 8.8 | 90, NA | NA | 5.68 | Ischemic Stroke | Left hemiparesis, acute stroke right internal capsule. MRA normal. | None | Yes (8.15) |
| 5.07 | No | No | 100 | 10.4 | 84, 93 | 1.25 | 1.83 | TIA | Left arm numbness (transient), normal MRI, MRA, MRV. | Pain crisis | Yes (6.05) |
| 11.84 | No | No | 109 | 8.5 | 92, NA | NA | 1.30 | TIA | Right face, arm, leg numbness (transient), MRI normal, MRA left ICA stenosis. | Febrile, elevated WBC, low Hb (7.2) | No |
| 8.33 | No | No | 108 | 7.4 | 138, 93 | 1.41 | 2.25 | Seizure | MRI, tiny foci of microvascular ischemia new since screening MRI, not meeting criteria for SCI, Normal MRA, MRV | None | Yes (9.82) |
TIA = transient ischemic attack, SBP = systolic blood pressure, Hb = hemoglobin, TCD = transcranial Doppler, SCI = silent cerebral infarct, MRI = magnetic resonance imaging, MRA = magnetic resonance angiography, MRV= magnetic resonance venography, ICA= internal carotic artery, WBC = white blood cell, ACS= acute chest syndrome, NA = not available
TCD values are the time averaged maximum velocity of the terminal portion of the internal carotid artery or proximal middle cerebral artery, whichever was greater.
Follow up TCD closest to neurological event
Two TIAs
Figure 1. Kaplan-Meier Plot.
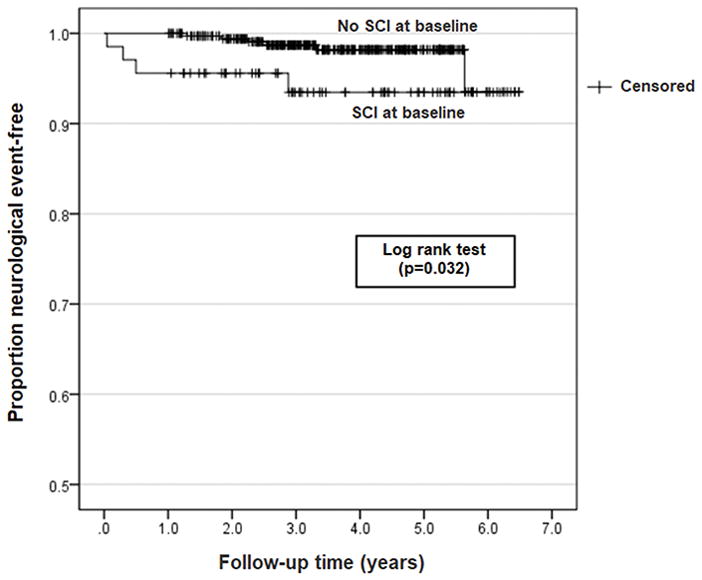
Time to first neurological event (stroke, seizure, transient ischemic attack TIA for children with normal transcranial Doppler ultrasound measurements (time averaged mean maximum velocity of < 200 cm/second, non-imaging or < 185 cm/second imaging technique) and with (n=68) and without silent cerebral infarcts (SCIs) on MRI (n=353).
Neurologists add value to detect subtle strokes in children believed to have silent cerebral infarcts
Due to the unique study design, we were able to compare the site hematologist’s neurological assessment of normal examination (an entry criterion) to the site pediatric neurologist’s examination in children with an infarct-like lesion on MRI in 67 of 68 children. Nearly 3% (2 of 67) of the children with a hematologist’s assessment of a normal examination would have been re-classified as having a stroke based on the site pediatric neurologist’s evaluation and neurology committee adjudication; neither was transfused and both had subsequent neurological events. Despite the central committee’s review, the site hematologists still considered these two individuals as having SCIs and not strokes, and elected not to put these children on regular blood transfusion therapy or hydroxyurea therapy. For our results to mimic clinical practice, we utilized the hematologist’s neurological examination and their classification of SCIs in our primary analysis. However, given the discordance between the pediatric hematologist’s and neurologist’s evaluation for stroke, we reanalyzed the data with the pediatric neurologist’s and neurology committee’s evaluation. Thus, of 68 children with SCI at baseline, two children were re-classified as having strokes and not a SCI. One of the children with a neurologist-identified overt stroke at baseline had a seizure and the other had an ischemic stroke during a pain crisis (Table 2). With exclusion of these two children from the SCI group, 3.0% (2 of 66) and 1.7% (6 of 353) developed neurological events with and without pre-existing SCIs, p=0.618 and the log rank test was p=0.447.
The site neurologists, without the benefit of the MRI of the brain, also had challenges in identifying a child with a stroke, when compared to the neurology committee that had access to both site neurologist’s examination and the MRI of the brain revealing the location of the cerebral infarct. The site neurologists graded 30% (20 of 67) of the neurological examinations as abnormal (PSOM >0). Site neurologists recorded significant concerns about two children with total PSOM >1.5; only one of the two children was confirmed as having a stroke. The child with stroke had both a subtle hemiparesis and cognitive difficulties. The other child was not a native English speaker and the examination was challenging with concerns regarding language and cognition; however, there were no focal motor or sensory findings, and a SCI-like lesion was not present on imaging. There were moderate concerns about two children each with total PSOM =1. In the two children, the neurology committee confirmed one child had a stroke based on subtle right hemiparesis, and one child had significant cognitive difficulties, but not a confirmed stroke. Site neurologists graded the remaining 16 children as having very mild abnormalities on examination (PSOM = 0.5 for cognitive or language concerns). The SIT Trial neurology committee did not adjudicate any of these 16 children as having a stroke.
Discussion
The goal of clinical trials for primary and secondary stroke prevention in children with SCA is to identify the subgroup of children at the highest risk for future neurological injury, and to determine whether an intervention (blood transfusion, hydroxyurea or both) prevents overt stroke or infarct recurrence. After completion of six clinical trials in children with SCA, children with strokes, SCIs, and abnormal TCD velocities have higher rates of neurological events when compared to children without strokes, SCIs and normal TCD velocities. However, we have less evidence to identify those at low risk for future neurological events because children believed to be at low risk for neurological events have been excluded from these controlled trials. We have demonstrated in a prospective multi-center observational study the lower incidence rate of overt neurological events (stroke, TIA, seizure) in children with normal TCD measurements and without SCIs, when compared to children with normal TCD measurements and SCIs. However, even in children with SCA considered to have the lowest stroke risk, their stroke incidence rate is still at least 100-times higher at 0.47 per 100 patient-years than the stroke incidence rate in the general population of children without SCA, 0.003 per 100 patient-years.20
The rate of all new neurological events for those children with SCIs and normal TCD measurements was similar to the rate in the group of children in the SIT Trial randomized cohort that did not receive treatment (observation group). In the observation arm of the SIT trial based on intention to treat analysis, the incidence rate of neurological events was 3.1 events per 100 patient-years (7 strokes, 2 TIAs, 0 seizures in 289 total patient-years) compared to the current analysis of 1.7 events per 100 patient-years (5 strokes, 3 TIAS and 2 seizures) in children with SCIs and normal TCD values. We also compared our findings to previously published studies of children with SCA (Figure 2) and show that even with normal TCD and MRI of the brain, children with SCA have a much higher rate of stroke than the general population of children.
Figure 2. Comparison of the incidence of new neurological events in different groups of children with SCA.
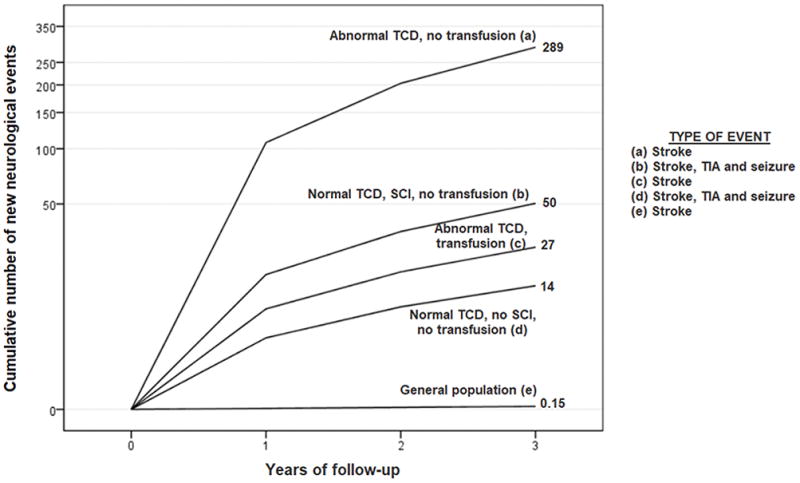
In a hypothetical cohort of 1,000 children followed for 3 years, we report the cumulative number of new neurological events. We compared data from the current study to published stroke rates from the STOP trial, children with abnormal transcranial Doppler (TCD) ultrasound measurement defined as: time averaged mean maximum velocity of ≥ 200 cm/second, treated and not treated with regular blood transfusion therapy and to the stroke rate in general population of children. The figure depicts the cumulative number of neurological events; all numbers are the cumulative events per 1,000 children followed for 3 years. The groups are (a) abnormal TCD velocities, no silent cerebral infarcts (SCIs) not on blood transfusion – 289 stroke events,5 (b) normal TCD velocities, SCIs, not on blood transfusion – 50 events -current study, events are stroke, TIA and seizure, (c) abnormal TCD, no SCI, on blood transfusion - 27 stroke events,5 (d) normal TCD velocities, no SCI, no transfusion - 14 events, current study - events are stroke, TIA and seizure, (e) children with strokes in the general population without SCA - 0.15 stroke events.20 Thus, even children with SCA in the lowest risk group for strokes (normal TCD velocities, no SCI, no transfusion), have approximately a 100-fold increase in risk of strokes when compared to the general population of children without SCA.
In children with SCA, a neurological examination to define a stroke is often challenging even for an experienced pediatric neurologist. In a child with SCA, an accurate assessment of the neurological status to determine the presence of stroke, SCIs, or neither requires a complete history, examination and MRI of the brain.21 In the SIT Trial, site neurologists were aware of the eligibility criterion excluding children with a stroke, but were unaware of MRI findings or at least location of the suspected SCIs when examining a child. Only members of the SIT Trial neurology committee had the full benefit of knowing the location of the SCI(s) along with the ability to review the neurological examination. In clinical practice, these data support the importance of a pediatric neurologist both examining the child, and reviewing the MRI findings in a child with silent cerebral infarct-like lesions, to distinguish between a child with SCI(s) and strokes. The experience in this observational study, was similar to that in the SIT Trial where the neurology committee later reclassified 7% of the children initially identified as having SCIs as having an overt stroke based on the neurology evaluation and review of MRI of the brain.8
The main point that the current study and the SIT Trial underscores is the importance of a pediatric neurology examination for all children with suspected SCIs because without a formal evaluation of all available data (history, neurological examination and review of the MRI of the brain), a defined proportion of children with strokes will be mis-classified as having SCI. A correct diagnosis of stroke or SCI is important because, if children with strokes are misclassified as having SCI(s), then parents may be less inclined to implement regular blood transfusion therapy for secondary stroke prevention. Thirteen children with SCIs must be treated to avoid one SCI recurrence.8 By comparison, with stroke, the number needed to treat to avoid one infarct recurrence drops to approximately four children, based on a pooled analysis of recent studies that revealed that the absolute risk of overt stroke recurrence with and without transfusion is approximately 2 per 100 patient-years and 29 per 100 patient-years, respectively.9
As anticipated, this ancillary study had several strengths and limitations. Our prospective multi-center cohort included the largest number of children with SCA followed for an average of 3 years. Thus, our results are generalizable to children with SCA and normal TCD measurements with and without SCIs, the former group (normal TCD and no SCIs) never having been evaluated prospectively in a multi-center study.
To reflect clinical practice, we utilized the hematologist’s neurological examination and their classification of SCIs. We excluded the research evaluation of the site neurologists that identified an abnormal neurological finding in 30% of the participants and the neurology committee’s adjudication, neither of which would be considered standard care clinical practice. The site neurologist did not have the benefit of reviewing the MRI when examining the patient, while the neurology committee had a panel of pediatric stroke experts to discuss the nuances of the case. However, when we used the site neurologist’s examination and neurology committee adjudication and reclassified two children with subtle neurological findings as having strokes, the incidence of neurological events was still nearly double that in the group with pre-existing SCIs and normal TCD velocities when compared to the group with no SCI and normal TCD velocities, but no longer statistically significant. Despite being the largest cohort of children ever evaluated to include both a pediatric hematologist’s and a pediatric neurologist’s neurological examination, the small number of new neurological events in our cohort is a weakness. However, based on the strengths of the study design (planned ancillary study of a NIH multi-center clinical trial, central adjudication of all MRIs of the brain, and standardized medical record extraction) our data provide compelling evidence that the absence of SCIs in children with normal TCD velocities have a lower risk of future neurological events when compared to children with SCI and normal TCD velocities.
Our analysis does not address the incidence of new SCIs inchildren with normal TCDs because we did not include new orprogressive SCIs. New or progressive SCIs can only be detected withbrain MRI, and follow-up MRIs were not routinely done. We elected to include children with TIAs and new onset seizures as evidence of at least transient ischemia of the brain because our patient populations were pre-selected to exclude children with prior neurological symptoms or epilepsy; thus, the presence of either or both would indicate a risk for concurrent or future ischemic brain injury. More data are needed on TIA in children with SCA using modern imaging and modern definitions of TIA to understand stroke-risk following TIA. Given the small number of children taking hydroxyurea at the time of the neurological event, no inference can be made regarding whether hydroxyurea decreased the incidence of neurological events in children with SCIs and normal TCD measurements. We only analyzed data in the non-randomized SIT cohort of children with both a brain MRI and normal TCD measurements. Presumably, children with only abnormal TCD measurements or those with both SCIs and abnormal TCD measurements had higher rates of future neurological events based on prior studies that included children with abnormal TCDs.14,22 However, the SIT Trial entry criteria excluded children with abnormal TCD values because they would have been offered regular blood transfusion therapy. Consequently, we have limited data in this population.
In children with SCA screened for participation in a clinical trial, and followed prospectively while receiving routine clinical care, we have shown those with normal TCD measurements and without SCIs have a significantly lower incidence of new neurological events (stroke, TIA or seizure) than children with SCIs and normal TCD measurements. Although children with no SCIs and normal TCD measurements represent the lowest risk group for future strokes, their rate of strokes is at least 100-times greater than the general population of children without SCA. Our results and those of the SIT Trial argue for at least one screening MRI of the brain during childhood to assess for infarct-like lesions and for stratification of future stroke risk in children with normal TCD velocities. Further, we provided strong evidence for the value of an evaluation by a pediatric neurologist to exclude strokes in children with infarct-like lesions on MRI of the brain that might otherwise be classified as SCIs.
Supplementary Material
Acknowledgments
SIT Trial, 5U01-NS042804, MRD, American Recovery Reinvestment ACT supplementary grant funded by NINDS 3U01NS042804-06S1, MRD. REDCap was funded by NCAT/NIH UL1 TR000445. We are also thank our families that participated in the trial, the coordinators and teams at study sites, and Cindy Terrill from Washington University School of Medicine who worked tirelessly on the planning and the day to day management of the trial.
Footnotes
Contributions
M.R.D, L. C. J: designed research, performed research, analyzed and interpreted data, wrote the first draft and critically revised the manuscript
M.J.R.: analyzed and interpreted data, performed statistical analysis, wrote the first draft and critically revised the manuscript
B.C., J.F.C., D.O.: collected data and critically revised the manuscript
B.F., F.J.K., E.R.M., M.M., R.C.M., S.M., M.J.N, T.L.M., M.R.P., S.S.: critically revised the manuscript
Conflict of Interest Disclosure
The authors declare no competing financial interests
References
- 1.DeBaun MR, Kirkham FJ. Central nervous system complications and management in sickle cell disease. Blood. 2016;127(7):829–838. doi: 10.1182/blood-2015-09-618579. [DOI] [PubMed] [Google Scholar]
- 2.Powars D, Wilson B, Imbus C, Pegelow C, Allen J. The natural history of stroke in sickle cell disease. Am J Med. 1978;65(3):461–471. doi: 10.1016/0002-9343(78)90772-6. [DOI] [PubMed] [Google Scholar]
- 3.Galadanci NA, Abdullahi SU, Tabari MA, et al. Primary stroke prevention in Nigerian children with sickle cell disease (SPIN): challenges of conducting a feasibility trial. Pediatr Blood Cancer. 2015;62(3):395–401. doi: 10.1002/pbc.25289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hulbert ML, McKinstry RC, Lacey JL, et al. Silent cerebral infarcts occur despite regular blood transfusion therapy after first strokes in children with sickle cell disease. Blood. 2011;117(3):772–779. doi: 10.1182/blood-2010-01-261123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke bytransfusions in children with sickle cell anemia and abnormal resultson transcranial Doppler ultrasonography. The New England journal of medicine. 1998;339(1):5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 6.Helton KJ, Adams RJ, Kesler KL, et al. Magnetic resonance imaging/angiography and transcranial Doppler velocities in sickle cell anemia: results from the SWiTCH trial. Blood. 2014;124(6):891–898. doi: 10.1182/blood-2013-12-545186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ware RE, Davis BR, Schultz WH, et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet. 2016;387(10019):661–670. doi: 10.1016/S0140-6736(15)01041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeBaun MR, Gordon M, McKinstry RC, et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N Engl J Med. 2014;371(8):699–710. doi: 10.1056/NEJMoa1401731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kassim AA, Galadanci NA, Pruthi S, DeBaun MR. How I treat and manage strokes in sickle cell disease. Blood. 2015;125(22):3401–3410. doi: 10.1182/blood-2014-09-551564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams RJ, Brambilla D Optimizing Primary Stroke Prevention in Sickle Cell Anemia Trial I. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med. 2005;353(26):2769–2778. doi: 10.1056/NEJMoa050460. [DOI] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lebensburger JD, Hilliard LM, McGrath TM, Fineberg NS, Howard TH. Laboratory and clinical correlates for magnetic resonance imaging (MRI) abnormalities in pediatric sickle cell anemia. Journal of child neurology. 2011;26(10):1260–1264. doi: 10.1177/0883073811405054. [DOI] [PubMed] [Google Scholar]
- 13.Prengler M, Pavlakis SG, Boyd S, et al. Sickle cell disease: ischemia and seizures. Annals of neurology. 2005;58(2):290–302. doi: 10.1002/ana.20556. [DOI] [PubMed] [Google Scholar]
- 14.Pegelow CH, Macklin EA, Moser FG, et al. Longitudinal changes in brain magnetic resonance imaging findings in children with sickle cell disease. Blood. 2002;99(8):3014–3018. doi: 10.1182/blood.v99.8.3014. [DOI] [PubMed] [Google Scholar]
- 15.Kinney TR, Sleeper LA, Wang WC, et al. Silent cerebral infarcts in sickle cell anemia: a risk factor analysis. The Cooperative Study of Sickle Cell Disease. Pediatrics. 1999;103(3):640–645. doi: 10.1542/peds.103.3.640. [DOI] [PubMed] [Google Scholar]
- 16.U. S. Department of Health and Human Services NIoH, National Heart, Lung, and Blood Institute. Report EP. 2014. Evidence Based Management of Sickle Cell Disease. [Google Scholar]
- 17.Kitchen L, Westmacott R, Friefeld S, et al. The pediatric stroke outcome measure: a validation and reliability study. Stroke. 2012;43(6):1602–1608. doi: 10.1161/STROKEAHA.111.639583. [DOI] [PubMed] [Google Scholar]
- 18.van Domburg R, Hoeks S, Kardys I, Lenzen M, Boersma E. Tools and techniques--statistics: how many variables are allowed in the logistic and Cox regression models? EuroIntervention. 2014;9(12):1472–1473. doi: 10.4244/EIJV9I12A245. [DOI] [PubMed] [Google Scholar]
- 19.Roberts DO, Covert B, Rodeghier MJ, et al. Randomization is not associated with socio-economic and demographic factors in a multi-center clinical trial of children with sickle cell anemia. Pediatr Blood Cancer. 2014;61(9):1529–1535. doi: 10.1002/pbc.25072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agrawal N, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Imaging data reveal a higher pediatric stroke incidence than prior US estimates. Stroke. 2009;40(11):3415–3421. doi: 10.1161/STROKEAHA.109.564633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glauser TA, Siegel MJ, Lee BC, DeBaun MR. Accuracy of neurologic examination and history in detecting evidence of MRI-diagnosed cerebral infarctions in children with sickle cell hemoglobinopathy. Journal of child neurology. 1995;10(2):88–92. doi: 10.1177/088307389501000203. [DOI] [PubMed] [Google Scholar]
- 22.Abboud MR, Yim E, Musallam KM, Adams RJ Investigators SIS. Discontinuing prophylactic transfusions increases the risk of silent brain infarction in children with sickle cell disease: data from STOP II. Blood. 2011;118(4):894–898. doi: 10.1182/blood-2010-12-326298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


