Abstract
Introduction:
Prenatal exposures to tobacco and cannabis are associated with combustible cigarette use. This study evaluated pathways from these prenatal exposures to adult electronic cigarette use. We tested whether there were indirect effects of these prenatal exposures via childhood behavior dysregulation, early tobacco use, and adolescent tobacco dependence.
Methods:
Telephone interviews were conducted with 427 adult offspring (22–33 years old) from 3 prenatal cohorts with trimester-specific data on exposures to tobacco, alcohol, and cannabis. The offspring were 59% Black and 41% White (61% female). Prenatal exposures included quantity/frequency of tobacco, alcohol, and cannabis use by mothers during the first trimester. Using logistic regression and structural equation modeling, we examined the effects of gestational exposures on adult electronic cigarette use via early cigarette use (prior to age 14), controlling for covariates of combustible and electronic cigarette use.
Results:
There were no effects of childhood behavioral dysregulation on electronic cigarette use. However, there was a significant indirect effect of prenatal exposures to tobacco and cannabis on electronic cigarette use via early adolescent combustible cigarette use and adolescent risk for tobacco dependence.
Conclusions:
One implication of these findings is that the inter-generational risk for tobacco use conferred via gestational exposures to tobacco and cannabis generalizes to novel products such as electronic cigarettes. These results have implications for public health, as more women use cannabis and co-use cigarettes and cannabis during pregnancy.
Keywords: Prenatal exposures, Prenatal tobacco, Electronic cigarettes, Tobacco dependence, Cannabis, Prenatal cannabis
1. Introduction
In the Population Assessment of Tobacco and Health (PATH) study, twice as many adults (18 and over) as youth (12–17 years old) reported electronic cigarette use in the past 30 days (Kasza et al., 2017). Other demographic correlates of electronic cigarette use include race/ethnicity, male sex, and educational attainment (Kasza et al., 2017; Chou et al., 2017). The best predictor of electronic cigarette use, however, is history of combustible cigarette use (Farsalinos et al., 2017; Glasser et al., 2016; Ramo et al., 2015; Vardavas et al., 2015). Most adults who have tried electronic cigarettes report that they used them to cut down or quit smoking combustible cigarettes (Glasser et al., 2016; Rutten et al., 2015). In the National Epidemiological Survey on Alcohol and Related Conditions (NESARC), electronic cigarette use in adults was associated with tobacco dependence (Chou et al., 2017), and in other population surveys with nationally representative samples, recent quitters were the most likely to have tried electronic cigarettes (Zhu et al., 2017). Thus, regular use of electronic cigarettes among adults likely represents smoking cessation attempts among the tobacco dependent.
Prenatal use of cannabis has increased 62% in the past decade (Brown et al., 2017), and prenatal co-use of cigarettes with cannabis is three times more common than prenatal cannabis use alone (Coleman-Cowger et al., 2017). Prenatal exposure to combustible cigarettes and cannabis are well-known risk factors for combustible cigarette use. Offspring prenatally exposed to tobacco are more likely to become combustible cigarette smokers (Agrawal et al., 2010; Cornelius et al., 2000, 2005; De Gennaet al., 2015; Goldschmidt et al., 2012) and to use multiple substances by adolescence (Goldschmidt et al., 2012) than those who were not exposed. They are also more likely to be tobacco dependent compared to offspring without prenatal exposure to tobacco (Buka et al., 2003; De Genna et al., 2017; Shenassa et al., 2015). Similarly, offspring prenatally exposed to cannabis are more likely to use combustible cigarettes and cannabis (Porath and Fried, 2005; Sonon et al., 2015) and become cannabis dependent (Sonon et al., 2015, 2016) than those not exposed. Therefore, it is likely that prenatal exposures to tobacco and cannabis will also predict electronic cigarette use, although this has not yet been demonstrated in the literature.
There may be indirect pathways from prenatal exposures to tobacco and cannabis and adult electronic cigarette use. Prenatal exposures to tobacco and cannabis are linked to childhood behavioral dysregulation. For example, there is a large literature linking prenatal tobacco exposure to externalizing behavior problems (Ashfordet al., 2008; Brion et al., 2010; Cornelius et al., 2007, 2011, 2012; Day et al., 2000; Orlebeke et al., 1997, 1999) that, in turn, predict early cigarette use (Brook et al., 2008; Lynskey and Fergusson, 1995; King et al., 2004). There are recent cross-sectional data linking externalizing behavior problems in adults to electronic cigarette use (Conway et al., 2017). Prenatal cannabis exposure has been linked to child depressive symptoms (Sonon et al., 2016; Gray et al., 2005), which are a correlate of adolescent combustible cigarette use (Brown et al., 1996; Escobedo et al., 1998; Windle and Windle, 2001). More recently, depressive symptoms have also been linked to electronic cigarette use in adolescents and college students (Bandiera et al., 2016, 2017; Lechner et al., 2017; Leventhal et al., 2016). Therefore, we hypothesized that there would be an indirect pathway from prenatal exposures to tobacco and cannabis to adult electronic cigarette use via childhood behavioral dysregulation.
Combustible cigarette users and those who have recently quit have the highest rates of electronic cigarette use (Zhu et al., 2017). Therefore, early and persistent combustible cigarette use may mediate the association between prenatal exposures to tobacco and cannabis and adult electronic cigarette use. We hypothesized that there would be an indirect pathway from prenatal exposures to adult electronic cigarette use via adolescent risk for tobacco dependence. To date, there are no prospective reports linking prenatal exposures to cigarettes and cannabis with adult electronic cigarette use.
The goal of this study was to investigate pathways from prenatal exposures to tobacco and cannabis to electronic cigarette use in young adult offspring. We used data collected across several decades from three prenatal cohorts designed to examine the long-term effects of prenatal substance use. We hypothesized that young adults with prenatal exposures to tobacco and cannabis would be more likely to have used electronic cigarettes, controlling for demographic covariates and other correlates of tobacco use, than those without such exposures. We tested direct and indirect pathways (via childhood behavioral dysregulation, early adolescent cigarette use, and tobacco dependence) from prenatal tobacco and cannabis exposure to adult electronic cigarette use with structural equation models.
2. Material and methods
Young adult offspring from three prenatal cohorts were contacted and asked to participate in the current study. The three original studies were part of a consortium of studies designed to determine the long-term effects of gestational exposures to tobacco, alcohol, and cannabis on offspring. Pregnant mothers reported on their first trimester substance use at their fourth month of gestation, and in two of the studies at the end of the second trimester and at delivery. In the third study, data on the second and third trimesters were collected at delivery. All prenatal phases of the studies were approved by the Magee-Womens Hospital IRB. All postnatal phases were approved by the University of Pittsburgh IRB. In all three studies, mothers and offspring were seen at 6, 10, 14, and 16 years postpartum for intensive assessments. All three studies recruited pregnant women from the same hospital and used many of the same measures and personnel. The young adult offspring from these three studies were recruited to participate in a brief telephone survey of electronic cigarette use for the current study.
2.1. Procedure
Offspring were mailed letters describing the current study and then recruited by telephone. If the participant was not immediately available to complete the telephone survey, an appointment was scheduled for a later date. The study was described to participants, who had the opportunity to ask questions about the study and then provide verbal consent. The survey took an average of 30 min to complete. In addition to questions about electronic cigarette, combustible cigarette and other substance use, participants were asked to provide basic demographic information.
2.2. Sample
The three original prenatal cohorts were comprised of mothers and offspring. Two studies included women 18+ years of age for studies of the effects of prenatal alcohol (AA006666, PI: Day) and cannabis exposures (DA003874, PI: Day) for a combined birth sample of 763 recruited from 1982 to 1985. For more information, see Day et al. (1989, 1990, 1994). The third cohort included adolescent mothers (⩽ 18 years of age) who were recruited from 1990 to 1995 to study the effects of prenatal alcohol and tobacco exposure (birth sample = 413; AA008284; DA009275, PI: Cornelius). For more information, see Cornelius et al. (1994, 1995). The adult mother cohorts were oversampled for alcohol and cannabis use, ensuring that at least half of those samples included women who drank at least 3 drinks/week (in the alcohol cohort) while pregnant or used cannabis at least 2 times/month while pregnant (the marijuana cohort). The adolescent mother cohort enrolled all pregnant adolescents at the clinic regardless of their substance use. There was a full range of gestational substance exposures in all three cohorts. A new dataset combining these birth cohorts was created to ensure adequate numbers of offspring exposed to prenatal substances to determine long-term effects. We circumvent much of the between-subject heterogeneity characteristic of merged cohort data because the participants from all three cohorts were drawn from the same hospital, assessed using the same measures, and interviewed by the same personnel (Curran and Hussong, 2009).
Of 1176 offspring in the combined birth cohorts, 427 were assessed for the current study as part of a study on electronic cigarette use. More female than male offspring were interviewed at this assessment. Their mothers were slightly older and more educated than the offspring who were not assessed. However, as seen in Table 1, these offspring did not differ from the offspring who were not seen with respect to gestational exposures to tobacco, alcohol, or cannabis.
Table 1.
Offspring from the birth cohorts who were and were not assessed in the current study.
| Not assessed (n = 749) |
Assessed (n = 427) |
p-value | |
|---|---|---|---|
| Maternal race (% Black) | 58.6 | 54.8 | ns |
| Maternal age (years) | 20.4 (4.7) | 21.1 (4.4) | p < 0.01 |
| Maternal education (years) | 11.0 (1.7) | 11.4 (1.6) | p < 0.01 |
| Offspring sex (% male) | 57.1 | 39.6 | p < 0.01 |
| First trimester cigarette use | 7.0 (10.1) | 7.0 (10.3) | ns |
| (mean cigarettes/day) | |||
| First trimester alcohol use | 0.54 (1.3) | 0.50 (1.1) | ns |
| (mean drinks/day) | |||
| First trimester cannabis use | 0.32 (0.9) | 0.26 (0.7) | ns |
| (mean joints/day) | |||
2.3. Measures
2.3.1. Demographic variables
Maternal race and offspring date of birth were available for each participant. Offspring reported their highest level of educational attainment.
2.3.2. Exposure to prenatal substance use
Mothers reported the quantity and frequency of cigarette, alcohol, and cannabis use during each trimester. These quantity and frequency data were used to calculate their average daily cigarettes, joints, and volume of alcohol. First trimester exposures were entered into the models because of higher rates of tobacco, alcohol, and cannabis use during this trimester. Average daily joints and average daily volume of alcohol were used in the analyses. First trimester cigarette use was dichotomized into less than 10 vs. 10 or more cigarettes/day. This threshold has been demonstrated to predict risk for adolescent tobacco dependence in the offspring (De Genna et al., 2017). Prenatal alcohol use was dropped from the multivariate models because it was not associated with early adolescent cigarette use or adult electronic cigarette use.
2.3.3. Small for gestational age
Birthweight and gestational age were recorded from the medical record. Small-for-gestational age (SGA weight < 10th percentile for the gestational age) was considered for the models because of previous reports of pathways from prenatal exposures to dysregulation in offspring via fetal growth (Godleski et al., 2016; Schuetze et al., 2018).
2.3.4. Childhood behavioral dysregulation
Behavioral dysregulation measured at age 10 was used to assess vulnerability to early cigarette use as part of an indirect pathway between prenatal tobacco and cannabis exposures and adult electronic cigarette use. Externalizing behavior problems were measured by the Externalizing Behavior Scale of the Child Behavior Checklist (CBCL) (Achenbach, 1991) that was completed by mothers at the age 10 follow-up visit. The consistency and validity of CBCL scores have been demonstrated across decades of research with a variety of samples (Achenbach and Rescorla, 2001). T-scores were used in the analysis. Impulsivity at age 10 was measured with maternal reports on the impulsivity scale of the Swanson, Nolan and Pelham questionnaire (SNAP) (Pelham and Bender, 1982). Depression in the offspring at age 10 was measured using the Children’s Depression Inventory (CDI) (Kovacs, 1992). The CDI, adapted from the Beck Depression Inventory, assesses recent general psychopathology over the last two weeks rather than clinical depression. It measures the presence of symptoms such as negative mood, low self-esteem, and anhedonia. Good internal consistency and validity have been reported for the CDI (Kovacs, 1992; Sitarenios and Stein, 2004). The total raw score was used in the analysis.
2.3.5. Maternal postnatal cigarette use
Mothers were asked about their combustible cigarette use at each assessment. We considered the role of postnatal maternal smoking in the pathways from gestational exposures to adult offspring electronic cigarette use by including maternal number of cigarettes smoked per day at the 14-year follow-up in the multivariate models.
2.3.6. Early cigarette use in offspring
Offspring were asked if they had ever tried cigarettes, including a puff, and if they had ever smoked an entire cigarette, at assessments from age 10 onward. Participants who responded that they had used combustible cigarettes prior to age 14 were coded as individuals who used cigarettes at an early age (1 = early smoker, 0 = not an early smoker).
2.3.7. Adolescent offspring risk for tobacco dependence
At age 16, adolescents completed the Fagerström Test for Nicotine Dependence (Heatherton et al., 1991). A score of 4 on this scale indicates moderate dependence in adults (Prokhorov et al., 2001). Adolescent offspring who scored 3 or higher were considered at risk for tobacco dependence.
2.3.8. History of electronic cigarette use
We developed the electronic cigarette use questionnaire after a review of the literature and pilot testing in the target demographic. The questionnaire contained core items recommended for the assessment of electronic cigarette use by a panel of experts from 15 countries (Pearson et al., 2017). Adult offspring were asked if they had ever tried an electronic cigarette/vaped (yes/no). Participants who replied positively were asked if they had ever used electronic cigarettes/vaped again since that first time (yes/no). Respondents who replied that they had used them again were asked if they had used electronic cigarettes/vaped in the past 30 days (yes/no).
2.4. Analytic approach
Bivariate associations among potential demographic covariates, gestational exposures, and adult electronic cigarette use were examined to create the most parsimonious models. Indirect pathways from prenatal exposures to tobacco and cannabis and adult use of electronic cigarettes were tested using indirect effects modeling in Mplus. The first model tested an indirect pathway from prenatal exposures to adult offspring electronic cigarette use via childhood behavioral dysregulation (externalizing behavior problems, depression, and impulsivity). The second model tested a pathway via adolescent tobacco dependence.
3. Results
3.1. Sample characteristics
Fifty-nine percent of the mothers were Black, and 41% were White. During the first trimester of pregnancy, 29% of the mothers smoked at least 10 cigarettes/day, 63% used any alcohol, and 34% used any marijuana.
Offspring participants were 39% male, and their ages ranged from 22 to 33 years old (M = 29.7 years, SD = 3.4). Twenty-eight percent used combustible cigarettes by age 14, and 9.5% were at risk for tobacco dependence by age 16. Offspring educational attainment by young adulthood ranged from 8 to 20 years (M = 13.4 years, SD = 1.9). Thirty-two percent of the offspring had used combustible cigarettes in the past 30 days. Among those who had ever used conventional cigarettes, the average age of initiation was 15.0 years (SD = 4, range = 7-28). One in five had used cannabis in the past 30 days. Twenty-eight percent had engaged in binge drinking (more than 4 drinks in 2 h for female and more than 5 drinks in 2h for males) in the past year (National Institute on Alcohol Abuse and Alcoholism, 2016).
3.2. Electronic cigarette use
Over a third (36%) of all participants had tried electronic cigarettes at an average age of 26.9 years old (SD = 3.7, range = 17–32). Only one participant had tried electronic cigarettes prior to trying combustible cigarettes. Seventeen percent of ever electronic cigarette users reported using them more than once. Only 5% reported any use in the past 30 days, so inferential analyses focused on the group who had used them more than once. Correlations among the variables are shown in Table 2. Electronic cigarette use was significantly related to maternal race, prenatal and postnatal tobacco exposure, offspring early cigarette use, risk for tobacco dependence at age 16, and educational attainment. It was not related to SGA, child impulsivity at age 10, or postnatal maternal cigarette use after controlling for demographic variables, so these variables were not considered in further analysis.
Table 2.
Correlations among variables and adult electronic cigarette use.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Adult offspring electronic cigarette use |
– | 0.18 | 0.09 | 0.005 | −0.04 | 0.02 | −0.08 | −0.03 | 0.02 | 0.06 | 0.12 | 0.17 | 0.17 | −0.12 |
| 2. Maternal race (white) | – | – | 0.33 | 0.005 | −0.13 | 0.09 | −0.13 | 0.00 | 0.06 | 0.06 | 0.25 | 0.21 | 0.21 | 0.20 |
| 3. Prenatal exposure to 10 cigarettes/day (1st trim) |
– | – | – | 0.06 | 0.07 | −0.06 | 0.04 | 0.11 | 0.07 | 0.15 | 0.56 | 0.08 | 0.20 | −0.05 |
| 4. Prenatal exposure to Alcohol (1st trimester) |
– | – | – | – | 0.12 | 0.00 | −0.02 | 0.00 | 0.03 | 0.06 | 0.06 | 0.02 | 0.13 | −0.10 |
| 5. Prenatal exposure to cannabis (1st trimester) |
– | – | – | – | – | −0.07 | 0.00 | 0.09 | 0.00 | 0.12 | 0.02 | 0.10 | −0.02 | −0.06 |
| 6. Offspring sex (male) | – | – | – | – | – | – | −0.10 | 0.12 | 0.03 | −0.10 | −0.05 | −0.06 | −0.10 | −0.03 |
| 7. Small for gestational age | – | – | – | – | – | – | – | −0.03 | 0.04 | 0.10 | 0.04 | 0.04 | 0.00 | −0.10 |
| 8. Child impulsivity at age 10 (SNAP) |
– | – | – | – | – | – | – | – | 0.56 | 0.16 | −0.04 | 0.12 | 0.02 | −0.18 |
| 9. Externalizing behavior problems-age 10 (CBCL) |
– | – | – | – | – | – | – | – | – | 0.17 | 0.04 | 0.11 | 0.13 | −0.16 |
| 10. Depressive symptoms at age 10 (CDI) |
– | – | – | – | – | – | – | – | – | – | 0.09 | 0.10 | 0.11 | −0.21 |
| 11. Maternal cigarette use 14 years post-partum |
– | – | – | – | – | – | – | – | – | – | – | 0.08 | 0.13 | −0.03 |
| 12. Offspring early cigarette use (by age 14) |
– | – | – | – | – | – | – | – | – | – | – | – | 0.33 | −0.10 |
| 13. Offspring risk for tobacco dependence (FTND > 3 by age 16) |
– | – | – | – | – | – | – | – | – | – | – | – | – | −0.20 |
| 14. Offspring current educational attainment |
– | – | – | – | – | – | – | – | – | – | – | – | – | – |
NB. Correlations ⩾ 0.09 in their absolute value are significant at p < .05.
3.3. Multivariate analyses
In the first structural equation model, pathways were tested between gestational exposures and childhood behavioral dysregulation to adolescent early cigarette use and adult use of electronic cigarettes, controlling for maternal race and offspring educational attainment (Fig. 1). The direct pathways from prenatal tobacco and prenatal cannabis exposures to adult electronic cigarette use were not significant, and there was no indirect effect of gestational exposures via childhood dysregulation. There was a significant indirect pathway (coef. = 0.06, S.E. = 0.03, p < 0.05) linking prenatal tobacco and cannabis exposures to adult electronic cigarette use via early adolescent combustible cigarette use.
Fig. 1.
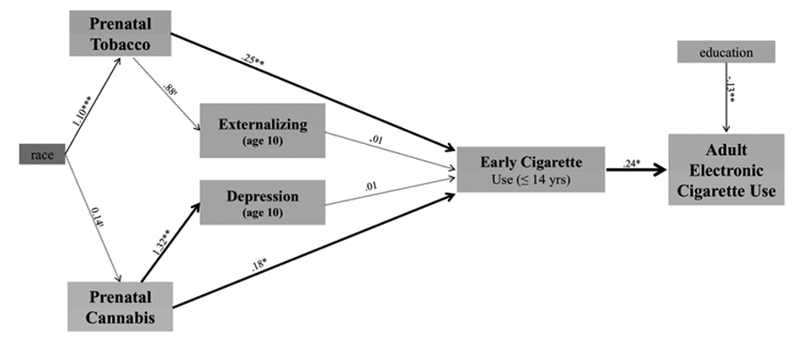
Pathways to electronic cigarette use through childhood behavioral dysregulation.
NB. tp < 0.10, *p < 0.05, **p < 0.01, ***p < 0.001; RMSEA = 0.11
In the second structural equation model, there were two significant indirect pathways (Fig. 2). One pathway linked prenatal exposures to tobacco and cannabis with adult electronic cigarette use via early adolescent combustible cigarette use and adolescent risk for tobacco dependence (coef. = 0.07, S.E. = 0.03, p < 0.05). There was a second pathway with an indirect effect of prenatal exposure to tobacco on adult electronic cigarette use via adolescent risk for tobacco dependence (coef. = 0.17, S.E. = 0.06, p < 0.01). There was no significant pathway between prenatal cannabis exposure and adolescent risk for tobacco dependence in this model.
Fig. 2.
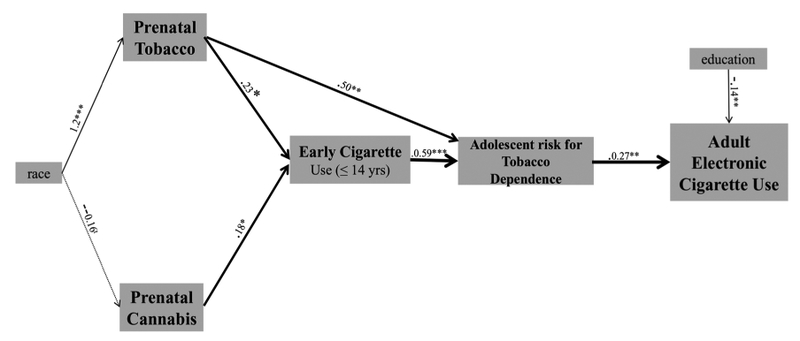
Pathways to electronic cigarette use through tobacco dependence.
NB. tp < 0.10, *p < 0.05, **p < 0.01, ***p < 0.001; RMSEA = 0.11
4. Discussion
This paper explored pathways from prenatal exposures to tobacco and cannabis to childhood and adolescent risk factors that may promote electronic cigarette use during adulthood. Our first model tested a hypothesis that electronic cigarette use would be predicted by gestational exposures to tobacco and cannabis due to their effects on childhood behavioral dysregulation and early adolescent cigarette use. The indirect pathways from gestational exposures to behavioral dysregulation and electronic cigarette use were not supported by the data. In this sample, there were direct effects of gestational tobacco and cannabis on adolescent risk for early cigarette use rather than indirect effects via childhood behavioral dysregulation. Youth who use electronic cigarettes may have an intermediate risk profile that lies somewhere between non-users and dual users who use combustible cigarettes and electronic cigarettes (Demissie et al., 2017; Dutra and Glantz, 2017; Wills et al., 2015). Thus, the use of electronic cigarettes may reflect self-regulation in the offspring who are using them to quit smoking combustible cigarettes or to adopt a product that they deem as less risky to their health. We tested this in post-hoc analysis and found that electronic cigarette use was more common in current compared to former smokers, suggesting that these were not successful quit attempts. Additionally, former smokers who had used electronic cigarettes more than once or in the past 30 days also reported that it had been fewer days since their last combustible cigarette; they stayed quit an average of 29 days compared to 68 days among former smokers who had not used electronic cigarettes recently (t = 3.95, p < 0.001).
The second model tested the hypothesis that electronic cigarette use would be predicted by gestational exposures to tobacco and cannabis due to their effects on adolescent risk for tobacco dependence. Indirect pathways from prenatal tobacco and cannabis exposures to electronic cigarette use via tobacco dependence were supported by the data. Individuals who were prenatally exposed to tobacco and cannabis were more likely to use combustible cigarettes by age 14, and this placed them at risk for tobacco dependence during adolescence and subsequent electronic cigarette use during young adulthood. The results of this analysis have public health implications, specifically for women who use tobacco and cannabis and who are pregnant or trying to become pregnant. These findings suggest that their offspring are not only at risk of using cigarettes early in adolescence but also of using other nicotine products such as electronic cigarettes.
Most adults who use electronic cigarettes report that they are using them because they want to cut back or quit smoking (Glasser et al., 2016; Rutten et al., 2015). In fact, the majority of young adults in this sample who had tried electronic cigarettes reported that they had used them for smoking reduction or cessation. Most individuals who used electronic cigarettes in the NESARC-III were also smokers (of combustible cigarettes), and electronic cigarette use was strongly associated with DSM-V nicotine use disorder (Chou et al., 2017). As individuals prenatally exposed to tobacco are more likely to use combustible cigarettes (Agrawal et al., 2010; Cornelius et al., 2000, 2005; De Genna et al., 2015; Goldschmidt et al., 2012) and become tobacco dependent (Buka et al., 2003; De Genna et al., 2017; Shenassa et al., 2015), it is not surprising that they will be more likely to use electronic cigarettes than unexposed individuals. In contrast to a study using national data from the US Current Population Survey-Tobacco Use Supplement (Zhu et al., 2017), we found that electronic cigarette use was more common in current than former smokers. This finding is supported by a systematic review and meta-analysis concluding that less quitting may be achieved by smokers who have used e-cigarettes (Kalkhoran and Glantz, 2016).
Although more attention has been paid to the long-term implications of prenatal tobacco exposure on tobacco use in offspring, the results of this study highlight the additional long-term impact of prenatal cannabis exposure. In both path models, prenatal cannabis exposure made an independent contribution to early combustible cigarette use, a significant predictor of electronic cigarette use. These findings are consistent with findings that individuals prenatally exposed to cannabis are at greater risk of both combustible cigarette and cannabis use (Porath and Fried, 2005; Sonon et al., 2015). The cannabis that pregnant women are using today is at least three times more potent than the cannabis used when these cohorts were recruited (Dujourdy and Besacier, 2017; El Sohly et al., 2016; Mehmedic et al., 2010), so prenatal cannabis exposure may have even greater effects today. Moreover, a wider range of cannabis products are now being used by women of reproductive age, including blunts, or cigars and little cigars that have been emptied and stuffed with cannabis (Lankenau et al., 2017; Krauss et al., 2017; Schauer et al., 2017). As legalization of cannabis spreads across the US and prenatal cannabis use becomes more common (Brown et al., 2017), it is important for pregnant women and their health care providers to be aware of the potential consequences of gestational cannabis exposure for future risk of tobacco dependence in offspring.
This is the first study linking prenatal tobacco and cannabis exposure to electronic cigarette use, but the results should be interpreted with caution due to several limitations. One, the electronic cigarette questionnaire developed for the purposes of this study included the same standard questions used in other epidemiological studies and contained the core questions recommended by electronic cigarette experts (Pearson et al., 2017), but reliability and validity were not established. Two, the prenatal exposures were measured using maternal report and were not verified with testing of urine or meconium samples. However, several steps were taken to ensure the validity of maternal report of substance use during pregnancy, including obtaining a NIH Certificate of Confidentiality, carefully training interviewers to use non-judgmental and non-leading questions, and interviewing the women privately and assuring them that their information would not become a part of their medical record. Moreover, self-report captures substance use across a longer time period than biological measures and makes it possible to consider different patterns of use over time (Richardson et al., 2006). Questions were asked about the quantity and frequency of mothers’ tobacco and cannabis use as well as their minimum and maximum use – questions that were developed to provide more accurate estimates of maternal substance use (Day and Robles, 1989). Three, some of the mothers in this study were recruited for higher levels of prenatal cannabis and/or alcohol use, so rates of prenatal use were higher than seen in the general population. Four, the results of this study may not generalize to individuals from rural areas or those of Hispanic and Asian American origin. Five, the sample collected for the supplemental study was disproportionately female and Black, and a low proportion of these young adults had used an electronic cigarette in the past 30 days. Thus, the results warrant replication in other, more diverse samples with adults who regularly use electronic cigarettes.
This paper extends the literature linking prenatal tobacco and cannabis exposures to risk for tobacco use and use of a newer nicotine delivery system: electronic cigarettes. As rates of prenatal cannabis use and co-use of cigarettes and cannabis continue to increase in the US, these findings have implications for public health. Maternal prenatal tobacco and cannabis use were associated with electronic cigarette use in offspring more than two decades after the exposure. Prospective, longitudinal data were leveraged to test hypotheses about childhood and adolescent risk factors and pathways from these gestational exposures to adult electronic cigarette use. Childhood behavioral dysregulation was not an important part of the pathway linking prenatal tobacco and cannabis exposure to electronic cigarette use. However, these findings provide evidence that prenatal exposures are linked to the adult use of products other than combustible cigarettes via risk for adolescent tobacco dependence.
Acknowledgements
The authors wish to thank Yolanda Duncan and Julie Moss for data collection, Katy Zeglen for recruitment, scheduling and editorial assistance, and Young Jhon for data management. All are employees of the University of Pittsburgh Medical Center (UPMC) and they were paid for their assistance with the study. The authors are also very grateful for the participation of the families in the Maternal Health Practices and Child Development (MHPCD) study.
Role of funding source
Funding for this study was provided by the National Institutes of Health (DA037209). The NIH had no role in the study design, data collection, analysis or interpretation of data, manuscript preparation or submission.
Footnotes
Contributors
NDG and MD designed the study and wrote the protocol. LG conducted the statistical analysis. NDG wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interest
No conflict declared.
References
- Achenbach TM, Rescorla LA, 2001. Manual for the ASEBA School-Age Forms and Profiles. University of Vermont, Research Center for Children, Youth, and Families; Burlington, VT. [Google Scholar]
- Achenbach T, 1991. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. University of Vermont Department of Psychiatry, Burlington, VT. [Google Scholar]
- Agrawal A, Scherrer JF, Grant JD, Sartor CE, Pergadia ML, Duncan AE, Madden PA, Haber JR, Jacob T, Bucholz KK, Xian H, 2010. The effects of maternal smoking during pregnancy on offspring outcomes. Prev. Med 50, 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford J, Van Lier PA, Timmermans M, Cuijpers P, Koot HM, 2008. Prenatal smoking and internalizing and externalizing problems in children studied from childhood to late adolescence. J. Am. Acad. Child Psychiatry 47, 779–787. [DOI] [PubMed] [Google Scholar]
- Bandiera FC, Loukas A, Wilkinson AV, Perry CL, 2016. Associations between tobacco and nicotine product use and depressive symptoms among college students in Texas. Addict. Behav 63, 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandiera FC, Loukas A, Li X, Wilkinson AV, Perry CL, 2017. Depressive symptoms predict current e-cigarette use among college students in Texas. Nicotine Tob. Res 9, 1102–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion MJ, Victora C, Matijasevich A, Horta B, Anselmi L, Steer C, Menezes AM,Lawlor DA, Davey Smith G, 2010. Maternal smoking and child psychological problems: disentangling causal and noncausal effects. Pediatrics 126, e57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook DW, Brook JS, Zhang C, Whiteman M, Cohen P, Finch SJ, 2008. Developmental trajectories of cigarette smoking from adolescence to the early thirties: personality and behavioral risk factors. Nicotine Tob. Res 10, 1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Lewinsohn PM, Seeley JR, Wagner EF, 1996. Cigarette smoking, major depression, and other psychiatric disorders among adolescents. J. Am. Acad. Child Psychiatry 35, 1602–1610. [DOI] [PubMed] [Google Scholar]
- Brown QL, Sarvet AL, Shmulewitz D, Martins SS, Wall MM, Hasin DS, 2017. Trends in marijuana use among pregnant and nonpregnant reproductive-aged women 2002–2014. JAMA 317, 207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buka SL, Shenassa ED, Niaura R, 2003. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: a 30-year prospective study. Am. J. Psychiatry 160, 1978–1984. [DOI] [PubMed] [Google Scholar]
- Chou SP, Saha TD, Zhang H, Ruan WJ, Huang B, Grant BF, Blanco C, Compton W, 2017. Prevalence, correlates, comorbidity and treatment of electronic nicotine delivery system use in the United States. Drug Alcohol Depend. 178, 296–301. [DOI] [PubMed] [Google Scholar]
- Coleman-Cowger VH, Schauer GL, Peters EN, 2017. Marijuana and tobacco co-use among a nationally representative sample of US pregnant and non-pregnant women: 2005–2014 National Survey on Drug Use and Health findings. Drug Alcohol Depend 177, 130–135. [DOI] [PubMed] [Google Scholar]
- Conway KP, Green VR, Kasza KA, Silveira ML, Borek N, Kimmel HL, Sargent JD, Stanton CA, Lambert E, Hilmi N, Reissig CJ, Jackson KJ, Tanski SE, Maklan D, Hyland AJ, Compton WM, 2017. Co-occurrence of tobacco product use, substance use, and mental health problems among adults: findings from Wave 1 (2013–2014) of the Population Assessment of Tobacco and Health (PATH) Study. Drug Alcohol Depend. 177, 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Geva D, Day NL, Cornelius JR, Taylor PM, 1994. Patterns and covariates of tobacco use in a recent sample of pregnant teenagers. J. Adolesc. Health 15, 528–535. [DOI] [PubMed] [Google Scholar]
- Cornelius MD, Taylor PM, Geva D, Day NL, 1995. Prenatal tobacco and marijuana use among adolescents: effects on offspring gestational age, growth, and morphology. Pediatrics 95, 738–743. [PubMed] [Google Scholar]
- Cornelius MD, Leech SL, Goldschmidt L, Day NL, 2000. Prenatal tobacco exposure: is it a risk factor for early tobacco experimentation? Nicotine Tob. Res 2, 45–52. [DOI] [PubMed] [Google Scholar]
- Cornelius MD, Leech SL, Goldschmidt L, Day NL, 2005. Is prenatal tobacco exposure a risk factor for early adolescent smoking?: a follow-up study. Neurotoxicol. Teratol 27, 667–676. [DOI] [PubMed] [Google Scholar]
- Cornelius M, Goldschmidt L, De Genna N, Day N, 2007. Smoking during teenage pregnancies: effects on behavioral problems in offspring. Nicotine Tob. Res 9, 739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, De Genna NM, Leech SL, Willford JA, Goldschmidt L, Day NL, 2011. Effects of prenatal cigarette smoke exposure on neurobehavioral outcomes in 10-year-old children of adolescent mothers. Neurotoxicol. Teratol 33, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Goldschmidt L, De Genna NM, Larkby C, 2012. Long-term effects of prenatal cigarette smoke exposure on behavior dysregulation among 14-year-old offspring of teenage mothers. Matern. Child Health J 16, 694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran PJ, Hussong AM, 2009. Integrative data analysis: the simultaneous analysis of multiple data sets. Psychol. Methods 14, 81–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day N, Robles N, 1989. Methodological issues in the measurement of substance use. Ann. N.Y. Acad. Sci 562, 8–13. [DOI] [PubMed] [Google Scholar]
- Day NL, Jasperse D, Richardson G, Robles N, Sambamoorthi U, Taylor P, Scher M, Stoffer D, Cornelius M, 1989. Prenatal exposure to alcohol: effect on infant growth and morphologic characteristics. Pediatrics 84, 536–541. [PubMed] [Google Scholar]
- Day NL, Richardson G, Robles N, Sambamoorthi U, Taylor P, Scher M, Stoffer D, Jasperse D, Cornelius M, 1990. Effect of prenatal alcohol exposure on growth and morphology of offspring at 8 months of age. Pediatrics 85, 748–752. [PubMed] [Google Scholar]
- Day NL, Richardson GA, Goldschmidt L, Robles NP, Taylor PM, Stoffer DS, Cornelius MD, Geva D, 1994. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol. Teratol 16, 169–175. [DOI] [PubMed] [Google Scholar]
- Day NL, Richardson GA, Goldschmidt L, Cornelius MD, 2000. Effects of prenatal tobacco exposure on preschoolers’ behavior. J. Dev. Behav. Pediatr 21, 180–188. [PubMed] [Google Scholar]
- De Genna NM, Goldschmidt L, Day NL, Cornelius MD, 2015. Prenatal and postnatal maternal trajectories of cigarette use predict adolescent cigarette use. Nicotine Tob. Res 18, 988–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Genna NM, Goldschmidt L, Day NL, Cornelius MD, 2017. Prenatal tobacco exposure, maternal postnatal nicotine dependence and adolescent risk for nicotine dependence: birth cohort study. Neurotoxicol. Teratol 61, 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demissie Z, Jones SE, Clayton HB, King BA, 2017. Adolescent risk behaviors and use of electronic vapor products and cigarettes. Pediatrics 139 (2), e20162921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujourdy L, Besacier F, 2017. A study of cannabis potency in France over a 25 year period (1992–2016). Forensic Sci. Int 272, 72–80. [DOI] [PubMed] [Google Scholar]
- Dutra LM, Glantz SA, 2017. E-cigarettes and national adolescent cigarette use: 2004–2014. Pediatrics 23, e20162450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC, 2016. Changes in cannabis potency over the last 2 decades (1995–2014): analysis of current data in the United States. Biol. Psychiatry 79, 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo LG, Reddy M, Giovino GA, 1998. The relationship between depressive symptoms and cigarette smoking in US adolescents. Addiction 93, 433–440. [DOI] [PubMed] [Google Scholar]
- Farsalinos KE, Poulas K, Voudris V, Le Houezec J, 2017. Prevalence and correlates of current daily use of electronic cigarettes in the European Union: analysis of the 2014 Eurobarometer survey. Intern. Emerg. Med 4, 1–7. [DOI] [PubMed] [Google Scholar]
- Glasser AM, Collins L, Pearson JL, Abudayyeh H, Niaura RS, Abrams DB, Villanti AC, 2016. Overview of electronic nicotine delivery systems: a systematic review. Am. J. Prev. Med 52, e33–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godleski SA, Eiden RD, Schuetze P, Colder CR, Huestis MA, 2016. Tobacco exposure and maternal psychopathology: impact on toddler problem behavior. Neurotoxicol. Teratol 57, 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt L, Cornelius MD, Day NL, 2012. Prenatal cigarette smoke exposure and early initiation of multiple substance use. Nicotine Tob. Res 14, 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KA, Day NL, Leech S, Richardson GA, 2005. Prenatal marijuana exposure: effect on child depressive symptoms at ten years of age. Neurotoxicol. Teratol 27, 439–448. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO, 1991. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br. J. Addict 86, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Kalkhoran S, Glantz SA, 2016. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet. Resp. Med 4 (2), 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasza KA, Ambrose BK, Conway KP, Borek N, Taylor K, Goniewicz ML, Cummings KM, Sharma E, Pearson JL, Green VR, Kaufman AR, Bansal-Travers M., Travers MJ, Kwan, Tworek C, Cheng YC, Yang L, Pharris-Ciurej N, van Bemmel DM, Backinger CL, Compton WM, Hyland AJ, 2017. Tobacco-product use by adults and youths in the United States in 2013 and 2014. N. Engl. J. Med 376, 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Iacono WG, McGue M, 2004. Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction 99, 1548–1559. [DOI] [PubMed] [Google Scholar]
- Kovacs M, 1992. Children’s Depression Inventory. Multi-Health Systems, Inc, Tonawanda, NY. [Google Scholar]
- Krauss MJ, Rajbhandari B, Sowles SJ, Spitznagel EL, Cavazos-Rehg P, 2017. A latent class analysis of poly-marijuana use among young adults. Addict. Behav 75, 159–165. [DOI] [PubMed] [Google Scholar]
- Lankenau SE, Fedorova EV, Reed M, Schrager SM, Iverson E, Wong CF, 2017. Marijuana practices and patterns of use among young adult medical marijuana patients and non-patient marijuana users. Drug Alcohol Depend. 170, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner WV, Janssen T, Kahler CW, Audrain-McGovern J, Leventhal AM, 2017. Bi-directional associations of electronic and combustible cigarette use onset patterns with depressive symptoms in adolescents. Prev. Med 96, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Strong DR, Sussman S, Kirkpatrick MG, Unger JB, Barrington-Trimis JL, Audrain-McGovern J, 2016. Psychiatric comorbidity in adolescent electronic and conventional cigarette use. J. Psychiatr. Res 73, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Fergusson DM, 1995. Childhood conduct problems, attention deficit behaviors, and adolescent alcohol, tobacco, and illicit drug use. J. Abnorm. Child Psychol 23, 281–302. [DOI] [PubMed] [Google Scholar]
- Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, Ross SA, Khan IA, El Sohly MA, 2010. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J. Forensic Sci 55,1209–1217. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism, 2016. Alcohol Facts and Statistics. The National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD: http://pubs.niaaa.nih.gov/publications/AlcoholFacts&Stats/AlcoholFacts&Stats.pdf. [Google Scholar]
- Orlebeke JF, Knol DL, Verhulst FC, 1997. Increase in child behavior problems resulting from maternal smoking during pregnancy. Arch. Environ. Health 52, 317–321. [DOI] [PubMed] [Google Scholar]
- Orlebeke JF, Knol DL, Verhulst FC, 1999. Child behavior problems increased by maternal smoking during pregnancy. Arch. Environ. Health 54, 15–19. [DOI] [PubMed] [Google Scholar]
- Pearson JL, Hitchman SC, Brose LS, Bauld L, Glasser AM, Villanti AC, McNeill A, Abrams DB, Cohen JE, 2017. Recommended core items to assess e-cigarette use in population-based surveys. Tob. Control 24, 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham W, Bender M, 1982. Peer relationships in hyperactive children. Description and treatment. Adv. Learn. Behav. Disabil 17, 560–567. [Google Scholar]
- Porath AJ, Fried PA, 2005. Effects of prenatal cigarette and marijuana exposure on drug use among offspring. Neurotoxicol. Teratol 27, 267–277. [DOI] [PubMed] [Google Scholar]
- Prokhorov AV, de Moor CA, Hudmon KS, Kelder SH, Conroy JL, Ordway N, 2001. Nicotine dependence, withdrawal symptoms, and adolescents’ readiness to quit smoking. Nicotine Tob. Res 3, 151–155. [DOI] [PubMed] [Google Scholar]
- Ramo DE, Young-Wolff KC, Prochaska JJ, 2015. Prevalence and correlates of electronic-cigarette use in young adults: findings from three studies over five years. Addict. Behav 41, 142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Huestis MA, Day NL, 2006. Assessing in utero exposure to cannabis and cocaine In: Bellinger DC (Ed.), Human Developmental Neurotoxicology. Taylor and Francis, New York, pp. 287–302. [Google Scholar]
- Rutten LJ, Blake KD, Agunwamba AA, Grana RA, Wilson PM, Ebbert JO, Okamoto J, Leischow SJ, 2015. Use of e-cigarettes among current smokers Associations among reasons for use, quit intentions, and current tobacco use. Nicotine Tob. Res 17, 1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer GL, Rosenberry ZR, Peters EN, 2017. Marijuana and tobacco co-administration in blunts, spliffs, and mulled cigarettes: a systematic literature review. Addict. Behav 64, 200–211. [DOI] [PubMed] [Google Scholar]
- Schuetze P, Eiden RD, Colder CR, Huestis MA, Leonard KE, 2018. Prenatal risk and infant regulation: indirect pathways via fetal growth and maternal prenatal stress and anger. Child Dev 89, e123–e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenassa ED, Papandonatos GD, Rogers ML, Buka SL, 2015. Elevated risk of nicotine dependence among sib-pairs discordant for maternal smoking during pregnancy: evidence from a 40-year longitudinal study. Epidemiology 26, 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitarenios G, Stein S, 2004. The use of the children’s depression inventory In: Maruish M (Ed.), The Use of Psychological Testing for Treatment Planning and Outcomes Assessment, 3rd ed Lawrence Erlbaum Associates, Mahwah, NJ, pp. 1–37. [Google Scholar]
- Sonon KE, Richardson GA, Cornelius JR, Kim KH, Day NL, 2015. Prenatal marijuana exposure predicts marijuana use in young adulthood. Neurotoxicol. Teratol 47, 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonon K, Richardson GA, Cornelius J, Kim KH, Day NL, 2016. Developmental pathways from prenatal marijuana exposure to Cannabis Use Disorder in young adulthood. Neurotoxicol. Teratol 58, 46–52. [DOI] [PubMed] [Google Scholar]
- Vardavas CI, Filippidis FT, Agaku IT, 2015. Determinants and prevalence of e-cigarette use throughout the European Union A secondary analysis of 26, 566 youth and adults from 27 countries. Tob. Control 24, 442–448. [DOI] [PubMed] [Google Scholar]
- Wills TA, Knight R, Williams RJ, Pagano I, Sargent JD, 2015. Risk factors for exclusive e-cigarette use and dual e-cigarette use and tobacco use in adolescents. Pediatrics 135 (1), e43–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M, Windle RC, 2001. Depressive symptoms and cigarette smoking among middle adolescents: prospective associations and intrapersonal and interpersonal influences. J. Consult. Clin. Psychol 69, 215–226. [PubMed] [Google Scholar]
- Zhu SH, Zhuang YL, Wong S, Cummins SE, Tedeschi GJ, 2017. E-cigarette use and associated changes in population smoking cessation: evidence from US current population surveys. B. Med. J 358, j3262. [DOI] [PMC free article] [PubMed] [Google Scholar]


