Abstract
Background
Optimal management of patients with intrahepatic cholangiocarcinoma (ICCA) and elevated CA19-9 remains undefined. We hypothesized CA19-9 elevation above normal indicates aggressive biology and that inclusion of CA19-9 would improve staging discrimination.
Methods
The National Cancer Data Base (NCDB-2010-2012) was reviewed for patients with ICCA and reported CA19-9. Patients were stratified by CA19-9 above/below normal reference range. Unadjusted Kaplan–Meier and adjusted Cox-proportional-hazards analysis of overall survival (OS) were performed.
Results
A total of 2,816 patients were included: 938 (33.3%) normal; 1,878 (66.7%) elevated CA19-9 levels. Demographic/pathologic and chemotherapy/radiation were similar between groups, but patients with elevated CA19-9 had more nodal metastases and less likely to undergo resection. Among elevated-CA19-9 patients, stage-specific survival was decreased in all stages. Resected patients with CA19-9 elevation had similar peri-operative outcomes but decreased long-term survival. In adjusted analysis, CA19-9 elevation independently predicted increased mortality with impact similar to node-positivity, positive-margin resection, and non-receipt of chemotherapy. Proposed staging system including CA19-9 improved survival discrimination over AJCC 7th edition.
Conclusion
Elevated CA19-9 is an independent risk factor for mortality in ICCA similar in impact to nodal metastases and positive resection margins. Inclusion of CA19-9 in a proposed staging system increases discrimination. Multi-disciplinary therapy should be considered in patients with ICCA and CA19-9 elevation.
Keywords: cholangiocarcinoma, CA19-9, biomarker, biologic resectability
INTRODUCTION
Intrahepatic cholangiocarcinoma (ICCA), also known as peripheral cholangiocarcinoma, is the second most common primary liver malignancy after hepatocellular carcinoma [1]. Although many patients present with either distant metastases or anatomically unresectable tumors with a historical survival of 6–12 months, aggressive surgical intervention can offer improved survival in selected patients with localized disease [2]. Assessment of resectability in ICCA has traditionally focused only on anatomic factors and the achievement of negative resection margins while preserving postoperative hepatic function [3]. Despite these criteria, long-term outcomes for ICCA after resection remain variable with median survival of 15–80 months and 5-year OS ranging from 14% to 40% [4]. The high recurrence rate and poor long-term prognosis associated with resected ICC reinforces the use of multimodality therapy [5], and patients identified pre-operatively with increased biologic risk may be better triaged for either neoadjuvant or adjuvant therapy to maximize patient outcomes [6,7]. For ICCA, previously identified important risk factors include: patient age, tumor size, multiple tumors or satellites, lymph node metastases, vascular invasion, positive margins, and pre-existing liver disease [8–10]. However, a definitive and quantifiable biologic risk factor to facilitate pre-operative identification of patients at highest risk of early post-operative recurrence remains lacking [11].
Originally found in the serum of metastatic gastrointestinal cancer patients in the 1970’s [12,13], Carbohydrate Antigen 19-9 (CA19-9) is an established biomarker in pancreatic and hepatobiliary malignancies [14–19] and has been suggested to play a mechanistic role in the formation of metastasis [20–23]. We have recently shown that any CA19-9 elevation above normal in patients with anatomically resectable pancreatic cancer is independently associated with decreased survival in a surgery-first strategy and that neoadjuvant systemic chemotherapy is the only treatment sequence that mitigates this increased biological risk [17]. Prior studies have explored the significance of CA19-9 elevation in ICCA as it relates to recurrence and decreased long-term survival after curative-intent resection [19,24], but these have all been institutional in nature with limited power. Given the relative rarity of cholangiocarcinoma, regardless of subtype, few institutions have sufficient clinical volume to accrue a large enough cohort of patients to effectively perform biomarker studies. Hence, the utility of CA19-9 as a triage biomarker for patients with ICCA remains unclear.
The current AJCC staging system for ICCA includes tumor, node, and metastasis categories to determine the summary stage with mixed results quantifying its prognostic value [25–27]. Consequently, the subject of staging quality in ICCA remains an area of active investigation [5,8,28–31] with a lack of consensus on how best to counsel, triage, and sequence therapy for these patients [11]. CA19-9 is a readily available and inexpensive measure of tumor biology, quantifiable in the pre-operative setting without invasive tests—making it an ideal parameter for use in a staging system. Because of its pro-metastatic mechanism, we hypothesized that any CA19-9 elevation in anatomically resectable ICCA indicates a biologically aggressive phenotype with worse prognosis and such patients should be considered for multidisciplinary therapy. We further hypothesized that inclusion of CA19-9 as a marker of biologically borderline resectability would improve staging quality over the current AJCC 7th edition staging. We sought to evaluate these hypotheses using a national hospital-based datasource.
METHODS
Methods used were similar to those used in our prior study of CA19-9 in pancreatic cancer [17] and are further detailed below. Our Institutional Review Board has deemed analysis of the NCDB PUF exempt from review. The NCDB contains over 30 million records of individual cancer cases collected by more than 1,500 Commission on Cancer (CoC) approved facilities across the United States (US) and is estimated to capture 70% of newly diagnosed cases of cancer in the US [32].
Patients with ICCA were identified using International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) topography (C22.0–22.1), and histology (8160) codes. Included patients were diagnosed and treated at the reporting facility. Patients diagnosed with multi-site cancer and those missing pathologic or follow-up data were excluded. Summary staging was assessed using the 7th edition AJCC staging manual. Curative intent surgery included surgery of primary site codes 20–26 (wedge resection); 30–38 (Lobectomy); 50–59 (extended lobectomy); 60–61 (hepatectomy); 65–66 (bile duct excision); and 75 (hepatectomy with transplantation). Patients with surgical codes 0 (no surgery), 10–17 (local tumor destruction), 90 (Surgery, NOS), and 99 (Unknown) were classified as not having curative intent surgery. A STROBE-compliant diagram showing patients included and excluded is provided in Figure 1.
Fig. 1.
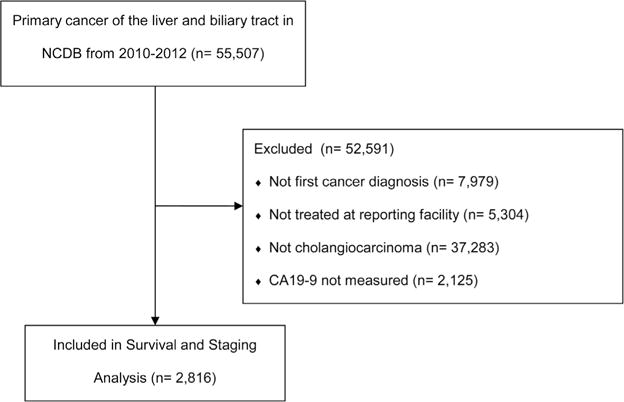
STROBE-compliant diagram of included and excluded patients.
CA19-9 is reported in the biliary NCDB PUF as site-specific-factor 12 and is the highest value documented in the medical record prior to treatment. The value is recorded continuously between 0 and 98.0 U/ml, and values in excess of 98.0 U/ml are clipped. Because of the clipped nature of the data, sensitivity analysis to determine an ideal cut-off level using this data source is not possible. Institutional threshold normal levels of CA19-9 vary from 35 to 55 U/ml because of multiple commercially available test assays. At most institutions, a CA19-9 level of 37 U/ml or less is considered normal, and we have used this level as the threshold, consistent with previous methods [17]. Prior to 2010, CA19-9 was not reported in the NCDB (<0.5% of cases per year). Therefore, cases prior to 2010 were excluded from the analysis. Bilirubin level is not reported in the NCDB PUF, making bilirubin correction impossible. Missing data were handled with indicator variables, and these are displayed in the tables.
Statistical Analysis
For intergroup comparisons, normally distributed continuous data were expressed as mean and standard deviation examined with the two-tailed student’s t-test. Non-normally distributed data were expressed as median and inter-quartile range and examined with the Mann–Whitney U test. Pearson’s chi-squared tests were used for uniformly distributed categorical variables and Fisher’s exact test was used for non-uniformly distributed categorical variables.
The primary study outcome was OS and secondary outcome was peri-operative mortality. Unadjusted survival analysis was performed using the method of Kaplan and Meier with survival defined as time from diagnosis to death or censor. Unadjusted survival estimates were compared with the log-rank test. NCDB does not provide data on progression or recurrence, therefore OS is reported. To estimate impact of CA19-9 level on survival, a multivariable Cox proportional hazards model adjusted for age, race, Charlson–Deyo comorbidity score, AJCC 7th edition stage, tumor size, node status, grade, presence of lympho-vascular invasion, margin status, receipt of radiation, chemotherapy, surgery, and type of facility. NCDB does not capture information on tumor multicentricity or multifocality, therefore, it was not possible to adjust for these pathologic covariates.
Staging was modified based on CA19-9 levels and patients were moved into new stages for comparison with AJCC 7th edition summary staging. In the proposed staging system, patients were considered to be in Proposed-Stage I if they were AJCC 7th edition Stage I with a normal CA19-9. Patients were considered Proposed-Stage II if they were AJCC 7th edition Stage I with elevated CA19-9 or AJCC 7th edition Stage II or III with normal CA19-9. Finally, patients were placed in Proposed-Stage III if they were AJCC 7th edition Stages II or III with elevated CA19-9. Patients with Stage IV disease were not altered. Unadjusted OS estimates were computed for Stages I–III patients under both staging systems, and the improvement in predictive ability using the Proposed-Stage compared to the AJCC 7th edition summary stage was assessed via the increase in the commonly used model assessment measures Concordance index and Goodman and Kruskal’s gamma statistic [33]. The net reclassification improvement (NRI) outlined by Pencina [34–37] has also become a popular means of assessing predictive improvement in survival models and was applied for this analysis. The nonparametric bootstrap procedure [38,39] was used to provide 95% confidence intervals for the predictive improvement under each of these measures, as suggested by Pencina [35]. Thus, a significance level of 0.05 was used for all comparisons. Statistical analysis was performed with R version 3.2.4 (“Very Secure Dishes”— R Foundation for Statistical Computing— Vienna, Austria www.r-project.org).
RESULTS
A total of 2,816 (57.8%) patients had CA19-9 reported in the database and were included; 2,125 (42.2%) had no reported CA19-9 and were excluded (Fig. 1). Unadjusted survival was similar between reported and unreported biomarker cohorts suggesting similar tumor biology independent of biomarker measurement (log rank P=0.945). During the course of the study, 2,150 (76.3%) patients had observed mortality and among the 23.6% of patients without observed mortality, the median length of follow-up was 22.7 months (IQR 13.2–33.6 months). Of those included, 938 (33.3%) had normal CA19-9 levels, and 1,878 (66.7%) had elevated levels. Overall demographics including age, race, Charlson–Deyo score, and year of diagnosis were similar, but patients with elevated CA19-9 were more likely to be male and to have their operation in the community (Table I). Pathologic comparisons were similar for grade, tumor size, and margin status, but patients with elevated CA19-9 were more likely to have higher stage disease due to nodal positivity (Table I). Patients with elevated CA19-9 were equally likely to receive chemotherapy and radiation but less likely to undergo surgery (83.0% vs. 67.0% having no surgery, P < 0.001 Table I).
TABLE I.
Cohort Demographics, Pathologic Characteristics, and Outcomes
| CA 19-9 ≤ 37, n = 938 | CA 19-9 > 37, n = 1,878 | P | |
|---|---|---|---|
| Median age (IQR) | 65.00 (56.00, 74.00) | 66.00 (57.00, 75.00) | 0.008 |
| Female sex | 53.1% | 47.4% | 0.005 |
| Race | 0.165 | ||
| Caucasian | 82.9% | 84.3% | |
| African American | 9.6% | 7.6% | |
| Other | 7.5% | 8.1% | |
| Charlson–Deyo score | 0.641 | ||
| 0 | 64.3% | 65.5% | |
| 1 | 23.6% | 22.0% | |
| 2+ | 12.2% | 12.5% | |
| Facility type | 0.015 | ||
| Community | 4.9% | 7.2% | |
| Comprehensive community | 29.9% | 33.0% | |
| Academic/research | 60.6% | 55.0% | |
| Integrated network | 4.6% | 4.7% | |
| Year of diagnosis | <0.628 | ||
| 2010 | 25.8% | 26.8% | |
| 2011 | 35.7% | 33.9% | |
| 2012 | 38.5% | 39.2% | |
| Stage (%) | <0.001 | ||
| Stage I | 18.2% | 10.3% | |
| Stage II | 14.4% | 12.2% | |
| Stage III | 7.3% | 6.0% | |
| Stage IV | 41.7% | 53.7% | |
| Stage unavailable | 18.4% | 17.7% | |
| Median tumor sizea | 5.0 | 5.4 | 0.110 |
| N1 statusa | 28.7% | 43.8% | <0.001 |
| High gradea | 32.0% | 35.7% | 0.516 |
| Lymphovascular invasiona | 38.3% | 46.5% | 0.200 |
| Positive margina | 14.7% | 20.4% | 0.165 |
| Radiation | 17.7% | 14.6% | 0.090 |
| Chemotherapy | 56.6% | 53.2% | 0.225 |
| Chemo surgery sequence | <0.001 | ||
| No surgery | 67.0% | 83.0% | |
| Surgery alone | 20.2% | 7.9% | |
| Neoadjuvant | 4.3% | 3.0% | |
| Surgery then adjuvant | 8.5% | 6.1% | |
| 30-day readmissiona | 6.2% | 9.1% | 0.058 |
| Median hospital stay (days)a | 9.5 | 11.2 | 0.143 |
| 30-day mortalitya | 4.2% | 4.5% | 0.110 |
| 90-day mortalitya | 6.2% | 13.2% | 0.004 |
| Median OS (months) | |||
| All patients | 14.6 | 6.6 | 0.003 |
| Resected patients | 47.8 | 22.6 | <0.001 |
| Interval survival (all patients) | |||
| 1-year | 54.9% | 34.6% | <0.001 |
| 2-year | 37.3% | 17.0% | <0.001 |
| 3-year | 26.5% | 11.1% | <0.001 |
| Interval survival (resected)a | |||
| 1-year | 84.7% | 71.2% | <0.001 |
| 2-year | 70.5% | 48.6% | <0.001 |
| 3-year | 50.7% | 40.8% | <0.001 |
Among resected patients, with evaluable data.
Among patients with elevated CA19-9, stage-specific survival was decreased in all stages (Fig. 2) with the largest difference in early stages. In the resected cohort, those with CA19-9 elevation had similar peri-operative outcomes (30-day-mortality 4.2% vs. 4.5%, P=0.110) but decreased intermediate and long-term survival (90-day mortality 13.2% with elevated CA19-9 vs. 6.2% with normal CA19-9, P < 0.001 Table I; median OS 22.6 months (elevated) vs. 47.8 months (normal), P < 0.001 Fig. 3). After adjustment for patient and tumor factors, CA19-9 elevation was independently associated with increased mortality hazard (HR 1.46 in the cohort of all patients; 1.76 in the resected cohort, both P < 0.001 Table II). The hazard ratio for CA19-9 elevation was thus similar in magnitude to that associated with node positivity (1.42, P = 0.01), positive margin resection (1.56, P = 0.004), and non-receipt of chemotherapy (1.40, P = 0.02).
Fig. 2.
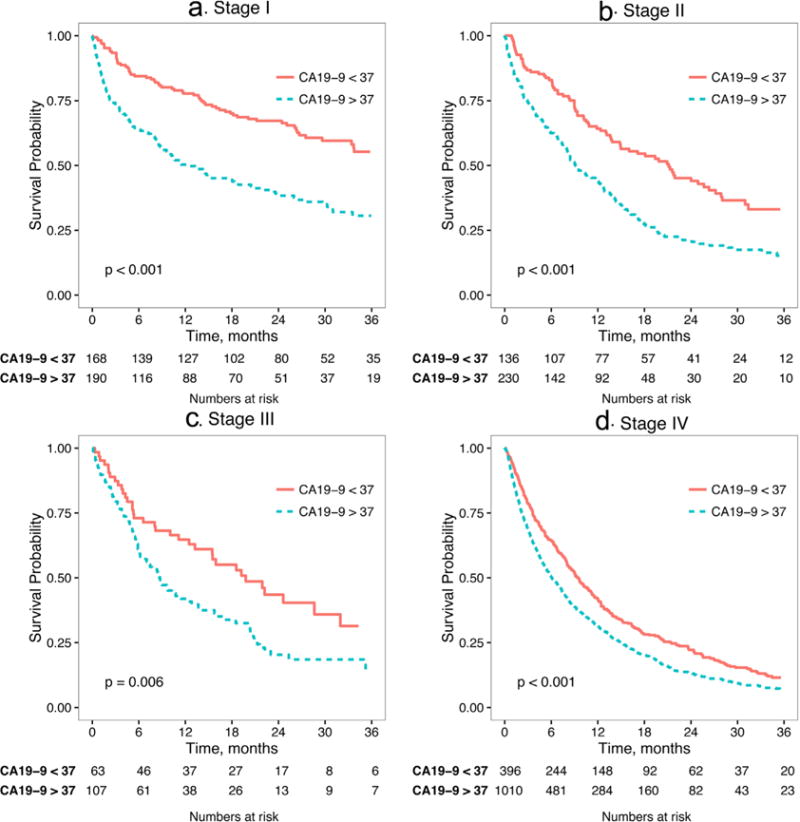
Stage-specific analysis of overall survival stratified by elevated/normal CA 19-9 level.
Fig. 3.
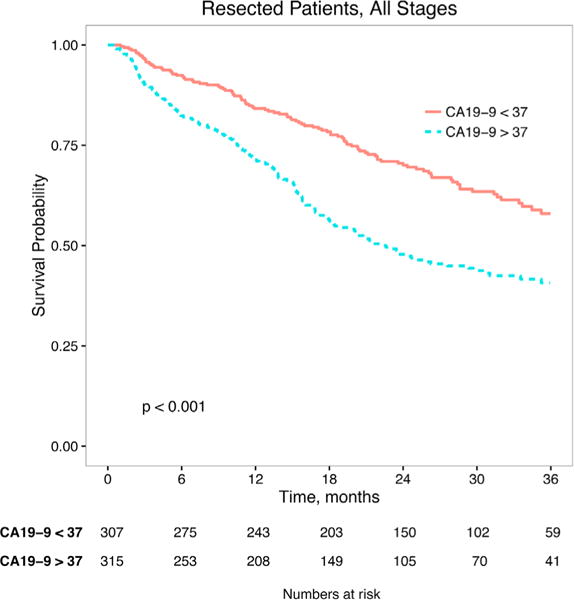
Overall survival of resected patients, stratified by elevated/normal CA19-9 level.
TABLE II.
Adjusted Cox Proportional Hazards Survival Model
| All patients (N = 2,816)
|
Resected patients (N = 625)
|
|||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P-value | HR | 95%CI | P-value | |
| CA 19–9 > 37 U/ml | 1.46 | (1.33, 1.61) | <0.001 | 1.72 | (1.33, 2.23) | <0.001 |
| Age > 65 years | 1.21 | (1.10, 1.32) | <0.001 | 1.27 | (0.99, 1.63) | 0.060 |
| Race (ref Caucasian) | ||||||
| African American | 1.07 | (0.91, 1.26) | 0.400 | 1.4 | (0.83, 2.36) | 0.210 |
| Other | 0.81 | (0.68, 0.96) | 0.010 | 0.7 | (0.43, 1.13) | 0.140 |
| Charlson–Deyo score (ref 0) | ||||||
| 1 | 1.19 | (1.07, 1.33) | 0.001 | 1.23 | (0.91, 1.67) | 0.170 |
| 2+ | 1.53 | (1.34, 1.75) | <0.001 | 1.53 | (1.06, 2.23) | 0.020 |
| Tumor size (ref <5 cm) | ||||||
| ≥5 cm | 1.26 | (1.12, 1.41) | <0.001 | 1.3 | (1.00, 1.68) | 0.050 |
| Unknown | 1.4 | (1.23, 1.58) | <0.001 | 1.53 | (0.80, 2.91) | 0.200 |
| Node status (ref no.) | ||||||
| N1 | 1.42 | (1.08, 1.86) | 0.010 | 1.39 | (0.91, 2.13) | 0.130 |
| Missing | 1.18 | (0.95, 1.46) | 0.130 | 0.89 | (0.66, 1.21) | 0.460 |
| Grade (ref low) | ||||||
| High | 1.64 | (1.43, 1.88) | <0.001 | 1.69 | (1.28, 2.22) | <0.001 |
| Unknown | 1.11 | (0.98, 1.25) | 0.100 | 0.93 | (0.62, 1.39) | 0.710 |
| Lymphovasc. invasion (ref absent) | ||||||
| Present | 1.32 | (1.06, 1.65) | 0.010 | 1.16 | (0.85, 1.57) | 0.350 |
| Unknown | 1.07 | (0.92, 1.25) | 0.390 | 0.94 | (0.68, 1.31) | 0.720 |
| Summary stage (ref stage I) | ||||||
| Stage II | 1.5 | (1.24, 1.83) | <0.001 | 2.12 | (1.40, 3.20) | <0.001 |
| Stage III | 1.42 | (1.12, 1.80) | 0.003 | 2.36 | (1.39, 4.01) | 0.001 |
| Stage IV | 1.82 | (1.53, 2.16) | <0.001 | 2.87 | (1.77, 4.64) | <0.001 |
| Stage unavailable | 1.81 | (1.48, 2.21) | <0.001 | 2.35 | (1.46, 3.79) | <0.001 |
| Surgical margins (ref negative) | ||||||
| Positive | N/A | N/A | N/A | 1.56 | (1.15, 2.13) | 0.004 |
| Unknown | N/A | N/A | N/A | 3.28 | (1.63, 6.61) | <0.001 |
| Chemotherapy (ref receipt of chemo) | ||||||
| Non-receipt | 2.22 | (2.02, 2.44) | <0.001 | 1.4 | (1.05, 1.88) | 0.020 |
| Missing | 1.74 | (1.26, 2.41) | <0.001 | 1.89 | (0.73, 4.92) | 0.190 |
| Radiation (ref receipt of radiation) | ||||||
| No radiation | 1.46 | (1.29, 1.66) | <0.001 | 0.93 | (0.67, 1.31) | 0.690 |
| Missing | 1.11 | (0.60, 2.07) | 0.740 | 0.56 | (0.13, 2.39) | 0.430 |
| No surgery (ref surgery) | 1.93 | (1.05, 3.52) | 0.030 | N/A | N/A | N/A |
| Facility type (ref community) | ||||||
| Comprehensive community | 0.97 | (0.81, 1.15) | 0.710 | 0.55 | (0.21, 1.42) | 0.220 |
| Academic/research | 0.75 | (0.63, 0.89) | <0.001 | 0.52 | (0.21, 1.31) | 0.170 |
| Integrated network | 0.8 | (0.61, 1.03) | 0.090 | 0.34 | (0.11, 1.05) | 0.060 |
Survival analysis after re-staging patients by the proposed staging system including CA19-9 as a marker of biologic resectability is shown in Figure 4. The new staging system had a concordance of 60.2% as opposed to 54.6% for the AJCC 7th edition staging, leading to an improvement of 5.5% (95%CI 3.7–7.6%). The Gamma statistic (essentially a measure of rank correlation) increased from 0.144 under the AJCC 7th edition staging to 0.321 under the new staging system, an increase of 0.177 (95%CI 0.121–0.239). Improvements in conditional concordance and Gamma (restricted to the reclassified set of observations) are similar to the improvement in concordance and Gamma on the entire sample (Supplemental Table SI). In order to evaluate NRI, one must specify a time horizon (H) for declaring an event. For example, if H = 12 months, then any deaths prior to 12 months are considered “events,” and those occurring after 12 months are “non-events.” With H = 12 months, the NRI results are summarized in Supplemental Table SI. The event NRI = 51.4%, non-event NRI = −31.2%, and NRI = event NRI + non-event NRI = 20.2% (95%CI 12.3–28.7%). An NRI of 20.2% indicates that the new staging system is effective at re-classifying events (upwards and not downwards), while less effective at re-classifying non-events (i.e., some upward reclassifying of non-events). As a whole, the NRI of 20.2% suggests that the new staging system leads to a substantial overall improvement in classification and corresponds with the visible depiction of staging improvement shown in Figure 4. The NRI estimates across varying values of H along with corresponding pointwise 95%CIs are shown in Supplemental Figure S1. The NRI stays relatively stable at approximately 20%, regardless of the value chosen for the event horizon.
Fig. 4.
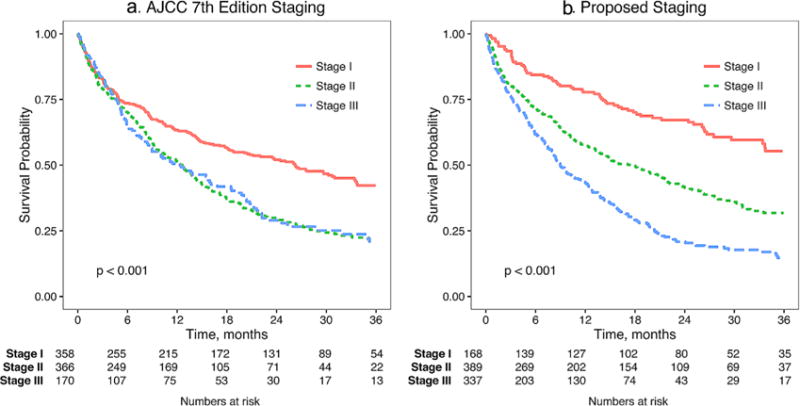
Comparison of AJCC 7th edition and proposed staging system including CA19-9.
DISCUSSION
We present here the largest analysis of CA19-9 levels in ICCA and the first to utilize a national dataset. Slightly more than half of patients presenting with ICCA had CA19-9 level reported in the NCDB. Any preoperative elevation of CA19-9 above normal is an independent negative prognostic indicator for ICCA patients adjusted for patient and tumor factors as well as receipt of surgery, chemotherapy, and radiation. The negative impact of high CA19-9 on prognosis is similar in magnitude to nodal metastases and positive margin resection—both of which are accepted predictors of poor outcome. Incorporation of CA19-9 as a marker of biologically aggressive disease improved the ability of a proposed novel staging system to correctly classify mortality risk, and this improvement was not a function of event horizon. The implications of such a change in staging are substantial for prognostic counseling, therapeutic triage, and treatment sequencing.
The fact that patients with elevated CA19-9 levels are less likely to undergo surgery than those with normal levels suggests that clinicians are already using CA19-9 in practice as a means for treatment selection. In spite of this, even in the cohort of patients undergoing curative-intent surgical resection, there is a persistent increase in adjusted mortality hazard associated with CA19-9 elevation, most marked in early stages of disease. The increased mortality due to CA19-9 elevation suggests that surgery—although necessary for long-term survival—is not sufficient as monotherapy. Additionally, the fact that non-receipt of chemotherapy is a persistent predictor of increased mortality—even in the resected cohort—indicates that chemotherapy remains an important therapeutic modality even after surgical resection (HR 1.4, P = 0.02 Table II) and supports the notion that a multidisciplinary approach to ICCA is critical to achievement of satisfactory long-term outcomes.
Although systemic cytotoxic chemotherapy options for ICCA have had limited efficacy with best results from the combination of gemcitabine and cisplatin [40], there is mounting evidence that improvement of long-term survival in patients with ICCA requires multidisciplinary therapy. Recent developments regarding use of chemoradiation in unresectable ICCA patients with disease control after initial systemic induction chemotherapy have demonstrated improvement in non-operative long-term outcomes, approaching the expected survival seen in surgical series [6,41]. Similar improvement in long-term oucomes with optimization of pre-operative sequencing have been seen in perihilar cholangiocarcinoma [42]. Due to the evolution of multidisciplinary management patterns for ICCA and advances in chemotherapy effectiveness, pre-operative identification of patients with the most aggressive disease—we believe identified by CA19-9 elevation at diagnosis—can maximize survival gained from therapy in addition to surgery [43,44]. Incorporation of this knowledge at the trial design stage can help researchers and clinicians identify the cohort of patients most likely to benefit from alternative treatment sequencing strategies.
Inclusion of CA19-9 in our proposed staging system increases discrimination. Our study is not the first effort to include CA19-9 in ICCA staging [5,8,28,45], but it is by far the largest and most well powered to evaluate such a change. A recent institutional study proposed updated ICCA staging [45] including CA19-9, but this study utilized a CA19-9 threshold of 1,000 U/ml and the institutional data analyzed had limited statistical power (N = 399 patients) to compare staging discrimination. The utilization of such a high level of CA19-9 as a trigger for re-staging leaves unaddressed an important clinical question which is: “what is best for a patient who presents with an CA19-9 above normal but not extremely high (>1,000 U/ml)?” Our analysis would suggest that this patient should be upstaged and managed in a multi-disciplinary manner.
The staging system presented here amplifies the power of pre-operative prognostic assessment through increased emphasis on tumor biology and decreased importance of tumor size and anatomy. This is an important step forward because as we learn more about tumor biology—particularly in aggressive cancers such as cholangiocarcinoma—having pre-operatively assessable biologic metrics will drive prognostic counseling and clinical management. Other parameters such as tumor grade, node status, and final pathologic analysis are certainly important predictors of survival— and our results confirm this. However, these parameters require expensive and invasive procedures (biopsy or surgical resection) in order to be adequately assessed. CA19-9 is assessable with a simple blood draw and is actionable for clinical decisions regarding non-surgical therapy and treatment sequence in the pre-operative setting. The data supporting such clinical decisions are currently lacking and trials will take years to show results, but utilization of a staging system which accounts for tumor biology can help maximize trial enrollment of the highest risk patients now. Finally, as seen in other aggressive cancers, the pre-operative identification of those with aggressive biology followed by neoadjuvant treatment can serve to select for surgical intervention only those patients who are most likely to derive benefit, which will help focus surgical resources on patients who need them the most while eliminating excess morbidity for those who would not benefit from surgery.
Limitations
Our study is limited by its retrospective and non-randomized nature, preventing the elimination of selection bias between treatment groups. We have attempted to adjust for intergroup differences with adjusted regression modeling and subgroup analysis of cohorts which are as homogenous as possible. However, the small number of patients receiving surgery and/or adjuvant therapies limit the size of this multivariable analysis, and unobserved confounding likely remains. It is not possible to adjust for biliary obstruction because the NCDB does not provide data regarding this, though jaundice is less frequent with intrahepatic compared to extrahepatic cholangiocarcinoma. There is likely a cohort of patients who have elevated CA19-9 due to biliary obstruction and not caused by aggressive disease. However, biliary obstruction—although it may contribute to treatment delay —is not in and of itself deadly and therefore is an unlikely source of the excess mortality observed. Removal of these patients with elevated CA19-9 due to obstruction—if they could be identified— would likely result in an even more dramatic difference in outcomes for the remaining patients with CA19-9 elevation, compared to those with normal levels.
CONCLUSION
We have demonstrated using national data adjusted for patient, tumor, and treatment factors that CA19-9 elevation at diagnosis is an independent predictor of increased mortality and decreased OS most marked in early stage ICCA. The increased mortality hazard is similar in impact to nodal metastases and positive resection margins—both accepted predictors of poor outcomes with resectional surgery. Our proposed staging system including CA19-9 more accurately stratifies OS by increasing the emphasis placed on biology and decreasing emphasis on anatomy. Quantification of the impact of elevated biomarker level on survival is informative for clinicians in understanding disease prognosis and has implications for patient counseling. Utilization of this staging system can help facilitate trial enrollment of the cohort of patients with the most aggressive disease who are most likely to benefit from multidisciplinary therapy. Additional studies are needed to further confirm and interpret the present findings with robust control for the presence of biliary inflammation, jaundice, the presence/absence of underlying liver disease, and peri-operative complications.
Supplementary Material
Acknowledgments
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used are derived from a de-identified NCDB participant user file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methods or the conclusions drawn from these data by the investigators. The authors gratefully acknowledge the support of the Mayo Clinic Department of Surgery and the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery as substantial contributors of resources to the project. Additionally, Dr. Bergquist acknowledges the support of the Mayo Clinic Clinician Investigator Training Program for salary support. Finally, we would like to thank the Society of Surgical Oncology for affording us the opportunity to present this work at their annual Cancer Symposium in March 2016. The Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery provides salary support for Dr. Habermann, Dr. Storlie, and Dr. Ivanics. Dr. Bergquist receives salary support from the Mayo Clinic Clinician Investigator Training program. Dr. Gores is supported by NIH R01 DK059427, DK041876, and DK063947. Dr. Roberts is supported by NIH R01 CA165076 and U01 DK082843. The conduct and presentation of this research was independent of the above funding sources. This work has not previously or concurrently been submitted for publication. The work was presented during the Society of Surgical Oncology Annual Cancer Symposium, Boston, MA March 2–5, 2016.
Grant sponsor: The Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery; Grant sponsor: Mayo Clinic Clinician Investigator Training Program; Grant sponsor: NIH; Grant numbers: R01, DK059427, DK041876, DK063947; Grant sponsor: NIH; Grant numbers: R01, CA165076, U01 DK082843.
Footnotes
Conflicts of interest: None.
AUTHORS’ CONTRIBUTIONS
Bergquist, Truty, Ivanics, Storlie, Habermann, Tee, and Groeschl contributed in the study design and conception. Bergquist, Storlie, Ivanics, Truty, and Habermann helped in analysis and interpretation of data. Initial drafting of manuscript was carried on by Bergquist, Ivanics, Groeschl, Tee, Habermann, and Truty. Bergquist, Ivanics, Groeschl, Tee, Storlie, Habermann, Smoot, Nagorney, Gores, Roberts, and Truty contributed in critical review, revision, and final approval of the manuscript.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Khan SA, Taylor-Robinson SD, Toledano MB, et al. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806–813. doi: 10.1016/s0168-8278(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 2.Khan SA, Thomas HC, Davidson BR, et al. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 3.Lang H, Sotiropoulos GC, Frühauf NR, et al. Extended hepatectomy for intrahepatic cholangiocellular carcinoma (ICC): When is it worthwhile? Single center experience with 27 resections in 50 patients over a 5-year period. Ann Surg. 2005;241:134–143. doi: 10.1097/01.sla.0000149426.08580.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lafaro KJ, Cosgrove D, Geschwind J-FH, et al. Multidisciplinary care of patients with intrahepatic cholangiocarcinoma: Updates in management. Gastroenterol Res Pract. 2015;2015:860861. doi: 10.1155/2015/860861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doussot A, Groot-Koerkamp B, Wiggers JK, et al. Outcomes after resection of intrahepatic cholangiocarcinoma: External validation and comparison of prognostic models. J Am Coll Surg. 2015;221:452–461. doi: 10.1016/j.jamcollsurg.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maithel SK, Gamblin TC, Kamel I, et al. Multidisciplinary approaches to intrahepatic cholangiocarcinoma. Cancer. 2013;119:3929–3942. doi: 10.1002/cncr.28312. [DOI] [PubMed] [Google Scholar]
- 7.Weber SM, Ribero D, O’Reilly EM, et al. Intrahepatic cholangiocarcinoma: Expert consensus statement. HPB (Oxford) 2015;17:669–680. doi: 10.1111/hpb.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou H, Jiang X, Li Q, et al. A simple and effective prognostic staging system based on clinicopathologic features of intrahepatic cholangiocarcinoma. Am J Cancer Res. 2015;5:1831–1843. [PMC free article] [PubMed] [Google Scholar]
- 9.Sakamoto Y, Kokudo N, Matsuyama Y, et al. Proposal of a new staging system for intrahepatic cholangiocarcinoma: Analysis of surgical patients from a nationwide survey of the Liver Cancer Study Group of Japan. Cancer. 2016;122:61–70. doi: 10.1002/cncr.29686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyder O, Marques H, Pulitano C, et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: An Eastern and Western experience. JAMA Surg. 2014;149:432–438. doi: 10.1001/jamasurg.2013.5168. [DOI] [PubMed] [Google Scholar]
- 11.Bartella I, Dufour J-F. Clinical diagnosis and staging of intrahepatic cholangiocarcinoma. J Gastrointestin Liver Dis. 2015;24:481–489. doi: 10.15403/jgld.2014.1121.244.chl. [DOI] [PubMed] [Google Scholar]
- 12.Koprowski H, Steplewski Z, Mitchell K, et al. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- 13.Koprowski H, Herlyn M, Steplewski Z, et al. Specific antigen in serum of patients with colon carcinoma. Science. 1981;212:53–55. doi: 10.1126/science.6163212. [DOI] [PubMed] [Google Scholar]
- 14.Berger AC, Meszoely IM, Ross EA, et al. Undetectable preoperative levels of serum CA 19-9 correlate with improved survival for patients with resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2004;11:644–649. doi: 10.1245/ASO.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 15.Barton JG, Bois JP, Sarr MG, et al. Predictive and prognostic value of CA 19-9 in resected pancreatic adenocarcinoma. J Gastrointest Surg. 2009;13:2050–2058. doi: 10.1007/s11605-009-0849-z. [DOI] [PubMed] [Google Scholar]
- 16.Hartwig W, Strobel O, Hinz U, et al. CA19-9 in potentially resectable pancreatic cancer: Perspective to adjust surgical and perioperative therapy. Ann Surg Oncol. 2013;20:2188–2196. doi: 10.1245/s10434-012-2809-1. [DOI] [PubMed] [Google Scholar]
- 17.Bergquist JR, Puig CA, Shubert CR, et al. Carbohydrate antigen 19-9 elevation in anatomically resectable, early-Stage pancreatic cancer is independently associated with decreased overall survival and an indication for neoadjuvant therapy: A national cancer database study. J Am Coll Surg. 2016;223:52–65. doi: 10.1016/j.jamcollsurg.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Kondo N, Murakami Y, Uemura K, et al. Prognostic impact of perioperative serum CA 19-9 levels in patients with resectable pancreatic cancer. Ann Surg Oncol. 2010;17:2321–2329. doi: 10.1245/s10434-010-1033-0. [DOI] [PubMed] [Google Scholar]
- 19.Kondo N, Murakami Y, Uemura K, et al. Elevated perioperative serum CA 19-9 levels are independent predictors of poor survival in patients with resectable cholangiocarcinoma. J Surg Oncol. 2014;110:422–429. doi: 10.1002/jso.23666. [DOI] [PubMed] [Google Scholar]
- 20.Kannagi R. Carbohydrate-mediated cell adhesion involved in hematogenous metastasis of cancer. Glycoconj J. 1997;14:577–584. doi: 10.1023/a:1018532409041. [DOI] [PubMed] [Google Scholar]
- 21.Koike T, Kimura N, Miyazaki K, et al. Hypoxia induces adhesion molecules on cancer cells: A missing link between Warburg effect and induction of selectin-ligand carbohydrates. Proc Natl Acad Sci USA. 2004;101:8132–8137. doi: 10.1073/pnas.0402088101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kannagi R, Izawa M, Koike T, et al. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci. 2004;95:377–384. doi: 10.1111/j.1349-7006.2004.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kannagi R. Carbohydrate antigen sialyl Lewis a–its pathophysiological significance and induction mechanism in cancer progression. Chang Gung Med J. 2007;30:189–209. [PubMed] [Google Scholar]
- 24.Chung MJ, Lee KJ, Bang S, et al. Preoperative serum CA 19-9 level as a predictive factor for recurrence after curative resection in biliary tract cancer. Ann Surg Oncol. 2011;18:1651–1656. doi: 10.1245/s10434-010-1529-7. [DOI] [PubMed] [Google Scholar]
- 25.Nathan H, Pawlik TM. Staging of intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol. 2010;26:269–273. doi: 10.1097/MOG.0b013e328337c899. [DOI] [PubMed] [Google Scholar]
- 26.Farges O, Fuks D, Treut Le Y-P, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: By the AFC-IHCC-2009 study group. Cancer. 2011;117:2170–2177. doi: 10.1002/cncr.25712. [DOI] [PubMed] [Google Scholar]
- 27.Ali SM, Clark CJ, Mounajjed T, et al. Model to predict survival after surgical resection of intrahepatic cholangiocarcinoma: The Mayo Clinic experience. HPB (Oxford) 2015;17:244–250. doi: 10.1111/hpb.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188–1195. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]
- 29.Li T, Qin L-X, Zhou J, et al. Staging, prognostic factors and adjuvant therapy of intrahepatic cholangiocarcinoma after curative resection. Liver Int. 2014;34:953–960. doi: 10.1111/liv.12364. [DOI] [PubMed] [Google Scholar]
- 30.Uenishi T, Ariizumi S, Aoki T, et al. Proposal of a new staging system for mass-forming intrahepatic cholangiocarcinoma: A multicenter analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2014;21:499–508. doi: 10.1002/jhbp.92. [DOI] [PubMed] [Google Scholar]
- 31.Hwang S, Lee Y-J, Song G-W, et al. Prognostic impact of tumor growth type on 7th AJCC staging system for intrahepatic cholangiocarcinoma: A single-center experience of 659 cases. J Gastrointest Surg. 2015;19:1291–1304. doi: 10.1007/s11605-015-2803-6. [DOI] [PubMed] [Google Scholar]
- 32.Raval MV, Bilimoria KY, Stewart AK, et al. Using the NCDB for cancer care improvement: An introduction to available quality assessment tools. J Surg Oncol. 2009;99:488–490. doi: 10.1002/jso.21173. [DOI] [PubMed] [Google Scholar]
- 33.Therneau TM, Grambsch PM. Modeling survival data: Extending the Cox model. 1st. New York: Springer-Verlag; 2000. [Google Scholar]
- 34.Pencina MJ, D’Agostino RB, D’Agostino RB, et al. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 35.Pencina MJ, D’Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med. 2010;48:1703–1711. doi: 10.1515/CCLM.2010.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pencina MJ, D’Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pencina KM, Pencina MJ, D’Agostino RB. What to expect from net reclassification improvement with three categories. Stat Med. 2014;33:4975–4987. doi: 10.1002/sim.6286. [DOI] [PubMed] [Google Scholar]
- 38.Storlie CB, Swiler LP, Helton JC, et al. Implementation and evaluation of nonparametric regression procedures for sensitivity analysis of computationally demanding models. Reliab Eng Syst Saf. 2009;94:1735–1763. [Google Scholar]
- 39.Davison AC, Hinckley DV. Bootstrap methods and their application. 1st. New York: Cambridge University Press; 1997. [Google Scholar]
- 40.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 41.Rogers JE, Nguyen V, Nogueras-Gonzalez GM, et al. Characterization of unresectable cholangiocarcinoma patients treated with or without chemoradiation. ASCO Meet Abstr. 2015;33:403. [Google Scholar]
- 42.Rea DJ, Rosen CB, Nagorney DM, et al. Transplantation for cholangiocarcinoma: When and for whom? Surg Oncol Clin N Am. 2009;18:325–337. doi: 10.1016/j.soc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Guion-Dusserre J-F, Lorgis V, Vincent J, et al. FOLFIRI plus bevacizumab as a second-line therapy for metastatic intrahepatic cholangiocarcinoma. World J Gastroenterol. 2015;21:2096–2101. doi: 10.3748/wjg.v21.i7.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang R, Wang B, Chen Y, et al. Efficacy of gemcitabine plus platinum agents for biliary tract cancers: A meta-analysis. Anticancer Drugs. 2013;24:871–877. doi: 10.1097/CAD.0b013e3283637292. [DOI] [PubMed] [Google Scholar]
- 45.Chaiteerakij R, Harmsen WS, Marrero CR, et al. A new clinically based staging system for perihilar cholangiocarcinoma. Am J Gastroenterol. 2014;109:1881–1890. doi: 10.1038/ajg.2014.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


