Abstract
Modification of messenger RNAs through a process called m6A methylation facilitates dynamic temporal regulation of RNA levels in neural precursor cells, enabling fine-tuning of developing neuronal circuits in the brain.
One of the most highly evolved structures in the human brain is the cerebral cortex1, which contains precisely structured layers of neurons that are involved in many aspects of cognition, including information processing and memory storage. During brain development, both the birth of neurons and their time-dependent wiring into complex functional circuits that span multiple neuronal layers must be carefully regulated to ensure normal cortical function. However, the molecular mechanisms that underlie these developmental processes remain mysterious. Writing in Cell, Yoon et al.2 report an analysis of gene regulation in the developing mouse cortex. Their work reveals a mechanism that mediates precise temporal control over gene expression to ensure proper function of neuronal precursors called radial glia.
The authors investigated a major player in gene regulation3–9 — the chemical attachment of a methyl group to a particular nitrogen atom of the nucleoside adenosine in messenger RNA. Such adenosine N6-methylation (dubbed m6A methylation) is catalysed in cells by Mettl3 and Mettl14 enzymes, among others7–9.
Yoon et al. set out to determine the role of m6A methylation in the nervous system. The authors analysed mice engineered either to lack the Mettl14 gene or to express lower than normal levels of Mettl3. They found that decreases in m6A methylation caused by these mutations increased levels of various messenger RNA molecules. The researchers then demonstrated that RNA m6A methylation causes enhanced RNA degradation by shortening the half-life of target mRNA species, limiting for how long the proteins that they encode can exert their effects in the cell. The affected transcripts mainly encode transcription factors and regulators of the cell cycle and neuronal differentiation. The authors found that, in wild-type embryos, RNA m6A methylation led to coordinated downregulation of target transcripts during crucial phases of development, indicating that dynamic adenosine modification enables temporal fine-tuning of mRNA levels.
How does disrupting RNA m6A methylation affect brain development? Yoon and colleagues showed that aberrant persistence of non-methylated transcripts in their mutant mice led to defects in the cell cycle in radial glia. These cells are not only neuronal precursors; they also act as scaffolds to guide migration of newly formed neurons to the appropriate layer of the cerebral cortex10–12, ensuring their proper integration into functional circuits during development. The authors found that delays in radial-glia divisions led to the cells’ abnormal persistence in the cortex after birth, and thereby disrupted cortical development. This caused disorganization of the finely layered architecture of the cortex, preventing normal circuit formation in cognition-related areas of the brain in mice.
Finally, the researchers extended their investigation to neuronal cells derived from human stem cells. They found that, as in mice, the development of human neuronal precursors is regulated by m6A methylation. Indeed, some of the human mRNA transcripts regulated by this mechanism have previously been associated with human brain disorders.
Yoon and colleagues’ findings together indicate that m6A methylation is an ‘uber-controller’ of mRNA stability across pathways that control the cell cycle and differentiation in radial glia. As a secondary consequence, this modification is a regulator of neurons. The work also opens up several avenues for future research. For instance, the researchers could not assess the cognitive consequences of the developmental disruption they observed, because mice died less than a month after birth. But this is an important area of potential investigation. Another question concerns whether the same mechanism might control the kinetics of decay in non-coding RNAs.
In addition, other roles for chemical modification of RNAs probably exist in the central nervous system (CNS), and it will be worthwhile dissecting these. More than 100 forms of chemical modification of RNA are documented to exist. Among the most prevalent are the methylation of adenosine and of the base cytosine, and the catalytic conversion of the nucleoside uridine to an isomer called pseudouridine. However, their functional roles in the brain are poorly understood13. Nonetheless, with previous work indicating that m6A methylation is a controller of cognitive function in the adult CNS, where it controls mRNA half-lives during the formation and storage of long-term memories14, there is a growing sense that a wide variety of roles for RNA chemical modification exists in the brain.
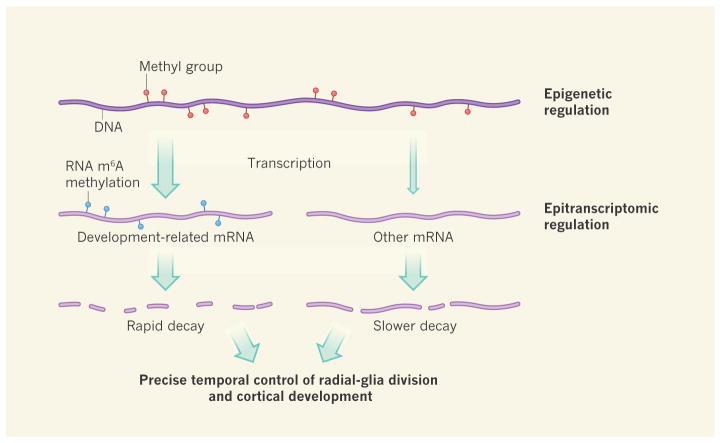
A major take-home message of Yoon and colleagues’ work is that cortical development is under dual layers of control (Fig. 1). The first layer, previously well described12, involves methylation of DNA and regulation of chromosome packaging — factors that modulate gene expression without altering the underlying DNA sequence. Such epigenetic regulation defines precise patterns of gene expression, regulated over time15. It is heritable over cell divisions because it relates to DNA, and has a role in maintaining cell identity over the lifespan of a cell lineage.
Figure 1. Two layers of transcriptional regulation in the mouse cortex.
The addition of methyl groups to DNA can lead to the modulation of gene-transcription levels (different levels indicated by size of arrow). Epigenetic regulatory mechanisms such as this, or chromosome packaging (not shown), which direct patterns of gene expression appropriate to a given cell type, are important in brain development12. Yoon et al.2 now demonstrate that development of the cortex of the embryonic mouse brain can also be regulated by the methylation of messenger RNA — specifically, of a nitrogen atom in the mRNA nucleoside adenosine. This m6A methylation reduces the half-lives of mRNAs, causing rapid decay of those involved in developmental regulatory pathways in neuronal precursor cells called radial glia. Such regulation is essential for precisely timed cell divisions and differentiation of radial glia, and so for normal cortical development.
By contrast, the second layer of control, m6A methylation, can be considered an epitran-scriptomic mechanism — one involving chemical modifications that alter RNA properties. m6A methylation controls the decay kinetics of individual transcripts and even of families of functionally related RNAs. In doing so, this mechanism allows finer temporal control of patterns of protein translation in individual cells as they undergo division and differentiation during key developmental time windows.
The ultimate output of this two-component epigenomic control system is a highly refined kinetic control of the translational output of the genome at a given time. It will not be surprising if such a system is soon uncovered in many of the other organs that undergo complex structural development.
References
- 1.Herculano-Houzel S, Manger PR, Kaas JH. Front Neuroanat. 2014;8:77. doi: 10.3389/fnana.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoon K, et al. Cell. 2017;171:877–889. doi: 10.1016/j.cell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Xiong X, Yi C. Nature Methods. 2016;14:23–31. doi: 10.1038/nmeth.4110. [DOI] [PubMed] [Google Scholar]
- 4.Zhao BS, Roundtree IA, He C. Nature Rev Mol Cell Biol. 2017;18:31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desrosiers RC, Friderici KH, Rottman FM. Biochemistry. 1975;14:4367–4374. doi: 10.1021/bi00691a004. [DOI] [PubMed] [Google Scholar]
- 6.Meyer KD, Jaffrey SR. Nature Rev Mol Cell Biol. 2014;15:313–326. doi: 10.1038/nrm3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patil DP, et al. Nature. 2016;537:369–373. doi: 10.1038/nature19342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, et al. Nature Cell Biol. 2014;16:191–198. doi: 10.1038/ncb2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batista PJ, et al. Cell Stem Cell. 2014;15:707–719. doi: 10.1016/j.stem.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 11.Breunig JJ, Haydar TF, Rakic P. Neuron. 2011;70:614–625. doi: 10.1016/j.neuron.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taverna E, Götz M, Huttner WB. Annu Rev Cell Dev Biol. 2014;30:465–502. doi: 10.1146/annurev-cellbio-101011-155801. [DOI] [PubMed] [Google Scholar]
- 13.Leighton LJ, et al. Genes Brain Behav. 2017 doi: 10.1111/gbb.12426. [DOI]
- 14.Widagdo J, et al. J Neurosci. 2016;36:6771–6777. doi: 10.1523/JNEUROSCI.4053-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sweatt JD. Neuron. 2013;80:624–632. doi: 10.1016/j.neuron.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]