Abstract
Background
The literature indicates that chromosome 6 is involved in balanced translocation and is involved in reproductive failure. This aim of this study was to explore the clinical features of chromosome 6 translocation in male carriers.
Material/Methods
We identified 10 patients who were carriers of chromosome 6 translocations and excluded the patients with varicocele, ejaculatory duct obstruction, and the other cause of infertility. The karyotype was analyzed using G-banding. A search for translocations on chromosome 6 involved in male infertility was performed using PubMed. We included cases of balanced chromosome 6 translocations involving adult men of fertile age and excluded those cases of live-born children, or those without breakpoints involving chromosome 6, or those with complex chromosomal translocations or chimeras.
Results
All 10 patients underwent genetic counseling for infertility. Semen analysis showed that 1 case had azoospermia, while 9 cases exhibited normal semen criteria. The respective partners of the 9 cases with normal semen parameters had a tendency to miscarry: 3 experienced spontaneous and induced abortion because of abnormal embryos; 3 experienced 3 incidents of spontaneous abortion, 2 experienced double spontaneous abortion, and 1 experienced biochemical pregnancy on 3 occasions. Most of the chromosome 6 breakpoints in translocation carriers obtained by the PubMed search were associated with spontaneous abortion.
Conclusions
Chromosome translocations involving chromosome 6 influence fertility status and lead to increased risk of miscarriage. Cytogenetic screening before opting for assisted reproductive technology and the breakpoints of chromosome 6 translocation should be considered for infertile male carriers.
MeSH Keywords: Chromosome Breakpoints; Cytogenetics; Infertility, Male
Background
Male-factor infertility is implicated in half of infertile couples [1]. Chromosomal aberrations are one of the most common causes of male infertility [2] and chromosomal disorders in non-obstructive azoospermia affect spermatogenesis [3]. The literature has demonstrated that structural chromosomal abnormalities in males can lead to abnormal sperm concentrations, influence fertility status, and lead to an increased risk of miscarriage [4–6]. Reduced fertility or a spouse experiencing recurrent miscarriage is the most common feature for the carriers of balanced translocations [7].
The specific chromosomes and breakpoints involved in translocations play an important role [7,8]. In some carriers, the location of a translocation breakpoint is closely related to an important gene, thus leading to spermatogenesis failure [9,10]. Previous studies indicate that chromosome 6 is involved in balanced translocation and is associated with reproductive failure [11–13]. There are important genes located on chromosome 6 that are associated with the complex and vital process of spermatogenesis. For example, the activator of cAMP-responsive element modulator in testis (ACT) gene was mapped to chromosome 6q16.1–16.3 and may be associated with the differentiation of spermatids into mature spermatozoa [14]. Furthermore, the sperm acrosomal membrane-associated protein 32 gene (SAMP32) located on chromosome 6q15–16.2 encodes a testis-specific protein that is involved in binding of sperm to the oocyte complex [15]. Genetic variants located within the human leukocyte antigen, Class II, DR alpha (HLA-DRA) region at 6p21.32 have been identified as risk factors for non-obstructive azoospermia [16].
Several previous publications by our group described the clinical features and genetic counseling of male patients with translocation breakpoints in chromosome 3, 4, and 5 [17–19]. This aim of this study was to explore the clinical features of chromosome 6 translocation in male carriers and genetic counseling for infertile carriers for these genetic anomalies.
Material and Methods
Subjects
Between July 2010 and December 2015, we recruited 5235 males experiencing infertility or receiving counseling from the Outpatient Department at the Center for Reproductive Medicine, First Hospital of Jilin University, Changchun, China. This study included all translocation cases involving chromosome 6 and excluded patients with varicocele, ejaculatory duct obstruction, and the other cause of infertility. For all patients, a clinical questionnaire, physical examination, and semen analysis were used according to previously described methods [20]. Their spouses had normal hormone levels. Chlamydia, Mycoplasma, and Ureaplasma detection was negative for these infertile couples. Abortions due to the female factor were excluded. The study was approved by the Ethics Committee of the First Hospital of Jilin University. Written informed consent was obtained from all study participants.
Cytogenetic analysis
Cytogenetic analysis was carried out for all patients. The protocol of blood sample collection, lymphocyte culture, chromosome preparation, and karyotype analysis were performed using previously described methods [20].
Analysis of identified balanced reciprocal translocations breakpoints
Balanced reciprocal translocations identified in chromosome 6 from infertile males were searched using PubMed on December 8, 2016. The keywords for PubMed searches were “chromosome/translocation/sperm” and “chromosome/translocation/abortion”. We included cases of balanced chromosome 6 translocations involving adult fertile-age men, and excluded those cases of live born children, or those without breakpoints involving chromosome 6, or those with complex chromosomal translocations, and chimeras. The relationships of translocation breakpoints with male infertility and recurrent pregnancy loss were analyzed.
Results
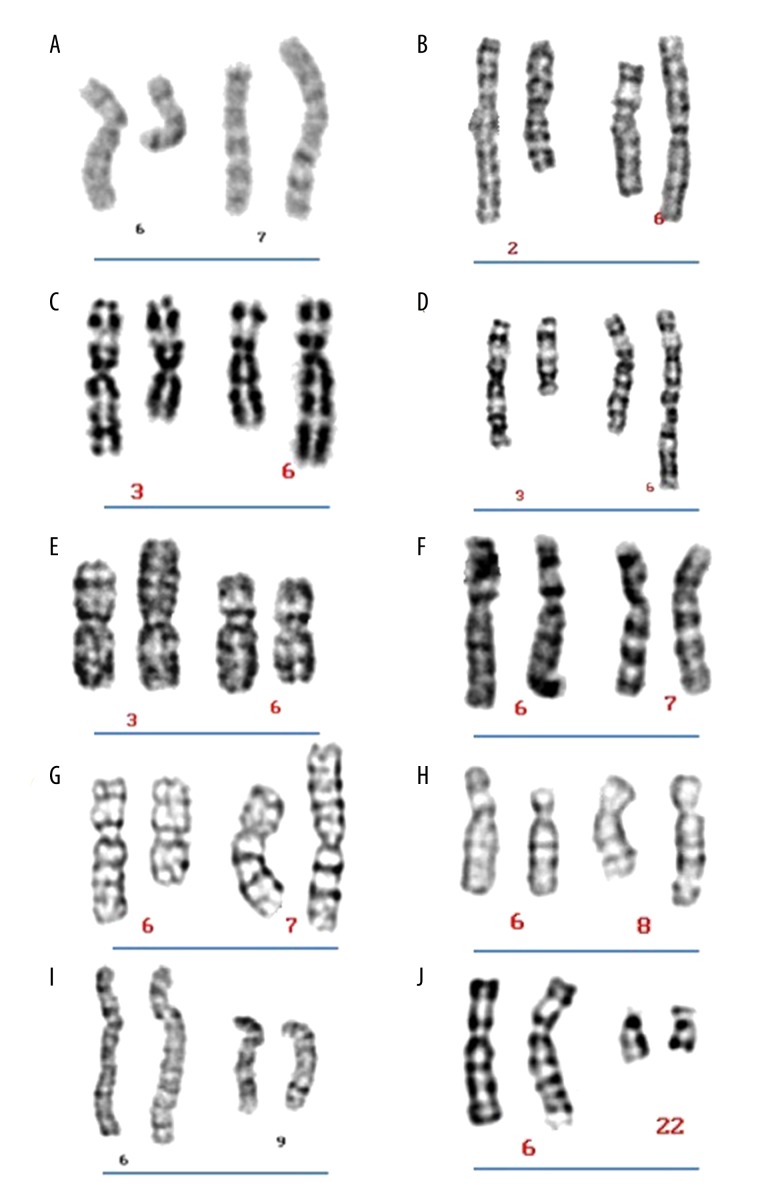
A total of 82 balanced reciprocal translocations carriers were detected among the 5235 male patients recruited for this study. Of these 82 translocation carriers, 10 patients (10/82; 12.2%) were carriers of a chromosome 6 translocation. The karyotypes obtained from these 10 cases are shown in Figure 1. All 10 patients underwent genetic counseling for infertility (mean age=32.8±4.9 years, normal phenotype). Testicular size was normal on physical examination. Semen analysis showed that 1 patient had azoospermia while 9 showed normal semen criteria. Of the 9 with normal semen parameters, their partners were able to conceive but experienced abortion: 3 partners experienced spontaneous and induced abortion because of abnormal embryos; 3 experienced 3 incidents of spontaneous abortion, 2 experienced double spontaneous abortion, and 1 experienced biochemical pregnancy on 3 occasions. The breakpoint at 6q15 was associated with pre-gestational infertility, while other breakpoints were related to gestational cases. Karyotype results from the 10 patients expressing chromosome 6 translocations are summarized in Table 1.
Figure 1.
Partial karyotypes of the 10 cases possessing chromosome 6 translocations.
Table 1.
Clinical and cytogenetic features of chromosome 6 translocation carriers.
| Infertility type | Sperm concentration (×106/ml) | Karyotype | Karyotype of spouse | Frequency of abortion | Figure No. |
|---|---|---|---|---|---|
| Pregestational | 0 | 46,XY,t(6;7)(q15;p15) | 46,XX | Nil | 1A |
| Gestational | 27 | 46,XY,t(2;6)(q21;p21) | 46,XX | 3 biochemical pregnancies | 1B |
| 44 | 46,XY,t(3;6)(q21;q25) | 46,XX | 2 spontaneous and 2 induced abortion of abnormal embryos | 1C | |
| 49 | 46,XY,t(3;6)(q12;q27) | 46,XX | 2 spontaneous abortions | 1D | |
| 61 | 46,XY,t(3;6)(q10;q10) | 46,XX | 2 spontaneous abortions | 1E | |
| 42 | 46,XY,t(6;7)(q25;p15) | 46,XX | 3 spontaneous abortions | 1F | |
| 99 | 46,XY,t(6;7)(q13;p15) | 46,XX | 3 spontaneous abortions | 1G | |
| 36 | 46,XY,t(6;8)(p21;q24) | 46,XX | 2 spontaneous and 1 induced abortion of abnormal embryos | 1H | |
| 46 | 46,XY,t(6;9)(q26;p13) | 46,XX | 3 spontaneous abortions | 1I | |
| 32 | 46,XY,t(6;22)(q27;q13) | 46,XX | 1 spontaneous and 1 induced abortion of abnormal embryos | 1J |
Table 1 also shows that breakpoints at locations 6p21, 6q25, and 6q27 were observed more than once. The breakpoints at 6p21, 6q10, 6q13, 6q25, 6q26, and 6q27 were associated with gestational infertility. Most of the chromosome 6 breakpoints in translocation carriers obtained in the PubMed search were associated with spontaneous abortion (Table 2). Analysis of previous reports showed that carriers of chromosome 6q15 exhibited pre-gestational infertility, while the carriers of the other breakpoints exhibited gestational infertility.
Table 2.
Clinical features and chromosome 6 breakpoints in translocation carriers reported in previous literature*.
| Karyotype | Breakpoints | Clinical findings | Reference |
|---|---|---|---|
| t(1;6) | 1q44; 6p11 | Abortion | Vozdova et al., 2013 [11] |
| t(1;6) | 1q25; 6q16 | Infertility | GadaSaxena et al., 2012 [29] |
| t(2;6) | 2q34; 6p24 | Recurrent spontaneous pregnancy loss | GadaSaxena et al., 2012 [29] |
| t(3;6) | 3q25.3; 6q11.2 | Recurrent fetal wastage | Fryns et al, 1998 [30] |
| t(3;6) | 3q28; 6q13 | Infertility | Mierla et al., 2015 [31] |
| t(3; 6) | 3q29; 6q21 | Recurrent pregnancy loss | Kochhar et al, 2013 [32] |
| t(4;6) | 4q31.3; 6q21 | Recurrent spontaneous pregnancy loss | GadaSaxena et al., 2012 [29] |
| t(4;6) | 4q33; 6q27 | Asthenospermia, abortion | Vozdova et al., 2013 [11] |
| t(4;6) | 4q31.3; 6q21 | Recurrent spontaneous pregnancy loss | GadaSaxena et al., 2012 [29] |
| t(4;6) | 4q23; 6q21 | Repeated spontaneous abortion | Ghazaey et al., 2015 [26] |
| t(5;6) | 5p13.3; 6q27 | Recurrent spontaneous pregnancy loss | GadaSaxena et al., 2012 [29] |
| t(6;7) | 6p22;7q22 | Four spontaneous abortions | Resim et al., 2013 [33] |
| t(6;7) | 6p22; 7q34 | Recurrent fetal wastage | Fryns et al, 1998 [30] |
| t(6;7) | 6q25; 7q34 | Abortion | Vozdova et al., 2013 [11] |
| t(6;7) | 6q15; 7p15 | Recurrent spontaneous abortion | Zhang et al., 2015 [13] |
| t(6;8) | 6p21; 8q24 | Recurrent spontaneous abortion | Zhang et al., 2015 [13] |
| t(6;8) | 6q21.3; 8q23.2 | Recurrent fetal wastage | Fryns et al, 1998 [30] |
| t(6;8) | 6q26; 8p12 | Normozoospermia | Godo et al., 2013 [8] |
| t(6;8) | 6q13; 8p12 | Recurrent spontaneous pregnancy loss | GadaSaxena et al., 2012 [29] |
| t(6;8) | 6p23; 8q12.2 | Repeated spontaneous abortion | Ghazaey et al., 2015 [26] |
| t(6;9) | 6q26; 9p13 | Recurrent spontaneous abortion | Zhang et al., 2015 [13] |
| t(6;10) | 6p21;10q26 | Two previous miscarriages | Vasilevska et al,. 2013 [34] |
| t(6;10) | 6p25; 10p11.2 | Repeated spontaneous abortion | Ghazaey et al., 2015 [26] |
| t(6;10) | 6q23; 10p13 | Early pregnancy loss | Li et al., 2012 [24] |
| t(6;11) | 6q15; 11p15 | Oligoazoospermia | Pernice et al., 2002 [9] |
| t(6;11) | 6p12.1; 11q25 | Recurrent spontaneous abortion | Celep et al., 2006 [35] |
| t(6;14) | 6q13; 14p10 | Oligoazoospermia | Li et al., 2012 [24] |
| t(6;14) | 6q24.2; 14q24.2 | Abortion | Vozdova et al., 2013 [11] |
| t(6;15) | 6p21; 15q26.1 | Recurrent fetal wastage | Fryns et al, 1998 [30] |
| t(6;15) | 6q25; 15q14 | Recurrent spontaneous pregnancy loss | GadaSaxena et al., 2012 [29] |
| t(6;16) | 6q26; 16p12 | Repeated spontaneous abortion | Ghazaey et al., 2015 [26] |
| t(6;21) | 6p21.1;21p13 | Azoospermia | Paoloni-Giacobino et al., 2000 [25] |
All breakpoints were listed from 6pter to 6qter to facilitate easy search of the publication.
Discussion
Whole-exome sequencing and array-comparative genome hybridization analyses have been increasingly applied in many medical fields in recent years, but these technologies fail to detect balanced reciprocal translocations or inversions, which sometimes have very detrimental effects on gene structure and function [21]. In contrast, karyotype analysis easily detects balanced reciprocal translocations and remains a powerful and cheap technological method [21]. This technology thus represents a powerful diagnostic tool and provides valuable information for genetic counseling of infertile males [22]. Balanced reciprocal translocation is one of the major chromosomal abnormalities in male infertility [6]. The incidence of balanced reciprocal translocation was reported to be approximately 1 in 625 (0.16%) in the general population [23]. In the present study, the frequency of balanced reciprocal translocations in infertile males was 1.57% (82/5235). Therefore, the incidence of balanced translocations was about 10 times higher in infertile men than in the general population.
In clinical practice, pre-gestational and gestational infertility are distinguished in infertile male patients [24]. Pre-gestational infertility patients exhibit abnormal semen parameters and their partners are not able to conceive. Gestational cases have partners who are able to conceive but have miscarriages. In particular, chromosome 6 translocation has often been associated with male infertility and recurrent pregnancy loss [12,25,26]. In the present study, 10 of our cases were identified as carriers of chromosome 6 translocations. Our findings showed the carriers of chromosome 6q15 exhibited pre-gestational infertility, while the carriers of the other breakpoints exhibited gestational infertility. Ni et al. [27] reported that low-frequency germline variants across locations 6p21.33 to 6p22.2 were associated with non-obstructive azoospermia in Han Chinese men, and disrupted the process of spermatogenesis. This finding is supported by the fact that the zinc finger gene ZNF165, which is mapped to 6p21, and the SAMP32 gene, mapped to 6q15–16.2, are both specifically expressed in the testes [15,28]. The carriers of the breakpoints at 6q13, 6q25, 6q26, and 6q27 had partners who had recurrent pregnancy loss. These results are consistent with previous reports [8,11,13,26,29]. In the literature, the breakpoints at 6p24, 6p23, 6p22, 6p21, 6p12.1, 6p11, 6q11.2, 6q13, 6q21.3, 6q21 6q25, 6q26, and 6q27 were associated with fetal abortion [11,26,29–35], and the breakpoints at 6q13, 6q15, and 6q21.1 were associated with oligoazoospermia or azoospermia [9,24,25]. Hence, it is noteworthy that another breakpoint involving balanced translocation should be considered in these pre-gestational individuals.
In genetic counseling, physicians should consider different breakpoints and different reproductive treatment options for the carriers of chromosome 6 translocations. Studies have shown that key genes associated with spermatogenesis are predominantly located on 6p21–22 and 6q15–16 [15,27,28]. Male patients with chromosome 6 breakpoints and pre-gestational infertility should be counseled regarding the potential application of intracytoplasmic sperm injection (ICSI). However, males with gestational infertility and their partners at risk of recurrent pregnancy loss should be counseled regarding the potential use of preimplantation genetic diagnosis (PGD) or prenatal diagnosis [11].
The limitations of this study included the small number of carriers of chromosome 6 translocations and the fact that we did not assess the specific molecular effects of the translocations we identified. However, this study is clinically useful because it provides additional information for male carriers of chromosome 6 translocation when seeking reproductive therapy.
Conclusions
We identified 10 patients who were carriers of chromosome 6 translocations. Combined with previous reports, the analysis of our new cases indicated that the breakpoints identified at location 6q15 exhibited pre-gestational infertility, while the other breakpoints exhibited gestational infertility. Chromosome translocations involving chromosome 6 influence fertility status and lead to an increased risk of miscarriage. Cytogenetic screening before opting for assisted reproductive technology and the breakpoints of chromosome 6 translocation should be considered for infertile male carriers.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by the Science and Technology Funds of the Education Department of Jilin Province, P.R. China (JJKH20170846KJ)
References
- 1.An G, Zou Z, Flannigan R, et al. Outcome of oocyte vitrification combined with microdissection testicular sperm extraction and aspiration for assisted reproduction in men. Med Sci Monit. 2018;24:1379–86. doi: 10.12659/MSM.909026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stouffs K, Seneca S, Lissens W. Genetic causes of male infertility. Ann Endocrinol (Paris) 2014;75:109–11. doi: 10.1016/j.ando.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Chu QJ, Hua R, Luo C, et al. Relationship of genetic causes and inhibin B in non-obstructive azoospermia spermatogenic failure. BMC Med Genet. 2017;18:98. doi: 10.1186/s12881-017-0456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao M, Pang H, Zhao YH, et al. Karyotype analysis in large sample cases from Shenyang Women’s and Children’s hospital: A study of 16,294 male infertility patients. Andrologia. 2017;49(4) doi: 10.1111/and.12649. [DOI] [PubMed] [Google Scholar]
- 5.Pastuszek E, Kiewisz J, Kulwikowska PM, et al. Sperm parameters and DNA fragmentation of balanced chromosomal rearrangements carriers. Folia Histochem Cytobiol. 2015;53:314–21. doi: 10.5603/fhc.a2015.0032. [DOI] [PubMed] [Google Scholar]
- 6.Suganya J, Kujur SB, Selvaraj K, et al. Chromosomal abnormalities in infertile men from Southern India. J Clin Diagn Res. 2015;9:GC05–10. doi: 10.7860/JCDR/2015/14429.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harton GL, Tempest HG. Chromosomal disorders and male infertility. Asian J Androl. 2012;14:32–39. doi: 10.1038/aja.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godo A, Blanco J, Vidal F, et al. Accumulation of numerical and structural chromosome imbalances in spermatozoa from reciprocal translocation carriers. Hum Reprod. 2013;28:840–49. doi: 10.1093/humrep/des431. [DOI] [PubMed] [Google Scholar]
- 9.Pernice F, Mazza G, Puglisi D, et al. Non-robertsonian translocation t(6;11) is associated with infertility in an oligoazoospermic male. Fertil Steril. 2002;78:192–94. doi: 10.1016/s0015-0282(02)03180-1. [DOI] [PubMed] [Google Scholar]
- 10.Bianco B, Christofolini D, Gava M, et al. Severe oligospermia associated with a unique balanced reciprocal translocation t(6;12)(q23;q24.3): Male infertility related to t(6;12) Andrologia. 2011;43:145–48. doi: 10.1111/j.1439-0272.2009.01020.x. [DOI] [PubMed] [Google Scholar]
- 11.Vozdova M, Oracova E, Kasikova K, et al. Balanced chromosomal translocations in men: relationships among semen parameters, chromatin integrity, sperm meiotic segregation and aneuploidy. J Assist Reprod Genet. 2013;30:391–405. doi: 10.1007/s10815-012-9921-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mierla D, Jardan D, Stoian V. Chromosomal abnormality in men with impaired spermatogenesis. Int J Fertil Steril. 2014;8:35–42. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Fan HT, Zhang QS, et al. Genetic screening and evaluation for chromosomal abnormalities of infertile males in Jilin Province, China. Genet Mol Res. 2015;14:16178–84. doi: 10.4238/2015.December.8.7. [DOI] [PubMed] [Google Scholar]
- 14.Palermo I, Litrico L, Emmanuele G, et al. Cloning and expression of activator of CREM in testis in human testicular tissue. Biochem Biophys Res Commun. 2001;283:406–11. doi: 10.1006/bbrc.2001.4805. [DOI] [PubMed] [Google Scholar]
- 15.Hao Z, Wolkowicz MJ, Shetty J, et al. SAMP32, a testis-specific, isoantigenic sperm acrosomal membrane-associated protein. Biol Reprod. 2002;66:735–44. doi: 10.1095/biolreprod66.3.735. [DOI] [PubMed] [Google Scholar]
- 16.Zhao H, Xu J, Zhang H, et al. A genome-wide association study reveals that variants within the HLA region are associated with risk for nonobstructive azoospermia. Am J Hum Genet. 2012;90:900–6. doi: 10.1016/j.ajhg.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang HG, Wang RX, Pan Y, et al. A report of nine cases and review of the literature of infertile men carrying balanced translocations involving chromosome 5. Mol Cytogenet. 2018;11:10. doi: 10.1186/s13039-018-0360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Wang R, Li L, et al. Translocation breakpoints of chromosome 3 in male carriers: A report of twelve cases and a review of the literature. Turk J Med Sci. 2018;48:150–56. doi: 10.3906/sag-1704-1. [DOI] [PubMed] [Google Scholar]
- 19.Zhang HG, Wang RX, Pan Y, et al. Translocation breakpoints of chromosome 4 in male carriers: Clinical features and implications for genetic counseling. Genet Mol Res. 2016;15(4) doi: 10.4238/gmr15049088. [DOI] [PubMed] [Google Scholar]
- 20.Zhang HG, Wang RX, Li LL, et al. Male carriers of balanced reciprocal translocations in Northeast China: Sperm count, reproductive performance, and genetic counseling. Genet Mol Res. 2015;14:18792–98. doi: 10.4238/2015.December.28.28. [DOI] [PubMed] [Google Scholar]
- 21.Pasquier L, Fradin M, Chérot E, et al. Karyotype is not dead (yet)! Eur J Med Genet. 2016;59:11–15. doi: 10.1016/j.ejmg.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Poli MN, Miranda LA, Gil ED, et al. Male cytogenetic evaluation prior to assisted reproduction procedures performed in Mar del Plata, Argentina. JBRA Assist Reprod. 2016;20:62–65. doi: 10.5935/1518-0557.20160015. [DOI] [PubMed] [Google Scholar]
- 23.Nonaka T, Ooki I, Enomoto T, et al. Complex chromosomal rearrangements in couples affected by recurrent spontaneous abortion. Int J Gynaecol Obstet. 2015;128:36–39. doi: 10.1016/j.ijgo.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Li D, Zhang H, Wang R, et al. Chromosomal abnormalities in men with pregestational and gestational infertility in northeast China. J Assist Reprod Genet. 2012;29:829–36. doi: 10.1007/s10815-012-9783-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paoloni-Giacobino A, Kern I, Rumpler Y, et al. Familial t(6;21)(p21.1;p13) translocation associated with male-only sterility. Clin Genet. 2000;58:324–28. doi: 10.1034/j.1399-0004.2000.580411.x. [DOI] [PubMed] [Google Scholar]
- 26.Ghazaey S, Keify F, Mirzaei F, et al. Chromosomal analysis of couples with repeated spontaneous abortions in northeastern Iran. Int J Fertil Steril. 2015;9:47–54. doi: 10.22074/ijfs.2015.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ni B, Lin Y, Sun L, et al. Low-frequency germline variants across 6p22.2-6p21.33 are associated with non-obstructive azoospermia in Han Chinese men. Hum Mol Genet. 2015;24:5628–36. doi: 10.1093/hmg/ddv257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tirosvoutis KN, Divane A, Jones M, et al. Characterization of a novel zinc finger gene (ZNF165) mapping to 6p21 that is expressed specifically in testis. Genomics. 1995;28:485–90. doi: 10.1006/geno.1995.1178. [DOI] [PubMed] [Google Scholar]
- 29.Gada Saxena S, Desai K, Shewale L, et al. Chromosomal aberrations in 2000 couples of Indian ethnicity with reproductive failure. Reprod Biomed Online. 2012;25:209–18. doi: 10.1016/j.rbmo.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Fryns JP, Buggenhout G. Structural chromosome rearrangements in couples with recurrent fetal wastage. Eur J Obstet Gynecol Reprod Biol. 1998;81:171–76. doi: 10.1016/s0301-2115(98)00185-7. [DOI] [PubMed] [Google Scholar]
- 31.Mierla D, Malageanu M, Tulin R, et al. Prevalence of chromosomal abnormalities in infertile couples in Romania. Balkan J Med Genet. 2015;18:23–30. doi: 10.1515/bjmg-2015-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kochhar PK, Ghosh P. Reproductive outcome of couples with recurrent miscarriage and balanced chromosomal abnormalities. J Obstet Gynaecol Res. 2013;39:113–20. doi: 10.1111/j.1447-0756.2012.01905.x. [DOI] [PubMed] [Google Scholar]
- 33.Resim S, Kadıoğlu A, Akman T, et al. Balanced chromosomal translocation of chromosomes 6 and 7: A rare male factor of spontaneous abortions. Balkan Med J. 2013;30:250–52. doi: 10.5152/balkanmedj.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasilevska M, Ivanovska E, Kubelka Sabit K, et al. The incidence and type of chromosomal translocations from prenatal diagnosis of 3800 patients in the Republic of Macedonia. Balkan J Med Genet. 2013;16:23–28. doi: 10.2478/bjmg-2013-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Celep F, Karagüzel A, Ozeren M, et al. The frequency of chromosomal abnormalities in patients with reproductive failure. Eur J Obstet Gynecol Reprod Biol. 2006;127:106–9. doi: 10.1016/j.ejogrb.2005.12.019. [DOI] [PubMed] [Google Scholar]