Abstract
Background
Reperfusion injury is one of the leading causes of myocardial cell death and heart failure. This study was performed to identify new candidate lipid biomarkers for the purpose of optimizing the diagnosis of myocardial ischemia reperfusion (I/R) injury, assessing the severity of myocardial I/R injury and trying to find the novel mechanism related to lipids.
Material/Methods
Forty patients who were diagnosed with ST-segment elevation myocardial infarction (STEMI) were randomly selected for this study. Serum samples from all the patients with STEMI were collected at 3 time periods: after STEMI diagnosis but prior to reperfusion (T0); and then at 2 hours (T2) and 24 hours (T24) after the end of the percutaneous coronary intervention procedure. Plasma lipidomics profiling analysis was performed to identify the lipid metabolic signatures of myocardial I/R injury using lipidomics.
Results
Sixteen types of potential lipid biomarkers at different time periods (T0, T2, T24) were identified by using lipidomics technology. The T0 time periods exhibited 16 differentially metabolized lipid peaks in the patients after STEMI diagnosis but prior to reperfusion. With the increase of reperfusion times, the contents of these 16 lipid biomarkers decreased gradually, but there was a 1.5- to 2-fold increase of those 16 lipid biomarkers contents at T2 compared with T24.
Conclusions
Lipidomics analysis demonstrated differential change before and after reperfusion, suggesting a potential role of some of these lipids as biomarkers for optimizing the diagnosis of myocardial I/R, as well as for therapeutic targets against myocardial I/R injury.
MeSH Keywords: Diagnosis, Lipid Metabolism, Myocardial Infarction, Myocardial Reperfusion Injury, Percutaneous Coronary Intervention
Background
Acute myocardial infarction (MI) remains a major cause of morbidity and mortality worldwide. Immediate and prompt revascularization with percutaneous coronary intervention (PCI) or thrombolysis can reduce acute myocardial ischemic injury, limit MI size, decrease in-hospital mortality, and improve the long-term outlook in survivors of the acute phase. However, reperfusion can itself induce cardiomyocyte death, known as myocardial reperfusion injury. In animal experiments, myocardial ischemia/reperfusion (I/R) injury may be responsible for up to 50% of the final infarct size.
At present, myocardial I/R injury diagnosis is generally made according to symptoms, irregular ECG, and subsequent significant increase in serum biochemical markers such as creatine kinase (CK), creatine kinase-MB (CK-MB), myoglobin, troponin I (cTnI), and troponin T (cTnT). However, the symptoms of myocardial I/R injury like chest pain are usually non-typical or absent and electrocardiogram (ECG) and myocardial enzyme changes are frequently indefinite or absent in the process of myocardial I/R injury.
Furthermore, widely used serum markers in myocardial I/R injury may be changed in numerous non-cardiac diseases, such as trauma, pulmonary embolism, chronic renal insufficiency, sepsis, seizures, hyperthermia, inflammatory conditions, and hyperthyroidism [1–5].
Therefore, earlier diagnosis of myocardial I/R injury and assessment of the injury extent may prevent disease progression, decrease the myocardial cell death, and then reduce mortality. Despite the great progress made in molecular biological studies and human genome research, there has not yet emerged specific diagnostic criteria well recognized by physicians in general. Currently, although reperfusion injury is one of the main causes of myocardial cell death and heart failure, the exact pathophysiological mechanism underlying myocardial I/R injury remains unclear.
The plasma contains thousands of lipids that participate in numerous of physiologic functions and are also involved in the pathological process. Lipids not only act as the essential materials of cell membranes and energy stores but are also linked to a variety of biological processes such as signaling molecules and interactions between cells. In addition, they are involved in many diseases such as diabetes, obesity, fatty liver, atherosclerosis, cancer, and Alzheimer disease. As a result, lipids have stimulated the emergence of the new field of lipidomics. If the plasma lipid profiles involved in early events of myocardial I/R injury can be identified by lipidomics technique, it may improve diagnosis and prognosis.
Lipidomics, as a branch of metabolomics, is a powerful approach to identify potential lipid biomarkers to reveal the pathogenic mechanisms of specific diseases and to provide therapeutic targets of human diseases. The new method of high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) is the most popular analytical technique for metabolite identification. LC-ESI-MS/MS has been successfully applied to discover new biomarkers for many diseases, including ovarian cancer [6], diabetes [7], hyperlipidemia [8], atherosclerosis [8], Alzheimer disease [9], and HIV [10]. So far, there has been no studies on the dynamic changes in lipids during the process of myocardial I/R injury, which may reveal how lipids participate in regulating myocardial I/R injury. The aim of the present study was to identify new candidate lipid biomarkers to optimize the diagnosis of myocardial I/R injury, assess the severity of myocardial I/R, and discover novel mechanisms related to lipids.
Material and Methods
AMI patients treated by PCI
This study was approved by the Research Ethics Committees of all relevant institutions. Written informed consents were provided by all participants.
In this study, 40 patients who were diagnosed with ST-segment elevation acute MI (STEMI) were randomly selected from June 2016 to October 2016 in the Department of Cardiology, Tianjin Medical University General Hospital. These patients, aged 42–75 years, were promptly treated by PCI after a definite diagnosis was made.
The inclusion criteria were based on STEMI diagnosis and final coronary angiography. STEMI patients were diagnosed to have acute ischemic-type chest pain symptoms, ST-segment elevation (at least 1 mm (0.1 mV) of ST-segment elevation in the limb leads, and at least 2-mm elevation in the precordial leads), and dynamic changes in ECG, high-sensitivity cardiac troponin T, and coronary angiography. The coronary angiography results indicated that at least 1 of the coronary arteries was 100% blocked.
We excluded patients with histories of old myocardial infarction, coronary revascularization, congestive heart failure, cardiomyopathy, pericardial disease, peripheral vascular disease, hematologic disease, cancer, metabolic disease, inflammatory conditions, congenital diseases, and liver/renal disease, and also patients who refused to sign the written informed consent. We also excluded patients without complete clinical information because statistical analysis could not be done without such information.
All patients were given a loading dose of 300 mg of clopidogrel, 100 or 200 mg of aspirin for antiplatelet therapy when the their STEMI diagnosis was made, 2000 U of intravenous heparins before coronary angiography, and then supplemental heparins at 70–100 U/kilogram body weight up to a total of 7000 U before PCI. Isosorbide dinitrate was also given by intracoronary injection before the final coronary angiographies and at other necessary times.
Serum sample collection and preparation
Serum samples from all the patients with STEMI were collected at 3 time periods before and after STEMI diagnosis and PCI treatment from elbow vein blood: the first, after STEMI diagnosis but prior to reperfusion (T0); the second, 2 hours after the PCI procedure was done (T2); and the third, 24 hours after the PCI procedure was done (T24). The serum samples collected from the participants at the 3 time periods were laid vertically for 1 hour at room temperature, naturally coagulated, and centrifuged at 3000 rpm for 15 minutes. Then, the supernatant was drawn into a 1.5-mL Eppendorf tube, which was stored at −80°C for mass spectrometry analysis.
LC-ESI-MS/MS lipid profiling
According to the method of Bligh and Dyer [11], we extracted the lipids from the plasma of patients with some modifications. We used 3 μL of each plasma sample for analysis. Each sample was centrifuged at 10 000 rpm for 20 minutes at room temperature on a table tube unit to pelletize the lipids before detection. For exact identification of all lipid species, we added exact amounts of internal standards, which were used for each class of lipid species. After serum centrifuging, the lipid extractions were redissolved in the solvents for HPLC injection. The solvents were chloroform/methanol/300 mM ammonium acetate in water (μL) at the rate of 360/840/44. HPLC-grade solvents were prepared for LC-ESI-MS/MS applications [12–15].
Statistics analysis
In this study, SPSS 17.0 software was applied for statistical analyses and a Proc Mixed analysis of variance-covariance was used for the data before the Tukey’s multiple comparisons test. The information is presented in the form of the mean ± standard error of the means, and differences were considered statistically significant when P values were lower than 0.05 and fold-change was larger than 1.5. After the chromatographic peak area was normalized, partial least squares-discriminant analysis (PLS-DA) was performed to construct plasma lipid metabolic profiles of ST-segment elevation acute myocardial infarction patients and controls.
Results
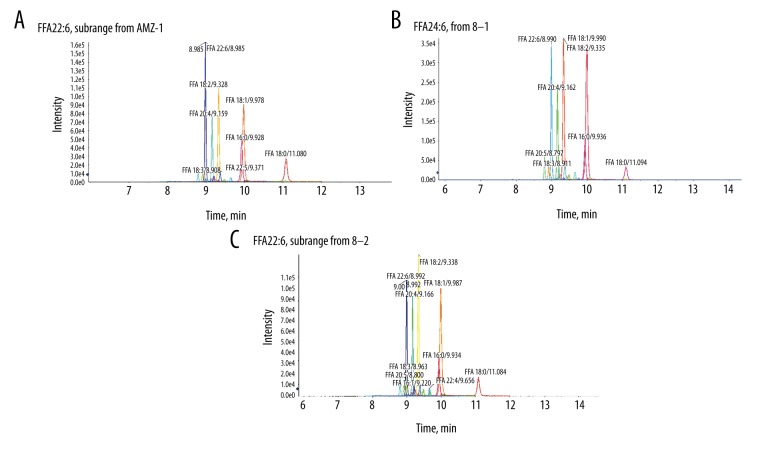
Table 1 shows the clinical characteristics of the study patients, their serum TC, LDL-C, and GLU and their SBP, DBP, HR, BMI, LV, and EF. Table 2 present the changes in concentrations of cardiac markers at the T0, T2, and T24 time periods. Table 3 displays 16 types of potential lipid biomarkers at different intervals (T0, T2, T24), and Figure 1 presents the representative XIC from T0 time periods (Figure 1A), T2 time periods (Figure 1B), and T24 time periods (Figure 1C).
Table 1.
Clinical characteristics of the study patients.
| Clinical characteristics | |
|---|---|
| Gender (Male/Famale) | 26/14 |
| Age (years) mean/range | 57.75/42–75 |
| Hypertension | 27 (67.5%) |
| Current smoker | 16 (40%) |
| Former smoker | 22 (55%) |
| SBP (mmHg) | 121.3±28.19 |
| DBP (mmHg) | 75.84±13.31 |
| HR (BPM) | 80.47±18.60 |
| BMI (Kg/m2) | 25.34±3.92 |
| LV (mm) | 48.37±3.59 |
| EF (%) | 53.26±5.40 |
| TC (mmol/L) | 4.77±0.62 |
| LDL-C(mmol/L) | 2.82±0.47 |
| GLU (mmol/L) | 5.71±1.15 |
The data are expressed as mean value ± standard deviation (SD) or number of patients. SBP – systolic blood pressure; DBP – diastolic blood pressure; HR – heart rate; BMI – body mass index; LV – left ventricle; EF – ejection fraction; TC – total cholesterol; LDL-C – low density lipoprotein-cholesterol; GLU – glucose; SD – stranded deviation.
Table 2.
Cardiac markers in acute myocardial infraction patients treated with percutaneous coronary intervention.
| Cardiac markers | T0 | T2 | T24 |
|---|---|---|---|
| CK(U/L) | 652.58±279.42 | 2831.79±2287.34 | 1648.11±1006.62 |
| CK-MB(U/L) | 86.36±73.99 | 259.00±179.64 | 158.33±82.06 |
| cTnT (ng/ml) | 0.76±0.88 | 2.04±1.73 | 1.35±1.10 |
Data are expressed as mean value ± standard deviation(SD), values are compared baseline to prior to PCI(T0) and 2 and 24 hours post-PCI(T2, T24)., CK – creatine kinase; CK-MB – creatine kinase isoenzyme MB; cTnT – cardiac troponin.
Table 3.
Identified lipid markers and their m/z.
| Lipid name | m/z | |
|---|---|---|
| FFA 14: 0 | Myristic acid | 228.37 |
| FFA 16: 0 | Palmitic acid | 256.42 |
| FFA 16: 1 | Palmitoleic acid | 268.43 |
| FFA 17: 0 | Heptadecanoic acid | 270.45 |
| FFA 18: 0 | Stearic acid | 284.48 |
| FFA 18: 1 | Oleic acid | 282.47 |
| FFA 18: 2 | Linoleic acid | 280.44 |
| FFA 18: 3 | γ-linolenic acid | 278.43 |
| FFA 20: 1 | Eicosenoic acid | 310.51 |
| FFA 20: 2 | Eicosadienoic acid | 308.5 |
| FFA 20: 3 | Eicosatrienoic acid | 320.51 |
| FFA 20: 4 | Arachidonic acid | 304.47 |
| FFA 20: 5 | Eicosapentaenoic acid | 302.56 |
| FFA 22: 4 | Hexadecyl azelaoyl phosphatidylcholine | 332.27 |
| FFA 22: 5 | Docosapentaenoic acid | 330.5 |
| FFA 22: 6 | Docosahexaenoic acid | 328.49 |
Figure 1.
A slice of data containing a contiguous m/z range extending across all RT is called an extracted ion chromatogram (XIC). Representative XIC from T0 time periods (A), T2 time periods (B), and T24 time periods (C). The different lipid metabolites detected were as follows: myristic acid, palmitic acid, palmitoleic acid, heptadecanoic acid, stearic acid, oleic acid, linoleic acid, γ-linolenic acid, eicosanoic acid, eicosadienoic acid, eicosatrienoic acid, arachidonic acid, eicosapentaenoic acid, hexadecyl azelaoyl phosphatidylcholine, docosapentaenoic acid, and docosahexaenoic acid.
Compared with the other 2 periods, the T0 time periods exhibited 16 differentially metabolized lipid peaks in the patients after STEMI diagnosis but prior to reperfusion. With reperfusion times, the contents of these 16 lipid biomarkers decreased gradually, but there was a 1.5- to 2-fold increase of those 16 lipid biomarkers contents at T2 compared with T24.
We further evaluated the association of PA profiles with reperfusion times. All cases were classified by different differentiation grade, and clinical stage. Statistical analysis showed that the concentrations of most fatty acids were significantly associated with clinical stage (P<0.05 in all cases). A score plot of the PCA model for the T0 time periods, T2 time periods, and T24 time periods sample data is shown in Figure 2. To improve the separation of the 3 time periods, we used PLS-DA to visualize the metabolic differences between them. The 3 time periods were well separated in the PLS-DA score plot (Figure 3), indicating that they have markedly different metabolic characteristics. Greater deviation of the variable from the origin was associated with a higher VIP value. The reliability of the constructed PLS-DA models was evaluated by the predicted variation, Q2, and the explained variation, R2, calculated by cross-validation. In general, R2 and Q2 should be N0.5 to give a significant biological model. In our established model, the values of R2 and Q2 were above 0.3 (R2X=0.419, R2Y=0.899, Q2=0.751), suggesting that this model is both good and reliable (Figure 4).
Figure 2.

PCA score plot for T0 time periods, T2 time periods, and T24 time periods; score plots displaying discrimination regarding T0 time periods (blue circles), T2 time periods (green circles), and T24 time periods (red circles) (R2=0.54861; Q2=0.30092).
Figure 3.
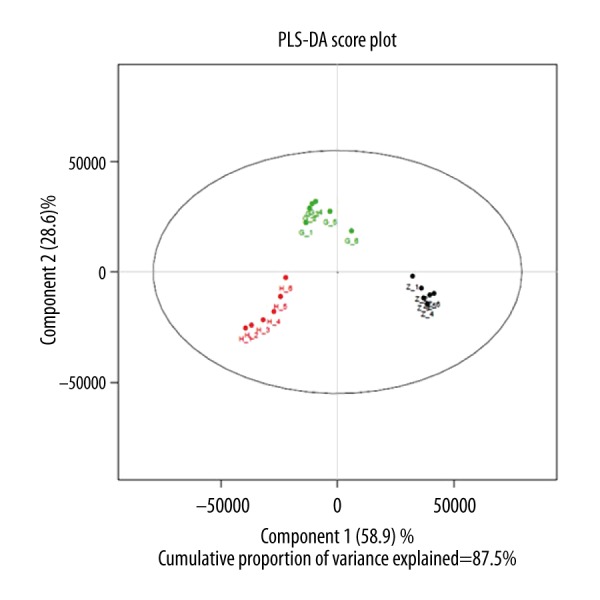
Score plot of PLS-DA for T0 time periods T2 time periods, and T24 time periods, score plots indicated the separation degree between T0 time periods (blue circles), T2 time periods (green circles), and T24 time periods (red circles) (R2X=0.419, R2Y=0.899, Q2=0.751).
Figure 4.
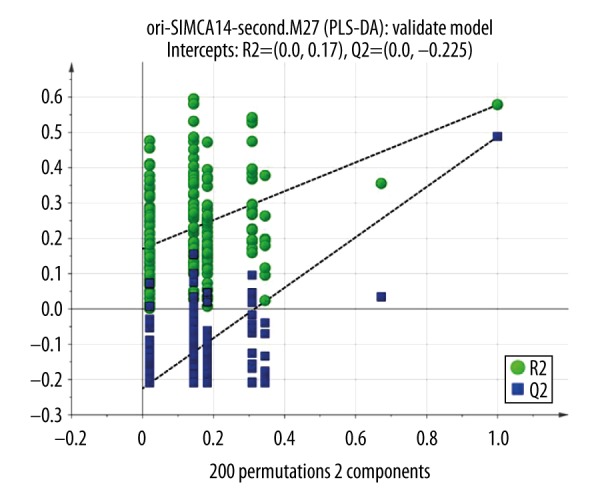
Validation of PLS-DA: a permutation test was conducted with 200 randomly initiated permutations in a PLS-DA model showing R 2 (green rounds) and Q 2 (blue rounds) values from the permuted analysis (left-bottom corner), and these are far lower than the associated initial values (right-top corner).
Discussion
Reperfusion after myocardial ischemia can induce cardiomyocyte death, known as myocardial reperfusion injury. The pathophysiological process of reperfusion involves the confluence of multiple pathways. Accumulating evidence suggests that the underlying mechanisms responsible for I/R injury include intracellular calcium overload, production of free oxygen radicals, oxidative stress, excessive reactive oxygen species (ROS) generation, immune cells, release of cytokines, inflammation [16], neutrophil infiltration and adhesion, and endothelial cell dysfunction. All of these pathological processes finally contribute to cardiomyocyte apoptosis, cell death, and myocardial necrosis, leading to decreased cardiac contractility and cardiac function [17,18]. Recent studies have focused on the role of lipids, which may play an important role during the pathological process of diseases.
Reperfusion of myocardial muscle after prolonged ischemia is associated with metabolic and functional abnormalities and eventual cell death. Lipid metabolic and functional abnormalities may be involved in the underlying pathological mechanism of myocardial I/R injury. Disorders in lipid metabolism play a major role in irreversible myocardial injury. In rat models of myocardial I/R injury, there was a 10- to 15-fold increase in various types of fatty acids released from myocardium [19–21], including arachidonic acid, palmitic acid, oleic acid, and linoleic acid. A high concentration of arachidonic acid released from cell membrane phospholipids under pathological conditions of I/R resulted in myocardial cell apoptosis and accelerated necrosis, which collectively accelerated the development of cardiac dysfunction and heart failure, and even caused sudden death [22,23]. But until now, there was no related report about the dynamic changes of plasma lipid in human body and no researchers had used lipidomics technique to investigate the differences in lipid metabolism during the pathological process of myocardial I/R injury.
Lipid biomarkers changing before and after reperfusion after AMI can be identified and developed into a rapid diagnostic test for myocardial I/R injury and also reveal the underlying mechanism of myocardial I/R injury. This may help researchers locate a novel cardioprotective target and decrease the myocardial damage caused by ischemia/reperfusion.
Our study identified 16 types of potential lipid biomarkers by bioinformatics analysis in the 3 time periods, and we also found dynamic changes of these lipid contents before and after reperfusion. Regarding these lipid metabolites, our results indicated that these 16 types of potential lipid biomarkers at different time periods (T0, T2, T24) were identified by using lipidomics technology. The T0 time periods exhibited 16 differentially metabolized lipid peaks in the patients after STEMI diagnosis but prior to reperfusion. With the increase of reperfusion times, the contents of these 16 lipid biomarkers decreased gradually, but there was a 1.5- to 2-fold increase of these 16 lipid biomarkers contents at T2 compared with T24. However, the concentrations of traditional cardiac markers indicators (CK, CK-MB, and cTnT) in T2 time period was the highest among the 3 time periods, and moreover the concentrations of these markers in T24 was higher than in T0 time periods. We compared the changes of lipid biomarkers with those of traditional indexes (CK, CK-MB, and cTnT) and found lipid biomarkers and traditional indicators, during the periods of STEMI but prior to reperfusion, were polarized in concentrations. While during the periods of post-reperfusion, the changing trend of lipid biomarkers in concentrations was consistent with that of traditional indicators.
Based on these time series analyses, the detected lipid metabolic biomarkers could be developed as the preliminary diagnoses of myocardial I/R injury patients. It is possible to infer that fatty acid metabolism disorders, especially the high level of fatty acids at early reperfusion, are the major lipid metabolism disorders in the pathological process of myocardial I/R injury. Many researchers have shown similar results in animal models, and they also found that the level of circulating fatty acids was elevated during reperfusion of the ischemic heart [24,25].
Experimental findings also suggest that energy metabolism disorders closely related with the abnormal metabolism of fatty acids form the major pathological basis for myocardial I/R injury.
Under normal conditions, the heart has a very high energy demand and mainly acquires energy from glucose oxidation and fatty acid β-oxidation, which provide 10–40% and 60–90% of energy, respectively [26]. Under normal aerobic conditions, most of the energy generated from the β-oxidation of long-chain fatty acids is required to support continuous contractile activity of the heart and for efficient cardiac pumping [27]. Fatty acid β-oxidation is responsible for 60–90% of adenosine triphosphate (ATP) production [28]. Cardiomyocytes failing to use either substrate in the presence of different situations such as ischemia or anoxia suggest abnormalities in oxidation at the cellular level. The alterations in cardiac energy generation contribute to the severity of ischemic injury.
Our results also showed that the level of circulating fatty acids rose rapidly when acute MI occurred. These findings are consistent with those of previous studies in animal models. Takahiro et al. found that acute myocardial ischemia could cause an increase in plasma fatty acids levels in mice [29].
When AMI occurs, the acute myocardial ischemia results in reduction of aerobic ATP formation in mitochondria, and further leads to a shift towards glucose and inhibition of fatty acid metabolism. Thus, the concentrations of plasma fatty acids were elevated during acute myocardial infarction [30]. All of these changes accelerate glycolysis and decrease cell pH, K (+) efflux, Ca (2+) accumulation, adenosine formation, cell swelling and rupture, cell apoptosis, and death.
Accumulation of fatty acids leads to myocardial cell membrane damage, promotes the occurrence of dysrhythmias, inhibits myocardial contractility, and enhances the myocardial oxygen consumption, without an increase in myocardial work.
The level of circulating fatty acids in the present study was elevated at T2 compared with T24. But compared with T0, the level of circulating fatty acids was reduced. We speculate that reperfusion after severe ischemia might lead to an increase in overall cardiac fatty acid β-oxidation rates and increasing accumulation of fatty acids in the ischemic myocardium, which reduce the level of overall circulating fatty acids.
During reperfusion after severe myocardial ischemia, when the blood flow is restored, the oxygen levels are also restored by reperfusion. Fatty acid β-oxidation is restored rapidly and dominates as the major pathway of mitochondrial oxidative metabolism [31–33], and results in a delay in intracellular pH recovery and cardiac by inhibiting glucose oxidation [34–36].
Exposing the heart to a high concentration of plasma circulating fatty acids and the alteration in the subcellular control of fatty acid oxidation, combined with a decrease of malonyl CoA levels, leads to an increase in overall cardiac fatty acid β-oxidation rates during reperfusion after ischemia, with a concomitant large decrease in glucose oxidation rates [37].
Animal studies have shown that the elevation of circulating fatty acids and increased cardiac fatty acid β-oxidation results in decreased cardiac function and efficiency, especially during reperfusion after severe myocardial ischemia. Elevated fatty acids may lead to a critical increase in oxygen requirements of the already compromised myocardium and infarct size [38], inhibiting the glucose utilization of ischemic myocardium, increasing accumulation of fatty acids in the ischemic myocardium, where they can cause a disorder in the electrical activity and conduction [39]. Lopaschuk et al. found that elevated circulating fatty acid oxidation can lead to decreasing cardiac efficiency by up to 30% [40]. Oliver et al. showed that the elevated level of free fatty acids was associated with a higher risk of severe heart block, ventricular arrhythmias, and sudden death [41,42]. Moreover, exposure of cells to elevated concentrations of fatty acids can cause oxidative damage to mitochondrial DNA. In another experiment, researchers reported fatty acid-induced apoptosis could be, at least in some cases, mediated through ROS generation [43].
In addition, the association between ROS production and the metabolism of fatty acids has been well known for 3 decades. Animal experiments have demonstrated that the elevated level of circulating free fatty acids can stimulate β-oxidation, and excessive fatty acid β-oxidation can lead to excessive generation of reactive oxygen that damages myocardial structure and function. We can test the level of ROS by testing the contents of malondialdehyde (MDA) in blood. Medium and high concentrations of ROS can induce cell apoptosis and even cause cell necrosis via oxidative stress [44–46].
Moreover, the lipidomics network analysis also revealed a number of interesting results regarding signal transduction function relationships that lipid biomarkers are involved in. Myristic acid, oleic acid, linoleic acid, γ-linolenic acid, arachidonic acid, timnodonic acid (EPA), palmitic acid, and hexadecyl azelaoyl phosphatidylcholine were demonstrated to mediate signal transduction by mechanisms related to peroxisome proliferator-activated receptors (PPARs) gene function [47–50]. PPARs signal pathways have been shown to play an important role in myocardial I/R injury [51]. Myristic, pentadecanoic, palmitic, and palmitoleic acids can result in down-regulation of TNF-α by activating PPAR in inflammatory diseases [52].
The experimental results as discussed reveal that elevated circulating fatty acids may induce cardiomyocytes apoptosis mediated by ROS generation and regulate PPARs signal pathways, which could be an underlying pathological mechanism of myocardial ischemia reperfusion injury. Therefore, the level of fatty acids may be seen as a risk factor of myocardial I/R injury, and the level of fatty acids can also be considered as a diagnostic indicator by which the extent of reperfusion injury can be assessed.
Conclusions
In summary, the lipidomics technique offers a highly effective approach to improve the under-diagnosis of myocardial I/R injury and detect biomarkers that can distinguish subsequent differentiation regarding before and after reperfusion. Our results indicate 16 potential biomarkers associated with myocardial I/R injury identified through data analysis. The detected lipid metabolic biomarkers, as predicative factors, have the potential to be applied to establish a classification model; this could be developed as the preliminary diagnoses of myocardial I/R injury patients. Our results also show that we can reduce the fatty acids level (lipid biomarkers) to prevent the development of myocardial I/R injury. These biomarkers also have potential to provide further insights into the pathological mechanisms of myocardial I/R injury.
Footnotes
Conflict of interest
None.
Source of support: This study was funded by the National Natural Science Foundation of China (Grant No. 81774016), the Science Foundation on Traditional Chinese Medicine/Integrative Medicine of the Tianjin Health and Family Planning Commission (Grant No. 2015147)
References
- 1.Bhayana V, Henderson AR. Biochemical markers of myocardial damage. Clin Biochem. 1995;28:1–29. doi: 10.1016/0009-9120(94)00065-4. [DOI] [PubMed] [Google Scholar]
- 2.Le Mar HJ, West SG, Garrett CR, et al. Covert hypothyroidism presenting as a cardiovascular event. Am J Med. 1991;91:549–52. doi: 10.1016/0002-9343(91)90194-3. [DOI] [PubMed] [Google Scholar]
- 3.Cohen LF, Mohabeer AJ, Keffer JH, et al. Troponin I in hypothyroidism. Clin Chem. 1996;42:1494–95. [PubMed] [Google Scholar]
- 4.Kumar M, Abrina VM, Chittimireddy S. Pulmonary embolism caused by delayed heparin-induced thrombocytopenia in a patient who received prophylactic LMWH. Am J Case Rep. 2012;13:118–21. doi: 10.12659/AJCR.883107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scalco RS, Chatfield S, Junejo MH, et al. McArdle disease misdiagnosed as meningitis. Am J Case Rep. 2016;17:905–8. doi: 10.12659/AJCR.900967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gadomska H, Grzechocińska B, Janecki J, et al. Serum lipids concentration in women with benign and malignant ovarian tumours. Eur J Obstet Gynecol Reprod Biol. 2005;120(1):87–90. doi: 10.1016/j.ejogrb.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Kong H, Guan Y, et al. Plasma phospholipid metabolic profiling and biomarkers of type 2 diabetes mellitus based on high-performance liquid chromatography/electrospray mass spectrometry and multivariate statistical analysis. Anal Chem. 2005;77(10):4108–16. doi: 10.1021/ac0481001. [DOI] [PubMed] [Google Scholar]
- 8.Clish CB, Davidov E, Oresic M, et al. Integrative biological analysisof the APOE*3-Leiden transgenic mouse. OMICS. 2004;8(1):3–13. doi: 10.1089/153623104773547453. [DOI] [PubMed] [Google Scholar]
- 9.Touboul D, Gaudin M. lipidomics of Alzheimer’s disease. Bioanalysis. 2014;6(4):541–61. doi: 10.4155/bio.13.346. [DOI] [PubMed] [Google Scholar]
- 10.Brügger B, Glass B, Haberkant P, et al. The HIV lipidome: A raft with an unusual composition. Proc Natl Acad Sci USA. 2006;103(8):2641–46. doi: 10.1073/pnas.0511136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bligh EG, Dyer WJ. A rapid method for total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–17. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 12.Yang K, Jenkins CM, Dilthey B, et al. Multidimensional mass spectrometry-based shotgun lipidomics analysis of vinyl ether diglycerides. Anal Bioanal Chem. 2015;407(17):5199–210. doi: 10.1007/s00216-015-8640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou X, Mao J, Ai J, et al. Identification of plasma lipid biomarkers for prostate cancer by lipidomics and bioinformatics. PLoS One. 2012;7(11):e48889. doi: 10.1371/journal.pone.0048889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anesi A, Guella G. A fast liquid chromatography-mass Spectrometry methodology for membrane lipid profiling through hydrophilic interaction liquid chromatography. J Chromatogr A. 2015;1384:44–52. doi: 10.1016/j.chroma.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 15.Zhang T, Chen S, Liang X, et al. Development of a mass-spectrometry-based lipidomics platform for the profiling of phospholipids and sphingolipids in brain tissues. Anal Bioanal Chem. 2015;407(21):6543–55. doi: 10.1007/s00216-015-8822-z. [DOI] [PubMed] [Google Scholar]
- 16.Wang HW, Liu HJ, Cao H, et al. Diosgenin protects rats from myocardial inflammatory injury induced by ischemia-reperfusion. Med Sci Monit. 2018;24:246–53. doi: 10.12659/MSM.907745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruisong M, Xiaorong H, Gangying H, et al. The Protective role of interleukin-33 in myocardial ischemia and reperfusion is associated with decreased HMGB1 expression and up-regulation of the P38 MAPK signaling pathway. PLoS One. 2015;10(11):e0143064. doi: 10.1371/journal.pone.0143064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vork MM, Glatz JFC, van der Vusse GJ. Release of fatty acid-binding protein and long-chain fatty acids from isolated rat heart after ischemia and subsequent calcium paradox. Mol Cell Biol. 1993;123(1–2):175–84. doi: 10.1007/BF01076490. [DOI] [PubMed] [Google Scholar]
- 19.Fang KM, Lee AS, Su MJ, et al. Free fatty acids act as endogenous ionophores, resulting in Na+ and Ca2+ influx and myocyte apoptosis. Cardiovasc Res. 2008;78(3):533–45. doi: 10.1093/cvr/cvn030. [DOI] [PubMed] [Google Scholar]
- 20.Maia RC, Culver CA, Laster SM. Evidence against calcium as a mediator of mitochondrial dysfunction during apoptosis induced by arachidonic acid and other free fatty acids. J Immunol. 2006;177(9):6398–404. doi: 10.4049/jimmunol.177.9.6398. [DOI] [PubMed] [Google Scholar]
- 21.Folmes CD, Sowah D, Clanachan AS, et al. High rates of residual fatty acid oxidation during mild ischemia decrease cardiac work and efficiency. J Mol Cell Cardiol. 2009;47(1):142–48. doi: 10.1016/j.yjmcc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Liedtke AJ, DeMaison L, Eggleston AM, et al. Changes in substrate metabolism and effects of excess fatty acids in reperfused myocardium. Circ Res. 1988;62(3):535–42. doi: 10.1161/01.res.62.3.535. [DOI] [PubMed] [Google Scholar]
- 23.Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: Enemy or ally? J Physiol. 2006;574(Pt 1):95–112. doi: 10.1113/jphysiol.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85(3):1093–129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 25.Neely JM, Morgan HE. Relationship between metabolism and energy balance of heart muscle. Annu Rev Physiol. 1974;36:413–59. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- 26.Neubauer S. The failing heart-an engine out of fuel. N Engl J Med. 2007;356(11):1140–51. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 27.Opie LH. Metabolism of the heart in health and disease. I. Am Heart J. 1968;76(5):685–98. doi: 10.1016/0002-8703(68)90168-3. [DOI] [PubMed] [Google Scholar]
- 28.Opie LH. Metabolismof the heart in health and disease. II. Am Heart J. 1969;77(1):100–122. doi: 10.1016/0002-8703(69)90135-5. [DOI] [PubMed] [Google Scholar]
- 29.Kambara T, Ohashi K, Shibata R. CTRP9 protein protects against myocardial injury following ischemia-reperfusion through AMP-activated protein kinase (AMPK)-dependent mechanism. J Biol Chem. 2012;287(23):18965–73. doi: 10.1074/jbc.M112.357939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopaschuk GD, Collins-Nakai R, Olley PM, et al. Plasma fatty acid levels in infants and adults after myocardial ischemia. Am Heart J. 1994;128(1):61–67. doi: 10.1016/0002-8703(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 31.Lopaschuk GD, Spafford MA, Davies NJ, et al. Glucose and palmitate oxidation in isolated working rat hearts reperfused after a period of transient global ischemia. Circ Res. 1990;66(2):546–53. doi: 10.1161/01.res.66.2.546. [DOI] [PubMed] [Google Scholar]
- 32.Liedtke AJ, Demaison L, Eggleston AM, et al. Changes in substrate metabolism and effects of excess fatty acids in reperfused myocardium. Circ Res. 1998;62(3):535–42. doi: 10.1161/01.res.62.3.535. [DOI] [PubMed] [Google Scholar]
- 33.Lerch R, Tamm C, Papageorgiou I, et al. Myocardial fatty acid oxidation during ischemia and reperfusion. Mol Cell Biochem. 1992;116(1–2):103–9. doi: 10.1007/BF01270576. [DOI] [PubMed] [Google Scholar]
- 34.Liu Q, Docherty JC, Rendell JC, et al. High levels of fatty acids delay the recovery of intracellular pH and cardiac efficiency in post-ischemic hearts by inhibiting glucose oxidation. J Am Coll Cardiol. 2002;39(4):718–25. doi: 10.1016/s0735-1097(01)01803-4. [DOI] [PubMed] [Google Scholar]
- 35.Liu B, Clanachan AS, Schulz R, et al. Cardiac efficiency is improved after ischemia by altering both the source and fate of protons. Circ Res. 1996;79(5):940–48. doi: 10.1161/01.res.79.5.940. [DOI] [PubMed] [Google Scholar]
- 36.Lopaschuk GD, Wambolt RB, Barr RL. An imbalance between glycolysis and glucose oxidation is a possible explanation for the detrimental effects of high levels of fatty acids during aerobic reperfusion of ischemic hearts. J Pharmacol Exp Ther. 1993;264(1):135–44. [PubMed] [Google Scholar]
- 37.Folmes CD, Clanachan AS, Lopaschuk GD. Fatty acid oxidation inhibitors in the management of chronic complications of atherosclerosis. Curr Atheroscler Rep. 2005;7(1):63–70. doi: 10.1007/s11883-005-0077-2. [DOI] [PubMed] [Google Scholar]
- 38.Vik-Mo H, Mjøs OD. Influence of free fatty acids on myocardial oxygen consumption and ischemic injury. Am J Cardiol. 1981;48(2):361–65. doi: 10.1016/0002-9149(81)90621-4. [DOI] [PubMed] [Google Scholar]
- 39.Kurien VA, Oliver MF. A metabolic cause of arrhythmias during acute myocardial hypoxia. Lancet. 1970;1(7651):813–15. doi: 10.1016/s0140-6736(70)92412-8. [DOI] [PubMed] [Google Scholar]
- 40.Lopaschuk GD, Ussher JR, Folmes CD, et al. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90(1):207–58. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 41.Oliver MF, Kurien VA, Greenwood TW. Relation between serum free fatty acids and arrhythmias and death after acute myocardial infarction. Lancet. 1968;1(7545):710–14. doi: 10.1016/s0140-6736(68)92163-6. [DOI] [PubMed] [Google Scholar]
- 42.Ravens KG, Jipp P. [Die freien plasmafettsäuren in der frühphase eines myocardinfarkts]. Arzneim Forsch. 1972;78:1831–35. [in German] [PubMed] [Google Scholar]
- 43.Cosgrove JP, Church DF, Pryor WA. The kinetics of the autoxidation of polyunsaturated fatty acids. Lipids. 1987;22(5):299–304. doi: 10.1007/BF02533996. [DOI] [PubMed] [Google Scholar]
- 44.Simon HU, Haj-Yehia H, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5(5):415–18. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 45.Bauer G. Signaling and proapoptotic functions of transformed cell-derived reactive oxygen species. Prostaglandins Leukot Essent Fat Acids. 2002;66(1):41–56. doi: 10.1054/plef.2001.0332. [DOI] [PubMed] [Google Scholar]
- 46.Fleury C, Mignotte G, Vayssière JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002;84(2–3):131–41. doi: 10.1016/s0300-9084(02)01369-x. [DOI] [PubMed] [Google Scholar]
- 47.Bodi V, Sanchis J, Morales JM, et al. Metabolomic profile of human myocardial ischemia by nuclear magnetic resonance spectroscopy of peripheral blood serum: A translational study based on transient coronary occlusion models. J Am Coll Cardiol. 2012;59(18):1629–41. doi: 10.1016/j.jacc.2011.09.083. [DOI] [PubMed] [Google Scholar]
- 48.Kliewer SA, Sundseth SS, Jones SA, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA. 1997;94(9):4318–23. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu HE, Lambert MH, Montana VG, et al. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell. 1999;3(3):397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- 50.Banner CD, Göttlicher M, Widmark E, et al. A systematic analytical chemistry/cell assay approach to isolate activators of orphan nuclear receptors from biological extracts: Characterization of peroxisome proliferator-activated receptor activators in plasma. J Lipid Res. 1993;34(9):1583–91. [PubMed] [Google Scholar]
- 51.Yue TL, Chen J, Bao W, et al. In vivo myocardial protection from ischemia/reperfusion injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2001;104(21):2588–94. doi: 10.1161/hc4601.099403. [DOI] [PubMed] [Google Scholar]
- 52.Debierre-Grockiego F, Schofield L, Azzouz N, et al. Fatty acids from Plasmodium falciparum down-regulate the toxic activity of malaria glycosylphosphatidylinositols. Infect Immun. 2006;74(10):5487–96. doi: 10.1128/IAI.01934-05. [DOI] [PMC free article] [PubMed] [Google Scholar]