Using intravenous thrombolysis in a stroke patient with cerebral microbleeds represents one of the most challenging clinical decisions in acute stroke neurology. In this setting, the implications of coexisting ischemic and hemorrhagic cerebrovascular disease(mixed cerebrovascular disease)1 must be confronted and urgently addressed. The clinical consequences of intervening or not intervening are profound.
What exactly are the consequences? The greatest concern, of course, is the risk of thrombolysis-induced intracerebral hemorrhage. This risk is explored in this issue of JAMA Neurology. In the study by Tsivgoulis et al,2 the presence of cerebral microbleeds was associated with substantial increased risk of symptomatic intracerebral hemorrhage. Among patients with any number of cerebral microbleeds, risk for symptomatic intracerebral hemorrhage increased more than 2-fold. Among patients with the highest number of microbleeds (more than 10), the risk increased 7- to 12-fold, and likelihood of symptomatic intracerebral hemorrhage increased 18- to 31-fold.
This increased risk of symptomatic intracerebral hemorrhage with mixed cerebrovascular disease will certainly give any clinician pause before using tissue plasminogen activator (tPA). Does presence of cerebral microbleeds represent a relative contraindication for tPA? An absolute contraindication for tPA? How does the contraindication depend on the number of microbleeds present?
At one level, hemorrhagic risk of thrombolysis in acute stroke is reasonably well understood. Activation of plasmin shifts the hemostatic balance toward fibrinolysis, with resultant mitigation of thrombus generation necessary to maintain vascular integrity. The vasculature is left relatively unprotected to the threat of ischemic injury to vessel integrity, and hemorrhage becomes more likely. At amore subtle level, the proteolytic consequences of plasmin activation include the well-described activation of matrix metalloproteinases, which further enhance ischemic injury to the vasculature.3 Therefore, hemorrhage in the setting of plasmin activation is no mystery.
But how does the presence of microbleeds fit in to the equation? Addressing this question requires confronting the issue of the underlying nature of cerebral microbleeds. We know that cerebral microbleeds are small regions of hemosiderin-iron demonstrable by magnetic resonance imaging. Microbleeds show a striking age distribution, present in more than 20% of older individuals but virtually absent in younger populations.4 Cerebral microbleeds have received enormous attention in the literature. Nevertheless, the nature of the underlying lesion of cerebral microbleeds has proved elusive.
The consensus view is that cerebral microbleeds are indicative of cerebral small-vessel disease, perhaps serving as biomarkers. While this view is likely correct, it is of limited value to explain the pathogenesis and characteristics of cerebral microbleeds. While no definitive answer can be provided at present, there is now sufficient evidence to create a plausible model for pathogenesis of cerebral microbleeds. The value of such a model relates to its dual role of being both hypothesis-generating and providing clinicians a framework for addressing the clinical conundrum created by presence of mixed cerebrovascular disease.
Brain Protection Against Hemorrhage
The brain has a unique hemostatic regulatory system, residing in the microvasculature at the capillary level and containing both structural and functional components. The presence of tight interendothelial junctions, while typically viewed as a molecular barrier, also provides protection against red blood cell extravasation beyond what is available in the peripheral vasculature. Pericytes, preferentially localized opposite interendothelial junctions, provide an additional structural barrier. Beyond these structural features, brain endothelial cells at the blood-brain barrier exhibit a highly characteristic restricted expression of antithrombotic and fibrinolytic molecules, combined with relative abundance of procoagulant factors.5
The net effect of these structural elements and functional features (restricted expression of antithrombotic molecules combined with upregulation of procoagulant factors) is to create a prothrombotic milieu in the brain microvasculature.5 The need for such a system may relate to the brain’s high blood flow/low resistance of the more proximal vasculature, placing capillary beds at risk for development of microhemorrhage (the pathological substrate of microbleeds). However, presence of high flow/low resistance are organ characteristics not limited to the brain. The kidney, for example, is another low-resistance organ and has blood flow estimated to be 7 times higher than the brain.6 The need for specific protection against brain hemorrhage likely goes well beyond the brain’s blood flow characteristics and may involve the unique role the central nervous system plays for the organism.
Pathologic Heterogeneity of Microbleeds: Primary and Secondary Processes
While the topography of cerebral microbleeds is understood to show variability depending on risk factors, including hypertension and cerebral amyloid angiopathy,4 the nature of the underlying lesions is still generally viewed as homogenous. This view appears to be incorrect. A more accurate view must account for the observations, now documented on multiple occasions, that microbleeds predict not only hemorrhagic phenomena as expected but also are predictive of ischemic stroke.1 This dual role of microbleeds in predicting both ischemic and hemorrhagic stroke provides strong indirect support to the concept of primary and secondary cerebral microbleeds. Primary microbleeds are the consequence of an initial direct disruption of the vasculature, while secondary microbleeds occur as a consequence of ischemic injury. The phenomenon of secondary microbleeds has been demonstrated by sequential magnetic resonance imaging studies of patients undergoing early ischemic injury, with microbleeds developing as a consequence.1
Within the conceptual framework of primary and secondary microbleeds, the dual predictive role for cerebral microbleeds becomes understandable. From the perspective of antithrombotic stroke prevention, the patient with primary microbleeds requires less, while the patient with secondary microbleeds may require more. From the perspective of acute thrombolysis, the risk of hemorrhage would appear to be far more substantial with primary microbleeds compared with the patient whose secondary microbleeds represent the consequences of prior ischemic injury.
Risk Factors and Microbleeds
As already noted, increasing age is the single most important risk factor for development of cerebral microbleeds. But age is clearly compounded by a series of important vascular comorbidities that are codeterminants of cerebral microbleeds. Both hypertension and cerebrala myloid angiopathy are widely appreciated risk factors for microbleeds.4 But 2 other risk factors are also important: chronic kidney disease7 and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL).8 While these latter 2 are less well known, they have a convincingly established relationship with cerebral microbleeds that offers important clues to the nature of the underlying lesion.
Microbleeds and White Matter Disease
White matter disease, subcortical lesions typically demonstrable on magnetic resonance fluid-attenuated inversion recovery (FLAIR) imaging, has long been shown to be highly predictive of cerebral microbleeds. The literature has reported this so consistently that the association is beyond dispute.9 The meaning of this association is less clear and relates in part to the uncertainty of the underlying lesions of white matter disease. Recent investigations have convincingly demonstrated that microinfarcts are a substantial component of white matter disease,10 providing additional clues to the nature of the microbleeds–white matter disease relationship.
Vascular Source of Microbleeds: Arterioles and Capillaries
Arteriolar smooth muscle cells are targets of all 4 of the major risk factors of microbleeds:hypertension, cerebralamyloid angiopathy, chronic kidney disease, and CADASIL. Smooth muscle cell loss is characteristic of the hypertensive arteriolar disease sometimes referred to as lipohyalinosis.11 Beta-amyloid has been shown to be toxic to smooth muscle cells in vitro, and pathologic analysis consistently shows smooth muscle cell loss in brain arterioles in cerebral amyloid angiopathy.12 Chronic kidney disease exhibits hyperphosphatemia, resulting in smooth muscle cell apoptosis and medial calcification.13 The hallmark of the vascular pathology of CADASIL is arteriolar smooth muscle injury.11 The consistent loss of smooth muscle cells and presence of arteriolar injury have the functional consequences of impaired autoregulation, with incapacity to accommodate alterations of systemic blood pressure.14,15
Capillary injury at the blood-brain barrier level is well described in hypertension,11 cerebralamyloid angiopathy,12 and CADASIL.11 Blood-brain barrier permeability is known to increase with aging.5 Blood-brain barrier function in chronic kidney disease is not yet well investigated.
Thrombolysis and Mechanisms of Cerebral Microbleeds
The hallmark of cerebral microbleeds appears to be arteriolar injury, targeted by all 4 of the major vascular comorbidities of microbleeds and resulting in the brain’s incapacity to appropriately regulate cerebral blood flow (Figure). Impairment of the distal capillary bed by vascular comorbidities of microbleeds creates the pathological substrate for red blood cell extravasation at the capillary level (microhemorrhage). The critical factor relevant to thrombolysis appears to be not the microbleeds themselves, but the arteriolar injury contributing to microhemorrhage development. The intracerebral hemorrhage developing in the context of thrombolysis is likely owing to ischemic necrosis, compounding the effects of pre-existing arteriolar injury.
Figure. A Vascular Neurobiology Model of Cerebral Microbleeds.
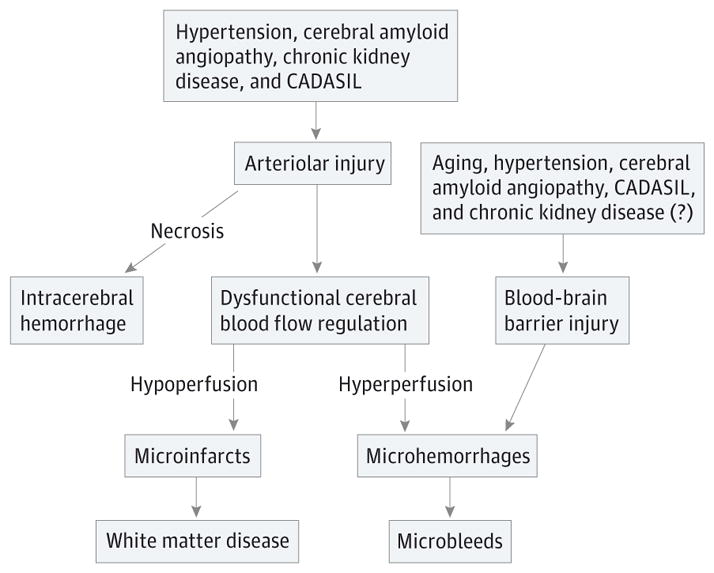
In this model of cerebral microbleeds, the 4 principal vascular comorbidities (hypertension, cerebral amyloid angiopathy, chronic kidney disease, and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy [CADASIL]) all affect brain arterioles. The consequences are impaired autoregulation (dysfunctional regulation of cerebral blood flow) producing incapacity to accommodate alterations in systemic blood pressure. Further arteriolar injury, ie, necrosis occurring spontaneously or induced by ischemia, results in intracerebral hemorrhage. Concurrently, blood-brain barrier alterations owing to aging, hypertension, cerebral amyloid angiopathy, CADASIL, and possibly chronic kidney disease, create the impaired capillary bed needed for red blood cell extravasation and development of microhemorrhage, the pathologic substrate of microbleeds.
The principal challenge for the clinician addressing microbleeds in acute ischemic stroke will be to distinguish primary from secondary microbleeds. It is the disseminated processes of primary microbleeds that create the substrate of the brain vulnerable to arteriolar ischemic necrosis and development of intracerebral hemorrhage. The more restricted process of prior ischemic injury producing secondary microbleeds will be of far less concern in this context. Ultimately, the number of cerebral microbleeds present will be less important than the nature of the process driving microbleed development.
Acknowledgments
Funding/Support: Dr Fisher is supported by National Institutes of Health grant NS20989.
Role of the Funder/Sponsor: The funding source had no role in the preparation, review, or approval of the manuscript and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Dr Fisher receives research grant support from Otsuka Pharmaceutical Company and Boehringer-Ingelheim and is a member of clinical events committees of Covidien.
References
- 1.Fisher M. Cerebral microbleeds: where are we now? Neurology. 2014;83(15):1304–1305. doi: 10.1212/WNL.0000000000000871. [DOI] [PubMed] [Google Scholar]
- 2.Tsivgoulis G, Zand R, Katsanos AH, et al. Risk of symptomatic intracerebral hemorrhage after intravenous thrombolysis in patients with acute ischemic stroke and high cerebral microbleed burden: a meta-analysis [published online April 18, 2016] JAMA Neurol. doi: 10.1001/jamaneurol.2016.0292. [DOI] [PubMed] [Google Scholar]
- 3.Jickling GC, Liu D, Stamova B, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. 2014;34(2):185–199. doi: 10.1038/jcbfm.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg SM, Vernooij MW, Cordonnier C, et al. Microbleed Study Group. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher MJ. Brain regulation of thrombosis and hemostasis: from theory to practice. Stroke. 2013;44(11):3275–3285. doi: 10.1161/STROKEAHA.113.000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman TG, Hall JE. Systemic hemodynamics and regional blood flow regulation. In: Izzo JL Jr, Sica DA, Black HR, editors. Hypertension. 4. Dallas, TX: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 7.Oh MY, Lee H, Kim JS, et al. Cystatin C, a novel indicator of renal function, reflects severity of cerebral microbleeds. BMC Neurol. 2014;14:127. doi: 10.1186/1471-2377-14-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JS, Kang CH, Park SQ, Choi HA, Sim KB. Clinical significance of cerebral microbleeds locations in CADASIL with R544C NOTCH3 mutation. PLoS One. 2015;10(2):e0118163. doi: 10.1371/journal.pone.0118163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher M. Cerebral microbleeds and white matter disease: separated at birth? Eur J Neurol. 2012;19(1):2–3. doi: 10.1111/j.1468-1331.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conklin J, Silver FL, Mikulis DJ, Mandell DM. Are acute infarcts the cause of leukoaraiosis? Brain mapping for 16 consecutive weeks. Ann Neurol. 2014;76(6):899–904. doi: 10.1002/ana.24285. [DOI] [PubMed] [Google Scholar]
- 11.Craggs LJ, Yamamoto Y, Deramecourt V, Kalaria RN. Microvascular pathology and morphometrics of sporadic and hereditary small vessel diseases of the brain. Brain Pathol. 2014;24(5):495–509. doi: 10.1111/bpa.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalaria RN. Cerebrovascular degeneration is related to amyloid-beta protein deposition in Alzheimer’s disease. Ann N Y Acad Sci. 1997;826:263–271. doi: 10.1111/j.1749-6632.1997.tb48478.x. [DOI] [PubMed] [Google Scholar]
- 13.Shroff RC, McNair R, Skepper JN, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J AmSoc Nephrol. 2010;21(1):103–112. doi: 10.1681/ASN.2009060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabayan B, Wijsman LW, Foster-Dingley JC, et al. Association of visit-to-visit variability in blood pressure with cognitive function in old age: prospective cohort study. BMJ. 2013;347:f4600. doi: 10.1136/bmj.f4600. [DOI] [PubMed] [Google Scholar]
- 15.Tarumi T, de Jong DL, Zhu DC, et al. Central artery stiffness, baroreflex sensitivity, and brain white matter neuronal fiber integrity in older adults. Neuroimage. 2015;110:162–170. doi: 10.1016/j.neuroimage.2015.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]


