Abstract
Purpose of review
Topical corticosteroid use in the setting of infectious keratitis has been a controversial issue. The aim of this review is to provide an update on the evidence for use of topical corticosteroids in addition to antibiotics in bacterial keratitis.
Recent findings
Judicious use of steroids is postulated to limit the inflammatory component of bacterial keratitis, but can theoretically retard healing. Three small randomized controlled trials and one large-scale trial, the Steroids for Corneal Ulcers Trial, have provided the most recent evidence to address this debate. Adjunctive topical corticosteroids initiated after at least 48 h of antibiotic usage in cases of culture-proven bacterial keratitis appear generally safe in the treatment of bacterial keratitis. They may be beneficial in cases of severe ulcers especially when initiated early in the course of the infection, in non-Nocardia ulcers, and in certain Pseudomonas ulcers.
Summary
Several randomized controlled trials have greatly contributed to our understanding of the controversy over steroid use in the management of bacterial keratitis. Future studies are needed to confirm subgroup analysis findings and define optimal timing, dosage, and the most appropriate treatment populations.
Keywords: bacterial keratitis, corneal ulcer, corticosteroid, Steroids for Corneal Ulcers Trial
INTRODUCTION
Bacterial keratitis is an infection of the cornea characterized by an area of suppurative stromal infiltration with an overlying epithelial defect. Symptoms are generally acute and include ocular pain, decreased vision, conjunctival injection, and photophobia. Even with early and aggressive treatment, this can be a vision-threatening condition and is a leading cause of monocular blindness worldwide [1]. It has been estimated that the incidence of all forms of infectious keratitis is 27.6 per 100 000 person-years [2], with bacterial keratitis affecting between 27 000 and 30 000 individuals in the United States annually [3]. In the developing world, the rate of infectious keratitis may be 10–70 times greater than those reported in the United States [1]. Major risk factors, which vary in importance by geography, include ocular trauma or surgery, ocular surface disease, and contact lens wear. Climate itself can dramatically affect the rate and type of keratitis, for example, fungal keratitis is more common in tropical regions than temperate zones.
The mainstay of treatment consists of early empiric broad-spectrum topical antibiotics, sometimes with narrowing of coverage based on culture and sensitivity results. The role of adjunctive topical corticosteroids has been controversial. Proponents of the use of steroids in bacterial keratitis claim that the inflammatory reaction to infection is responsible for much of the tissue destruction and subsequent scarring seen after corneal ulceration. They feel that controlling the inflammation along with the infection will lead to improved clinical outcomes. Opponents to the use of steroids are concerned that suppressing the local immune system may lead to poor wound healing and potentiate bacterial activity. In this study, we will review the evidence for use of adjunctive topical corticosteroids in bacterial keratitis.
RATIONALE FOR CORTICOSTEROID USE
Infection and inflammation play dual roles in both the pathogenesis and clinical sequelae of bacterial keratitis, providing the main rationale for adjunctive use of topical corticosteroids in addition to antibiotics. Although bacteria induce corneal injury, the host inflammatory response to the infection further undermines corneal integrity to prevent healing and ultimately lead to scarring. As T cells and macrophages respond to the bacterial invasion, they produce cytokines such as IL-1 and tumor necrosis factor to facilitate neutrophil migration and degranulation [4]. In particular, platelet-activating factor upregulates metalloproteinases, which can cause further stromal necrosis [5]. These complex host–microbial interactions can ultimately lead to significant corneal thinning and contribute to corneal scarring.
Corticosteroids have been used for their anti-inflammatory effect in various other corneal conditions to decrease haze and scarring, including after refractive surgery and in the treatment of herpes simplex stromal keratitis [6]. They exert their effect through decreasing inflammatory factors, such as prostaglandins, inducing vasoconstriction, and decreasing neovascularization [5]. The goal of using steroids in addition to antibiotics for bacterial keratitis is to simultaneously target the infection and limit the host inflammatory response, ultimately mitigating corneal opacification.
REASONS AGAINST CORTICOSTEROID USE
The concern that corticosteroid administration can potentiate bacterial infection and lead to severe corneal thinning with possible stromal melt, is the primary rationale against its use in infectious keratitis. Animal studies have shown that corneal wound strength can be decreased in the setting of topical steroid administration after corneal injury [7,8]. Additionally, several infectious causes are often cited as particularly worrisome in the context of steroid use. For example, a potential prospective study of topical prednisolone sodium phosphate administration for culture-positive bacterial keratitis was halted after one patient with a Pseudomonas ulcer who was improving after 5 days of fortified antibiotics therapy dramatically worsened after 2 days of steroid use, eventually requiring hospital admission and penetrating keratoplasty [9]. Another concern is that steroids may be started inappropriately in fungal or acanthamoeba infections, which are thought to be exacerbated by topical steroid use. Furthermore, these organisms are all more prevalent with contact lens use, which is on the rise as a risk factor for infectious keratitis in many developed countries [2,10–13].
KEY STUDIES
For decades, the debate over the use of adjunctive corticosteroids for the treatment of bacterial keratitis has persisted. In 1990, a randomized but unmasked trial conducted in South Africa by Carmichael et al. [14] included 40 cases and demonstrated no statistically significant difference in final visual acuity, healing rate, or complication rate between the antibiotic only and antibiotic plus corticosteroid groups. Two patients in the steroid group did not receive full treatment (one patient because of descemetocele formation the morning after admission, and another because of progressive corneal thinning at 12 days), and healing rates were calculated only with a subset of data (those with persistent epithelial defects or who had received therapy other than protocol medications were excluded from the analysis). The sample size was small and may have been insufficient to find a true effect and they did not analyze using intention-to-treat methodology. Retrospective studies thereafter found possible associations of steroid use with failure of medical therapy and larger ulcer size in contact lens users [15,16]. Nonetheless, neither a 2002 review of the literature by Wilhelmus [17] nor a 2009 Cochrane review [18] could definitively conclude whether the adjuvant use of topical steroids in bacterial keratitis conferred benefit or harm. Since then, there have been three additional randomized trials. Blair et al. [19] showed in a study of 30 patients randomized to either gatifloxacin or gatifloxacin plus dexamethasone that there was no difference in residual ulcer size at 10 weeks based on digital photographs, visual acuity, or time to healing. There was also no difference in adverse events. However, patient enrollment was lower than the expected, precalculated target of 54. In 2009, a randomized controlled trial was published both to assess the effect of adjunctive topical corticosteroids, as well as to determine the sample size for a larger trial [20]. This single-center, double-masked prospective trial included 42 patients with culture-confirmed bacterial keratitis at Aravind Eye Hospital in India, and patients were randomized to receive either topical prednisolone phosphate or placebo in addition to topical moxifloxacin. The authors found that patients in the steroid group reepithelialized more slowly (hazard ratio 0.47, 95% confidence interval 0.23–0.94), but there was no significant difference in best spectacle-corrected visual acuity (BSCVA) or infiltrate/scar size at 3 weeks or 3 months. However, the authors also calculated that to have 80% power to detect a two-line difference in acuity, 360 cases would be required. Based on results of this pilot study, the Steroids for Corneal Ulcers Trial (SCUT) was initiated and published in 2012, and remains the largest randomized controlled trial on the topic to date [21]. In a recent review by Tallab and Stone [22], the authors concluded that, based on SCUT, there was level I evidence for a small benefit with the use of steroids in culture-proven non-Nocardia bacterial keratitis.
STEROIDS FOR CORNEAL ULCERS TRIAL AND ITS SUBGROUP ANALYSES
SCUT was a randomized, double-masked, placebo-controlled trial, which sought to determine whether there is a benefit to the use of adjunctive topical corticosteroids bacterial keratitis. Five-hundred study participants with culture-proven bacterial keratitis were enrolled at Aravind Hospital in India, the University of San Francisco, and Dartmouth. At least 48 h after initiation of appropriate antibiotics, topical prednisolone phosphate 1% was started at four times daily for 1 week, twice daily for 1 week, and once daily for 1 week. The primary outcome was BSCVA at 3 months. Secondary outcomes included infiltrate/scar size, time to reepithelialization, and rate of perforation. There was no difference between the steroid and placebo groups in 3-month visual acuity, scar size, time to reepithelialization, or rate of perforation.
Although the main study showed that on average, steroids do not make a difference, several subgroup analyses suggest that there may be a role for steroids in the treatment of certain categories of bacterial ulcers. In a prespecified subgroup analysis of severe ulcers defined as vision of counting fingers or worse, steroid-treated ulcers had 0.17 Logarithm of the Minimum Angle of Resolution (LogMAR) (1.7 lines) better visual acuity at 3 months compared with placebo. Similarly, those with central ulcers had 0.20 LogMAR (two lines) better visual acuity at 3 months compared with placebo [21].
A subsequent analysis also showed that early initiation of steroid 2 or 3 days after starting antibiotics may lead to improved BSCVA at 3 months compared with those receiving steroid more than 3 days after starting antibiotics [23▪]. The early steroid treatment group performed 1.1 lines better than the placebo group (P =0.01), an effect driven primarily by patients with severe ulcers (vision worse than counting fingers at enrollment). Another important subgroup analysis looked at study participants with Nocardia infection (Fig. 1). Nocardia, a common cause of infectious keratitis in South Asia, comprised 11% of the SCUT study cohort (55/500). Study participants with Nocardia ulcers experienced two lines less in BSCVA improvement compared with non-Nocardia patients (P =0.001) [24▪]. Additionally, patients with Nocardia infection who received adjunctive corticosteroids had 0.40-mm larger infiltrate/scar size on average at 3 months (P =0.03), though no difference in BSCVA (P =0.21).
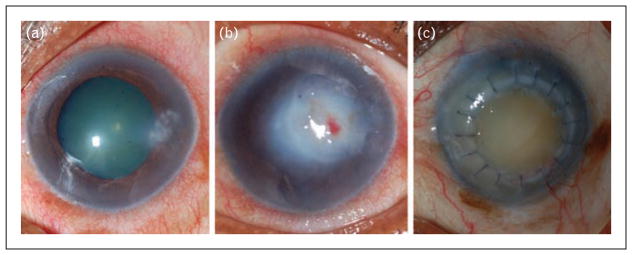
FIGURE 1.
A 50-year-old female agricultural worker whose corneal ulcer was culture positive for Nocardia and was assigned to the steroid treatment arm. (a) At presentation, her visual acuity was LogMAR 0.28 (Snellen ~20/30); (b) at 3 months, her visual acuity was LogMAR 1.8 (Snellen ~20/1200) and no improvement with contact lens overrefraction; (c) by 12 months, she underwent therapeutic penetrating keratoplasty with resulting LogMAR visual acuity 1.8 (Snellen 20/1200) and no improvement with contact lens overrefraction.
At the 12-month SCUT follow-up, there was still no overall difference between the steroid and placebo groups in prespecified outcomes [25▪▪]. However, in a subgroup analysis looking at non-Nocardia ulcers, there was one line of visual acuity improvement in the corticosteroid group over the placebo group at 12 months (P =0.02). Moreover, use of corticosteroids was associated with 0.47-mm larger mean scar size among Nocardia ulcers (P =0.02), whereas no significant difference in scar size was seen in non-Nocardia ulcers. It is possible that other slow growing or atypical bacterial similar to Nocardia also do not fare well with steroids, whereas other, more typical fast-growing bacteria will have improved outcomes with the use of adjuvant steroids.
Pseudomonas aeruginosa is an important cause of corneal ulceration worldwide and is associated with contact lens use in developed countries (Fig. 2). In the 110 study patients with confirmed P. aeruginosa infection, there appeared to be no overall benefit or harm in adding adjuvant steroids. There was no increase in adverse events such as increased infiltrate size, delayed epithelialization, or corneal perforation in those receiving steroids [26▪]. When classified as classically cytotoxic (18 patients), classically invasive (56 patients), or atypical (27 patients) based on PCR, patients in the invasive subgroup achieved a 2.5-line greater improvement in BSCVA at 3 months in the steroid-treated arm (P =0.04) [27▪].
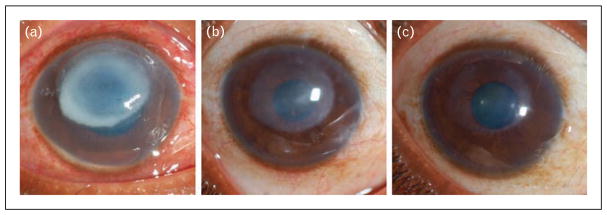
FIGURE 2.
A 30-year-old female agricultural worker whose corneal ulcer was culture positive for Pseudomonas aeruginosa, and PCR analysis revealed atypical genotype. She was assigned to the steroid treatment arm and received topical steroid after 48 h. (a) At presentation, her visual acuity was LogMAR 1.8 (Snellen ~20/1200); (b) at 3 months, her visual acuity has improved to ~20/140; (c) and by 12 months, the scar has almost resolved and visual acuity improved to 20/26 (20/24 with contact lens overrefraction).
FUTURE DIRECTIONS
It must be kept in mind that the study populations of the four randomized controlled trials, where the majority of patients were recruited from developing countries, may be very different from patients typically seen in the United States. In particular, the SCUT trial recruited 485 of 500 patients from India, and the vast majority of patients were manual laborers with foreign body-induced infections, with only eight contact lens wearers. Comparatively, in developed countries, contact lens wear can account for 20–50% of bacterial keratitis [12,28–31]. However, in these patients, Pseudomonas is often the most commonly isolated organism as it was in the SCUT [12,28–30,32]. Future studies could focus on culture proven non-Nocardia keratitis, large central ulcers, or early initiation of steroids – subgroups where steroids may confer a benefit. The optimal regimen and length of steroid also warrants further exploration.
CONCLUSION
The role of adjunctive topical corticosteroid use in addition to topical antibiotic application for the treatment of bacterial keratitis has been a controversial topic. Steroids are thought to limit inflammation-induced tissue destruction and scarring, but they can also retard healing and potentiate the infectious process. Although three small randomized trials have shed light on this dilemma, none was sufficiently powered. A fourth study, the SCUT is the largest randomized controlled trial to date and enrolled 500 patients primarily from India. Its results suggest that adjunctive topical corticosteroid initiated after at least 48 h of antibiotic usage in cases of culture-proven bacterial keratitis appears safe. Steroids may be beneficial in cases of severe ulcers especially when initiated early in the course of the infection, in non-Nocardia ulcers, and in certain Pseudomonas ulcers. Future randomized trials are needed to confirm the findings from these subgroup analyses.
KEY POINTS.
In the setting of bacterial keratitis, adjunctive use of topical steroids have been controversial as they are thought to limit inflammation-induced tissue destruction and scarring, but may also retard healing and potentiate the infectious process.
Two small, randomized trials of 40 and 42 patients found no difference in visual acuity or adverse events in patients treated with steroid versus placebo.
A third, large-scale randomized controlled trial, the SCUT, found that adjunctive topical corticosteroids initiated after at least 48 h of antibiotic usage in cases of culture-proven bacterial keratitis did not affect BSCVA at 3 months, infiltrate/scar size, time to reepithelialization, or rate of perforation.
This study indicated that steroids may confer a benefit in BSCVA in patients with baseline visual acuity of count fingers or worse vision and those with central ulcers.
Steroids may also be beneficial in non-Nocardia ulcers when initiated early in the course of the infection.
Footnotes
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 2.Jeng BH, Gritz DC, Kumar AB, et al. Epidemiology of ulcerative keratitis in Northern California. Arch Ophthalmol. 2010;128:1022–1028. doi: 10.1001/archophthalmol.2010.144. [DOI] [PubMed] [Google Scholar]
- 3.Thylefors B, Negrel AD, Pararajasegaram R, et al. Global data on blindness. Bull World Health Organ. 1995;73:115–121. [PMC free article] [PubMed] [Google Scholar]
- 4.Chusid MJ, Davis SD. Polymorphonuclear leukocyte kinetics in experimentally induced keratitis. Arch Ophthalmol. 1985;103:270–274. doi: 10.1001/archopht.1985.01050020122034. [DOI] [PubMed] [Google Scholar]
- 5.Hindman HB, Patel SB, Jun AS. Rationale for adjunctive topical corticosteroids in bacterial keratitis. Arch Ophthalmol. 2009;127:97–102. doi: 10.1001/archophthalmol.2008.504. [DOI] [PubMed] [Google Scholar]
- 6.Wilhelmus KR, Gee L, Hauck WW, et al. Herpetic Eye Disease Study. A controlled trial of topical corticosteroids for herpes simplex stromal keratitis. Ophthalmology. 1994;101:1883–1895. doi: 10.1016/s0161-6420(94)31087-6. [DOI] [PubMed] [Google Scholar]
- 7.McDonald TO, Borgmann AR, Roberts MD, et al. Corneal wound healing I. Inhibition of stromal healing by three dexamethasone derivatives. Invest Ophthalmol. 1970;9:703–709. [PubMed] [Google Scholar]
- 8.Sugar J, Chandler JW. Experimental corneal wound strength. Arch Ophthalmol. 1974;92:248–249. doi: 10.1001/archopht.1974.01010010256018. [DOI] [PubMed] [Google Scholar]
- 9.Cohen EJ. The case against the use of steroids in the treatment of bacterial keratitis. Arch Ophthalmol. 2009;127:103–104. doi: 10.1001/archophthalmol.2008.503. [DOI] [PubMed] [Google Scholar]
- 10.Chew HF, Yildiz EH, Hammersmith KM, et al. Clinical outcomes and prognostic factors associated with Acanthamoeba keratitis. Cornea. 2011;30:435–441. doi: 10.1097/ICO.0b013e3181ec905f. [DOI] [PubMed] [Google Scholar]
- 11.Stapleton F, Keay LJ, Sanfilippo PG, et al. Relationship between climate, disease severity, and causative organism for contact lens-associated microbial keratitis in Australia. Am J Ophthalmol. 2007;144:690–698. doi: 10.1016/j.ajo.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 12.Yildiz EH, Airiani S, Hammersmith KM, et al. Trends in contact lens-related corneal ulcers at a tertiary referral center. Cornea. 2012;31:1097–1102. doi: 10.1097/ICO.0b013e318221cee0. [DOI] [PubMed] [Google Scholar]
- 13.Lichtinger A, Yeung SN, Kim P, et al. Shifting trends in bacterial keratitis in Toronto: an 11-year review. Ophthalmology. 2012;119:1785–1790. doi: 10.1016/j.ophtha.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 14.Carmichael TR, Gelfand Y, Welsh NH. Topical steroids in the treatment of central and paracentral corneal ulcers. Br J Ophthalmol. 1990;74:528–531. doi: 10.1136/bjo.74.9.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miedziak AI, Miller MR, Rapuano CJ, et al. Risk factors in microbial keratitis leading to penetrating keratoplasty. Ophthalmology. 1999;106:1166–1170. doi: 10.1016/S0161-6420(99)90250-6. [DOI] [PubMed] [Google Scholar]
- 16.Wang JC, Su D, Lim L. Contact lens microbial keratitis and prior topical steroid use: a disaster in the making? Ann Acad Med Singapore. 2004;33:484–488. [PubMed] [Google Scholar]
- 17.Wilhelmus KR. Indecision about corticosteroids for bacterial keratitis: an evidence-based update. Ophthalmology. 2002;109:835–842. doi: 10.1016/s0161-6420(02)00963-6. [DOI] [PubMed] [Google Scholar]
- 18.Suwan-Apichon O, Reyes JM, Herretes S, et al. Topical corticosteroids as adjunctive therapy for bacterial keratitis. Cochrane Database Syst Rev. 2007;4:CD005430. doi: 10.1002/14651858.CD005430.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blair J, Hodge W, Al-Ghamdi S, et al. Comparison of antibiotic-only and antibiotic-steroid combination treatment in corneal ulcer patients: double-blinded randomized clinical trial. Can J Ophthalmol. 2011;46:40–45. doi: 10.3129/i10-054. [DOI] [PubMed] [Google Scholar]
- 20.Srinivasan M, Lalitha P, Mahalakshmi R, et al. Corticosteroids for bacterial corneal ulcers. Br J Ophthalmol. 2009;93:198–202. doi: 10.1136/bjo.2008.147298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinivasan M, Mascarenhas J, Rajaraman R, et al. Corticosteroids for bacterial keratitis: the Steroids for Corneal Ulcers Trial (SCUT) Arch Ophthalmol. 2012;130:143–150. doi: 10.1001/archophthalmol.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tallab RT, Stone DU. Corticosteroids as a therapy for bacterial keratitis: an evidence-based review of ‘who, when and why’. Br J Ophthalmol. 2016 doi: 10.1136/bjophthalmol-2015-307955. pii: bjophthalmol-2015-307955 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23▪.Ray KJ, Srinivasan M, Mascarenhas J, et al. Early addition of topical corticosteroids in the treatment of bacterial keratitis. JAMA Ophthalmol. 2014;132:737–741. doi: 10.1001/jamaophthalmol.2014.292. A SCUT subanalysis, which showed that early initiation of steroid may lead to improved BSCVA at 3 months. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24▪.Lalitha P, Srinivasan M, Rajaraman R, et al. Nocardia keratitis: clinical course and effect of corticosteroids. Am J Ophthalmol. 2012;154:934–939. doi: 10.1016/j.ajo.2012.06.001. A SCUT subanalysis, which showed Nocardia patients fared worse than non-Nocardia patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25▪▪.Srinivasan M, Mascarenhas J, Rajaraman R, et al. The steroids for corneal ulcers trial (SCUT): secondary 12-month clinical outcomes of a randomized controlled trial. Am J Ophthalmol. 2014;157:327–333. doi: 10.1016/j.ajo.2013.09.025. An update on SCUT 12-month results showed similar findings as the initial 3-month outcomes; however, Nocardia patients fared worse with steroids, in contrast with non-Nocardia patients, who achieved better BSCVA with steroids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26▪.Sy A, Srinivasan M, Mascarenhas J, et al. Pseudomonas aeruginosa keratitis: outcomes and response to corticosteroid treatment. Invest Ophthalmol Vis Sci. 2012;53:267–272. doi: 10.1167/iovs.11-7840. A SCUT subanalysis, which showed that Pseudomonas ulcers in the steroid group did not do better compared with controls, but also did not have increased incidence of adverse events. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27▪.Borkar DS, Fleiszig SM, Leong C, et al. Association between cytotoxic and invasive Pseudomonas aeruginosa and clinical outcomes in bacterial keratitis. JAMA Ophthalmol. 2013;131:147–153. doi: 10.1001/jamaophthalmol.2013.778. A SCUT subanalysis that classified Pseudomonas organisms as classically cytotoxic, classically invasive, or atypical based on PCR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni N, Nam EM, Hammersmith KM, et al. Seasonal, geographic, and antimicrobial resistance patterns in microbial keratitis: 4-year experience in eastern Pennsylvania. Cornea. 2015;34:296–302. doi: 10.1097/ICO.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 29.Keay L, Edwards K, Naduvilath T, et al. Microbial keratitis predisposing factors and morbidity. Ophthalmology. 2006;113:109–116. doi: 10.1016/j.ophtha.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Bourcier T, Thomas F, Borderie V, et al. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87:834–838. doi: 10.1136/bjo.87.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gebauer A, McGhee CN, Crawford GJ. Severe microbial keratitis in temperate and tropical Western Australia. Eye (Lond) 1996;10:575–580. doi: 10.1038/eye.1996.133. [DOI] [PubMed] [Google Scholar]
- 32.Alexandrakis G, Alfonso EC, Miller D. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology. 2000;107:1497–1502. doi: 10.1016/s0161-6420(00)00179-2. [DOI] [PubMed] [Google Scholar]