Abstract
Cooperative catalysis enables the direct enantioselective α-allylation of linear prochiral esters using 2-substituted allyl electrophiles. Critical to the successful development of the method was the recognition that metal-centered reactivity and the source of enantiocontrol are independent. This feature is unique to simultaneous catalysis events and permits logical tuning of the supporting ligands without compromising enantioselectivity.
Keywords: alkylation, C1-ammonium enolate, palladium, enantioselective, Lewis base
Two is better than one!
Cooperative catalysis enables the direct enantioselective α-allylation of aryl and vinyl acetic acid esters using 2-substituted allyl electrophiles. Critical to the successful development of the method was the recognition that metal-centered reactivity and the source of enantiocontrol are independent, which permits logical tuning of the supporting ligands without compromising enantioselectivity.

Transition metal-catalyzed allylic alkylation is a well-established and powerful method for asymmetric carbon–carbon bond formation.[1] A hallmark of these reactions is the high levels of enantiocontrol imparted by chiral ligands, and implicit in their design is the ability to change the metal and/or ligands to tune reactivity and selectivity. However, despite significant advances numerous challenges remain, including the ability to logically regulate metal reactivity without compromising enantioselection. Herein, we demonstrate that independent modulation of metal-centered reactivity and enantiocontrol elements via cooperative catalysis can, in a rational manner, overcome structure-based substrate reactivity limitations during Pd-catalyzed allylic alkylation reactions. This permits the use of 2-substituted electrophiles, a hitherto unreactive structural class in this process.
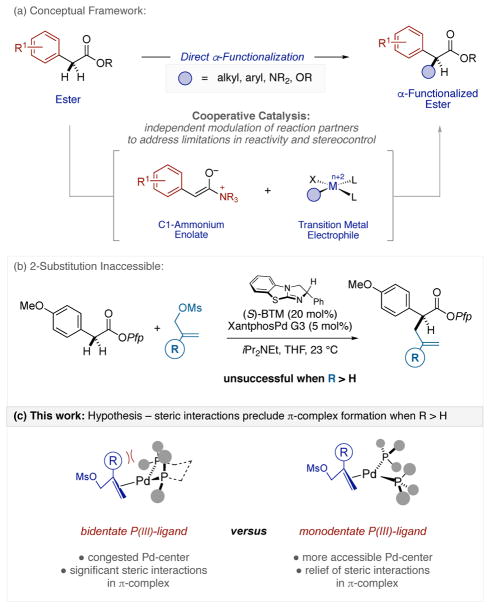
We have embraced cooperative catalysis[2] as a general design framework for the direct catalytic asymmetric α-functionalization of acyclic esters (Figure 1a).[3] This construct unites C1-ammonium enolates with transition metal electrophiles and has addressed key challenges associated with inter alia Lewis base turnover,[4–6] the locus of enantiocontrol, and the preservation of enolizable stereocenters in the products. Despite the useful substrate scope and high efficiency of this process,[3] attempts to accommodate substitution on the central carbon of the allyl partner completely shut down the reaction (Figure 1b). Although the decreased reactivity of 2-substituted allyl halides and acetates toward oxidative ionization by Pd(0) has been described,[7] our recovery of the highly electrophilic allyl mesylates was unexpected. Examination of molecular models led us to speculate on the development of deleterious non-bonding interactions between the 2-substitutent of the electrophile and a pseudoaxially positioned Ph-ring of the large Xantphos ligand (Figure 1c). This could disrupt formation of the necessary π-complex en route to π(allyl)Pd(II) formation. Accordingly, we posited that monodentate ligands would relieve such interactions and re-engage the cooperative process.
Figure 1.
(a) Conceptual framework for the direct α-functionalization of linear esters via cooperative catalysis; (b) 2-substituted electrophiles are unreactive using previously established conditions; (c) This work: alter ligand based modulation of Pd-reactivtiy independent of enantiocontrol.
While tuning metal reactivity by modification of associated ligands is a common and effective strategy, doing so with logical maintenance of enantiocontrol (e.g. bidentate versus monodentate ligands) is not.[8] The postulated mechanistic scenario upon which our cooperative catalysis is predicated permits the separation of these two critical features.[3]
Using our previously established conditions employing Birman’s benzotetramisole (BTM)[9] as the Lewis base catalyst, we began by evaluating the effect of a representative range of monodentate and bidentate phosphine ligands possessing different steric and electronic profiles (Table 1). As a control, re-evaluation of Xantphos using this in situ catalyst formation protocol was performed. Using this procedure, allylated product was obtained albeit in low yield and with very poor enantioselectivity (Entry 1). As expected, other bidentate phosphines also perform poorly (Entries 2–4). Electron-rich monodentate phosphines were also ineffective (Entries 5–7). Moving to tri(2-furyl)phosphine provided the necessary reactivity and gave the allylated product in excellent yield and with encouraging levels of enantioselection (Entry 8). The related tri(2-thienyl)phosphine ligand furnished the product in reproducibly higher enantioselectivity, albeit at the expense of yield (Entry 9). Further assessment of solvent and reaction time gave the product in excellent yield and enantioselectivity (Entries 10–14). Although the unique efficacy of tri(2-furyl) and tri(2-thienyl)-phosphine ligands on reactivity is currently unknown we expect the low steric demand and significant π-accepting character of these ligands[10] translates to more facile formation of Pd(0)/π-complexes as necessary progenitors to oxidative addition.
Table 1.
Reaction Optimization.

| |||||
|---|---|---|---|---|---|
| Entrya | PR3 | (mol%) | Solvent | Yield [%]b | erc |
| 1 | Xantphos | 5 | THF | 14 | 58:42 |
| 2 | DPEphos | 5 | THF | 0 | -- |
| 3 | dppf | 5 | THF | 0 | -- |
| 4 | dppe | 5 | THF | 12 | -- |
| 5 | PCy3 | 10 | THF | 0 | -- |
| 6 | P(o-tolyl)3 | 10 | THF | 0 | -- |
| 7 | P(p-OMePh)3 | 10 | THF | 0 | -- |
| 8 | P(2-furyl)3 | 10 | THF | 90 | 85:15 |
| 9 | P(2-thienyl)3 | 10 | THF | 65 | 90:10 |
| 10 | P(2-thienyl)3 | 20 | THF | 77 | 90:10 |
| 11 | P(2-thienyl)3 | 20 | 2-MeTHF | 61 | 80:20 |
| 12 | P(2-thienyl)3 | 20 | CPME | 56 | 90:10 |
| 13 | P(2-thienyl)3 | 20 | Dioxane | 92 | 95:5 |
| 14d | P(2-thienyl)3 | 20 | Dioxane | 98e | 95:5 |
Reactions performed on a 0.1 mmol scale.
Yields determined by 1H NMR by comparison with an internal standard (1,2,4,5-tetramethyl-benzene).
Determined by chiral HPLC analysis.
24 h reaction time.
Isolated yield.
Pfp = pentafluorophenyl, Ms = methanesulfonyl.
Having established effective conditions a variety of 2-substituted allyl mesylates were evaluated. The reaction was remarkably general and tolerant of a wide range of substituents including alkyl (1–4), vinyl (5), acetylenyl (6), halide (7–8), and aryl substitution (9–10). In each case the product esters could be isolated and stored for prolonged periods without loss of enantioselectivity. Although Pfp and related aryl esters can be easily derivatized without loss of enantioenrichment,[3,5b,11] we nonetheless sought to increase the synthetic utility of our process further. In concert with additional assessment of the 2-aryl substituent scope, we have also developed an in situ amine addition protocol to produce amides. Primary, secondary and branched amine amines react readily to provide functionalized amides in high yields and enantiopurity (11–18). Reduction to the corresponding alcohol 19 using LiAlH4 was also straightforward. Finally, we also evaluated the scope of the Pfp ester nucleophile (Scheme 2). As expected, a range of substitution was possible illustrating the insensitivity of the nucleophile toward steric and electronic modification (20–31). Significantly, α-vinyl acetic acid Pfp esters (32–33) are also competent nucleophiles.[12]
Scheme 2.
Nucleophile Scope.
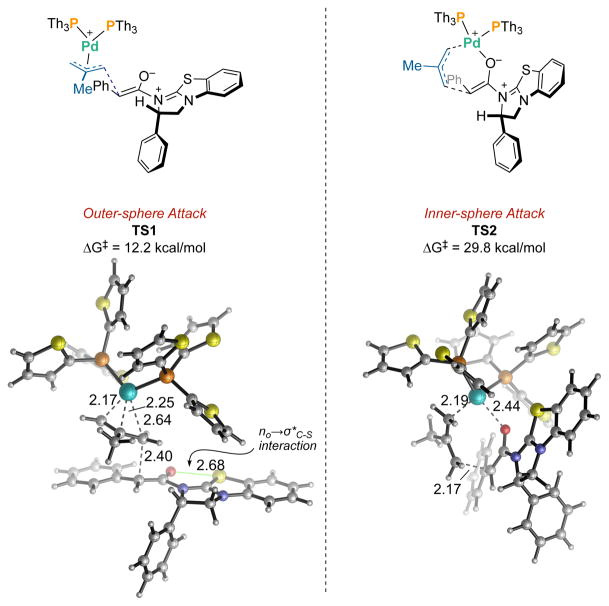
In an effort to understand the manner in which the two catalytic cycles intersect we initiated a computational interrogation of both the postulated enolate ligation and the nature of stereoinduction.[13] In our original report we posited the intermediacy of C1-ammonium enolate-ligated π-(allyl)Pd(II) species,[3] which was inspired by Lectka’s stabilization of quinuclidine-derived C1-ammonium enolates by Pd(II) and Ni(II) Lewis acids.[14] We also provided a tentative yet predictive model for enantioselection that is based on Smith’s model for isothiourea-derived C1-ammonium enolate preorganization.[15] Our density functional theory (DFT) calculations revealed that the (Z)-O-C1-ammonium enolate is conformationally rigid due to a stabilizing nO→σ*C-S interaction[16] as well as steric effects (see SI for details). This is in line with the findings of others[15a] and is consistent with our previously proposed induction model in which the π-(allyl)Pd complex attacks the less hindered face of the enolate. We then investigated both inner- and outer-sphere nucleophile addition to the π-(allyl)Pd complex (Figure 2).[17] The outer-sphere pathway (TS1) requires a relatively low barrier of 12.2 kcal/mol. In contrast, the inner-sphere addition (TS2) from an O-enolate ligated π-(allyl)Pd complex is highly disfavored due to steric repulsion between the C1-ammonium enolate and the ancillary ligands on Pd. This support for an outer-sphere mechanism reinforces the generality of our cooperative design where the transition metal and Lewis base catalysts are fully independent.
Figure 2.
DFT studies. Outer-sphere (TS1) and inner-sphere (TS2) transition states. DFT calculations were performed at the M06/SDD–6311+G(d,p)/SMD(THF)//B3LYP/SDD–6-31G(d) level of theory. See SI for details.
In conclusion, we have demonstrated the separation of enantiocontrol and metal-centered reactivity in Pd-catalyzed allylic alkylation using linear prochiral ester nucleophiles. This is complementary to classical ligand-only modification in Pd-catalyzed allylic alkylation and permits a wide range of 2-substituted allyl electrophiles to be employed in the enantioselective synthesis of α-branched esters. Electrophiles of this structural type have previously exhibited poor reactivity. Beyond simply broadening the repertoire of cooperative catalysis, this study documents and describes the unique capacity of simultaneous catalysis events to confront reactivity and stereocontrol limitations in a modular fashion. Such flexibility is typically beyond a single catalyst.
Supplementary Material
Scheme 1.
Electrophile Scope: Direct work up giving Pfp esters (top), and amine addition giving amides or alcohol (bottom).
Acknowledgments
We gratefully acknowledge Indiana University, NIH (R01GM121573), ACS-PRF (55734-DNI) and NSF (CHE-1654122) for generous financial support. We thank Dr. Maren Pink and Dr. Chun-Hsing Chen (IU) for X-ray crystallography. This project was partially supported by the IU Vice Provost for Research through the Research Equipment Fund.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Dedication ((optional))
Supporting information for this article is given via a link at the end of the document.
Contributor Information
Kevin J. Schwarz, Department of Chemistry, Indiana University, 800 East Kirkwood Avenue, Bloomington, IN 47405 (USA).
Dr. Colin M. Pearson, Department of Chemistry, Indiana University, 800 East Kirkwood Avenue, Bloomington, IN 47405 (USA).
Gabriel A. Cintron-Rosado, Department of Chemistry, University of Pittsburgh Pittsburgh, PA 15260 (USA)
Prof. Peng Liu, Department of Chemistry, University of Pittsburgh Pittsburgh, PA 15260 (USA).
Prof. Thomas N. Snaddon, Department of Chemistry, Indiana University, 800 East Kirkwood Avenue, Bloomington, IN 47405 (USA).
References
- 1.For reviews, see: Hethcox JC, Shockley SE, Stoltz BM. ACS Catal. 2016;6:6207–6213. doi: 10.1021/acscatal.6b01886.Weaver JD, Recio A, III, Grenning AJ, Tunge JA. Chem Rev. 2011;111:1846–1913. doi: 10.1021/cr1002744.Trost BM, Crawley ML. Chem Rev. 2003;103:2921–2944. doi: 10.1021/cr020027w.Trost BM, Van Vranken DL. Chem Rev. 1996;96:395–422. doi: 10.1021/cr9409804.
- 2.For selected reviews, see: Peters R, editor. Cooperative Catalysis: Designing Efficient Catalysts for Synthesis. Wiley-VCH; Weinheim: 2015. Afewerki S, Córdova A. Chem Rev. 2016;116:13512. doi: 10.1021/acs.chemrev.6b00226.Chen DF, Han ZY, Zhou X-L, Gong LZ. Acc Chem Res. 2014;47:2365. doi: 10.1021/ar500101a.Du Z, Shao Z. Chem Soc Rev. 2013;42:1337. doi: 10.1039/c2cs35258c.Allen AE, MacMillan DWC. Chem Sci. 2012;3:633–658. doi: 10.1039/C2SC00907B.Ma JA, Carhard D. Angew Chem Int Ed. 2004;43:4566–4583. doi: 10.1002/anie.200300635.
- 3.Schwarz KJ, Amos JA, Klein JC, Do D, Snaddon TN. J Am Chem Soc. 2016;138:5214–5217. doi: 10.1021/jacs.6b01694. [DOI] [PubMed] [Google Scholar]
- 4.For reviews, see: Morrill LC, Smith AD. Chem Soc Rev. 2014;43:6214–6226. doi: 10.1039/c4cs00042k.Paull DH, Weatherwax A, Lectka T. Tetrahedron. 2009;65:6771–6803. doi: 10.1016/j.tet.2009.05.079.France S, Guerin DJ, Miller SJ, Lectka T. Chem Rev. 2003;103:2985–3012. doi: 10.1021/cr020061a.
- 5.For other examples of C1-ammonium enolates in combination with transition metal catalysis, see: Spoehrle SS, West TH, Taylor JE, Slawin AMZ, Smith AD. J Am Chem Soc. 2017;139:11895–11902. doi: 10.1021/jacs.7b05619.Jiang Z, Beiger JJ, Hartwig JF. J Am Chem Soc. 2017;139:87–90. doi: 10.1021/jacs.6b11692.Lu X, Ge L, Cheng C, Chen J, Cao W, Wu X. Chem Eur J. 2017;23:7689–7693. doi: 10.1002/chem.201701741.Song J, Zhang ZJ, Gong LZ. Angew Chem Int Ed. 2017;56:5212–5216. doi: 10.1002/anie.201700105.Song J, Zhang ZJ, Chen SS, Fan T, Gong LZ. J Am Chem Soc. 2018;140:3177–3180. doi: 10.1021/jacs.7b12628.
- 6.For a comprehensive review, see: Hartley WC, O’Riordan TJC, Smith AD. Synthesis. 2017;49:3303–3310.Matviitsuk A, Greenhalgh MD, Barrios Antunez D-J, Slawin AMZ, Smith AD. Angew Chem Int Ed. 2017;56:12282–12287. doi: 10.1002/anie.201706402.Lee SY, Neufeind S, Fu GC. J Am Chem Soc. 2014;136:8899–8902. doi: 10.1021/ja5044209.
- 7.(a) Hussain MM, Walsh PJ. Angew Chem Int Ed. 2010;49:1834–1837. doi: 10.1002/anie.200905399. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Organ MJ, Arvanitis EA, Dixon CE, Cooper JT. J Am Chem Soc. 2002;124:1288–1294. doi: 10.1021/ja011508k. [DOI] [PubMed] [Google Scholar]; (c) van Laren MW, Diederen JJH, Elsevier CJ. Adv Synth Catal. 2001;343:255–259. [Google Scholar]
- 8.Kamer PCJ, van Leeuwen PWNM, editors. Phosphorus(III) Ligands in Homogeneous Catalysis: Design and Synthesis. John Wiley & Sons; 2012. [Google Scholar]
- 9.(a) Liu P, Yang X, Birman VB, Houk KN. Org Lett. 2012;14:3288–3291. doi: 10.1021/ol301243f. [DOI] [PubMed] [Google Scholar]; (b) Bumbu VD, Birman VB. J Am Chem Soc. 2011;133:13902–13905. doi: 10.1021/ja2058633. [DOI] [PubMed] [Google Scholar]; (c) Yang X, Lu G, Birman VB. Org Lett. 2010;12:892–895. doi: 10.1021/ol902969j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Birman VB, Li X. Org Lett. 2006;8:1351–1354. doi: 10.1021/ol060065s. [DOI] [PubMed] [Google Scholar]
- 10.Anderson NG, Keay BA. Chem Rev. 2001;101:997–1030. doi: 10.1021/cr000024o. [DOI] [PubMed] [Google Scholar]
- 11.(a) Gayo LM, Suto MJ. Tetrahedron Lett. 1996;37:4915–4918. [Google Scholar]; (b) Green M, Berman J. Tetrahedron Lett. 1990;31:5851–5852. [Google Scholar]; (c) Kisfaludy L, Roberts JE, Johnson RH, Mayers GL, Kovacs J. J Org Chem. 1970;35:3563–3565. doi: 10.1021/jo00835a086. [DOI] [PubMed] [Google Scholar]
- 12.α-Alkyl acetic acid esters are not competent in this reaction. However, the use of α-vinyl nucleophiles would, following reduction of the alkene, provide such products. For other examples of α-vinyl acetic acid derived C1-ammonium enolates, see: (a) ref 5e; Arokianathar JN, Frost AB, Slawin AMZ, Stead D, Smith AD. ACS Catal. 2018;8:1153–1160.Morrill LM, Smith SM, Slawin AMZ, Smith AD. J Org Chem. 2014;79:1640–1655. doi: 10.1021/jo402591v.
- 13.Thus far we have been unsuccessful in our efforts to computationally interrogate the ligand effects on the efficiency of π-complex formation due to the unknown role/effect of either the nucleofuge (MsO–) or the solvent. Nonetheless, calculations reveal little energetic difference with respect to the supporting ligand (Xantphos versus tri(2-thienyl)phosphine) in the oxidative addition and subsequent C–C bond formation steps. See supporting information for further details.
- 14.(a) Erb J, Paull DH, Dudding T, Belding L, Lectka T. J Am Chem Soc. 2011;133:7536–7546. doi: 10.1021/ja2014345. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Paull DH, Scerba MT, Alden-Danforth E, Widger LR, Lectka T. J Am Chem Soc. 2008;130:17085–17094. doi: 10.1021/ja806818a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) West TH, Walden DM, Taylor JE, Brueckner AC, Johnston RC, Cheong PHY, Lloyd-Jones G, Smith AD. J Am Chem Soc. 2017;139:4366–4375. doi: 10.1021/jacs.6b11851. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) West TH, Daniels DSB, Slawin AMZ, Smith AD. J Am Chem Soc. 2014;136:4476–4479. doi: 10.1021/ja500758n. [DOI] [PubMed] [Google Scholar]
- 16.Pascoe DJ, Ling KB, Cockroft SL. J Am Chem Soc. 2017;139:15160–15167. doi: 10.1021/jacs.7b08511.For the importance of such interactions in drug design, see: Beno BR, Yeung K-S, Bartberger MD, Pennington LD, Meanwell NA. J Med Chem. 2015;58:4383–4438. doi: 10.1021/jm501853m.
- 17.For an instructive computational study, see: Keith JA, Behenna DC, Sherden N, Mohr JT, Ma S, Marinescu SC, Nielsen RJ, Oxgaard J, Stotlz BM, Goddard WA., III J Am Chem Soc. 2012;134:19050–19060. doi: 10.1021/ja306860n.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.