Abstract
Many breast cancer survivors report a loss of sexual desire and arousability, consonant with the new DSM-V category of female sexual interest/arousal disorder. The cause of decreased sexual desire and pleasure after treatment for cancer is unknown. One possibility is that cancer, or treatment for cancer, damages brain circuits that are involved in reward-seeking. To test the hypothesis that brain reward systems are involved in decreased sexual desire in breast cancer survivors, we used functional magnetic resonance imaging (fMRI) to compare brain responses to erotica and other emotional stimuli in two groups of women previously treated for breast cancer with chemotherapy: those who were distressed about a perceived loss of sexual desire and those who may have had low desire, but were not distressed about it. Women distressed about their desire had reduced brain responses to erotica in the anterior cingulate and dorsolateral prefrontal cortex, which are part of the brain reward system. This study is the first to demonstrate, in cancer survivors, that problems with sexual desire/arousability are associated with blunted brain responses to erotica in reward systems. Future research is necessary to determine whether brain responses differ as a result of chemotherapy, hormone therapy, and menopausal status. This may contribute to the development of new, evidence-based interventions for one of the most prevalent and enduring side effects of cancer treatment.
Keywords: sexual dysfunction, breast cancer, fMRI, anterior cingulate, reward-processing, chemotherapy
After treatment for breast cancer, many women complain they have lost desire for sex and feel little pleasure with sexual stimulation. Some describe the change as feeling like a switch was flipped in their brain setting sex to “off.” Low desire for sex is typically reported by 50% to 80% of women after breast cancer (Dizon, 2009; Panjari, Bell, & Davis, 2011). Prevalence varies because most samples are relatively small and researchers use different measures of sexual dysfunction. In contrast, in a recent population-based study of healthy, postmenopausal women, only a third complained of low sexual desire (Trompeter, Bettencourt, & Barrett-Connor, 2012). It is clear that sexual dysfunction is more common in women treated with chemotherapy, regardless of initial menopausal status, and does not differ according to type of breast surgery, such as breast conservation versus mastectomy (Alder et al., 2008; Ganz, Rowland, Meyerowitz, & Desmond, 1998; Schover et al., 1995). In fact, in a prospective survey of 304 premenopausal women treated for ductal carcinoma in situ with breast surgery, and in some cases with radiation therapy or tamoxifen, but who did not have chemotherapy, sexual function and body image remained normal acrossl8 months of follow-up (Bober, Giobbie-Hurder, Emmons, Winer, & Partridge, 2013). Yet, a number of cohort studies also have found that sexual function is more likely to be disrupted if cancer treatment causes abrupt and continuing premature ovarian failure, whether the culprit is chemotherapy (Berglund, Nystedt, Bolund, Sjoden, & Rutquist, 2001; Burwell, Case, Kaelin, & Avis, 2006; Ochsenkühn et al., 2011; Panjari et al., 2011), bilateral oophorectomy (Finch et al., 2011; Sayakhot, Vincent, Deeks, & Teede, 2011), or use of a gonadotropin-releasing hormone agonist (Berglund et al., 2001).
The cause for loss of desire remains unclear. An early hypothesis was that a drop in circulating androgens in women in ovarian failure accounted for low desire, yet androgen levels in breast cancer survivors are not significantly correlated with self-reported sexual desire (Alder et al, 2008; Greendale, Petersen, Zibecchi, & Ganz, 2001; Speer et al, 2005), nor was replacement testosterone effective in restoring desire in women with breast cancer in a randomized trial (Barton et al, 2007). Another hypothesis is that loss of desire for many women is a reaction to the painful experiences with sex that occur because of vulvovaginal atrophy (Burwell et al, 2006; Fobair et al, 2006; Schover, 2008), a sequence of dysfunctions that has also been observed in healthy, postmenopausal women (Avis et al, 2009). Since few trials are longitudinal, however, and the reaction to sexual pain can occur rapidly, it is difficult to ascertain whether pain is the cause of loss of desire. An often overlooked factor is that many breast cancer survivors use medications that blunt sexual desire to manage symptoms such as mood changes, hot flashes, pain, and fatigue (Dizon, 2009; Suppli et al., 2011).
A hypothesis that has not been explored is that chemotherapy directly damages brain circuits involved in sexual desire. Although controversy still exists about prevalence and severity of cognitive changes after chemotherapy for breast cancer, a number of studies have documented persistent deficits, especially in tasks involving working memory, executive functions, and attention (Collins, Mackenzie, Tasca, Scherling, & Smith, 2012; de Ruiter et al., 2012; Koppelmans et al., 2012; Wefel, Lenzi, Theriault, Davis, & Meyers, 2004). Affective processes, including those related to the expression of sexual desire, are functionally integrated with and supported by the same brain networks that support cognition (Lang & Bradley, 2009; Lang, Bradley, & Cuthbert, 1997; Pessoa, 2008). For example, in healthy participants, brain regions including the extended visual system (posterior parietal and occipital cortex) and the amygdala are activated both by tasks that demand attention and working memory and by the presentation of images that evoke an emotional response, whether pleasant or unpleasant (Lang et al, 1998; Morris et al, 1998; Pessoa, McKenna, Gutierrez, & Ungerleider, 2002; Sabatinelli, Bradley, Fitzsimmons, & Lang, 2005). Furthermore, pleasant stimuli (including erotic images) activate specific brain circuits associated with reward processing, such as the anterior cingulate, medial prefrontal cortex, and ventral striatum (Sabatinelli, Bradley, Lang, Costa, & Versace, 2007; Walter et al., 2008). Brain imaging studies testing attention and executive functions in breast cancer survivors suggest that chemotherapy has a negative impact on the same brain regions (de Ruiter et al., 2011; Kesler, Kent, & O’Hara, 2011). Thus, cancer treatments affecting frontal cortical and subcortical networks could diminish the brain’s ability to respond to stimuli that would normally trigger sexual desire.
One challenge is that sexual desire is difficult to define or measure. In recent years, a major controversy has been whether women’s desire for sex can be separated from the subjective pleasure that occurs when physical and mental cues lead to sexual arousal. Many researchers believe that sexual desire and subjective sexual arousal are part of a single continuum but others continue to view them as separate entities (Bitzer, Giraldi, & Pfaus, 2013). DSM-V (American Psychiatric Association, 2013) now groups desire and arousal together in defining Female Sexual Interest/Arousal Disorder. Another debate is whether most women experience a linear sequence of spontaneous desire leading to sexual arousal, or whether it is common for women to engage in sex because of social motivation, so that pleasant sexual sensations lead to desire for sex, which in turn amplifies erotic excitement in a circular fashion (Hayes, 2011). Women’s reports of sexual desire across varying situations do not support either DSM-IV (American Psychiatric Association, 1994) or DSM-V diagnostic criteria for hypoactive desire disorder (Sarin, Amsel, & Binik, 2013). In particular, many women reporting a lack of interest in partner sex or masturbation acknowledge fairly frequent erotic thoughts and fantasies.
A model of sexual desire based closely on pharmacological and brain research with both animals and humans includes both excitatory and inhibitory components (Pfaus, 2009). Excitation involves the hypothalamic and limbic systems. Steroid hormones prime the brain to respond to sexual cues. Premenopausal women report somewhat greater sexual desire at the time of ovulation, and functional magnetic resonance imaging (fMRI) of brain responses to sexual stimuli has confirmed a greater response during ovulation, although not in all studies (Stoleru, Fonteille, Cornells, Joyal, & Moulier, 2012). Neurotransmitters, including dopamine, norepinephrine, melanocortin, and oxytocin, interact to stimulate sexual arousal and attention to sexual stimuli (Pfaus, 2009). Areas particularly important to desire include the medial preoptic area (mPOA), the nucleus accumbens (NAcc), and the ventral tegmental area (VTA). As Holstege and Huynh (2011) point out, women’s sexual arousal not only involves focusing on positive cues, but also on inhibiting areas of the brain that would normally be alert to nonsexual cues. Inhibitory mechanisms involve endogenous opioids, endocannabinoids, and serotonin.
Neuroimaging studies of women exposed to erotic stimuli are consistent with Pfaus’ model (Stoleru et al, 2012). Brain areas showing activation in fMRI studies of women viewing erotic stimuli include the extended visual system, the insula, the orbitofrontal and medial prefrontal cortices, the anterior cingulate cortex, and the ventral striatum. Few brain imaging studies have compared women with normal versus low sexual desire. However, two recent studies found that women with low sexual desire show reduced activation in frontal and parietal areas relative to women with normal sexual desire (Arnow et al, 2009; Bianchi-Demicheli et al, 2011). The goal of the present study was to collect preliminary data to evaluate whether breast cancer survivors distressed about loss of desire/arousability after chemotherapy show differences in brain activity from nondistressed women when viewing erotic and other emotional stimuli.
Methods
All study-related procedures were approved by the University of Texas MD Anderson Cancer Center’s Institutional Review Board.
Participants
Forty-five eligible potential participants were identified and phone screened. Four women declined to participate due to lack of interest or difficulty with scheduling; eight cancelled or did not show up to their scheduled appointment. Four women that did not receive chemotherapy were not included in the analyses. Of the twenty-nine women with a history of chemotherapy enrolled in the study, two were excluded due to excessive motion artifact in their fMRI data, resulting in a final sample size of 27 women. Women were eligible for the study if they were between 40 and 60 years of age, were at least one year post-menopause, had been previously diagnosed with Stage 0 - III breast cancer or with noninvasive breast carcinoma, were currently free of known cancer, and were living within the greater Houston metropolitan area. Women were ineligible if they had any active cancer treatment in the previous 12 months, took any form of hormonal therapy or hormone replacement therapy in the previous 12 months, were using psychotropic medication, had a history of seizures or seizure disorder, had uncorrected vision impairments, had a current or past psychiatric or substance abuse disorder (except smoking), had any condition affecting their safety in the MRI (e.g., metal implants, claustrophobia), or could not communicate fluently in English. Participants were compensated $60 for their participation.
A list of potential participants was obtained from two databases at the University of Texas MD Anderson Cancer Center. Initial eligibility was assessed using information such as age, cancer stage, and recent treatment. A letter about the study was sent to potential participants with an opportunity to opt out of a follow-up phone call. Screening by phone was conducted by a female staff member. During the telephone interview, we collected basic demographic and health history information and assessed sexual desire using the Decreased Sexual Desire Screener (DSDS; Clayton et al, 2009), an instrument designed for use in studies of pharmacologic agents to treat low sexual desire in women. We used the first four questions from this questionnaire to classify participants. These items asked whether the participant 1) was satisfied with her level of sexual desire prior to her cancer diagnosis, 2) had noticed a decrease in her level of sexual desire since her cancer diagnosis, 3) was bothered by decreased sexual desire, and 4) would like her level of sexual desire to increase. We divided the participants in this study into two groups. The group distressed about low desire (DSDS-yes) consisted of 11 women who answered “yes” to all four questions on the DSDS. The nondistressed group (DSDS-no) consisted of 16 women who answered “no” to at least one of the questions. The most common negative answer in the nondistressed group (for 15 out of 16 participants) was to question #3 (“Are you bothered by your decreased level of sexual desire or interest?”).
Stimuli
We measured brain responses to emotional stimuli using a picture-viewing procedure that has been previously used in our laboratory (Versace et al., 2011) and elsewhere (Bradley et al., 2003). Sixty pictures were selected from the International Affective Picture System (Lang, Bradley, & Cuthbert, 2008). The pictures belonged to five categories, with 12 pictures each showing neutral people (NEU), erotic couples (ERO), romantic couples (ROM), mutilations (MUT), and sad scenes (SAD). Because some women with breast cancer may have negative emotions triggered by viewing erotic pictures of breasts, the ERO images did not include views of the whole breast that showed the nipples. The IAPS pictures used for each category were: NEU: 2102, 2200, 2210, 2305, 2383, 2393, 2396, 2593, 2595, 2630, 7550, 9070; ERO: 4604, 4650, 4658, 4659, 4668, 4669, 4676, 4677, 4691, 4698; ROM: 4624, 4625, 4700; MUT: 3000, 3030, 3051, 3053, 3060, 3069, 3080, 3100, 3110, 3120, 3130, 3170; SAD: 2205, 2455, 2490, 2590, 2700, 2703, 2800, 2900, 9429, 9520, 9530, 9926.
Procedure
Upon arrival at the laboratory, participants provided written, informed consent to participate. Then, using a computer, they completed several questionnaires: the Brief Symptom Inventory (a measure of anxiety an depressive symptoms; Derogatis, 2001), the NEO-Five Factor Inventory (NEO-FFI; a measure of personality traits; McCrae & Costa, 2004), the Sexual Arousal and Desire Inventory (SADI; Toledano & Pfaus, 2006), the Menopausal Sexual Interest Questionnaire (MSIQ; Rosen, Lobo, Block, Yang, & Zipfel, 2004), the Dyadic Adjustment Scale (DAS; Sabourin, Valois, & Lussier, 2005), and the Female Sexual Function Index (FSFI; Wiegel, Meston, & Rosen, 2005).
Following questionnaire assessment, participants entered the fMRI scanner and completed the picture-viewing procedure. The pictures were presented using an MR-compatible system (ESys/fMRI, Invivo, Gainesville, FL) running E-Prime software (Psychology Software Tools, Pittsburgh, PA), such that they subtended a visual angle of 30° horizontally and 23° vertically. Before starting the picture presentation task, the participants were instructed to look at the pictures while the scanner measured their brain’s reaction. To minimize the effect of picture presentation order on the results, each participant was randomly assigned to one of five picture presentation sequences. In all sequences, the pictures were presented in a random order with respect to category (NEU, ERO, ROM, MUT, and SAD) with the restriction that no more than 2 pictures of the same category were presented consecutively. Picture presentation was divided into two blocks of 30 pictures each, with a small break between the blocks. Each picture was presented for 5 s, followed by an intertrial interval ranging from 15–20 s. Each picture-viewing block lasted approximately 12 minutes.
During the picture presentation blocks, fMRI data were recorded using a 3.0 T Discovery MR750, 32-channel MRI system with high order shim (GE Healthcare, Milwaukee, WI). The blood-oxygenation-level-dependent (BOLD) signal was measured using a T2*-weighted, echo- planar, parallel imaging protocol with a 2.5 s repetition time, 25 ms echo time, and 90° flip angle. Parallel imaging used the Array Spatial Sensitivity Encoding Technique (ASSET) with an acceleration factor of 2. Data were collected as 58 contiguous 3-mm coronal slices with a 160 mm × 160 mm field-of-view, 64 × 64 acquisition matrix, right-left phase encoding direction, and 2.5 mm × 2.5 mm in-plane resolution. This resulted in full brain coverage with a spatial resolution of 2.5 × 2.5 × 3 mm. For each picture-viewing block, the first two volumes were discarded to allow magnetization to reach steady-state, followed by collection of 286 volumes that were time-locked to stimulus presentation. After the second picture-viewing block, a 3- dimensional, high-resolution, T1-weighted structural MRI was completed for purposes of mapping the BOLD data to brain anatomy (5.5 ms repetition time, 2.1 ms echo time, 400 ms inversion time, 20° flip angle, and 1 mm3 voxel size).
Following the MRI scan, participants used a computer to provide subjective ratings of the pictures used in the study. Affective valence (pleasure) and arousal ratings were collected using the Self-Assessment Manikin (SAM; Lang, 1980), a visual-analog scale with 9 points. Then, participants were thanked for their participation, debriefed, compensated for their time, and dismissed.
Statistical Analysis
Self-Report Measures
Questionnaire data were reported as means and standard errors for the entire sample and for the normal desire and low desire groups separately. Two-sample t-tests were used to compare scores between the two groups. The subjective ratings of the pictures (SAM ratings) were analyzed using group (nondistressed, versus distressed about low desire) × picture category (NEU, ERO, ROM, MUT, SAD) Analysis of Variance (ANOVA).
fMRI Data
fMRI data were analyzed using BrainVoyager QX, version 2.4 (Brain Innovation, Maastricht, The Netherlands; Goebel, Esposito, & Formisano, 2006). Following pre-processing, our analysis focused on identifying brain regions with 1) larger BOLD responses to emotional cues than neutral cues in all subjects and 2) differences in responses to emotional versus neutral stimuli between the nondistressed and distressed sexual desire groups.
For each subject and picture-viewing block, the raw fMRI data were preprocessed using the following steps: slice-timing correction using cubic spline interpolation, motion correction using a six-parameter rigid-body transformation with trilinear interpolation, spatial smoothing with a three-dimensional Gaussian spatial filter (5-mm full-width-at-half-maximum), and temporal filtering (high pass: 0.02 Hz). Two subjects who had estimated head motion parameters of > 3 mm translation and/or > 3° rotation in any direction were excluded from further analyses. The pre-processed images from each picture-viewing block were co-registered to the subject’s anatomical MRI using a nine-parameter (translation, rotation, scale) gradient-based affine alignment, and transformed into standard stereotaxic (Talairach-Toumoux) space (3-mm isotropic voxels) through manual identification of reference points in the anatomical images.
For each subject, the Talairach-transformed fMRI images for both picture-viewing blocks were entered into a voxelwise general linear model (Mumford & Nichols, 2009). This model included a term for the predicted BOLD response for each picture category (NEU, ERO, ROM, MUT, SAD), obtained by convolving a boxcar function representing the stimulus timing with a canonical hemodynamic response function. Estimates of head motion parameters obtained during pre-processing and the overall mean signal level for each picture-viewing block were included in this model as nuisance parameters. All terms in the model were treated as fixed effects.
Parameter estimates (β) were computed using ordinary least-squares estimation.
To identify brain regions with greater responses to emotional pictures than to neutral pictures, we used a mixed-effects general linear model. The β values for each subject and picture category were entered into this model. Each picture category was modeled as a fixed effect and subject was modeled as a random effect. A contrast of parameter estimates and its associated t- statistic was computed for the difference in response to neutral and emotional pictures (contrast coefficients of [−4, 1, 1, 1, 1] for [NEU, ERO, ROM, MUT, SAD], respectively). Clusters of voxels that exceeded a statistical threshold of t(26) > 3.07 (p < .005) and cluster-size threshold of 1000 mm3 were labeled as significantly active.
To identify brain regions where BOLD responses to emotional versus neutral stimuli differed between participants who were nondistressed or distressed about low sexual desire, the β values for each subject and picture category were entered into a 2 × 5 voxelwise ANOVA. In this ANOVA, group (distressed versus nondistressed) was a between subjects factor, and picture category (NEU, ERO, ROM, MUT, SAD) was a within-subjects factor. Thus, a significant group × picture category interaction reflects a difference in reactivity to at least one of the picture categories between subjects in the two groups. The F statistic for this interaction term was thresholded at F(4,100) > 3.96 (p < .005) with a minimum cluster size of 50 mm3.
In each region that showed a significant group × picture category interaction, we conducted follow-up analyses to identify the specific picture categories that contributed to between-group differences. We conducted four ANOVAs with group (distressed versus nondistressed) as a between-subjects factor and picture category (NEU, emotional) as a within- subjects factor. Each ANOVA tested one of the emotional picture categories (ERO, ROM, MUT, and SAD). Thus, a significant interaction in each follow-up test reflects a between-groups difference in activation to the specific category of emotional cue (relative to neutral cues).
Results
Self-Report Measures
Participant demographics and questionnaire scores are shown in Table 1. The distressed and nondistressed groups (as defined by the DSDS screener) did not differ significantly on any questionnaire measure, including FSFI and MSIQ subscales measuring sexual desire (ps > .05). Responses suggested that both groups were below the norm in sexual desire, although the distressed group had somewhat poorer sexual function. The two groups also did not differ in their subjective ratings of valence and emotional arousal of the IAPS images used in the experiment (for both valence and emotional arousal the group × picture category interactions were non-significant; Fs[4,100] < 1, ps > .1). Both groups rated erotic and romantic images as most pleasant, followed by neutral, sad, and mutilation (main effect of picture category F[4,100] = 165.29, p< .0001; linear trend F[1,25] = 271.57, p< .0001). Erotica and mutilations were rated as most emotionally arousing, and neutral pictures as least arousing (F[4,100] = 7.26, p < .0001; quadratic trend F[1,25] = 18.66,p < .0005).
Table 1:
Demographics and Self-Report Measures
| Measure | Not distressed about desire (n = 16) | Distressed about desire (n = 11) | All Participants (n = 27) | |
|---|---|---|---|---|
| n | n | n | ||
| Race/Ethnicity | ||||
| White | 8 | 10 | 18 | |
| African American | 8 | 0 | 8 | |
| Asian | 0 | 1 | 1 | |
| Mean (SD) | Mean (SD) | Group Difference (p) |
Mean (SD) | |
| Age (years) | 53.56 (3.26) | 54.73 (2.83) | .35 | 54.04 (3.09) |
| Time since end of treatment (months) | 83 (61) | 61 (47) | .33 | 74 (56) |
| BSI - Total Score | 4.00 (6.50) | 5.82 (7.80) | .52 | 4.74 (6.97) |
| NEO - Neuroticism | 14.19(7.84) | 19.36 (8.32) | .11 | 16.30 (8.30) |
| NEO - Extroversion | 31.50 (6.36) | 27.73 (4.82) | .11 | 29.96 (5.98) |
| NEO - Openness | 27.50 (4.38) | 28.45 (7.34) | .68 | 27.89 (5.66) |
| NEO - Agreeableness | 35.69 (6.93) | 35.36 (5.30) | .90 | 35.56 (6.20) |
| NEO - Conscientiousness | 36.88 (5.20) | 38.00 (4.00) | .55 | 37.33 (4.70) |
| SADI - Total Score | 161.1 (41.89) | 130.1 (47.43) | .09 | 148.44 (46.02) |
| MSIQ - Sexual Desire | 10.06 (6.95) | 7.55 (4.01) | .29 | 9.04 (5.97) |
| MSIQ – Sexual Satisfaction | 6.56(2.56) | 5.45 (2.07) | .24 | 6.11 (2.39) |
| DAS - Total Score | 4.63 (3.86) | 6.55 (3.50) | .20 | 5.41 (3.78) |
| FSFI - Total Score | 19.18 (10.05) | 13.44 (7.60) | .12 | 16.84 (9.42) |
| SAM Ratings - Valence | ||||
| ERO | 7.02 (1.38) | 7.33 (1.62) | .60 | 7.17(1.50) |
| ROM | 6.60(1.47) | 7.07 (0.87) | .35 | 6.83 (1.17) |
| NEU | 5.09 (0.41) | 5.12 (0.36) | .85 | 5.11 (0.38) |
| SAD | 2.39(1.09) | 2.63 (0.92) | .55 | 2.51 (1.01) |
| MUT | 1.35 (0.82) | 1.17 (0.25) | .49 | 1.26 (0.54) |
| Sam Ratings - Emotional Arousal | ||||
| ERO | 5.83 (1.84) | 6.37 (2.05) | .49 | 6.10(1.95) |
| ROM | 4.76(1.29) | 4.99 (1.80) | .70 | 4.88 (1.55) |
| NEU | 3.66 (1.33) | 3.90 (1.46) | .66 | 3.78 (1.40) |
| SAD | 4.21 (1.75) | 5.14 (1.84) | .20 | 4.68 (1.80) |
| MUT | 5.36 (2.75) | 6.24 (2.57) | .41 | 5.80 (2.66) |
Group difference p values were obtained from a two-sample t-test comparing the normal and low desire groups.
fMRI Data
Overall, BOLD responses were larger to emotional pictures than neutral pictures in the occipital lobes, posterior parietal lobes, inferior parietal lobule, anterior cingulate cortex, and dorsolateral prefrontal cortex (Table 2). In these regions, BOLD responses were largest in response to erotic pictures and smallest in response to neutral pictures (Figure 1).
Table 2:
Brain Areas with Larger Responses to Emotional Pictures than Neutral Pictures
| Center of Gravity (mm) |
|||||||
|---|---|---|---|---|---|---|---|
| Brain Area | Hem | BA | x | y | z | Size (mm3) | t(26)Mmax |
| Posterior Parietal and Occipital Lobes | L | 39 | −38 | −70 | 17 | 19,595 | 5.97 |
| R | 31 | 30 | −68 | 23 | 33,585 | 7.48 | |
| Inferior Parietal Lobule | L | 40 | −57 | −32 | 30 | 2,975 | 4.90 |
| R | 40 | 52 | −36 | 31 | 1,067 | 4.97 | |
| Anterior Cingulate Gyrus | R | 32 | −2 | 38 | 12 | 2,234 | 5.27 |
| Dorsolateral Prefrontal Cortex | R | 6 | 44 | −1 | 31 | 1,085 | 5.53 |
Note: These brain areas were identified from a whole-brain, voxelwise contrast of responses to emotional (Erotic, Romantic, Mutilation, and Sad) versus neutral pictures in all 27 participants. The statistical threshold for this analysis was a voxelwise p < .005 and a minimum cluster size of 1000 mm3. Hem = Hemisphere; L = Left; R = Right; x,y,z = Talairach coordinates for the location of the center of gravity of the activation cluster, in mm; Size = volume of the activation cluster; t(26)max = Maximum t statistic observed in the activation cluster for the contrast between emotional and neutral pictures.
Figure 1.
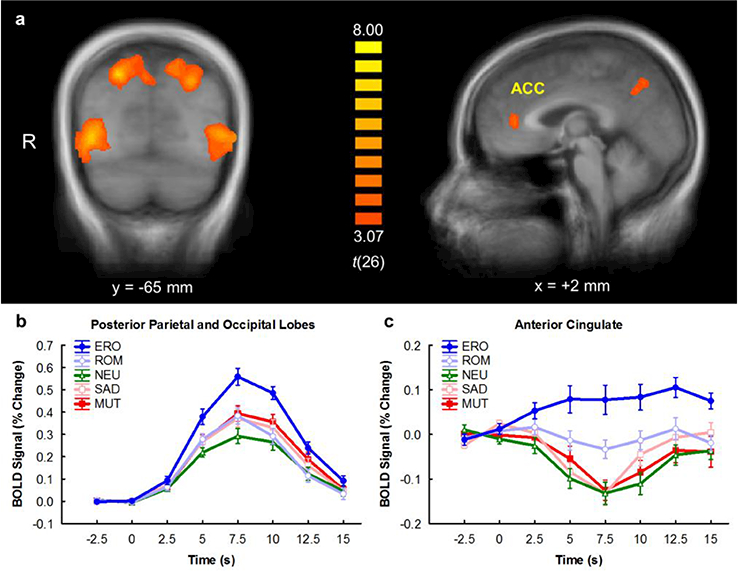
a) Brain areas with significantly larger BOLD responses to emotional stimuli than neutral stimuli. b) Time course of BOLD responses to each picture category in the occipital and posterior parietal lobes. c) Time course of BOLD responses to each picture category in the anterior cingulate cortex. x and y refer to slice locations in Talairach space of the sagittal and coronal slice, respectively. t(26) refers to the t-statistic for the emotional versus neutral contrast. MUT = mutilations, SAD = sad scenes, NEU = neutral, ROM = romance, ERO = erotica. On the time axis, 0 s indicates picture onset. Error bars indicate the mean ± 1 standard error. The time course of activation was expressed as the percent change from the region’s mean level of activation across the entire session. For each trial, the time course was baseline-corrected by subtracting the mean signal measured from 2.5 s before through 2.5 s after picture onset from each volume (Versace et al, 2011). Trials were then averaged across picture categories and participants and plotted above.
To compare brain responses between participants distressed versus nondistressed about low sexual desire, we computed a group (nondistressed versus distressed) × picture category (NEU, ERO, ROM, MUT, SAD) voxelwise ANOVA. The group × picture category interaction was statistically significant in the anterior cingulate (Figure 2), dorsolateral prefrontal cortex, inferior occipital gyrus, superior temporal gyrus, and posterior insula (Table 3). These areas overlap with those that showed larger responses to emotional pictures than neutral pictures overall (Table 2). Follow-up ANOVAs in the anterior cingulate indicated that the nondistressed group showed larger BOLD responses to ERO (F[l, 25] = 11.56,p < .01) and ROM (F[l, 25] = 10.55,P < .01) pictures, relative to neutral pictures, than the distressed group. The two groups did not differ in their reactions to MUT (F[l, 25] =3.60, p = .07) and SAD (F[l, 25] < 1, p > .1) pictures, relative to neutral pictures. Follow-up ANOVAs in the other brain regions showed a similar pattern of results.
Figure 2.
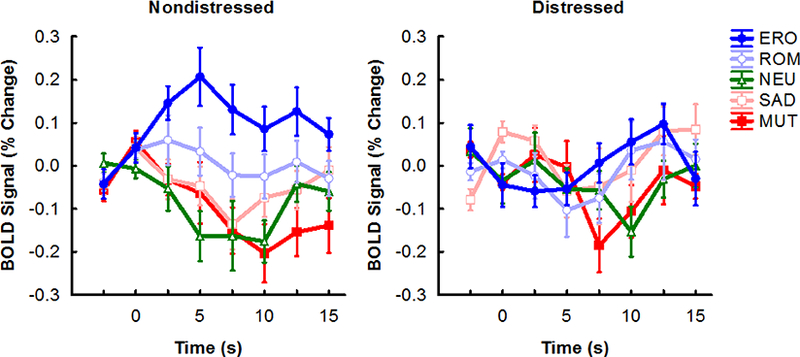
Time course of BOLD responses to each picture category in the anterior cingulate cortex in the nondistressed (n = 16) and distressed (n = 11) groups. MUT = mutilations, SAD = sad scenes, NEU = neutral, ROM = romance, ERO = erotica. On the time axis, 0 s indicates picture onset. Error bars indicate the mean ± 1 standard error. The time course for each picture category was computed as described in Figure 1.
Table 3:
Brain Areas showing a Significant Sexual Desire × Picture Category Interaction
| Center of Gravity (mm) |
|||||||
|---|---|---|---|---|---|---|---|
| Brain Area | Hem | BA | x | y | z | Size (mm3) | F(4,100)max |
| Inferior Occipital Gyrus | R | 17 | 12 | −88 | −6 | 487 | 5.28 |
| Superior Temporal Gyrus | R | 39 | 37 | −58 | 28 | 95 | 4.99 |
| Anterior Cingulate Gyrus | R | 24 | 3 | 34 | 10 | 81 | 5.74 |
| R | 32 | 26 | 35 | 9 | 50 | 4.92 | |
| Middle Frontal Gyrus | R | 8 | 46 | 14 | 45 | 689 | 5.66 |
| Posterior Insula | L | 13 | −28 | −32 | 20 | 83 | 4.79 |
Note: These brain areas were identified from a whole-brain, voxelwise Analysis of Variance (ANOVA) with distressed versus nondistressed about low sexual desire as a between-subjects factor and Picture Category (Neutral, Erotic, Romantic, Mutilation, and Sad) as a within-subjects factor. The statistical threshold for this analysis was a voxelwise p < .005 and a minimum cluster size of 50 mm3. Hem = Hemisphere; L = Left; R = Right; x,y,z = Talairach coordinates for the location of the center of gravity of the activation cluster, in mm; Size = volume of the activation cluster; F(4, 100)max = Maximum F statistic observed in the activation cluster for the Sexual Dysfunction × Picture Category interaction.
Discussion
Many breast cancer survivors report a loss of sexual desire and arousability. We hypothesized that this change may result from a chemotherapy-induced dysfunction in brain reward systems. Thus, our goal was to collect preliminary data about brain responses to erotic and other emotional stimuli in breast cancer survivors who were treated with chemotherapy. Breast cancer survivors with distressed about a loss of sexual desire had blunted responses to erotic and romantic pictures within brain reward circuits, including the anterior cingulate gyrus and dorsolateral prefrontal cortex. Although our findings are limited by the nature of our sample, they support the hypothesis that reduced sexual desire in breast cancer survivors could be the result of abnormal functioning in brain reward systems.
In healthy women with no cancer history, viewing of erotic stimuli results in increased activation in the anterior cingulate, medial prefrontal cortex, and nucleus accumbens (Sabatinelli et al, 2007; Stoleru et al, 2012; Walter et al, 2008). Thus, these brain regions are thought to be involved in the desire for (or “wanting” of) sex, rather than the experience of pleasure during sex (Arnow et al., 2009; Bianchi-Demicheli et al., 2011; Georgiadis & Kringelbach, 2012; Pfaus, 2009). The nucleus accumbens, anterior cingulate, and medial prefrontal cortex all receive dopaminergic input from the ventral tegmental area. This neural network is thought to form a “seeking” system that motivates the pursuit of reward. In fact, this brain system is activated during anticipation of other forms of natural (e.g., food), conditioned (e.g., money), or pharmacological (e.g., drugs of abuse) rewards (Engelmann et al., 2012; Kuhn & Gallinat, 2011; Sescousse, Caldu, Segura, & Dreher, 2013). Our finding that women who are dissatisfied with their desire for sex show decreased responses to erotica in the anterior cingulate is consistent with its involvement in sexual desire. Interestingly, we only found blunted brain responses to pleasant stimuli (i.e., erotic, and romantic images) and not to other categories of motivationally relevant stimuli, such as mutilations or sad pictures. Thus, the between groups difference in reaction to erotic cues cannot be attributed to a generalized difference in emotional reactivity, but appears to be specifically related to reward processing.
Due to the cross-sectional nature of our study, and the lack of a group of women treated for breast cancer who did not receive chemotherapy, we can only draw limited conclusions. The between-group differences observed here may be a consequence of cancer-related treatment or may have existed prior cancer diagnosis or treatment. However, brain imaging studies of cognitive deficits in breast cancer survivors suggest that chemotherapy has a negative impact on frontal neural networks responsible for attention and executive functions (de Ruiter et al., 2011; Kesler et al., 2011). Importantly, these are the same brain regions that are specifically activated by appetitive stimuli, including erotica (Sabatinelli et al., 2007). Thus, cancer treatments affecting frontal cortical and subcortical networks could diminish emotional processes, such as sexual desire. Future longitudinal studies including both pre- and post-treatment fMRI sessions are necessary to clarify the role of chemotherapy in altering brain responses to erotic cues and sexual desire.
Another challenge is separating the effects of chemotherapy, menopausal status, and self- reported sexual desire on reactivity to erotic stimuli. Although 7 participants in our study had been menopausal at breast cancer diagnosis (3 in the distressed and 4 in the nondistressed group), all were currently menopausal either because of cancer treatment or normal aging. A recent comparison of premenopausal women on versus off oral contraceptives suggested that serum estradiol does not change women’s fMRI responses to intense erotic videos or photos, but decreased levels did attenuate brain responses when women were given the expectation that the next image would be erotic (Abler et al., 2013). Future studies, ideally using a prospective design, should compare BOLD responses to erotic images in women who maintain menstrual cycles after chemotherapy with an age-matched group in premature ovarian failure. Another important comparison is with women who do not have chemotherapy or hormonal therapy as part of the treatment for breast cancer. It is difficult to find such women, given that even those with early stage disease typically have adjuvant systemic therapies. However, women with ductal carcinoma in situ (DCIS) sometimes have only local therapy, and could be a comparison group for future studies. Our limited funding did not allow us to include a DCIS group in this pilot study. A recent survey of sexually active survivors of treatment for DCIS found normal levels of sexual function and body image, even though almost half were postmenopausal (Bober et al., 2013). Premenopausal women whose breast cancer treatment does not cause premature ovarian failure also report better sexual function than those who become menopausal (Ochsenkühn et al., 2011).
Another limitation of this study is that women who were dissatisfied with their desire according to the DSDS (Clayton et al., 2009) may not have met all diagnostic criteria for female Sexual Interest/Arousal Disorder (American Psychiatric Association, 2000), but still were distressed about their perceived loss of desire since breast cancer treatment. In addition, the two groups did not differ significantly on total scores or desire subscale scores on two questionnaires designed to assess female sexual desire, the FSFI and MSIQ. In fact, scores for women in the nondistressed group would indicate abnormally poor sexual function according to the norms for these tests (Rosen et al., 2004; Wiegel et al., 2005). This suggests that blunted brain responses to erotica might only be present in the most extreme cases of sexual dysfunction, such as in women who not only report low desire/arousability, but are also distressed about the problem. Since societal norms about female sexuality are often contradictory and confusing, distress has been considered as central to a diagnosis of low desire in women since DSM-IV-TR, and is reported by over 80% of women who met DSM-IV-TR criteria for a desire disorder (Derogatis, Clayton, Lewis-D’Agostino, Wunderlich, & Fu, 2008). Distress may be even more important when a medical illness and treatment cause physiological damage to a woman’s ability to enjoy sex. Future research should address the quantitative relationship between sexual dysfunction measured using a variety of scales and brain responses to erotic cues.
Notwithstanding these limitations, our results provide initial evidence that breast cancer survivors with very low sexual desire have blunted brain responses in frontal regions while viewing erotic and romantic images. Dysfunctions in the brain reward systems may contribute to the high incidence of sexual dysfunction in breast cancer survivors. Efforts to find a drug that would increase women’s desire for sex have thus far been largely unsuccessful (Bradford, 2013). Further study of the mechanisms that reduce sexual desire and arousability in breast cancer survivors may foster the development of new, evidence-based interventions for one of the most prevalent and enduring side effects of cancer treatment.
Acknowledgments
This research was supported by funds from the University Cancer Foundation and the Duncan Family Institute for Cancer Prevention and Risk Assessment via the Cancer Survivorship Research Seed Money Grants at the University of Texas MD Anderson Cancer Center to Francesco Versace.
The University of Texas MD Anderson Cancer Center Breast Cancer Management System Database was consulted to obtain the initial list of potential participants.
The authors wish to thank Lauren Baker, Jerell Jones, Cheryl Mize, and Jennifer Ng for
their help in data collection.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abler B, Kumpfmuller D, Gron G, Walter M, Stingl J, & Seeringer A (2013). Neural correlates of erotic stimulation under different levels of female sexual hormones. PloS one, 8, e54447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder J, Zanetti R, Wight E, Urech C, Fink N, & Bitzer J (2008). Sexual dysfunction after premenopausal stage I and II breast cancer: do androgens play a role? J Sex Med, 5, 1898–1906. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and Statistical Manual of Mental Disorders, 4th Edition Washington, D.C.: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and Statistical Manual of Mental Disorders. (4th Revised ed.) Washington, D.C.: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association. [Google Scholar]
- Arnow BA, Millheiser L, Garrett A, Lake PM, Glover GH, Hill KR et al. (2009). Women with hypoactive sexual desire disorder compared to normal females: a functional magnetic resonance imaging study. Neuroscience, 158, 484–502. [DOI] [PubMed] [Google Scholar]
- Avis NE, Brockwell S, Randolph JF Jr., Shen S, Cain VS, Ory M, & Greendale GA (2009). Longitudinal changes in sexual functioning as women transition through menopause: results from the Study of Women’s Health Across the Nation. Menopause, 16, 442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton DL, Wender DB, Sloan JA, Dalton RJ, Balcueva EP, Atherton PJ et al. (2007). Randomized controlled trial to evaluate transdermal testosterone in female cancer survivors with decreased libido; North Central Cancer Treatment Group protocol N02C3. J Natl.Cancer Inst, 99, 672–679. [DOI] [PubMed] [Google Scholar]
- Berglund G, Nystedt M, Bolund C, Sjoden PO, & Rutquist LE (2001). Effect of endocrine treatment on sexuality in premenopausal breast cancer patients: a prospective randomized study. J.Clin.Oncol, 19, 2788–2796. [DOI] [PubMed] [Google Scholar]
- Bianchi-Demicheli F, Cojan Y, Waber L, Recordon N, Vuilleumier P, & Ortigue S (2011). Neural bases of hypoactive sexual desire disorder in women: an event-related FMRI study. J Sex Med, 8, 2546–2559. [DOI] [PubMed] [Google Scholar]
- Bitzer J, Giraldi A, & Pfaus J (2013). Sexual desire and hypoactive sexual desire disorder in women. Introduction and overview. Standard operating procedure (SOP Part 1). J Sex Med, 10, 36–49. [DOI] [PubMed] [Google Scholar]
- Bober SL, Giobbie-Hurder A, Emmons KM, Winer E, & Partridge A (2013). Psychosexual functioning and body image following a diagnosis of ductal carcinoma in situ. J Sex Med, 10, 370–377. [DOI] [PubMed] [Google Scholar]
- Bradford A (2013). Listening to placebo in clinical trials for female sexual dysfunction. J Sex Med, 10, 451–459. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, & Desai P (2003). Activation of the visual cortex in motivated attention. Behavior Neuroscience, 117, 369–80. [DOI] [PubMed] [Google Scholar]
- Burwell SR, Case LD, Kaelin C, & Avis NE (2006). Sexual problems in younger women after breast cancer surgery. J Clin Oncol, 24, 2815–2821. [DOI] [PubMed] [Google Scholar]
- Clayton AH, Goldfischer ER, Goldstein I, Derogatis L, Lewis-D’Agostino DJ, & Pyke R (2009). Validation of the decreased sexual desire screener (DSDS): a brief diagnostic instrument for generalized acquired female hypoactive sexual desire disorder (HSDD). J Sex Med, 6, 730–738. [DOI] [PubMed] [Google Scholar]
- Collins B, Mackenzie J, Tasca GA, Scherling C, & Smith A (2012). Cognitive effects of chemotherapy in breast cancer patients: a dose-response study. Psychooncology. [DOI] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G et al. (2012). Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum Brain Mapp, 33, 2971–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter ΜB, Reneman L, Boogerd W, Veltman DJ, van Dam FS, Nederveen AJ et al. (2011). Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp, 32, 1206–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis L, Clayton A, Lewis-D’Agostino D, Wunderlich G, & Fu Y (2008). Validation of the female sexual distress scale-revised for assessing distress in women with hypoactive sexual desire disorder. J Sex Med, 5, 357–364. [DOI] [PubMed] [Google Scholar]
- Derogatis LR (2001). Brief Symptom Inventory 18: Administration, Scoring, and Procedures Manuel Minneapolis, MN: NCS Pearson. [Google Scholar]
- Dizon DS (2009). Quality of life after breast cancer: survivorship and sexuality. Breast J, 15, 500–504. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y et al. (2012). Neural substrates of smoking cue reactivity: A meta-analysis of fMRI studies. Neuroimage, 60, 252–262. doi: 10.1016/j.neuroimage.2011.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch A, Metcalfe KA, Chiang JK, Elit L, McLaughlin J, Springate C et al. (2011). The impact of prophylactic salpingo-oophorectomy on menopausal symptoms and sexual function in women who carry a BRCA mutation. Gynecol.Oncol, 121, 163–168. [DOI] [PubMed] [Google Scholar]
- Fobair P, Stewart SL, Chang S, D’Onofrio C, Banks PJ, & Bloom JR (2006). Body image and sexual problems in young women with breast cancer. Psychooncology, 15, 579–594. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Rowland JH, Meyerowitz BE, & Desmond KA (1998). Impact of different adjuvant therapy strategies on quality of life in breast cancer survivors. Recent Results Cancer Res, 152, 396–411. [DOI] [PubMed] [Google Scholar]
- Georgiadis JR & Kringelbach ML (2012). The human sexual response cycle: brain imaging evidence linking sex to other pleasures. Prog.Neurobiol, 98, 49–81. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, & Formisano E (2006). Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp, 27, 392–401. doi : 10.1002/hbm.20249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greendale GA, Petersen L, Zibecchi L, & Ganz PA (2001). Factors related to sexual function in postmenopausal women with a history of breast cancer. Menopause, 8, 111–119. [DOI] [PubMed] [Google Scholar]
- Hayes RD (2011). Circular and linear modeling of female sexual desire and arousal. J Sex Res, 48, 130–141. [DOI] [PubMed] [Google Scholar]
- Holstege G & Huynh HK (2011). Brain circuits for mating behavior in cats and brain activations and de-activations during sexual stimulation and ejaculation and orgasm in humans. Horm.Behav, 59, 702–707. [DOI] [PubMed] [Google Scholar]
- Kesler SR, Kent JS, & O’Hara R (2011). Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol, 68, 1447–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, & Schagen SB (2012). Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol, 30, 1080–1086. [DOI] [PubMed] [Google Scholar]
- Kuhn S & Gallinat J (2011). Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur.J Neurosci, 33, 1318–1326. doi: 10.1111/j.l460-9568.2010.07590.x [DOI] [PubMed] [Google Scholar]
- Lang PJ (1980). Behavioral treatment and bio-behavioral assessment: Computer applications In Sidowski JB, Johnson JH, & Williams TA (Eds.), Technology in mental health care delivery systems (pp. 119–137). Norwood, NJ: Ablex. [Google Scholar]
- Lang PJ & Bradley ΜM (2009). Emotion and the motivational brain. Biological Psychology, 84, 437–450. doi: 10.1016/j.biopsycho.2009.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (1997). Motivated attention: Affect, activation, and action In Lang PJ, Simons RF, & Balaban M (Eds.), Attention and orienting: Sensory and motivational processes (pp. 97–136). Mahwah, NJ: Lawrence Erlbaum. [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN International affective picture system (IAPS): Affective ratings of pictures and instruction manualTechnical Report A-8 2008. Gainesville, FL, University of Florida. [Google Scholar]
- Lang PJ, Bradley ΜM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, & Nangia V (1998). Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology, 35, 199–210. [PubMed] [Google Scholar]
- McCrae RR & Costa PT (2004). A contemplated revision of the NEO Five-Factor Inventory. Personality and Individual Differences, 36, 587–596. [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, & Dolan RJ (1998). A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain, 121 (Pt 1), 47–57. [DOI] [PubMed] [Google Scholar]
- Mumford JA & Nichols T (2009). Simple group fMRI modeling and inference. Neuroimage, 47, 1469–1475. doi: 10.1016/j.neuroimage.2009.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsenkühn R, Hermelink K, Clayton AH, von SV, Gallwas J, Ditsch N et al. (2011). Menopausal status in breast cancer patients with past chemotherapy determines long-term hypoactive sexual desire disorder. J Sex Med, 8, 1486–1494. [DOI] [PubMed] [Google Scholar]
- Panjari M, Bell RJ, & Davis SR (2011). Sexual function after breast cancer. J Sex Med, 8, 294–302. [DOI] [PubMed] [Google Scholar]
- Pessoa L (2008). On the relationship between emotion and cognition. Nat.Rev Neurosci, 9, 148–158. doi: 10.1038/nrn2317 [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, & Ungerleider LG (2002). Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences of the United States of America, 99, 11458–11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaus JG (2009). Pathways of sexual desire. J Sex Med, 6, 1506–1533. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Lobo RA, Block BA, Yang HM, & Zipfel LM (2004). Menopausal Sexual Interest Questionnaire (MSIQ): a unidimensional scale for the assessment of sexual interest in postmenopausal women. J.Sex Marital Ther, 30, 235–250. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmons JR, & Lang PJ (2005). Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage, 24, 1265–1270. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Lang PJ, Costa VD, & Versace F (2007). Pleasure rather than salience activates human nucleus accumbens and medial prefrontal cortex. J Neurophysiol, 98, 1374–1379. [DOI] [PubMed] [Google Scholar]
- Sabourin S, Valois P, & Lussier Y (2005). Development and validation of a brief version of the dyadic adjustment scale with a nonparametric item analysis model. Psychological Assessment, 17, 15–27. [DOI] [PubMed] [Google Scholar]
- Sarin S, Amsel RM, & Binik YM (2013). Disentangling Desire and Arousal: A Classificatory Conundrum. Arch Sex Behav. [DOI] [PubMed] [Google Scholar]
- Sayakhot P, Vincent A, Deeks A, & Teede H (2011). Potential adverse impact of ovariectomy on physical and psychological function of younger women with breast cancer.Menopause, 18, 786–793. [DOI] [PubMed] [Google Scholar]
- Schover LR (2008). Premature ovarian failure and its consequences: vasomotor symptoms, sexuality, and fertility. J.Clin.Oncol, 26, 753–758. [DOI] [PubMed] [Google Scholar]
- Schover LR, Yetman RJ, Tuason LJ, Meisler E, Esselstyn CB, Hermann RE et al. (1995). Partial mastectomy and breast reconstruction. A comparison of their effects on psychosocial adjustment, body image, and sexuality. Cancer, 75, 54–64. [DOI] [PubMed] [Google Scholar]
- Sescousse G, Caldu X, Segura B, & Dreher JC (2013). Processing of primary and secondary rewards: A quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav.Rev, 37, 681–696. [DOI] [PubMed] [Google Scholar]
- Speer JJ, Hillenberg B, Sugrue DP, Blacker C, Kresge CL, Decker VB et al. (2005). Study of sexual functioning determinants in breast cancer survivors. Breast J, 11, 440–447. [DOI] [PubMed] [Google Scholar]
- Stoleru S, Fonteille V, Cornelis C, Joyal C, & Moulier V (2012). Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: a review and meta-analysis. Neurosci Biobehav.Rev, 36, 1481–1509. [DOI] [PubMed] [Google Scholar]
- Suppli NP, Deltour I, Damkjaer LH, Christensen J, Jensen AB, Kroman NT et al. (2011). Factors associated with the prescription of antidepressive medication to breast cancer patients. Acta Oncol, 50, 243–251. [DOI] [PubMed] [Google Scholar]
- Toledano R & Pfaus J (2006). The Sexual Arousal and Desire Inventory (SADI): a multidimensional scale to assess subjective sexual arousal and desire. J Sex Med, 3, 853–877. [DOI] [PubMed] [Google Scholar]
- Trompeter SE, Bettencourt R, & Barrett-Connor E (2012). Sexual activity and satisfaction in healthy community-dwelling older women. American Journal of Medicine, 125, 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Engelmann JM, Jackson EF, Costa VD, Robinson JD, Lam CY et al. (2011). Do brain responses to emotional images and cigarette cues differ? An fMRI study in smokers. Eur.J Neurosci, 34, 2054–2063. doi: 10.1111/j.1460-9568.2011.07915.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Bermpohl F, Mouras H, Schiltz K, Tempelmann C, Rotte M et al. (2008). Distinguishing specific sexual and general emotional effects in fMRI-subcortical and cortical arousal during erotic picture viewing. Neuroimage, 40, 1482–1494. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Lenzi R, Theriault RL, Davis RN, & Meyers CA (2004). The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer, 100, 2292–2299. [DOI] [PubMed] [Google Scholar]
- Wiegel M, Meston C, & Rosen R (2005). The female sexual function index (FSFI): cross- validation and development of clinical cutoff scores. J.Sex Marital Ther, 31, 1–20. [DOI] [PubMed] [Google Scholar]


