Abstract
IMPORTANCE
Identifying patients with infectious keratitis who are at risk of experiencing a poor outcome may be useful to allocate resources toward high-risk patients, particularly in resource-poor settings.
OBJECTIVE
To determine baseline patient and ulcer characteristics that predict a high risk of developing corneal perforation and/or the need to undergo therapeutic penetrating keratoplasty (TPK).
DESIGN, SETTING, AND PARTICIPANTS
This is a secondary analysis of Mycotic Ulcer Treatment Trial II, a multicenter, double-masked, placebo-controlled randomized clinical trial that enrolled 240 patients with smear-positive filamentous fungal corneal ulcers who enrolled between May 2010 and August 2015. Participants had a baseline visual acuity of 20/400 or worse and were randomized to receive oral voriconazole or a placebo (all participants received topical voriconazole, 1%). After 39 participants (16.3%) were enrolled, topical natamycin, 5%, was also added.
MAIN OUTCOMES AND MEASURES
The primary outcome of this secondary analysis was the rate of corneal perforation or the need to undergo TPK.
RESULTS
The mean (SD) age at enrollment was 49 (13) years, 104 participants (43.3%) were women, and all were of Southeast Asian descent. The presence of hypopyon at baseline indicated 2.28 times the odds of the patient developing corneal perforation and/or needing TPK (95% CI, 1.18–4.40; P = .01). Study participants whose infiltrate involved the posterior one-third had a 71.4% risk of developing corneal perforation and/or needing TPK. For each 1-mm increase in the geometric mean of the infiltrate, there was 1.37 (95% CI, 1.12–1.67; P = .002) increased odds of developing perforation and/or needing TPK. Other clinical features such as visual acuity, baseline culture positivity, type of filamentous fungal organism and duration of symptoms, and demographic characteristics, such as sex and occupation, were not significant predictors in the multivariable regression analysis.
CONCLUSIONS AND RELEVANCE
These results suggest that risk stratification from baseline ulcer characteristics can identify those at highest risk for developing corneal perforation and/or needing TPK.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00996736
Corneal perforation is a common complication of severe fungal keratitis that occurs in up to 50% of cases.1–3 Therapeutic penetrating keratoplasty (TPK) may be performed during a period of acute infection because of a patient’s lack of response to medical therapy or for tectonic support. The graft survival for these transplantations is inferior when compared with transplantations that are performed for optical indications, and complications are common.4,5 The Mycotic Ulcer Treatment Trial II (MUTT II) was a randomized clinical trial that found no benefit of adjuvant oral voriconazole in reducing the risk of perforation and/or TPK in severe filamentous fungal keratitis except for Fusiarum species ulcers.3,6 In this study, we investigate baseline demographic and clinical features in MUTT II that predict the eventuation to perforation and/or TPK. Identifying high-risk patients early may be useful to appropriately allocate resources in resource-poor settings.
Methods
The methods for MUTT II have been previously outlined.7 Briefly, patients who presented with a smear-positive filamentous fungal corneal ulcer and a visual acuity worse than 20/400 (logMAR 1.3) were randomized to receive oral voriconazole or a placebo. All patients received topical voriconazole, 1%, and after the results of MUTT I became available, topical natamycin, 5%, was also administered to all patients. Enrollment centers included hospitals in the Aravind Eye Care System (Madurai, Pondicherry, Tirunelveli, or Coimbatore, India), Lumbini Eye Hospital and Bharatpur Eye Hospital in Nepal, and the University of California–San Francisco. Exclusion criteria included coinfection with bacteria, Acanthamoeba or herpes, an impending perforation, being younger than 16 years, poor visual acuity in the other eye (<20/200), a weight less than 40 kg, or known liver disease or pregnancy. The primary outcome of the trial was the rate of perforation and/or the need for TPK.
Institutional review board approval was obtained at the University of California–San Francisco, the Aravind Eye Care System, the Dartmouth-Hitchcock Medical Center Committee for the Protection of Human Subjects, and Nepal Netra Jyoti Sangh. The trial conformed to the Declaration of Helsinki and written informed consent was obtained from all participants.
In this post hoc secondary analysis, we first assessed associations between baseline patient and ulcer characteristics and risk for the patient developing perforation and/or needing TPK in univariate logistic regression models. Statistically significant predictors (P < .05) were included in a subsequent multivariable model. Multivariable model selection was determined using a backward-stepwise regression with a cutoff P value of less than .20 for covariates. All analyses were conducted from March 1, 2017, to March 10, 2017, using Stata, version 13.0 (Stata Corp).
Results
A total of 240 patients were enrolled in Southeast Asia between May 24, 2010, and November 23, 2015, and 122 (50.8%) developed a full-thickness corneal perforation or needed to undergo TPK. Univariate analyses comparing baseline characteristics of those who developed perforation and/or required TPK compared with those who did not are outlined in Table 1.
Table 1.
Baseline Characteristics of Those Who Developed Corneal Perforation or Underwent Therapeutic Penetrating Keratoplasty
| TPK (n = 122) | No TPK (n = 118) | OR (95% CI) | P Valuea | |
|---|---|---|---|---|
| Sex, No. (%)b | ||||
| Male | 70 (57.9) | 65 (55.5) | 0.94 (0.56–1.57) | .82 |
| Female | 51 (42.1) | 52 (44.4) | ||
| Age, median (IQR), y | 53.5 (42–63) | 51.5 (45–60) | 1.01 (0.99–1.03) | .31 |
| Occupation, No. (%) | ||||
| Agriculture | 64 (52.5) | 77 (65.3) | 0.59 (0.35–0.99) | .045c |
| Nonagricultured | 58 (47.5) | 41 (34.7) | ||
| Trauma/injury, No. (%) | 53 (43.4) | 63 (53.4) | 0.98 (0.77–1.24) | .88 |
| Vegetative matter/wood | 36 (29.5) | 38 (32.2) | ||
| Metal/othere | 27 (22.1) | 37 (31.4) | ||
| Unknown object | 2 (1.6) | 4 (3.4) | ||
| Affected eye, No. (%) | ||||
| Right | 80 (65.5) | 59 (50.0) | 0.80 (0.53–1.21) | .29 |
| Visual acuity, logMAR, median (IQR) | 1.7 (1.70–1.80) | 1.66 (1.22–1.80) | 1.17 (1.08–1.27) | <.001c |
| Infiltrate/Scar, median (IQR), mmf | 6.07 (5.00–6.94) | 5.00 (4.90–5.92) | 1.51 (1.26–1.81) | <.001c |
| Hypopyon, No. (%), mm | ||||
| No | 22 (18.3) | 43 (37.7) | 2.70 (1.52–4.82) | .001c |
| <0.5 | 19 (15.8) | 20 (17.5) | ||
| ≥0.5 | 79 (65.8) | 51 (44.7) | ||
| % of Depth, No. (%) | ||||
| >0–33 | 18 (15.0) | 40 (33.9) | 2.25 (1.56–3.24) | <.001c |
| >33–67 | 46 (38.3) | 56 (47.5) | ||
| >67–100 | 55 (45.8) | 22 (18.6) | ||
| Epithelial defect, median (IQR), mmf | 5.02 (3.88–6.08) | 4.36 (3.07–5.23) | 1.23 (1.06–1.42) | .005c |
| Organism | ||||
| Fusarium | 42 | 30 | 1.05 (0.83–1.34) | .69 |
| Aspergillus | 31 | 32 | ||
| Other | 31 | 30 | ||
| Duration of symptoms, median (IQR), d | 10.00 (7.00–15.00) | 9.50 (5.00–15.25) | 0.99 (0.97–1.02) | .56 |
| Systemic disease, No. (%)g | 9 (7.4) | 17 (14.4) | 0.47 (0.20–1.10) | .09 |
Abbreviations: IQR, interquartile range; OR, odds ratio; TPK, therapeutic penetrating keratoplasty.
Univariate analysis to determine whether baseline characteristic predicts the development of corneal perforation or the need for therapeutic penetrating keratoplasty.
Two patients were missing values for sex.
Statistically significant difference in univariate analysis and added to multivariate logistic regression model.
Includes unemployed and retired.
Includes dust, battery acid, ash, cement, fingernail, stick, cow or buffalo tail, goat horn, and insect.
Geometric mean of the longest diameter and longest perpendicular to that diameter in millimeters.
Includes diabetes mellitus, asthma, and hypertension.
Results of the final multivariable model are listed in Table 2. The presence of hypopyon at baseline indicated 2.28 times the odds of the patient developing a corneal perforation and/or needing TPK (95% CI, 1.18–4.40; P = .01). Infiltrate depth was graded as (1) no infiltrate, (2) infiltrate involving the anterior one-third of the stroma, (3) infiltrate involving up to two-thirds of the stroma, and (4) infiltrate involving the deepest third of the cornea. For each step increase in the infiltrate depth as measured at baseline there was 1.69 times the odds of the patient developing a full-thickness corneal perforation and/or needing TPK (95% CI, 1.12–2.53; P = .01). Study participants whose infiltrate involved the posterior third of the stroma had a 71.4% risk of developing a corneal perforation and/or needing TPK.
Table 2.
Multivariate Logistic Regression Model Predicting Perforation and/or Therapeutic Penetrating Keratoplasty
| Baseline Characteristic | OR (95% CI) | P Valuea |
|---|---|---|
| Infiltrate depthb | 1.69 (1.12–2.53) | .01 |
| Infiltrate and/or scar sizec | 1.37 (1.12–1.67) | .002 |
| Visual acuity, logMAR | 1.06 (0.97–1.16) | .14 |
| Agricultural occupation | 0.59 (0.33–1.08) | .09 |
| Hypopyond | 2.28 (1.18–4.40) | .01 |
Abbreviation: OR, odds ratio.
Global P value of the model is <.001.
Categorical variable: no infiltrate, infiltrate in anterior one-third of the stroma, up to two-thirds stromal depth, or involving posterior one-third of the stroma.
Geometric mean of the longest diameter and longest perpendicular to that diameter in millimeters.
Binary variable indicating presence or absence of hypopyon at baseline visit.
The infiltrate size was measured by taking the geometric mean of the longest diameter and longest perpendicular to that diameter in millimeters. The median baseline infiltrate size was 5.37 mm (interquartile range, 4.47–6.64). For each 1-mm increase in the geometric mean of the infiltrate there was 1.37 (95% CI, 1.12–1.67; P = .002) increased odds of the patient developing perforation and/or needing TPK. Study participants with a baseline infiltrate size more than a geometric mean of 6.63 mm (the highest quartile seen in MUTT II) had a 66.7% risk of developing perforation and/or needing TPK.
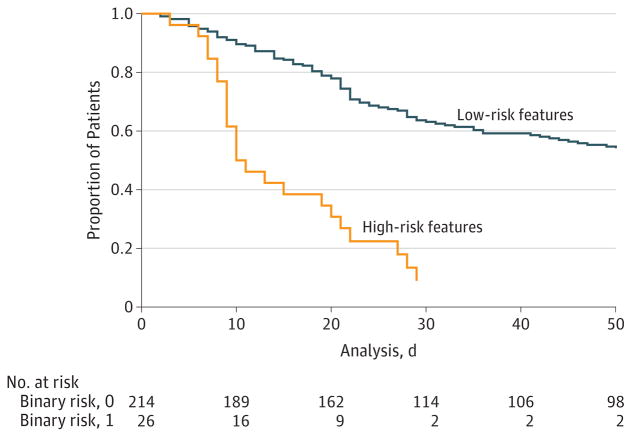
Baseline visual acuity was generally poor, with a median of logMAR 1.7 (interquartile range, 1.42–1.8). For each 1-line increase (worse) in baseline visual acuity, there was 1.06 times the odds of the patient developing perforation, although this was not statistically significant (95% CI, 0.81–4.65; P = .14). An agricultural occupation was no longer significantly protective in the multivariable analysis (odds ratio, 0.59; 95% CI, 0.33–1.08; P = .09). The Figure shows Kaplan-Meier survival curves comparing time with perforation and/or TPK among those with high-risk baseline ulcer features, defined as an infiltrate geometric mean size of more than 6.63 mm, an infiltrate involving the posterior one-third of the stroma, and the presence of a hypopyon compared with all other study participants.
Figure. Time to Perforation or Therapeutic Penetrating Keratoplasty in Patients With High-Risk Features at Baseline.
High-risk is defined as an infiltrate geometric mean size of more than 6.63 mm, an infiltrate involving the posterior one-third of the stroma, and a hypopyon. Low-risk includes all other patients in the Mycotic Ulcer Treatment Trial II.
Discussion
This study suggests that stratification of severe fungal corneal ulcers based on clinical examination at baseline can select patients who are at the highest risk of experiencing a poor outcome. In our multivariable model, baseline infiltrate size and depth, as well as the presence of hypopyon, were the most associated with a patient developing corneal perforation and/or needing TPK. Other clinical features, such as visual acuity, epithelial defect size, baseline culture positivity, type of filamentous fungal organism, and duration of symptoms, were not statistically significant predictors in our study population. Demographics such as age, sex, and occupation were also not significant risk factors.
Therapeutic penetrating keratoplasty for fungal keratitis has been associated with worse vision-related quality of life; however, risk factors for the development of this complication have not been well characterized.8 One retrospective case-control study of microbial keratitis of all types found several characteristics that predicted poor outcomes in univariate analyses, including older age, delay in referral, steroid use, poor vision, positive microbiology, fungal infection, large ulcer size, central location, the presence of hypopyon, prior perforation or descemetocele, and limbal involvement.9 Another study that looked at pathology and the culture of TPK buttons found that deeper infiltrates, a heavy fungal load, and Fusarium species were risk factors for a patient developing a perforation.10 A prospective study of less severe filamentous fungal ulcers found that only baseline epithelial defect size was a statistically significant predictor of perforation in multivariable analysis.7
It has been well established that outcomes of therapeutic grafts fare worse than those for optical indications.4,11 The optimal timing of TPK is unknown, and some have argued that eliminating the infection surgically early could potentially improve clinical outcomes.12–14 This could be an important area of future research if those at highest risk of requiring surgery could be identified early.
Strength and Limitations
The robust prospective data collection in several smear positive filamentous fungal ulcers is a strength of this study. Limitations include the fact that all study participants were enrolled in Southeast Asia; therefore, it is possible that organisms in this area exhibit different characteristics than those in other regions. Most infections were related to agricultural exposure and not contact lenses, as seen in most developed countries.
Conclusions
Risk stratification from baseline characteristics of large, severe fungal ulcers can identify those who are at highest risk of developing a corneal perforation and/or needing TPK. This is useful to allocate resources toward high-risk patients, particularly in resource-poor settings.
Key Points.
Question
Do baseline clinical features predict the risk of developing corneal perforation and/or undergoing therapeutic penetrating keratoplasty in infectious keratitis?
Findings
In this post hoc analysis of patients in the Mycotic Ulcer Treatment Trial II, we found that a baseline presence of hypopyon and infiltrate depth and size were statistically significant predictors of corneal perforation and/or the need for therapeutic penetrating keratoplasty.
Meaning
Identifying patients who are at high risk of experiencing a poor outcome may be useful to allocate resources, particularly in resource-poor settings.
Acknowledgments
Funding/Support: This work was supported by grants U10 EY018573 (Drs Lietman and Acharya) and K23 EY025025 (Dr Rose-Nussbaumer) from the National Eye Institute and grants from That Man May See, the Harper/Inglis Trust, the South Asia Research Foundation, and Research to Prevent Blindness (Drs Lietman and Acharya). Natamycin and voriconazole were donated by Alcon and Pfizer, respectively.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Role of the Funder/Sponsor: The sponsoring organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Group Information: The Mycotic Ulcer Treatment Group includes the following members: Aravind Eye Hospital, Madurai, Tamil Nadu, India: N. Venkatesh Prajna, MD (principal investigator), Prajna Lalitha, MD, Muthiah Srinivasan, MD, FRCOphth, MA, Manoranjan Das, Rajarathinam Karpagam, Malaiyandi Rajkumar, S. R. Sumithra, and C. Sundar; Aravind Eye Hospital, Coimbatore, Tamil Nadu, India: Revathi Rajaraman, MD (site director), Anita Raghavan, MD, and P. Manikandan, MPhil; Aravind Eye Hospital, Pondicherry, Tamil Nadu, India: K. Tiruvengada Krishnan, MD (site director), and N. Shivananda; Aravind Eye Hospital, Tirunelveli, Tamil Nadu, India: R. Meenakshi, MD (site director), J. Bharathi and E. Raja; Bharatpur Eye Hospital, Chitwan, Nepal: Byanju Raghunandan, MD (site director), Kamal Bahadur Khadka, Ranjeet Shah, and Anju Ligal; Francis I. Proctor Foundation, University of California, San Francisco: Thomas M. Lietman, MD (principal investigator), Nisha R. Acharya, MD (principal investigator), Stephen D. McLeod, MD, Jennifer Rose-Nussbaumer, MD, John P. Whitcher, MD, MPH, Travis C. Porco, PhD, MPH, Salena Lee, OD, Vicky Cevallos, MT(ASCP), Brett L. Shapiro, MD, Catherine E. Oldenburg, MPH, Kieran S. O’Brien, MPH, and Kevin C. Hong, BA; Lumbini Eye Institute, Bhairahawa, Nepal: Sushila Patel MD (site director), Salma K. C. Rai, Bel Bahadur Thapa, Binita Bhattarai, Ramesh C. Giri, Abhijeet Sarkar, Santosh Ghimire, Krishna Kunwar, Roji Yadav, Srijana S. Gautam, Sandeep Bashyal, Rojina Begam, and Amar Gautam; Data and Safety Monitoring Committee: Marian Fisher, PhD (chair), Anthony Aldave, MD, Donald Everett, MA, Jacqueline Glover, PhD, K. Ananda Kannan, MD, Steven Kymes, PhD, and Ivan Schwab, MD; Coordinating Center, Francis I. Proctor Foundation, University of California, San Francisco: Thomas M. Lietman, MD (principal investigator), Nisha R. Acharya, MD (principal investigator), Stephen D. McLeod, MD, Jennifer Rose-Nussbaumer, MD, John P. Whitcher, MD, MPH, Travis C. Porco, PhD, MPH, David Glidden, PhD, Salena Lee, OD, Kathryn Ray, MA, Vicky Cevallos, MT(ASCP), Brett L. Shapiro, MD, Catherine E. Oldenburg, MPH, Kieran S. O’Brien, MPH, and Kevin C. Hong, BA; Project Office, National Eye Institute, Rockville, Maryland: Donald Everett, MA; Photography Reading Center, Dartmouth Medical School, Lebanon, New Hampshire: Michael E. Zegans, MD, and Christine M. Kidd, PhD.
Author Contributions: Dr Lietman and Ms Ray had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Patel, Sah, Srinivasan, Oldenburg, McLeod, Zegans.
Acquisition, analysis, or interpretation of data: Prajna, Tiruvengada, Rajaraman, Patel, Das, Ray, Oldenburg, McLeod, Zegans, Acharya, Lietman, Rose-Nussbaumer.
Drafting of the manuscript: Patel, Sah, Zegans, Rose-Nussbaumer.
Critical revision of the manuscript for important intellectual content: Prajna, Tiruvengada, Rajaraman, Patel, Srinivasan, Das, Ray, Oldenburg, McLeod, Acharya, Lietman.
Statistical analysis: Ray, Oldenburg, Rose-Nussbaumer.
Obtained funding: Zegans, Lietman.
Administrative, technical, or material support: Prajna, Tiruvengada, Rajaraman, Patel, Das, Oldenburg, Acharya.
Supervision: Prajna, Patel, Sah, Das, Oldenburg, McLeod, Acharya, Lietman.
References
- 1.Whitcher JP, Srinivasan M. Corneal ulceration in the developing world—a silent epidemic. Br J Ophthalmol. 1997;81(8):622–623. doi: 10.1136/bjo.81.8.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79(3):214–221. [PMC free article] [PubMed] [Google Scholar]
- 3.Prajna NV, Krishnan T, Rajaraman R, et al. Mycotic Ulcer Treatment Trial II Group. Effect of oral voriconazole on fungal keratitis in the Mycotic Ulcer Treatment Trial II (MUTT II): a randomized clinical trial. JAMA Ophthalmol. 2016;134(12):1365–1372. doi: 10.1001/jamaophthalmol.2016.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan DT, Janardhanan P, Zhou H, et al. Penetrating keratoplasty in Asian eyes: the Singapore Corneal Transplant Study. Ophthalmology. 2008;115(6):975–982 e1. doi: 10.1016/j.ophtha.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 5.Bajracharya L, Gurung R. Outcome of therapeutic penetrating keratoplasty in a tertiary eye care center in Nepal. Clin Ophthalmol. 2015;9:2299–2304. doi: 10.2147/OPTH.S92176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prajna NV, Krishnan T, Rajaraman R, et al. Mycotic Ulcer Treatment Trial II Group. Effect of oral voriconazole on fungal keratitis in the Mycotic Ulcer Treatment Trial II (MUTT II) JAMA Ophthalmol. 2016;134(12):1365–1372. doi: 10.1001/jamaophthalmol.2016.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prajna NV, Krishnan T, Mascarenhas J, et al. Predictors of outcome in fungal keratitis. Eye (Lond) 2012;26(9):1226–1231. doi: 10.1038/eye.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose-Nussbaumer J, Prajna NV, Krishnan T, et al. Mycotic Ulcer Treatment Trial Group. Risk factors for low vision related functioning in the Mycotic Ulcer Treatment Trial: a randomised trial comparing natamycin with voriconazole. Br J Ophthalmol. 2016;100(7):929–932. doi: 10.1136/bjophthalmol-2015-306828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miedziak AI, Miller MR, Rapuano CJ, Laibson PR, Cohen EJ. Risk factors in microbial keratitis leading to penetrating keratoplasty. Ophthalmology. 1999;106(6):1166–1170. doi: 10.1016/S0161-6420(99)90250-6. [DOI] [PubMed] [Google Scholar]
- 10.Vemuganti GK, Garg P, Gopinathan U, et al. Evaluation of agent and host factors in progression of mycotic keratitis: a histologic and microbiologic study of 167 corneal buttons. Ophthalmology. 2002;109(8):1538–1546. doi: 10.1016/s0161-6420(02)01088-6. [DOI] [PubMed] [Google Scholar]
- 11.Ayalew M, Tilahun Y, Holsclaw D, et al. Penetrating keratoplasty at a tertiary referral center in Ethiopia: indications and outcomes. Cornea. 2017;36(6):665–668. doi: 10.1097/ICO.0000000000001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forster RK, Rebell G. Therapeutic surgery in failures of medical treatment for fungal keratitis. Br J Ophthalmol. 1975;59(7):366–371. doi: 10.1136/bjo.59.7.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koçluk Y, Sukgen EA. Results of therapeutic penetrating keratoplasty for bacterial and fungal keratitis [published online October 8, 2016] Int Ophthalmol. doi: 10.1007/s10792-016-0372-7. [DOI] [PubMed] [Google Scholar]
- 14.Sharma N, Jain M, Sehra SV, et al. Outcomes of therapeutic penetrating keratoplasty from a tertiary eye care centre in northern India. Cornea. 2014;33(2):114–118. doi: 10.1097/ICO.0000000000000025. [DOI] [PubMed] [Google Scholar]