Abstract
Poultry has been identified as a reservoir of foodborne enteric pathogens and antimicrobial resistant bacteria. The objective of this study was to describe and compare antimicrobial resistant isolates from an Ontario broiler chicken farm-level baseline project (2003 to 2004) to the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) Ontario abattoir and retail surveillance data from 2003, and to the most recent (2015) CIPARS Ontario chicken surveillance data in order to assess the impact of an industry-wide policy change in antimicrobial use. Ceftiofur resistance (TIO-R) prevalence in Salmonella decreased by 7% on farm between 2003 and 2004 and 2015. During the same timeframe, TIO-R E. coli prevalence decreased significantly by 16%, 11%, and 8% in farm, abattoir, and retail samples, respectively. Gentamicin resistant (GEN-R) E. coli, however, increased by 10% in farm and 15% in retail-derived isolates, and trimethoprim-sulfamethoxazole resistant (TMSm-R) E. coli increased significantly by 20%, 18%, and 5% in farm, abattoir, and retail isolates, respectively. Similarly, ciprofloxacin-resistant (CIP-R) Campylobacter spp. significantly increased in retail isolates by 11% and increased in farm (33%) and abattoir isolates (7%). The decrease in TIO-R Salmonella/E. coli in recent years is consistent with the timing of an industry-led intervention eliminating the preventive use of ceftiofur, a third generation cephalosporin and class of antimicrobials deemed critically important to human medicine. The rise in GEN-R and TMSm-R prevalence is indicative of recent shifts in antimicrobial use. Our study highlights the importance of integrated surveillance in detecting emerging trends and determining the efficacy of interventions to improve food safety.
Résumé
La volaille a été identifiée comme étant un réservoir d’agents pathogènes entériques d’origine alimentaire et de bactéries résistantes aux antimicrobiens. L’objectif de la présente étude était de décrire et comparer des isolats résistants aux antimicrobiens provenant d’une ferme ontarienne de poulets à griller obtenus dans le cadre d’un projet de base (2003 à 2004) aux données de surveillance de 2003 du Programme intégré canadien de surveillance de la résistance aux antimicrobiens (PICRA) d’abattoir et de ventes au détail en Ontario, et aux plus récentes données de surveillance (2015) du PICRA Ontario pour la volaille afin d’évaluer l’impact d’un changement à l’ensemble de l’industrie dans l’utilisation des antimicrobiens. La prévalence de la résistance au cefiofur (TIO-R) de Salmonella a diminué de 7 % sur la ferme entre 2003 à 2004 et 2015. Durant ce même intervalle de temps, la prévalence de TIO-R de E. coli diminua de manière significative de 16 %, 11 %, et 8 % dans les échantillons provenant de la ferme, de l’abattoir et de la vente au détail, respectivement. Toutefois, les E. coli résistant à la gentamicine (GEN-R) ont augmenté de 10 % et 15 % dans les échantillons pris à la ferme et de la vente au détail, respectivement. Les E. coli résistants au trimethoprime-sulfaméthoxazole (TMSm-R) ont augmenté de manière significative par 20 %, 18 %, et 5 % dans les isolats de la ferme, de l’abattoir et de la vente au détail, respectivement. Les isolats de Campylobacter spp. résistants au ciprofloxacin (CIP-R) augmentèrent de manière significative dans les échantillons de vente au détail (11 %), ceux de la ferme (33 %) ainsi que ceux de l’abattoir (7 %). La diminution de la TIO-R chez Salmonella/E. coli au cours des dernières années concorde avec une intervention menée par l’industrie d’éliminer l’utilisation en prévention du ceftiofur, une céphalosporine de troisième génération qui est une classe d’antimicrobiens considérée d’importance critique en médecine humaine. L’augmentation de la prévalence de GEN-R et de TMSm-R est indicative d’un changement récent dans l’utilisation des antimicrobiens. Notre étude fait ressortir l’importance d’un programme intégré de surveillance pour détecter les tendances émergentes et déterminer l’efficacité des interventions pour améliorer la salubrité alimentaire.
(Traduit par Docteur Serge Messier)
Introduction
Contamination of food with Salmonella and Campylobacter remains a significant food safety issue among Canadians (1,2), and resistant Salmonella and Campylobacter strains pose an additional food safety threat. The Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) monitors antimicrobial resistance (AMR) in the food chain in Canada. The flow of products from hatching egg production to retail in Canada is vertically coordinated but not integrated, unlike other countries. The CIPARS has current surveillance activities to detect AMR in the farm, abattoir, and retail stages of the broiler chicken production chain. Of particular concern is the detection of bacterial strains resistant to antimicrobials deemed critically important to human medicine by the World Health Organization (WHO), such as the third generation cephalosporins [e.g., ceftiofur (TIO), ceftriaxone (CRO)] and fluoroquinolones [e.g., ciprofloxacin (CIP)] (3). In 2003 in Quebec, there was a strong association between ceftiofur administered through in ovo or subcutaneous injection (TIOinj) use at the broiler chicken hatcheries and prevalence of ceftiofur resistant (TIO-R) E. coli from clinically normal slaughtered chickens at the abattoir (4). In Ontario, abattoir and retail surveillance during the same period also found changes in prevalence of TIO-R E. coli and Salmonella; however, there were no corresponding antimicrobial use (AMU) data available to explain these findings (5).
In the last decade, there has been increasing public awareness about microbiological food safety. This has resulted in on-farm food safety enhancements (6) and other farm-based initiatives. Vaccination against Salmonella is one such strategy that has been used in laying hens to reduce the duration, shedding, and potential for re-infection (7,8). Controlling Salmonella in the breeder sector also reduces the potential for downstream contamination throughout the poultry production chain. In Ontario, serovar-specific control practices are being utilized in breeder flocks. These strategies include termination of flocks found positive for Salmonella enterica enterica serovar Enteritidis and S. Typhimurium DT104, as well as vaccination against S. Enteritidis, S. Typhimurium, S. Heidelberg, and S. Kentucky (9).
Veterinarians in Ontario routinely diagnose bacterial diseases in the field and there is evidence that poultry pathogens have persisted (e.g., avian pathogenic E. coli, Clostridium perfringens) and emerged (e.g., Enterococcus cecorum) (10). Availability of antimicrobials in Canada for therapy of these diseases may become more limited due to AMR development or decreased access. Limited access may result from poultry industry-led or government initiatives designed to address the issue of AMR (11,12). In May 2014, the Canadian poultry industry, which is managed nationally by 4 producer-led marketing agencies, chose voluntarily to eliminate the preventive use of antimicrobials that are deemed highly important to human medicine by Health Canada’s Veterinary Drug Directorate (13) and critically important to human medicine by the WHO (e.g., TIO, CIP) (3,14) in the broiler chicken and turkey sectors. These antimicrobials are not labelled for use in broiler chickens in Canada but can be used when prescribed extra-label by a veterinarian. This initiative was extended to the broiler breeder sector in May 2015 (15).
Although CIPARS has tracked changes in the occurrence of antimicrobial resistance in Salmonella, E. coli, and Campylobacter in chickens at slaughter and chicken meat at retail since 2002 to 2003 (5,16), CIPARS did not have a broiler chicken farm component for comparison to abattoir and retail data until 2013. There was, however, a farm-based broiler chicken pathogen project conducted in Ontario in 2003 to 2004, which had many methodological similarities to the current CIPARS broiler-farm component. The objective of the present study was to describe the farm-level Salmonella serovar distribution and AMR among E. coli, Salmonella, and Campylobacter isolates from samples collected from Ontario broiler flocks as part of a farm-level pathogen baseline project conducted in 2003 to 2004 and compare these data to the CIPARS Ontario abattoir and retail surveillance data from 2003. In the context of industry-wide changes in antimicrobial use policy, a second objective was to compare the 2003 to 2004 farm-level baseline project data to the most recent (2015) CIPARS Ontario farm, abattoir, and retail surveillance data.
Materials and methods
Broiler chicken sampling
Ontario broiler farm study (OBFS) farm isolates, 2003 to 2004
In this paper, the 2003 to 2004 OBFS refers to an Ontario Ministry of Agriculture, Food and Rural Affairs-funded farm-level Salmonella and Campylobacter baseline research project conducted in Ontario broiler flocks. Flocks were selected from a list of broiler chicken producers that volunteered in the OBFS. The study is described in detail elsewhere (17). In brief, various barn environment (e.g., clean litter and surface swabs in bird contact areas) and bird-associated samples (fecal samples, cloacal swabs, and meconium swabs from chick pads) were collected from single flocks on 90 commercial broiler chicken farms in Ontario between May 2003 and May 2004. Equal numbers of farms were sampled from the 9 administrative districts of the Chicken Farmers of Ontario. One flock (defined as a group of birds hatched at the same time, delivered, and reared in the same barn or pen/floors) per farm (a registered establishment in Ontario and may have multiple barns in the premise) was sampled twice. The first sampling occurred on the day a new crop of chicks from the hatchery (chick placement) was delivered and then a second sampling was conducted 1 to 7 d before the flock was sent for slaughter (pre-harvest). Of the 90 flocks initially sampled, pooled fecal and environmental samples from 50 flocks were frozen in 2 mL aliquots and stored at −82°C for possible future analysis.
The CIPARS farm, abattoir, and retail samples
In 2015, the CIPARS farm program collected samples using a similar methodology to that described. Briefly, to ensure representativeness, the following farm inclusion and exclusion criteria were utilized: farms needed to be Safe, Safer, Safest [i.e., a comprehensive food safety and biosecurity program monitored by the Chicken Farmers of Canada (6)] compliant, representative of the hatcheries and feedmills supplying chicks and feeds in Ontario, and representative of the veterinary practice profile of the participating veterinarian. Samples were also collected at placement (environmental and chick pad swabs) and pre-harvest (fecal). In the CIPARS abattoir component, cecal samples were collected from clinically normal slaughtered birds in all 7 federally registered Ontario abattoirs processing commercial broiler chickens. In the CIPARS retail component, fresh chicken meat (legs and/or thighs bone-in with skin-on) were collected from grocery stores/butcher shops from population weighted, randomly selected census divisions in Ontario (18).
Bacterial isolation and antimicrobial susceptibility testing
For the recovery of Salmonella, fecal samples were enriched in buffered peptone water (BPW) (1:10 ratio) and incubated at 35°C for 24 h. A 0.1-mL loopful of BPW broth was inoculated into a modified semi-solid Rappaport Vassiliadis (MSRV) plate and incubated at 42°C for 24 to 72 h. For E. coli, a loopful of BPW broth was inoculated on MacConkey agar and incubated at 35°C for 24 h. Further isolation and characterization of each organism has been previously described (18). Archived Salmonella isolates from the OBFS had been stored at −82°C at the Office Internationale des Epizooties Reference Laboratory for Salmonellosis, Salmonella Typing Laboratory, National Microbiology Laboratory (NML) at Guelph [formerly the Laboratory for Foodborne Zoonoses (LFZ)], Public Health Agency of Canada, Guelph, Ontario, and were grown in Mueller-Hinton agar followed by phenotypic susceptibility testing using microbroth dilution methods. Generic E. coli isolates were cultured from aliquots of pooled fecal samples and fecal and chick paper pad samples using routine E. coli culture methodology (18) at the University of Guelph and transferred to NML at Guelph for antimicrobial susceptibility testing.
Campylobacter isolates from the OBFS were originally processed by the Agri-food Laboratory, University of Guelph. Briefly, environmental swab samples were enriched in Rosef’s broth (microaerophilically, temperature was adjusted in a stepwise manner at 30°C, then to 37°C, then at 43°C for a total of 48 to 72 h). The fecal samples were directly plated onto Campy-Charcoal media and were incubated in a microaerophilic environment at 37°C for 72 h, after further characterization, the resulting isolates were stored in Brucella broth with 15% glycerol at −82°C (17). In 2015, these broth cultures were transported on dry ice to the NML at St. Hyacinthe (formerly LFZ), PHAC, St. Hyacinthe, Quebec for antimicrobial susceptibility testing. All isolates were also typed by polymerase chain reaction (PCR) according to routine CIPARS protocols (18).
Minimum inhibitory concentrations (MIC) for the 3 bacterial organisms were determined using an automated broth microdilution and the Clinical and Laboratory Standard Institute (CLSI) M7-A8 standards and breakpoints when available (18). The E. coli and Salmonella isolates were susceptibility tested using routine CLSI protocols and the CMV2AGNF plate (Sensititre; Trek Diagnostic Systems, West Sussex, England) designed by the National Antimicrobial Resistance Monitoring System (NARMS) of the United States. All Campylobacter isolates were also susceptibility tested using routine CIPARS protocols and the NARMS CAMPY plates (Sensititre; Trek Diagnostic Systems) (18).
Antimicrobial use
Farm and hatchery level AMU and other farm-level operational and biosecurity information were collected for the CIPARS farm program using questionnaires administered by the participating veterinarian to the producer (18). The AMU information was not available for the OBFS.
Statistical data analysis
Analysis was done using computer software (Stata 13; StataCorp, College Station, Texas, USA). At the isolate level, for each organism, data were dichotomized into susceptible (including intermediate susceptibility) or resistant using CIPARS breakpoints. If no CLSI interpretative criteria were available for a specific antimicrobial/organism combination, breakpoints were based on the distribution of MIC and harmonized with those of the NARMS (18). Resistance prevalence estimates were then adjusted for clustering at the flock level using generalized estimating equations (GEE) with a binary outcome, logit-link function, and exchangeable correlation structure. Null binomial response models were run for each antimicrobial and from each null model, the intercept (β0) and 95% confidence intervals (CI) were used to calculate population-averaged prevalence estimates using the formula [1 + exp(−β0)]−1.
Temporal analysis
TIO, gentamicin (GEN), trimethoprim-sulfamethoxazole (TMSm), and CIP resistance data from the 2003 to 2004 OBFS, and CIPARS data from 2003 abattoir and 2003 retail components were compared to the 2015 CIPARS data from farm, abattoir, and retail using logistic regression models (asymptotic or exact models depending on prevalence of the outcome variable). Models were developed with year as a categorical independent variable and using P ≤ 0.05 for significance (i.e., marked by the use of the words “significant” or “significantly” throughout the text). Trends in the percentages of total Salmonella of 5 selected Salmonella serovars, resistance prevalence and AMU were also analyzed descriptively. Hereafter, significant changes in resistance prevalence are simply referred to as changes in resistance. The percent change in the proportion of multi-class resistance [i.e., resistance to 1 or more of the 7 antimicrobial classes included in the CMV2AGNF panel (18)] between 2003 and 2004 OBFS and the 2015 CIPARS pre-harvest farm isolates were also determined. Temporal changes in the use of antimicrobials administered via injection such as TIOinj, GEN (GENinj) and lincomycin-spectinomycin (LSinj), and trimethoprim-sulfadiazine administered via feed (TMSfeed) were determined using the same logistic regression models described above.
Limitations of this present analysis
Detailed risk factor analysis for Salmonella- and Campylobacter-positive flock or sample status (OBFS, CIPARS broiler farm program) and risk factor analysis for resistance to each of the antimicrobials tested (e.g., AMU-AMR associations) are beyond the scope of this paper but were described in detail elsewhere (17) and in future analysis.
Results
Salmonella (n = 692) from the 2003 to 2004 OBFS
A total of 150 chick placement Salmonella isolates from 38 flocks and 542 pre-harvest Salmonella isolates from 49 flocks had associated sampling information and barn identifiers, and, therefore, were susceptibility tested. At chick placement, S. Heidelberg was the most frequently isolated serovar from Salmonella recovered from meconium and cloacal swab specimens (44%, 38/87 isolates) and the barn environment Salmonella isolates (65%, 41/63). Similarly, at pre-harvest, S. Heidelberg was the most frequently isolated serovar from fecal samples or litter (40%, 170/421) and the barn environment (47%, 57/121). There were other non-typhoidal Salmonella serovars with public health significance (e.g., S. Enteritidis, S. Infantis, S. Newport, S. Thompson, and S. Typhimurium) detected but at relatively lower proportions (< 1% to 3%). The total numbers of unique serovars identified were similar in both farm sampling visits (chick placement, n = 16 serovars; pre-harvest, n = 17 serovars).
Table I summarizes the results of susceptibility testing of Salmonella isolates to individual antimicrobials. Between chick placement and pre-harvest visits, prevalence of resistance significantly (P ≤ 0.05) increased among beta-lactam antimicrobials: amoxicillin-clavulanic acid (AMC), TIO, CRO, and cefoxitin (FOX) but remained stable for ampicillin (AMP; Table I). Salmonella Heidelberg accounted for the majority of the TIO-R Salmonella isolates observed at chick placement (96%, 26/27 TIO-R Salmonella) and pre-harvest (76%, 62/82 TIO-R Salmonella). No S. Kentucky isolates recovered at chick placement were resistant to any of AMC, TIO, CRO, or FOX, but accounted for 11/82 TIO-R Salmonella isolates at pre-harvest. The remaining isolates that exhibited AMC, TIO, FOX, and CRO resistance at pre-harvest were S. Infantis (9/82). Relatively infrequent (≤ 5%) GEN-R Salmonella was noted in both sampling visits. There were no chick placement or pre-harvest isolates resistant to azithromycin (AZM), CIP, nalidixic acid (NAL), or TMSm. The prevalence of resistance to chloramphericol (CHL), sulfisoxazole (SSS), streptomycin (STR), and tetracycline (TET) was < 25% and in some cases declined (P ≤ 0.05 for STR and TET) between the chick placement and pre-harvest stage (Table I).
Table I.
Percentage of resistance to antimicrobials among Salmonella and Escherichia coli, from a 2003 to 2004 Ontario broiler farm study.
| Organism sampling stage | Salmonella spp. | Escherichia coli (generic) | ||
|---|---|---|---|---|
|
|
|
|||
| Chick placement (n = 150) % Positive (LCL-HCL) |
Pre-harvest (n = 542) % Positive (LCL-HCL) |
Chick placement (n = 118) % Positive (LCL-HCL) |
Pre-harvest (n = 153) % Positive (LCL-HCL) |
|
| Antimicrobial | ||||
| Amoxicillin-clavulanic acid (AMC) | 9 (4–21) | 16 (9–27)a↑ | 17 (10–29) | 24 (16–34)a↑ |
| Ceftiofur (TIO) | 9 (4–21) | 15 (8–27)a↑ | 13 (6–24) | 22 (14–31)a↑ |
| Ceftriaxone (CRO) | 9 (4–21) | 15 (8–27)a↑ | 15 (8–27) | 22 (14–32)a↑ |
| Ciprofloxacin (CIP) | ND | ND | ND | ND |
| Ampicillin (AMP) | 27 (17–41) | 27 (18–39) | 32 (22–45) | 38 (29–47) |
| Azithromycin (AZI) | ND | ND | ND | ND |
| Cefoxitin (FOX) | 9 (4–21) | 15 (8–27)a↑ | 15 (8–27) | 22 (14–31) |
| Gentamicin (GEN) | 5 (2–12) | 4 (1–9) | 13 (6–27) | 7 (4–13)a↓ |
| Nalidixic acid (NAL) | 0 (0–5) | 0 (0–1) | 4 (1–14) | 0 (0–4) |
| Streptomycin (STR) | 21 (12–34) | 17 (9–28)a↓ | 23 (16–32) | 33 (24–43)a↑ |
| Trimethoprim-sulfamethoxazole (TMSm) | ND | ND | 2 (0–11) | 3 (1–9) |
| Chloramphenicol (CHL) | 3 (1–10) | 3 (1–11) | 3 (1–9) | 3 (1–9) |
| Sulfisozaxole (SSS) | 11 (5–23) | 6 (3–13) | 23 (14–36) | 18 (11–26) |
| Tetracycline (TET) | 19 (10–33) | 13 (7–25)a↓ | 42 (30–54) | 53 (42–64)a↑ |
Significant (P ≤ 0.05) farm-level sampling stage difference; arrows indicate the direction of the change between chick placement and pre-harvest sampling.
The percent positive values were adjusted to account for multiple samples collected per farm.
LCL — lower confidence limits; HCL — upper confidence limits; ND — resistant to the antimicrobial was not detected in all of the isolates tested.
Escherichia coli (generic) (n = 271) from the 2003 to 2004 OBFS
Antimicrobial resistance among E. coli isolated from pooled cloacal/chick pad specimens from chick placement visits (n = 118; 39 flocks) and pooled fecal samples from pre-harvest visits (n = 153; 50 flocks) are summarized in Table I. Between chick placement and pre-harvest, resistance to the beta-lactam antimicrobials significantly increased (e.g., 13% to 22%, P ≤ 0.05; Table I). In contrast, GEN-R E. coli significantly decreased from chick placement (13%) to pre-harvest (7%, P ≤ 0.05; Table II). Consistent with the Salmonella results, there were no CIP-R or NAL-R isolates. Resistance to other antimicrobials was low (2% TMSm, 3% CHL), moderate (23% for STR, SSS), and high (43% for TET). When the prevalence of resistance among chick placement isolates was compared to pre-harvest isolates, the change in prevalence significantly increased (P ≤ 0.05) for STR and TET (Table I).
Table II.
Recovery of Escherichia coli, Salmonella spp., and Campylobacter spp. from broiler chickens in the Ontario broiler farm study and on-farm, abattoir and retail CIPARS program.
| Stage of sampling | Year | Escherichia coli | Salmonella | Campylobacter | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| % | Positive/total samples or farms | % | Positive/total samples or farms | % | Positive/total samples or farms | ||
| OBFS, chick placement | 2003/04 | NA | NA | 49% | 44/90 farmsa | 2% | 2/90 farmsa |
| OBFS, pre-harvest | 2003/04 | NA | NA | 69% | 64/90 farmsa | 26% | 24/90 farmsa |
| Farm, chick placement | 2013 | 85% | 64/75 | 17% | 13/75c | NA | NA |
| 2014 | 87% | 65/75 | 3% | 2/75c | NA | NA | |
| 2015 | 88% | 66/75 | 9% | 7/75c | NA | NA | |
| Farm, pre-harvest | 2013 | 100% | 120/120 | 54% | 65/120c | 17% | 20/120 |
| 2014 | 99% | 166/168 | 25% | 42/168c | 21% | 35/168 | |
| 2015 | 99% | 195/196 | 54% | 106/196c | 18% | 36/196 | |
| Abattoir | 2003 | 97% | 182/187 | 21% | 66/136 | NA | NA |
| 2004 | 99% | 178/179 | 22% | 60/271 | NA | NA | |
| 2005 | 100% | 216/217 | 20% | 57/282 | NA | NA | |
| 2006 | 98% | 58/59 | 31% | 91/296 | NA | NA | |
| 2007 | 98% | 61/62 | 28% | 90/317 | NA | NA | |
| 2008 | 98% | 63/64 | 35% | 110/314 | NA | NA | |
| 2009 | 100% | 66/66 | 31% | 99/320 | 14% | 7/50 | |
| 2010 | 98% | 50/51 | 29% | 72/246 | 16% | 40/246 | |
| 2011 | 100% | 52/52 | 24% | 50/211 | 14% | 29/211 | |
| 2012 | 100% | 51/51 | 27% | 55/205 | 20% | 41/206 | |
| 2013 | 100% | 57/57 | 25% | 59/240c | 18% | 43/235 | |
| 2014 | 100% | 58/58 | 15% | 33/226c | 27% | 61/226 | |
| 2015 | 98% | 53/54 | 15% | 35/236c | 18% | 42/237 | |
| Retail | 2003 | 95% | 137/144 | 16% | 27/167 | 47% | 78/166 |
| 2004 | 95% | 150/158 | 17% | 54/315 | 45% | 143/315 | |
| 2005 | 95% | 145/153 | 9% | 26/303 | 40% | 120/303 | |
| 2006 | 97% | 152/156 | 12% | 36/311 | 34% | 104/311 | |
| 2007b | 98% | 157/161 | 54% | 172/320 | 37% | 117/320 | |
| 2008 | 96% | 150/156 | 45% | 139/311 | 39% | 121/311 | |
| 2009 | 95% | 155/164 | 43% | 142/328 | 31% | 101/328 | |
| 2010 | 86% | 100/116 | 39% | 90/232 | 28% | 64/232 | |
| 2011 | 93% | 137/147 | 40% | 119/294 | 24% | 71/293 | |
| 2012 | 92% | 107/116 | 44% | 102/232 | 39% | 87/226 | |
| 2013 | 93% | 110/118 | 39% | 89/231c | 35% | 83/234 | |
| 2014 | 92% | 144/157 | 24% | 75/312c | 25% | 78/312 | |
| 2015 | 91% | 69/76 | 17% | 26/151c | 26% | 40/151 | |
In this study a flock was deemed positive if at least one of the environmental and bird samples collected tested positive.
Methodological enhancement to the recovery of Salmonella was initiated.
Samples were from progenies of Ontario broiler breeders vaccinated with Salmonella.
CIPARS — Canadian Integrated Program for Antimicrobial Resistance Surveillance; OBFS — Ontario broiler farm study (2003 to 2004).
NA — Not available.
Campylobacter (n = 302) from the 2003 to 2004 OBFS
There was only 1 Campylobacter isolate isolated at a chick placement visit from a water sample from the drinker line. This isolate was susceptible to all 9 antimicrobials tested. The pre-harvest fecal isolates that originated from 22 flocks consisted of C. jejuni (87%, 262/302) and E. coli (13%, 40/302). Infrequent (< 7%) resistance for each of telithromycin (TEL), AZM, clindamycin (CLI), and erythromycin (ERY) was noted. There were no isolates resistant to CIP, GEN, NAL, or florflenicol (FLR). The most frequent resistance was to TET at 54%.
Salmonella prevalence and serovar distribution comparing 2003 to 2004 OBFS and CIPARS farm, abattoir, and retail surveillance
Prevalence of Salmonella positive flocks rather than prevalence of Salmonella positive samples was reported in the OBFS (Table II) in which a flock was considered positive if at least one of the samples collected tested positive by bacterial isolation (17). In the CIPARS farm data, the percentage of positive samples in 2013 to 2015 ranged from 24% to 54% (Table II). At the abattoir, the percentage of Salmonella positive samples ranged from 15% to 35% (peaked in 2008) and at retail, it ranged from 17% to 54%. Overall, prevalence of positive samples in both abattoir and retail sampling appeared to decrease.
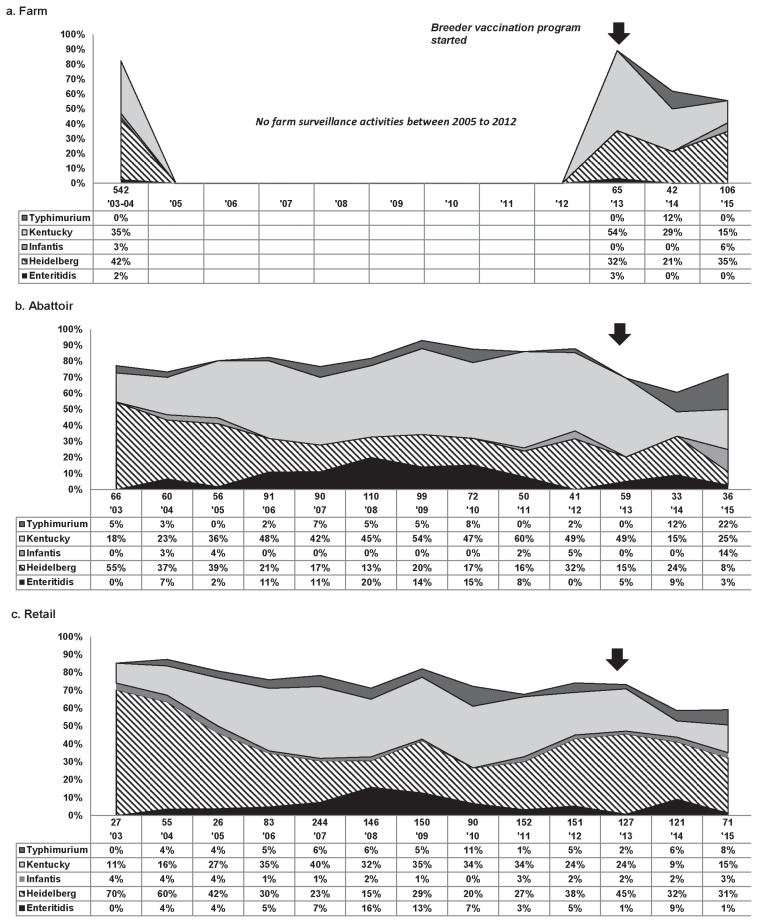
Farm — Figure 1a summarizes the temporal variations in prevalence of selected serovars among total Salmonella. Serovars that contributed to ≤ 2% of all isolates were not included in the figure. From 2003 to 2004, in the OBFS S. Heidelberg was the most frequently isolated serovar, followed by S. Kentucky. There were no farm level data between 2005 and 2012, but in 2013, when the CIPARS broiler farm program was initiated, S. Kentucky was the most prevalent serovar isolated followed by S. Heidelberg. This ranking was unchanged in 2014, but in 2015, the ranking shifted with S. Heidelberg moving back into the number 1 ranking serovar.
Figure 1.
Temporal variations in percentage of total Salmonella for 5 Salmonella serovars, farm to retail, from 2003 to 2015. Arrows signify the initiation of an enhanced food safety programs including Salmonella vaccination protocol in broiler breeder flocks; thus, most of the chicks placed in sentinel farms surveyed by CIPARS (2013–2015) were from vaccinated breeders. Methodological enhancement to the recovery of Salmonella was initiated in 2007 (18).
Abattoir. Similar to the 2003 to 2004 OBFS, in 2003 the top 2 serovars detected at abattoir for CIPARS samples were S. Heidelberg (55%) and S. Kentucky (18%) (Figure 1b). Salmonella Heidelberg remained more prevalent than S. Kentucky until 2006 when S. Kentucky took over the top spot. This 2006 ranking stayed consistent until 2014 when S. Heidelberg again became the most prevalent serovar. In 2015, S. Kentucky (25%) occupied the top spot while S. Heidelberg (8%) ranked 4th.
Retail. In 2003, S. Heidelberg (70%) and S. Kentucky (11%) were the top 2 serovars isolated from retail chicken, which is consistent with the 2003 to 2004 OBFS and abattoir surveillance (Figure 1c). Salmonella Heidelberg remained in the top position, followed by S. Kentucky, until 2006 when similar to abattoir, S. Kentucky prevalence surpassed S. Heidelberg. This order was maintained until 2012 when these 2 serovars again flipped and S. Heidelberg moved back to the top position. Salmonella Heidelberg has remained the most common serovar since 2012 (Figure 1c).
Escherichia coli and Campylobacter spp. prevalence.. Recovery rates were relatively stable across all CIPARS surveillance components (Table II).
Data integration: Farm, abattoir, and retail surveillance and AMU
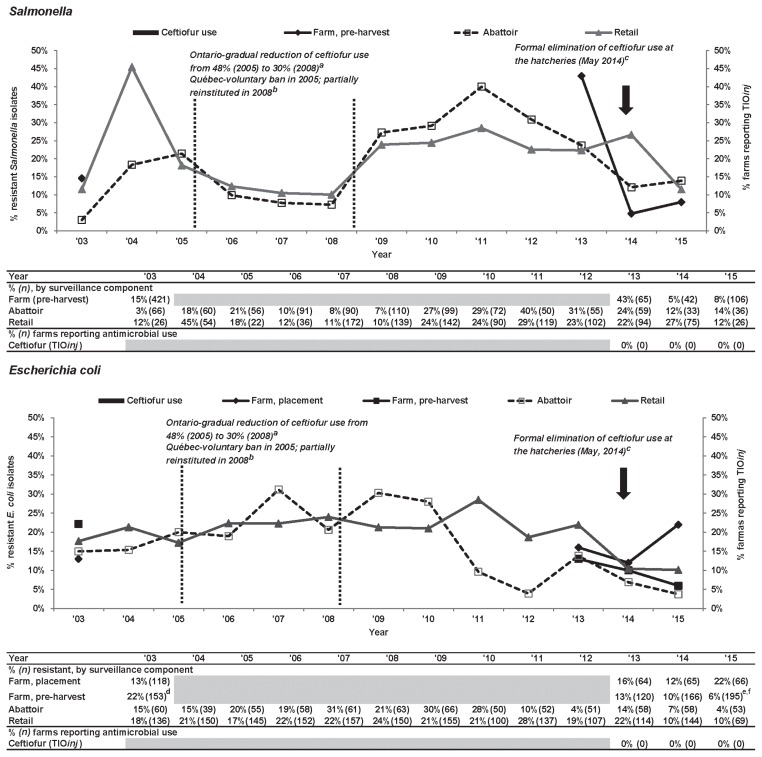
Ceftiofur. Figure 2 summarizes resistance to TIO among Salmonella and E. coli from Ontario broiler chicken samples collected in multiple CIPARS surveillance components and the OBFS. Ceftiofur resistance in isolates from farm, abattoir, and retail samples fluctuated over time. Trends in TIO-R Salmonella at abattoir and retail show an initial peak in 2004 to 2005, followed by decline to 2008, then increase to 2011, then gradual decline to 2015. Pre-harvest farm TIO-R Salmonella increased from 15% in 2003 to 2004 to 43% in 2013 (first CIPARS surveillance year on farm), then declined to 8% in 2015. Comparing point estimates from the start (2003 to 2004) and end (2015) of the observation period, TIO-R Salmonella was similar among retail isolates (12%), increased among abattoir isolates (2003: 3%, 2015: 14%) and modestly decreased among farm isolates (2003 to 2004: 15%, 2015: 8%). In 2015, the prevalence of TIO resistance was most common among S. Heidelberg from chickens at pre-harvest farm (89%, 8/9), abattoir (20%, 1/5), and retail (67%, 2/3). Other TIO resistant serovars included S. Typhimurium (1 farm isolate, 2 abattoir isolates), S. Infantis (1 retail isolate), S. Kentucky (1 abattoir isolate), and S. Schwarzengrund (1 abattoir isolate). Unlike in 2003 to 2004, none of the more recent S. Kentucky isolates from CIPARS farm or retail surveillance exhibited resistance to TIO.
Figure 2.
Temporal variations in ceftiofur resistance among Salmonella and Escherichia coli isolates from farm to retail, from 2003 to 2015.
a Dr. Rachel Ouckama and Dr. Cynthia Philippe, Salmonella isolations: Historical OHSFP trends. Presented at the Ontario Association of Poultry Practitioners’ workshop. May 9, 2009. Guelph, Ontario.
b Dutil et al (5).
c Bold arrows represent the industry voluntary elimination of the preventive use of ceftiofur that was formally announced in 2013 and fully implemented in 2014.
d Significant (P ≤ 0.05) farm-level sampling stage difference; level increased at pre-harvest.
e Significant (P ≤ 0.05) farm-level sampling stage difference; level decreased at pre-harvest.
f Significant temporal difference (P ≤ 0.05), level decreased between 2003 and 2015. Temporal analysis result is unavailable in the Ontario abattoir data due to small sample size.
Grey-shaded areas indicate no surveillance activities.
TIOinj — ceftiofur injection at the hatchery.
Among E. coli (Figure 2), TIO-R prevalence fluctuated over time in all surveillance components with a gradual increase in abattoir and retail isolates from 2010 to 2011 followed by a general decline to 2015. In pre-harvest farm isolates, there was a significant (P ≤ 0.05) drop in TIO-R E. coli from 22% in 2003 to 2004 to 6% in 2015; among placement farm isolates there was an increase from 13% in 2003 to 2004 to 22% in 2015.
Antimicrobial use information was unavailable prior to the initiation of the CIPARS farm surveillance program, but in 2013 to 2015, there were no broiler producers that reported TIOinj at the hatchery or any third-generation cephalosporins at the farm.
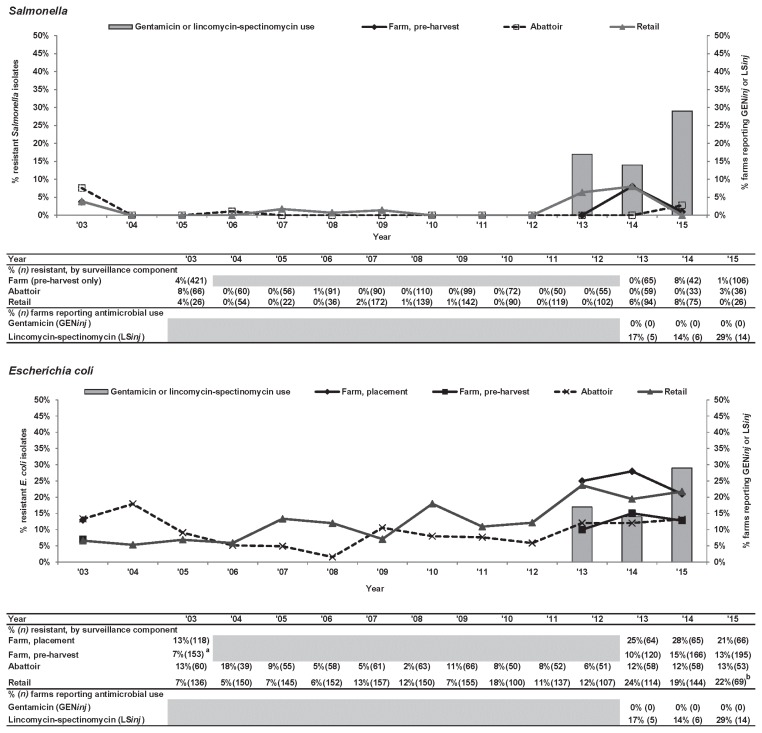
Gentamicin. GEN-R Salmonella was generally low (< 10%) in all CIPARS surveillance components and the OBFS (Figure 3). However, among E. coli, there was an increase; GEN-R E. coli isolates were detected from farm samples, with a modestly increased trend across all surveillance components between 2003 to 2004 and 2015. The only significant (P ≤ 0.05) increase in GEN-R E. coli was seen at retail with an increase from 7% to 22% between 2003 and 2015.
Figure 3.
Temporal variations in gentamicin resistance among Salmonella and Escherichia coli isolates from farm to retail, from 2003 to 2015.
a Significant (P ≤ 0.05) farm-level sampling stage difference; level decreased at pre-harvest.
b Significant temporal difference (P ≤ 0.05), level decreased between 2003 and 2015. Temporal analysis result is unavailable in the Ontario abattoir data due to small sample size.
Grey-shaded areas indicate no surveillance activities.
GENinj — gentamicin injection at the hatchery; LSinj — lincomycin-spectinomycin injection at the hatchery.
Through the CIPARS farm program, Ontario broiler producers occasionally (2.5%, 3/121 flocks surveyed in 2013 to 2015) reported the use of aminoglycoside antimicrobials (i.e., the same class as GEN) (e.g., apramycin, neomycin). The reported use of a related antimicrobial, spectinomycin, an aminocyclitol, combined with lincomycin, a lincosamide (LSinj), though not significant, increased from 17% to 29% of the broiler producers in Ontario participating in the CIPARS farm program (Figure 3).
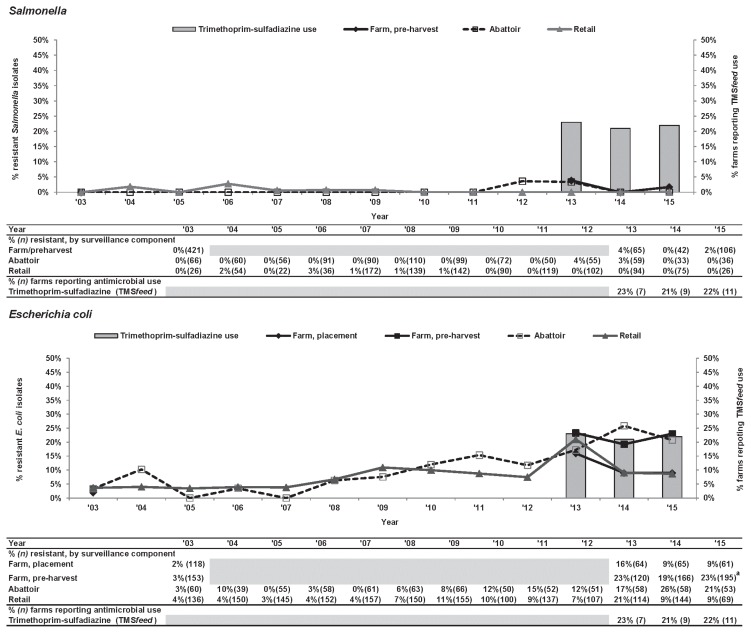
Trimethoprim-sulfamethoxazole.Similar to GEN, an overall increasing trend in TMSm-R Salmonella was noted across all surveillance components. There was a significant (P ≤ 0.05) increase in TMSm-R E. coli at pre-harvest farm samples from 3% to 23% (Figure 4). Between sampling visits (chick placement and pre-harvest), resistance prevalence for TMSm increased in contrast to GEN, where prevalence consistently decreased between sampling visits from 2013 to 2015 surveillance years and in 2003 to 2004 (P ≤ 0.05) as previously described (Table I).
Figure 4.
Temporal variations in trimethoprim-sulfamethoxazole resistance of Salmonella and Escherichia coli isolates from farm to retail, from 2003 to 2015.
a Significant temporal difference (P ≤ 0.05); level increased between 2003 and 2015. Temporal analysis is unavailable in the Ontario abattoir data due to small sample size.
Grey-shaded areas indicate no surveillance activities.
TMSfeed — trimethoprim-sulfadiazine use in feed.
Trimethoprim-sulfadiazine administered via feed (TMSfeed) was reported by 21% to 23% (no significant temporal change noted) of broiler producers in Ontario participating in the CIPARS Farm program between 2013 and 2015.
Campylobacter from the 2003 to 2004 OBFS compared to CIPARS Ontario farm, abattoir, and retail surveillance
In 2003, CIP-R Campylobacter was detected in 4% of the retail isolates but no resistant isolates were detected in pre-harvest farm isolates obtained from the 2003 to 2004 OBFS (Figure 5). At the abattoir (Campylobacter testing started in 2010), CIP-R Campylobacter was first detected in 2011. Figure 5 shows a trend toward increasing CIP-R prevalence; however, a significant difference was noted only for retail (4% to 15%, P ≤ 0.05).
Figure 5.
Temporal variations in ciprofloxacin resistance among Campylobacter, farm to retail, from 2003 to 2015.
a Significant temporal difference (P ≤ 0.05), level increased between 2003 and 2015. Temporal analysis is unavailable in the Ontario abattoir data due to small sample size.
Grey-shaded areas indicate no surveillance activities.
ENRwater — enrofloxacin use via the drinking water; use of this drug is deemed extra-label use in poultry in Canada.
No producers reported the use of fluoroquinolones [enrofloxacin (ENR) and danofloxacin, veterinary fluoroquinolones available for use in food animals in Canada, but not labelled for use in broiler chickens] in the CIPARS Ontario flocks surveyed between 2013 and 2015.
Multiclass resistance from the 2003 to 2004 OBFS compared to CIPARS Ontario farm, preharvest isolates
As summarized in Table III, resistance in pre-harvest farm Salmonella isolates shifted between 2003 and 2004 and 2015. There was a 6% increase in the proportion of susceptible isolates and 10% increase (P ≤ 0.05) in isolates resistant to one class. These corresponded to a decrease in 2 to 3 classes (6% decrease), 4 to 5 classes (7% decrease), and 6 to 7 classes (3% decrease). Among E. coli, results were different from those observed for Salmonella. Between 2003 and 2004 and 2015, the percentage of isolates increased in 2 multiclass resistance categories (2 to 3 classes: 9% increase and 4 to 5 classes: 4% increase), and decreased in 2 other resistance categories: (susceptible to all: 7% decrease and 1 to 2 classes: 5% decrease). Campylobacter isolates also showed a shift in the proportion of susceptible isolates. Of importance to note is the emergence of isolates exhibiting the CIP-NAL-TET multidrug resistance pattern (19% of total isolates). These isolates contributed to the overall increase in the proportion of isolates that were resistant to 2 to 3 classes of antimicrobials (27% increase between 2003 to 2004 and 2015, P ≤ 0.05). In the same timeframe, there was also a decrease in the percentage of isolates that were susceptible to all antimicrobials tested (17% decrease) and the percentage of isolates resistant to the 1 to 2 classes (10% decrease).
Table III.
Changes in multi-class antimicrobial resistance prevalence among Salmonella, Escherichia coli, and Campylobacter isolated from chicken farms at pre-harvest.
| Number of antimicrobial classes in the resistance pattern | Salmonella | Escherichia coli | Campylobacter | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| OBFS n = 542 |
CIPARS n = 106 |
% change | OBFS n = 153 |
CIPARS n = 95 |
% change | OBFS n = 302 |
CIPARS n = 36 |
% change | |
| 0 | 66% | 73% | 6% | 33% | 25% | −7% | 45% | 28% | −17% |
| 1 | 0% | 11% | 10%a | 23% | 18% | −5% | 49% | 40% | −10% |
| 2–3 | 22% | 16% | −6% | 36% | 45% | 9% | 6% | 33% | 27%a |
| 4–5 | 7% | 0% | −7% | 8% | 12% | 4% | 0% | 0% | 0% |
| 6–7 | 3% | 0% | −3% | 0% | 0% | 0% | 0% | 0% | 0% |
Significant temporal change for this multi-class resistance category (P ≤ 0.05) between the OBFS and CIPARS.
OBFS — Ontario broiler farm study (2003 to 2004); CIPARS — Canadian Integrated Program for Antimicrobial Resistance Surveillance, broiler chicken farm program (2015 surveillance year).
Percent change is the difference in the proportion of isolates in the resistance pattern between 2003 and 2004 and 2015. The results above were adjusted to account for multiple samples per flock.
Discussion
Our study assessed AMR prevalence among Salmonella, E. coli, and Campylobacter isolates from Ontario broiler chicken farms from 2003 to 2004; because of study design similarities to subsequent CIPARS farm-level surveillance, these results were considered as baseline farm-level AMR data and complemented early CIPARS abattoir and retail results. This paper also serves as a valuable reference point, in light of upcoming industry and federal legislations concerning AMU to reduce AMR threats in both animals and humans in Canada (11,12,19). Overall, sampling frame and design varied between farm, abattoir, and retail surveillance. However, the sampling methodology was designed to generate data representative of Ontario broiler chicken farms [farms were selected based on certain inclusion and exclusion criteria, and distributed geographically throughout Ontario (17,18)], slaughtered chickens (chickens were sampled proportional to slaughter volume from all federally registered abattoirs in Ontario) and retail meats (chicken meat sampled from randomly selected geographic areas within Ontario selected based on population). Further, the microbiology methodology was consistent allowing the integration of these surveillance data, which is important in facilitating the detection of emerging food safety threats and assessing the efficacy of interventions.
The 2003 to 2004 OBFS identified several Salmonella serovars implicated in human infections in Canada including S. Enteritidis, S. Typhimurium, S. Heidelberg, S. Newport, S. Infantis, and S. Hadar in Ontario broiler chicken flocks. There was a gap (2004 to 2012) in farm-based surveillance for broiler chickens, but another enteric surveillance program, FoodNet Canada (formerly C-EnterNet), also detected these serovars in broiler chicken farms located within an Ontario sentinel site (Region of Waterloo, Ontario) between 2006 and 2011 (2). Our data combined with FoodNet Canada indicates that the relative proportion of these serovars shifted over time and in recent surveillance years, the prevalence of certain serovars, particularly those that exhibit resistance to antimicrobials (e.g., TIO-R S. Heidelberg) or implicated in food-borne outbreaks (e.g., S. Enteritidis) in Ontario has decreased in the broiler chicken population (20). The shift in serovar distribution may be a result of ongoing enhancements to farm food safety programs, e.g., vaccinations, monitoring (9). It is important to note that susceptible and antimicrobial-resistant Salmonella were detected from chick placement samples in both the OBFS and CIPARS farm programs. Although the re-use of litter is not practiced in Canadian broiler flocks, potential carry-over from previous flock or vertical-transmission may explain the recovery of Salmonella at chick placement. At the very minimum, dry-cleaning prior to chick placement is recommended and a full washing and disinfection are required once a year as per the Safe, Safer, Safest program (6),
During the 2003 surveillance year, TIO-R Salmonella was prevalent (3% to 15%) across all surveillance components. Hatchery AMU data are not available prior to 2003 because there were no farm-level information available elsewhere (e.g., flock sheets) and CIPARS farm surveillance was not yet operational, so it is unknown exactly when the hatcheries began using TIOinj in Ontario. Passive surveillance of clinical Salmonella isolates submitted to NML at Guelph between 1994 and 1999 detected TIO-R Salmonella in turkeys and cattle but not in chickens (21). In 2002, a study of retail meats originating from Ontario detected FOX and CRO resistant E. coli carrying beta-lactamase conferring gene, blaCMY2 (22). Although no AMU information are available for that time for Ontario, a Quebec study conducted during the same timeframe (2003 to 2004), reported in ovo TIOinj in 76% of the broiler flocks sampled (4). Quebec hatcheries implemented a voluntary withdrawal of TIOinj in 2005 to 2006 that resulted in a rapid drop in TIO-R S. Heidelberg from retail chicken (62% to 7%; P < 0.001) (5). In 2007 the Quebec poultry industry partially reinstituted the use of TIOinj, which may explain the re-emergence of TIO-R S. Heidelberg in that province (5). Ontario retail chicken data showed a similar TIO-R Salmonella trend, although there was no hatchery or farm-level data to characterize TIOinj use reductions. The CIPARS broiler farm surveillance data for Ontario between 2013 and 2015 indicates no reported TIOinj at the hatchery level. As previously described, the poultry industry formally eliminated the preventive use of antimicrobials considered of very high importance to human medicine (including third generation cephalosporins) in broiler chickens and turkeys in May 2014. Anecdotal information from the poultry industry suggests that some hatcheries started reducing or eliminating the TIOinj as early as 2010, and although there is no AMU data to corroborate this, the fact that none of the CIPARS flocks in Ontario reported any TIOinj use at the hatchery in 2013 is consistent with a possible earlier AMU practices change. Historically (2003 to 2013 CIPARS data), a high proportion of S. Heidelberg and S. Kentucky chicken isolates exhibited resistance Percent change is the difference in the proportion of isolates in the resistance pattern between 2003 and 2004 and 2015. The results above were adjusted to account for multiple samples per flock. to TIO (20). The decreasing proportion of S. Heidelberg/TIO-R S. Heidelberg isolates and increasing proportion of S. Kentucky/TIO-susceptible S. Kentucky isolates may also explain the shift towards susceptibility to TIO among Salmonella spp. overall. The more recent S. Heidelberg prevalence and their AMR profile are similar to historic data (23,24). Overall, decreasing prevalence of TIO-R isolates is indicative of the impact of AMU and food safety interventions against Salmonella in Ontario broiler chickens but emerging GEN-R and TMS-R isolates will need to be monitored.
Surveillance of E. coli, monitored as an indicator organism, showed similar results to Salmonella for resistance to TIO. As for Salmonella, [FOX resistant and carried the blaCMY2 plasmid (25)] the TIO-R E. coli isolates from this present study exhibited an AMC-TIOFOX-CRO resistance pattern, consistent with isolates harboring the blaCMY2 gene, a plasmid-mediated AmpC beta-lactamase conferring gene in E. coli (26) found in Canadian (25) and American chickens (27). The use of TIOinj in the early stages of the bird’s life has been associated with the emergence of TIO-R E. coli that can persist throughout the life of the broiler flock or throughout the production period for laying hens (28,29). As previously described, the high proportion of chicks/hatching eggs medicated with TIOinj in Quebec hatcheries in 2003 to 2004 was significantly associated with the detection of TIO-R E. coli in broiler chickens (4). The voluntary withdrawal resulted in a rapid drop in TIO-R E. coli retail isolates (34% to 6%; P < 0.0001), in addition to a drop in TIO-R S. Heidelberg as previously described (5). Ceftiofur resistance among E. coli showed a decreasing trend and reached ≤ 2003 levels in 2015. The CIPARS farm program continues to detect TIO-R E. coli from chick pads/meconium and in pre-harvest broilers although there is no reported TIOinj use at the hatcheries in Ontario. In Japan, there was a gradual decrease in cefazolin and cefotaxime/TIO resistance 2 y after the withdrawal of TIOinj use in hatcheries (30). The less frequent resistance observed in the Canadian isolates may be suggestive of a transitional period in gut bacteria and the environment (31), such as a shift from highly resistant to susceptible gut flora. Other potential contributing factors may include contamination from the upper levels of the production pyramid, maintenance of resistant strains in the environment (e.g., litter was disposed but no cleaning and disinfection practiced between flock cycles), the use of related antimicrobials (e.g., aminopenicillins), and international/interprovincial movement of poultry products. The finding of beta-lactam resistant E. coli in CIPARS chick placement samples is suggestive of vertical transmission of beta-lactam resistant strains or genes/plasmid transfer from upper levels of production as described in the literature (32–34). As evidenced by the presence of resistant isolates found on barn environmental surface swabs in CIPARS farm surveillance and in other similar studies (35,36), beta-lactam resistant E. coli are being maintained in the environment and, therefore, can act as a reservoir for resistant genes/plasmids. These findings highlight the importance of proper cleaning, disinfection, and down-time or rest periods between flocks in order to reduce potential carry-over to newly placed chicks.
Another notable observation was an increase in GEN-R E. coli. This trend in GEN resistance appears consistent with the reported use of GENinj, or a related antimicrobial, spectinomycin. In Quebec, the trend in GEN-R prevalence among clinical E. coli isolates corresponded with LSinj use in broiler production. Molecular characterization of aminoglycoside and aminocyclitol genes also indicated that the use of this drug can co-select for GEN resistance (37). Trimethoprim-sulfamethoxazole resistance prevalence was also identified as an emerging trend. Similar to the trend observed for GEN resistance, TMSm-R increase corresponded with the reported use of TMSfeed. Although this antimicrobial drug combination is not labelled for use in broilers, in the CIPARS farm surveillance program, it was reportedly used by broiler producers for the treatment of respiratory and septicemic diseases in neonatal and growing broilers. The emergence of TMSm-R in Ontario broiler chicken E. coli isolates is potentially due to combination of co-selection by sulfonamides (i.e., exposure to sulfadiazine present in TMSfeed or sulfaquinoxaline and sulfamethoxazole via water) and trimethoprim (exposure to trimethoprim present in TMSfeed) (38). Because of these emerging AMR trends, the level of on-farm use requires ongoing monitoring. The association between use at the hatchery and farm and other hatchery/farm level risk factors collected through the CIPARS farm questionnaire needs to be evaluated.
Depending on the antimicrobial and the timeframe, there was a shift in the prevalence of resistance among E. coli detected between chick placement and pre-harvest. Factors that could influence this shift include antimicrobial use/selection pressure in the industry overall and specifically during the broiler grow-out period, the bird’s intestinal microbiome (39,40), and the microbiome’s interaction with multiple factors such as host immunity and diet (39,40). The use of multiple antimicrobials concurrently or successively throughout the grow-out period has been shown to result in the development of resistant isolates with complex multiclass resistance patterns that could subsequently displace susceptible microflora (41,42). This may explain the shift towards resistance to multiple classes of antimicrobials among E. coli. Thus, careful consideration of treatment options and frequency of use are important in reducing overall AMR in targeted bacteria in broiler chickens. Further molecular investigation is also warranted to better understand the dissemination and transfer of resistance, co-selection and cross-selection properties as they pertain to transferrable mobile genetic elements, such as plasmids.
Campylobacter, one of the major causes of foodborne infections in humans, which is a reportable disease in Ontario (43), has been regularly detected from Ontario chicken and chicken meats. Ciprofloxacin resistant Campylobacter was first observed by CIPARS in the western provinces in 2007 and peaked in 2009 (16), but this study found that resistant strains are now relatively common in Ontario broiler chickens. With no reported use of fluoroquinolones at the farm level in Canada, it is unclear how CIP-R Campylobacter emerged in Ontario broiler chicken flocks. The use of ENR was not captured by the CIPARS farm program because of the surveillance timeframe or that usage was relatively low (i.e., therapeutic/metaphylactic use for infections unresponsive to currently available drugs). Potential sources such as cross-contamination from other production animals or wildlife colonized by CIP-R C. jejuni strains, and other potential farm-level sources, as identified in a recent systematic review (44) should be considered in future research. Fluoroquinolone-resistant Campylobacter in broiler flocks has been shown to persist after the cessation of fluoroquinolone use in the poultry industry and may require additional interventions to reduce the prevalence in the poultry production chain (45,46).
In summary, resistance profiles and Salmonella serovar distribution in broiler chickens in Ontario have shifted over time; towards increased prevalence of resistance to GEN, TMS, CIP, and decreased prevalence of resistance to TIO. The continued decreasing resistance trend to beta-lactams among Salmonella and E. coli isolates suggests that reduction in resistance can, in some cases, occur relatively quickly after the introduction of an AMU reduction policy. Additionally, the changes in the proportional prevalence of Salmonella serovars with public health significance (e.g., decrease in S. Heidelberg, S. Enteritidis, S. Typhimurium) is consistent with the idea that pathogen control programs, such as vaccination in upper levels of the production pyramid and food safety enhancement throughout the food chain, are working. However, the increase in resistance to GEN and TMSm in E. coli and Salmonella and CIP resistance in Campylobacter may signal emerging trends, and warrants ongoing monitoring and consideration with respect to prudent use practices. The reason for the increasing trend in CIP-R Campylobacter is unknown. This study highlights the importance of integrated surveillance in detecting emerging threats and determining the efficacy of interventions. The data also serve as a valuable reference point in light of upcoming changes in policy for AMU in animals in Canada.
Acknowledgments
The authors thank the Chicken Farmers of Ontario and the producers who supported the research/surveillance program through their participation (data and sampling collection). We also acknowledge funding support from the Ontario Ministry of Agriculture, Food and Rural Affairs and the Public Health Agency of Canada (PHAC). We are grateful to Dr. Danielle Daignault and Andrea Desruisseau and the other CIPARS microbiology technicians, NML at St. Hyacinthe, and NML at Guelph, PHAC, for conducting the susceptibility testing of Campylobacter spp. and E. coli and Salmonella isolates, respectively. We acknowledge the Agri-food Laboratory, University of Guelph (Dr. Carlos Leon-Velarde), for processing the stored samples and coordinating the transfer of isolates, Sarah Martz and Lindsey Lebert (University of Guelph) for E. coli culture, and Dr. Rebecca Irwin for her guidance. Finally, we thank the Ontario poultry industry ad hoc AMU/AMR review committee and the industry broiler health working group for the technical discussions and valuable input to the manuscript.
References
- 1.Thomas MK, Murray R, Flockhart L, et al. Estimates of foodborne illness-related hospitalizations and deaths in Canada for 30 specified pathogens and unspecified agents. Foodborne Pathog Dis. 2015;12:820–827. doi: 10.1089/fpd.2015.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flockhart L, Pintar K, Cook A, et al. Distribution of Salmonella in humans, production animal operations and a watershed in a FoodNet canada sentinel site. Zoonoses Public Health. 2016:1–12. doi: 10.1111/zph.12281. [DOI] [PubMed] [Google Scholar]
- 3.Collignon PC, Conly JM, Andremont A, McEwen SA, Aidara-Kane A World Health Organization Advisory Group, Bogota Meeting on Integrated Surveillance of Antimicrobial Resistance (WHO-AGISAR) World Health Organization ranking of antimicrobials according to their importance in human medicine: A critical step for developing risk management strategies to control antimicrobial resistance from food animal production. Clin Infect Dis. 2016;63:1087–1093. doi: 10.1093/cid/ciw475. [DOI] [PubMed] [Google Scholar]
- 4.Boulianne M, Arsenault J, Daignault D, Archambault M, Letellier A, Dutil L. Drug use and antimicrobial resistance among Escherichia coli and Enterococcus spp. isolates from chicken and turkey flocks slaughtered in Quebec, Canada. Can J Vet Res. 2015;80:49–59. [PMC free article] [PubMed] [Google Scholar]
- 5.Dutil L, Irwin R, Finley R, et al. Ceftiofur resistance in Salmonella enterica serovar Heidelberg from chicken meat and humans, Canada. Emerg Infect Dis. 2010;16:48–54. doi: 10.3201/eid1601.090729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chicken Farmers of Canada. Chicken Farmers of Canada’s on-farm food safety assurance program manual, 2014 Safe, Safer, Safest. CFC. 2014:1–96. [Google Scholar]
- 7.Gast RK. Serotype-specific and serotype-independent strategies for preharvest control of food-borne Salmonella in poultry. Avian Dis. 2007;51:817–828. doi: 10.1637/8090-081807.1. [DOI] [PubMed] [Google Scholar]
- 8.Gast RK. Paratyphoid infections. In: Swayne DE, Glisson JR, McDougald LR, Nolan LK, Suarez DL, Nair V, editors. Diseases of Poultry. 13th ed. Ames, Iowa: John Wiley and Sons; 2013. pp. 693–706. [Google Scholar]
- 9.OBHECC. Ontario Broiler Hatching Eggs and Chicks Commission Regulation E-12, 2008. Framework for certification, testing, treatment and disposal of hatching eggs, chicks and breeders for Salmonella Enteritidis and Salmonella Typhimurium DT104. 2008:29–35. [Google Scholar]
- 10.Boerlin P, Nicholson V, Brash M, et al. Diversity of Enterococcus cecorum from chickens. Vet Microbiol. 2012;157:405–411. doi: 10.1016/j.vetmic.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Chicken Farmers of Canada. Responsible antimicrobial use in the Canadian chicken and turkey sector V2 [updated 2016] [Last accessed May 2, 2018]. Available from: http://www.chickenfarmers.ca/wp-content/uploads/2015/12/AMU-Booklet-June-2015-EN.pdf.
- 12.Government of Canada. Federal Action Plan on Antimicrobial Resistance and Use in Canada: Building on the Federal Framework for Action [updated 2015] [Last accessed May 2, 2018]. Available from: http://healthycanadians.gc.ca/publications/drugs-products-medicaments-produits/antibiotic-resistance-antibiotique/action-plandaction-eng.php.
- 13.Health Canada. Veterinary Drugs Directorate’s Categorization of Antimicrobial Drugs Based on Importance in Human Medicine version-april 2009 [updated 2009 September 23] [Last accessed May 2, 2018]. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/antimicrobial-resistance/categorization-antimicrobial-drugs-based-importancehuman-medicine.html.
- 14.World Health Organization — WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR) Critically important antimicrobials for human medicine 5th revision [updated 2017] [Last accessed May 2, 2018]. Available from: http://www.who.int/foodsafety/publications/antimicrobials-fifth/en/
- 15.Chicken Farmers of Canada. The Antimicrobial Use Reduction Strategy. Sep 22, 2017. [Last accessed May 2, 2018]. Available from: https://www.chickenfarmers.ca/2017/09/the-antimicrobial-use-reductionstrategy-2/
- 16.Agunos A, Leger D, Avery B, et al. Ciprofloxacin-resistant Campylobacter spp. in retail chicken in western Canada. Emerg Infect Dis. 2013;19:1121–1124. doi: 10.3201/eid1907.111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arsenault R. MSc thesis. Guelph, Ontario: University of Guelph; 2005. Campylobacter and Salmonella positive commercial broiler chicken farms in Ontario and associated risk factors; pp. 1–272. [Google Scholar]
- 18.Public Health Agency of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance 2014 Report, Chapter 1. Design and methods [updated 2015] [Last accessed May 2, 2018]. Available from: https://www.canada.ca/en/public-health/services/surveillance/canadian-integrated-program-antimicrobial-resistance-surveillancecipars/cipars-2013-annual-report.html.
- 19.Government of Canada. Food and Drugs Act. Regulations amending the food and drug regulations (Veterinary drugs-antimicrobial resistance) [Last accessed May 2, 2018];2017 151(10) Available from: http://www.gazette.gc.ca/rp-pr/p2/2017/2017-05-17/html/sor-dors76-eng.php. [Google Scholar]
- 20.Public Health Agency of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance 2014 Report, Chapter 2. Antimicrobial Resistance. [Last accessed May 2, 2018]. [updated 2015] Available from: https://www.canada.ca/en/public-health/services/surveillance/canadian-integrated-program-antimicrobial-resistance-surveillancecipars/cipars-2013-annual-report.html.
- 21.Allen KJ, Poppe C. Occurrence and characterization of resistance to extended-spectrum cephalosporins mediated by beta-lactamase CMY-2 in Salmonella isolated from food-producing animals in Canada. Can J Vet Res. 2002;66:137–144. [PMC free article] [PubMed] [Google Scholar]
- 22.Forward KR, Matheson KM, Hiltz M, Musgrave H, Poppe C. Recovery of cephalosporin-resistant Escherichia coli and Salmonella from pork, beef and chicken marketed in Nova Scotia. Can J Infect Dis Med Microbiol. 2004;15:226–230. doi: 10.1155/2004/695305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwin RJ, McEwen SA, Clarke RC, Meek AH. The prevalence of verocytotoxin-producing Escherichia coli and antimicrobial resistance patterns of nonverocytotoxin-producing Escherichia coli and Salmonella in Ontario broiler chickens. Can J Vet Res. 1989;53:411–418. [PMC free article] [PubMed] [Google Scholar]
- 24.Poppe C, Irwin RJ, Messier S, Finley GG, Oggel J. The prevalence of Salmonella Enteritidis and other Salmonella spp. among canadian registered commercial chicken broiler flocks. Epidemiol Infect. 1991;107:201–211. doi: 10.1017/s0950268800048822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin LC, Weir EK, Poppe C, Reid-Smith RJ, Boerlin P. Characterization of blaCMY-2 plasmids in Salmonella and Escherichia coli isolates from food animals in Canada. Appl Environ Microbiol. 2012;78:1285–1287. doi: 10.1128/AEM.06498-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22:161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.United States Food and Drugs Administration. [Last accessed May 2, 2018]. [updated 2017] Available from: https://www.fda.gov/downloads/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/UCM528861.pdf.
- 28.Baron S, Jouy E, Larvor E, Eono F, Bougeard S, Kempf I. Impact of third-generation-cephalosporin administration in hatcheries on fecal Escherichia coli antimicrobial resistance in broilers and layers. Antimicrob Agents Chemother. 2014;58:5428–5434. doi: 10.1128/AAC.03106-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baron S, Jouy E, Touzain F, et al. Impact of the administration of a third-generation cephalosporin (3GC) to one-day-old chicks on the persistence of 3GC-resistant Escherichia coli in intestinal flora: An in vivo experiment. Vet Microbiol. 2016;185:29–33. doi: 10.1016/j.vetmic.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Hiki M, Kawanishi M, Abo H, et al. Decreased resistance to broad-spectrum cephalosporin in Escherichia coli from healthy broilers at farms in Japan after voluntary withdrawal of ceftiofur. Foodborne Pathog Dis. 2015;12:639–643. doi: 10.1089/fpd.2015.1960. [DOI] [PubMed] [Google Scholar]
- 31.Ranjitkar S, Lawley B, Tannock G, Engberg RM. Bacterial succession in the broiler gastrointestinal tract. Appl Environ Microbiol. 2016;82:2399–2410. doi: 10.1128/AEM.02549-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bortolaia V, Bisgaard M, Bojesen AM. Distribution and possible transmission of ampicillin- and nalidixic acid-resistant Escherichia coli within the broiler industry. Vet Microbiol. 2010;142:379–386. doi: 10.1016/j.vetmic.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 33.Agerso Y, Jensen JD, Hasman H, Pedersen K. Spread of extended spectrum cephalosporinase-producing Escherichia coli clones and plasmids from parent animals to broilers and to broiler meat in a production without use of cephalosporins. Foodborne Pathog Dis. 2014;11:740–746. doi: 10.1089/fpd.2014.1742. [DOI] [PubMed] [Google Scholar]
- 34.Zurfluh K, Wang J, Klumpp J, Nuesch-Inderbinen M, Fanning S, Stephan R. Vertical transmission of highly similar bla CTX-M-1-harboring IncI1 plasmids in Escherichia coli with different MLST types in the poultry production pyramid. Front Microbiol. 2014;5:519. doi: 10.3389/fmicb.2014.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laube H, Friese A, von Salviati C, Guerra B, Rosler U. Transmission of ESBL/AmpC-producing Escherichia coli from broiler chicken farms to surrounding areas. Vet Microbiol. 2014;172:519–527. doi: 10.1016/j.vetmic.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Laube H, Friese A, von Salviati C, et al. Longitudinal monitoring of extended-spectrum-beta-lactamase/AmpC-producing Escherichia coli at German broiler chicken fattening farms. Appl Environ Microbiol. 2013;79:4815–4820. doi: 10.1128/AEM.00856-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chalmers G, Cormiera AC, Nadeau M, Côté G, Reid-Smith RJ, Boerlin P. Determinants of virulence and of resistance to ceftiofur, gentamicin, and spectinomycin in clinical Escherichia coli from broiler chickens in Québec, Canada. Vet Microbiol. 2017;203:149–157. doi: 10.1016/j.vetmic.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Bean DC, Livermore DM, Hall MC. Plasmid imparting sulfonamide resistance in Escherichia coli: Implications of persistence. Antimicrob Agents Chemother. 2009;53:1088–1093. doi: 10.1128/AAC.00800-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brisbin JT, Gong J, Sharif S. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim Health Res Rev. 2008;9:101–110. doi: 10.1017/S146625230800145X. [DOI] [PubMed] [Google Scholar]
- 40.Pan D, Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.da Costa PM, Belo A, Gonçalves J, Bernardo F. Field trial evaluating changes in prevalence and patterns of antimicrobial resistance among Escherichia coli and Enterococcus spp. isolated from growing broilers medicated with enrofloxacin, apramycin and amoxicillin. Vet Microbiol. 2009;139:284–292. doi: 10.1016/j.vetmic.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 42.da Costa PM, Bica A, Vaz-Pires P, Bernardo F. Effects of antimicrobial treatment on selection of resistant Escherichia coli in broiler fecal flora. Microb Drug Resist. 2008;14:299–306. doi: 10.1089/mdr.2008.0859. [DOI] [PubMed] [Google Scholar]
- 43.Public Health Ontario. Surveillance Reports. [Last accessed May 2, 2018]. [updated 2017] Available from: http://www.publichealthontario.ca/en/eRepository/Reportable_Disease_Trends_in_Ontario_2014.pdf.
- 44.Agunos A, Waddell L, Leger D, Taboada E. A systematic review characterizing on-farm sources of Campylobacter spp. for broiler chickens. PLoS One. 2014;9:e104905. doi: 10.1371/journal.pone.0104905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price LB, Johnson E, Vailes R, Silbergeld E. Fluoroquinolone-resistant Campylobacter isolates from conventional and antibiotic-free chicken products. Environ Health Perspect. 2005;113:557–560. doi: 10.1289/ehp.7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price LB, Lackey LG, Vailes R, Silbergeld E. The persistence of fluoroquinolone-resistant Campylobacter in poultry production. Environ Health Perspect. 2007;115:1035–1039. doi: 10.1289/ehp.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]