Abstract
Objective
GO-COLITIS aimed to measure the effectiveness of subcutaneous golimumab in tumour necrosis factor-α antagonist–naive patients with moderate to severe ulcerative colitis (UC) despite conventional treatment.
Design
GO-COLITIS was an open label, single arm, phase 4 study with a pragmatic design which reflected UK clinical practice. Adult patients were eligible if diagnosed with UC ≥3 months, partial Mayo score (PMS) 4–9. Patients received subcutaneous golimumab induction (200 mg initially and 100 mg at week 2) followed at week 6 by 50 mg or 100 mg (depending on weight) every 4 weeks until week 54 with a 12-week follow-up. Efficacy was measured by PMS at baseline, week 6, 30, 54 and 66. Health-related quality of life (HRQoL; Inflammatory Bowel Disease Questionnaire (IBDQ) and EuroQol Group 5 Dimensions Health Questionnaire (EQ-5D)) was assessed at baseline, week 6 and week 54. All safety adverse events (AEs) were recorded.
Results
207 patients were enrolled and 205 received golimumab (full analysis set (FAS)205). At week 6, 68.8% (95% CI 62.0% to 75.1%) and 38.5% (95% CI 31.8% to 45.6%) of patients were in response and remission, respectively, using PMS. At the end of the induction phase, 140/141 patients in clinical response continued into the maintenance phase (Maintenance FAS). Sustained clinical response through week 54 was achieved in 51/205 (24.9%) of the FAS205 population and 51/140 (36.4%) of the Maintenance FAS population. Statistically significant improvements from baseline to week 6 were observed for the IBDQ total score and for each IBDQ domain score (bowel symptoms, emotional function, systemic symptoms and social function), as well as the EQ-5D index score and associated visual analogue scale score (p<0.0001). Improvement of HRQoL was sustained through week 54. Serious AEs leading to treatment discontinuation occurred in 8.8% of patients.
Conclusion
In this study measuring patient-reported outcomes in patients with moderate to severe UC, golimumab induced and maintained response as measured by PMS and significantly improved quality of life measures.
Trial registration number
NCT02092285; 2013-004583-56.
Keywords: ulcerative colitis, tnf-alpha, Ibd
Summary box.
What is already known about this subject?
The randomised, placebo-controlled Programme of Ulcerative colitis Research Studies Utilising an Investigational Treatment (PURSUIT) studies demonstrated the efficacy of golimumab in inducing and maintaining continuous clinical response over 54 weeks.
What are the new findings?
Efficacy and safety of golimumab in moderate to severe ulcerative colitis (UC) is confirmed in this large (n=205) study whose pragmatic design reflected clinical practice in the UK. Early response to golimumab induction was higher, but sustained response in the induction responders was lower compared with the randomised controlled PURSUIT trials. Overall, 1-year sustained response rates in PURSUIT-M and GO-COLITIS are similar for the full analysis set, despite very different trial designs.
Well-executed observational real-world UC studies are needed to translate the findings of complex randomised controlled registration trials into clinical practice.
How might it impact on clinical practice in the foreseeable future?
GO-COLITIS assessed golimumab effectiveness by partial Mayo score, quality of life, and safety analysis. Patient-reported stool frequency and rectal bleeding can be used to monitor disease activity in moderate to severe UC.
The results of GO-COLITIS support the use of golimumab as per its European Medicines Agency indication: for treatment of moderately to severely active UC in adult patients who have had an inadequate response to conventional therapy including corticosteroids and 6-mercaptopurine or azathioprine or who are intolerant to or have medical contraindications for such therapies.
Introduction
Ulcerative colitis (UC) is a chronic inflammatory disease characterised by inflammation of the colon with increased stool frequency and rectal bleeding. Conventional therapies, including mesalazine, corticosteroids, immunomodulators and surgery are established treatments of UC. However, there remains an unmet need for sustained disease control preventing bowel damage and colectomy while improving patients’ quality of life (QoL).1The antitumour necrosis factor (TNF) therapies with infliximab, adalimumab and golimumab have been approved for the treatment of moderately to severely active UC in adults.2 Early response to induction and mucosal healing with infliximab are associated with long-term benefit and lower colectomy rates.3
Treatment with subcutaneous (SC) golimumab, a human monoclonal anti-TNF antibody, was approved in the European Union) based on two phase 3 clinical studies (Programme of Ulcerative colitis Research Studies Utilising an Investigational Treatment (PURSUIT)-SC, a 6-week SC induction study and PURSUIT-Maintenance (PURSUIT-M), a 54-week SC maintenance study), which demonstrated the efficacy and safety of golimumab subcutaneous injection in moderately to severely active patients with UC who were refractory to or intolerant of conventional UC therapies.4 5
GO-COLITIS was an open-label, single-arm, phase 4 study reflecting clinical practice in the UK aimed at assessing the efficacy and safety of golimumab in inducing and maintaining clinical response and improving QoL over 54 weeks in patients with moderate to severe UC.
Methods
Patients
GO-COLITIS was conducted in 29 UK centres. Eligible patients (≥18 years) had UC for ≥3 months, a diagnosis of moderate to severe disease at baseline (defined as Partial Mayo Score (PMS) 4–9), were anti-TNF naive and had a baseline rectal bleeding subscore ≥1. All patients received golimumab induction treatment according to the Summary of Product Characteristics (ie, they had responded inadequately to, had failed to tolerate or had medical contraindications for conventional therapy, defined as azathioprine/6-mercaptopurine and corticosteroids).6 Responders to induction entered the 54-week maintenance phase. Exclusion criteria were prior surgery for UC or likely need for surgery during the study, ischaemic or fulminant colitis, toxic megacolon, pathogenic bowel infection, inflammatory bowel disease (IBD) unclassified, evidence of Crohn’s disease, UC confined to proctitis (distal 15 cm or less) or treatment within 2 weeks of study inclusion with rectal corticosteroids or 5-aminosalicylates (5-ASAs). Stable dosing of 5-ASAs and corticosteroids was required for ≥2 weeks before baseline and for ≥4 weeks before baseline for azathioprine or 6-mercaptopurine. Patients could be discontinued from the study for any reason and were not to be replaced; mandatory discontinuations were made for patients meeting the criteria for treatment failure (lack of therapeutic effect, prohibited change in UC medication, formation of a stoma or colectomy). All patients provided written informed consent.
Study design and treatment
During the 6-week golimumab induction phase, patients received SC golimumab on day 0 (200 mg) and day 14 (100 mg). Those responding to induction, assessed by PMS change from baseline at week 6, were eligible to receive maintenance SC golimumab 50 or 100 mg (dependent on body weight as per SmPC) every 4 weeks (Q4W)±5 days, for a total of 54 weeks of treatment, with a 12-week follow-up at the Investigator’s discretion. Dose adjustment was not allowed.
Study evaluations
Disease activity was assessed at screening, baseline, week 6, 30, 54 and 66 using PMS. Clinical response was defined as a decrease in PMS of ≥2 points and ≥30% from baseline, plus either a decrease in the rectal bleeding subscore of ≥1 point or an absolute rectal bleeding subscore of 0 or 1. Clinical remission was defined as a PMS ≤2 and no individual subscore >1. Data for endoscopy was collected whenever the procedure was performed; however, endoscopy was not mandated.
Health-related quality of life (HRQoL) was assessed at baseline, week 6 and week 54 using IBDQ and EuroQol Group 5 Dimensions Health Questionnaire (EQ-5D).7 8 Routine laboratory assessments were made at baseline, week 6, 30 and 54. All adverse events (AEs) were recorded at study visits.
Data analysis
The primary endpoint of GO-COLITIS was the proportion of patients maintaining a clinical response (ie, sustained clinical response) through week 54, as assessed by PMS measured at weeks 6, 30 and 54. Secondary endpoints included clinical response at week 6, response and remission rates at week 54, proportion of patients with normal QoL (defined as IBDQ score >170), change in HRQoL from baseline at week 6 and 54) and safety.
A sample size of 200 patients was calculated to provide a 95% CI for the induction response rate of 43%–57% and for the overall 1 year sustained response rate of 19%–32% based on the PURSUIT trial results.4 5
Efficacy and HRQoL were assessed for all patients (full-analysis set (FAS)). Safety was assessed for all enrolled patients who received at least one golimumab injection. Patients with missing PMS subscores were excluded from analysis of PMS change from baseline and treated conservatively in other analyses. Comparisons in the mean change from baseline in PMS, PMS subscores, IBDQ and EQ-5D data were made using the paired t-test. Factors possibly related to response outcomes were explored using summary statistics, graphical methods and logistic regression.
Results
Patients
Between July 2014 and February 2015, 207 patients enrolled in GO-COLITIS with 205 patients receiving golimumab (figure 1). The median (range) age was 35 (18–79) years; 60% of patients were male (table 1). Baseline mean (SD) PMS was 6.4 (1.4); mean (SD) full Mayo score (FMS) (n=92) was 8.7 (1.7). Overall, 203 patients received both golimumab induction doses. Concomitant medications at baseline included 5-ASAs (70.7%), corticosteroids (39.0%), azathioprine (45.4%), 6-mercaptopurine (9.3%) and methotrexate (2.9%) (table 1).
Figure 1.
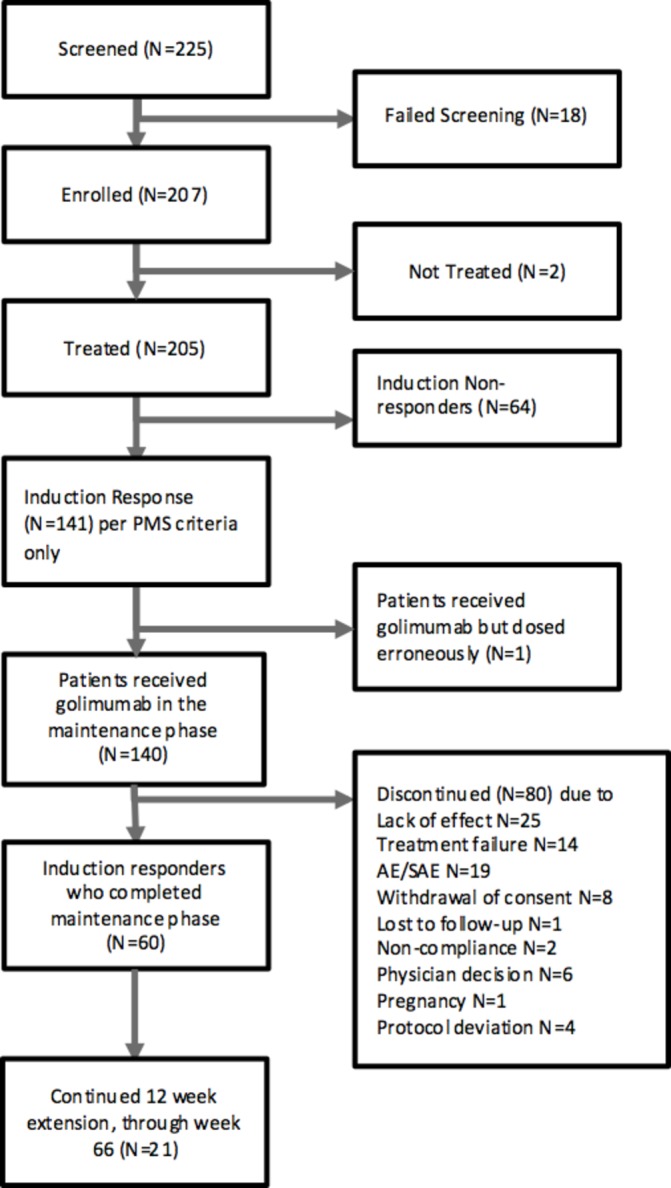
Consolidated Standards of Reporting Trials diagram. *Two patients were not treated as a result of post-consent adverse events.
Table 1.
Demographics and baseline disease characteristics
| Characteristic | All patients (n=205) |
| Sex, n (%) | |
| Female | 82 (40.0) |
| Male | 123 (60.0) |
| Median (range) age, years | 35.0 (18.0–79.0) |
| Race, n (%) | |
| White | 166 (81.0) |
| Asian | 28 (13.7) |
| Multiracial* | 6 (2.9) |
| Black or African American | 5 (2.4) |
| Median (range) disease duration, y ears | 3.9 (0.3–38.8) |
| Disease extent | |
| Left-sided colitis | 97 (47.3) |
| Pancolitis (extensive) | 64 (31.2) |
| Unknown | 44 (21.5) |
| Mean (SD) partial Mayo score | 6.4 (1.40) |
| Mean (SD) full Mayo score (n=92) | 8.7 (1.65) |
| Stool frequency score (SFS), n (%) | |
| SFS 0 (Normal) | 3 (1.5) |
| SFS 1 (1–2 stools more than normal) | 16 (7.8) |
| SFS 2 (3–4 stools more than normal) | 64 (31.2) |
| SFS 3 (≥5 stools more than normal) | 122 (59.5) |
| Rectal bleeding score (RBS), n (%) | |
| RBS 0 (No blood seen)† | 6 (2.9) |
| RBS 1 (Streaks of blood with stool less than half of the time) | 77 (37.6) |
| RBS 2 (Obvious blood with stool most of the time) | 94 (45.9) |
| RBS 3 (Blood alone passed) | 28 (13.7) |
| Endoscopic findings (n=92), n (%) | |
| Mild disease‡ | 5 (5.4) |
| Moderate disease§ | 58 (63.0) |
| Severe disease¶ | 29 (31.5) |
| Physician global assessment (PGA), n (%) | |
| PGA 0 (Normal) | 1 (0.5) |
| PGA 1 (Mild disease) | 1 (0.5) |
| PGA 2 (Moderate disease) | 150 (73.2) |
| PGA 3 (Severe disease) | 53 (25.9) |
| Concomitant medications, n (%) | |
| 5-Aminosalicylates | 145 (70.7) |
| Azathioprine | 93 (45.4) |
| Corticosteroids | 80 (39.0) |
| 6-Mercaptopurine | 19 (9.3) |
| Methotrexate | 6 (2.9) |
*Defined as have ≥2 of American Indian or Alaska native, black or African-American, Native Hawaiian or other Pacific islander, White or Asian.
†The six patients with rectal bleeding subscore 0 were included in the analysis; no patient selection was performed for the analysis.
‡Mild disease = erythema, decreased vascular pattern, mild friability.
§Moderate disease = marked erythema, absent vascular pattern, friability, erosions.
¶Severe disease = spontaneous bleeding, ulceration.
Efficacy analysis
The primary analysis population for the evaluation of efficacy during the maintenance period was the FAS205 consisting of patients who received at least 1 dose of golimumab. Patients who were responders at week 6 and continued into the maintenance phase were used as a secondary population for evaluation of rates of clinical response and remission (Maintenance FAS).
Clinical efficacy
In the induction phase, 68.8% (141/205, 95% CI 62.0% to 75.1%) and 38.5% (79/205, 95% CI 31.8% to 45.6%) of patients were in clinical response and clinical remission at week 6, respectively. At the end of the induction phase, 140/141 patients in clinical response continued into the maintenance phase; one patient in clinical response after the remission phase did not receive maintenance medication. Sustained clinical response through week 54 (primary endpoint) was achieved by 36.4% (51/140, 95% CI 28.5% to 45.0%) of induction responders (Maintenance FAS population) and 24.9% (51/205, 95% CI 19.1% to 31.4%) of all patients (FAS205 population) (table 2). Clinical remission was achieved by 25.7% of the Maintenance FAS population (36/140, 95% CI 18.7% to 33.8%) and 17.6% (36/205, 95% CI 12.6% to 23.5%) of the FAS205 population.
Table 2.
Patients meeting partial Mayo score response criteria at week 6, 30 and 54
| Response at | FAS (n=205) n (%) (95% CI) | Maintenance FAS (n=140) n (%) (95% CI) |
| Week 6 | 141 (68.8%) (62.0% to 75.1%) | |
| Week 30 | 79 (38.5%) (31.8% to 45.6%) | 79 (56.4%) (47.8% to 64.8%) |
| Week 54 | 52 (25.4%) (19.6% to 31.9%) | 52 (37.1%) (29.1% to 45.7%) |
| Both weeks 30 and 54 | 51 (24.9%) (19.1% to 31.4%) | 51 (36.4%) (28.5% to 45.0%) |
FAS, full analysis set.
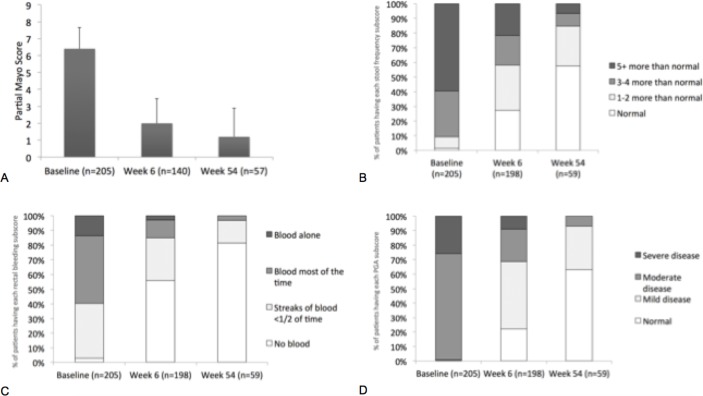
Golimumab induction resulted in improvement from baseline to week 6 in PMS (mean change, –3.2; SD 2.4; p<0.0001; figure 2A). From baseline to week 54, mean PMS, stool frequency score (SFS), rectal bleeding score (RBS) and physician global assessment (PGA) scores decreased from 6.4 to 1.2, from 2.5 to 0.6, from 1.7 to 0.2 and from 2.2 to 0.4, respectively. The proportions of patients with normal partial Mayo subscores of SFS, RBS and PGA at week 6 and week 54 were increased compared with baseline (figure 2B–D). In the Maintenance FAS population, 11/62 (17.7%) patients using corticosteroids at baseline were able to taper corticosteroids after induction and 9/62 (14.5%) were off corticosteroids at week 54.
Figure 2.
Mean (SD) change from baseline to week 6 and week 54 in partial Mayo score (A), and the proportions of patients by severity grade for the (B) stool frequency subscore, (C) rectal bleeding subscore and (D) physician global assessment.
From baseline (n=103) and week 6 (n=87) to week 54 (n=40), median C-reactive protein (CRP) decreased from 5.0 to 2.0 mg/L and from 4.0 to 2.0 mg/L, respectively. Measurement of calprotectin level was not mandated and measurements from baseline are only available for seven patients at week 54; the data is not presented here. Among disease baseline characteristics tested, only disease extent was a significant predictor of treatment response as measured by PMS at week 54 (see online Supplementary material for results).
bmjgast-2018-000212supp001.docx (81.8KB, docx)
At week 54, 52/140 of the Maintenance FAS population were in clinical response and entered the 12-week follow-up; 21 patients continued and 31 patients discontinued golimumab therapy. At week 66, 48 patients were in clinical response; 34.3% of the Maintenance FAS population and 23.4% of the FAS205 population. Of the patients who continued golimumab therapy, 100% remained in clinical response at week 66. Of the patients who discontinued golimumab therapy at week 54, 27/31 patients (87.1%) maintained clinical response.
Health-related quality of life
During induction, statistically significant improvements from baseline to week 6 were observed for IBDQ total score (mean increase, 45.2; p<0.0001, n=192), with 142/195 (72.8%) patients having ≥20 point improvement in the IBDQ total score. At week 54, mean IBDQ (SD) total score in the Maintenance FAS population was 186.2 (SD 27.1) with a mean change from baseline of 66.8 (n=59) (table 3). Median IBDQ at week 54 was 192.0, indicating that over 50% of patients who completed the study had normal QoL at week 54. Statistically significant improvements from baseline to week 6 were observed for the EQ-5D index score (mean increase, 0.1; p<0.0001) and the EQ-5D health state Visual Analogue Scale score (mean increase, 15.6 mm; p<0.0001). At week 54, mean EQ-5D (SD) was 0.9 (0.2), with a mean (SD) change from baseline of 0.2 (0.3). Mean EuroQol-Visual Analogue Scale (SD) at week 54 was 76.7 (20.5), with a mean (SD) change from baseline of 27.8 (27.7).
Table 3.
Mean (SD) change from baseline in IBDQ total score, EQ-5D and EQ-VAS at week 6 and week 54
| Baseline, mean (SD) | Week 6, mean (SD) | Week 54, mean (SD) | Change from baseline to week 54 (Maintenance FAS) | |
| IBDQ total score | ||||
| N | 202 | 140 | 59 | 59 |
| Mean (SD) | 115.9 (32.4) | 173.4 (30.0) | 186.2 (27.1) | +66.8 (36.7) |
| Median | 111 | 177.0 | 192.0 | +71.0 |
| EQ-5D index score | ||||
| N | 201 | 140 | 60 | 58 |
| Mean (SD) | 0.7 (0.2) | 0.8 (0.2) | 0.9 (0.2) | +0.2 (0.3) |
| Median | 0.7 | 0.8 | 1.0 | +0.2 |
| EQ-VAS | ||||
| N | 195 | 140 | 59 | 56 |
| Mean (SD) | 46.9 (22.6) | 67.1 (23.6) | 76.7 (20.5) | +27.8 (27.7) |
| Median | 50.0 | 72.0 | 80.0 | +29.0 |
EQ-5D, EuroQol Group 5 Dimensions Health Questionnaire; EQ-VAS, EuroQol-Visual Analogue Scale; FAS, full analysis set; IBDQ, Inflammatory Bowel Disease Questionnaire.
Safety
Treatment-emergent AEs and serious AEs were reported in 174 (84.9%) and 49 (23.9%) patients, respectively. Thirty-one of 49 serious AEs were recorded as gastrointestinal disorders, 28 of which were recorded as UC. Serious AEs leading to treatment discontinuation were reported in 18 patients (8.8%). Three serious AEs (1.5%) were reported to be treatment related; septicaemia (×2) and miscarriage. One patient (0.5%) died as a result of hospital-acquired pneumonia; causality was assessed as not related to study drug.
Two patients (1%) needed colectomy during the induction phase and seven patients (3.4%) during the maintenance phase. Three pregnancies were reported; all three patients discontinued the trial.
Discussion
GO-COLITIS was the first pragmatically designed open-label study to assess the efficacy and safety of golimumab SC for induction and maintenance therapy in patients with moderate to severe UC in the UK. At week 6, 68.8% (95% CI 62.0% to 75.1%) and 38.5% (95% CI 31.8% to 45.6%)%) of patients were in response and remission, respectively, using PMS. Sustained clinical response through week 54 was achieved in 24.9% of all patients (n=205) and 36.4% of week-6 responders (n=140).
Clinical response and remission rates in the induction phase of GO-COLITIS are greater than the week-6 FMS clinical response rate (51.0%) and remission rate (17.8%) observed following golimumab 200/100 mg induction in PURSUIT-SC.5 Several smaller real-world studies with golimumab in UC have also reported high response and remission rates. In a UK study of ‘real-world’ effectiveness of golimumab, clinical response was achieved in 52% (23/44) and clinical remission in 34% (15/44) of patients at a median of 12 weeks, with a clinical benefit seen in 50% of patients who underwent dose escalation.9 Taxonera et al (n=142) reported week-8 clinical response and remission rate by PMS of 75.4% and 43.9%, respectively, in anti-TNF naive patients with UC.10 Sustained clinical benefit reported by Taxonera et al at 1 year was 57.7%, comparable with reports of 1 year response of 63.0% by Bressler et al.11 Both Taxonera and Bressler studies were retrospective studies that allowed dose adjustment of golimumab, which increased golimumab persistence on drug. In Taxonera et al, 31 (22%) patients with secondary loss of response had dose adjustment and 22/31 (71%) regained response to golimumab. Whereas in Bressler et al, dose optimisation occurred in 3.6% of patients. In PURSUIT-M, dose optimisation was considered treatment failure and not achieving continuous clinical response. We can therefore best compare our results with PURSUIT-M. Higher induction response and remission rates in GO-COLITIS may be related to the use of PMS rather than FMS as efficacy measure at week 6. Alternatively, the high initial response rate may result from the intrinsic bias of such an open label study: however, patients treated in the ‘real world’ all have knowledge of their therapy and so the bias is no greater than in routine clinical care. Lower maintenance response and remission rates reported in GO-COLITIS may be related to the differences in study design between GO-COLITIS and PURSUIT-M. Close follow-up with 4-weekly clinic visits in the PURSUIT-M randomised controlled trial compared with the observational design of GO-COLITIS with only two clinic visits may influence outcomes. Still, overall 51/205 (24.9%) of patients achieved sustained clinical response from baseline to week 54 in GO-COLITIS compared with 24% (47% continuous clinical response in 51% responders to induction) in PURSUIT-M.
Improvements in all three domains of PMS were illustrated by the increased proportions of patients with normal scores at week 6 and week 54 compared with baseline, particularly for stool frequency and rectal bleeding subscores, the two patient-reported components of the PMS (PRO2). PMS and PRO2 have previously been shown to perform as well as FMS to identify patient perceived clinical response.12 Furthermore, PRO2 was as accurate as PMS and FMS in monitoring continuous clinical response of patients in PURSUIT-M trial13: because PMS can accurately predict FMS, endoscopy may not always be essential for assessment of UC disease activity in clinical trials.14 Endoscopy to assess clinical response is not a standard practice in all UK centres and was not required in GO-COLITIS; however, it is recommended by Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) consensus as the objective measure of mucosal inflammation in UC.15 Rectal bleeding, which is associated with mucosal inflammation and correlates with Mayo endoscopy subscore, can be considered a surrogate marker of endoscopic activity.16
Clinical trial indices for assessing UC disease activity may not always align with the symptoms considered important by patients, such as cramping or anxiety related to the effects of disease activity (eg, rectal bleeding or incontinence),17 indicating a need for more patient-centred assessment of disease activity. In GO-COLITIS, golimumab induction significantly improved general and disease-specific QoL, including bowel symptoms, emotional function, systemic symptoms and social function. At week 6, 142/195 (72.8%) of patients exceeded the IBDQ increase cut-off (≥20 points) previously proposed as representative of patient-defined improvement in an assessment of UC clinical endpoints.18 Fast improvement of PROs is important for adherence to therapy and STRIDE recommends restoring QoL in patients with UC through resolution of rectal bleeding and normalisation of bowel habit.15
No new safety signals were observed in GO-COLITIS. The overall incidence of AEs of 84.9% in our study is comparable to that reported in PURSUIT-M (72.7%). The incidence of serious AEs in PURSUIT-M and GO-COLITIS was 8.4% and 23.9%. Higher rates of AEs and serious AEs in GO-COLITIS may reflect the patient population recruited to the study.
A final observation was that of the 21 patients who were in response at week 54 and continued on golimumab therapy after week 54; none had relapsed by week 66. Of the 31 who discontinued golimumab, four (13%) had lost response by week 66. The decision to stop treatment at 54 weeks may have been driven by National Institute for Health and Care Excellence guidance which states that the requirement for continued therapy should be reviewed after 12 months of treatment. Clinicians and patients should consider carefully whether to discontinue golimumab once remission is achieved. However, further investigation is required to determine the effect of golimumab withdrawal after 1 year in patients responding to treatment.
Strengths of GO-COLITIS include the large number of patients (n=205), the pragmatic design and the dosing consistent with the product label.6 Limitations include the early assessment at 6 weeks, following two doses (200 mg and 100 mg), which may have excluded the so-called ‘delayed’ responders from entering the maintenance phase.19 Efficacy and QoL results may have been subject to observer bias because of the open-label treatment assignment. PMS measures are subjective: stool frequency and rectal bleeding are subjective for the patient, while PGA is subjective for the physician. Calprotectin measurement is not routine practice, during follow-up, in many UK centres and according to STRIDE, the biomarkers calprotectin and CRP are adjunctive measures of inflammation, not a therapeutic target.15 Therapeutic drug monitoring is not a routine practice in the UK and was not measured in this study: indeed, the assay is still not available for clinical use. The exposure–response relationship of golimumab in UC has previously been established.20
In conclusion, in this study patients with moderate to severe UC experienced meaningful improvements from baseline to week 6 and 54 in PMS. The observed improvements in generic and disease-specific QoL provide further evidence for the importance of assessing PROs in patients with UC. Golimumab treatment was generally well tolerated, and no new safety signals were identified.
Footnotes
Contributors: CSJP, SS, GG, HT, CW and PMI contributed to the design of the study. CSJP, SS, DRG, PJH and PMI collected the data. PJH, GG, AR, HT and CW analysed the data and all authors contributed to the interpretation of the data. CSJP wrote the article and all authors critically reviewed and approved the final version of the manuscript.
Funding: GO-COLITIS was sponsored by MSD UK, Hoddesdon, UK.
Competing interests: CSJP acted as a paid advisor to MSD, Abbvie, Dr Falk, Ferring, Janssen, Napp, Pfizer, Shield, Takeda and Vifor and Dr Falk. Study grants received from Shield. SS holds research grants from Takeda, AbbVie, Warner Chilcott, Ferring, Biohit and Celgene, served on the advisory boards of Takeda, AbbVie, Merck, Ferring, Pharmacocosmos, Warner Chilcott, Janssen, Falk Pharma, Biohit, TriGenix, Cellgene and Tillots Pharma and has received speakers fees from AbbVie, Warner Chilcott, Janssen, Takeda, Tillots Pharma and Falk Pharma. DRG received speaker honoraria/travel grants from MSD, Abbvie, Ferring, Janssen, Vifor, Tillots and Dr Falk. PJH served as a speaker for Abbvie, Ferring, Janssen, MSD, Takeda, Tillotts, Warner Chilcott. GG and AR are employees (and shareholders) of MSD. HT carried out statistical consultancy work for MSD as an independent statistician. CW was an employee of MSD. PI received honoraria for acting in an advisory capacity for or speaking on behalf of Abbvie, MSD, Takeda, Janssen, Falk, Ferring, Shire, Tillotts, VH2, Topivert, Pfizer, Lilly and Samsung Bioepis. Research grants received from Takeda, MSD and Janssen.
Patient consent: Not required.
Ethics approval: The study protocol and its amendments (8259-032-03) were approved by an independent ethics committee (Committee North West – Liverpool Central of the National Research Ethics Service) and the Medicines and Healthcare Products Regulatory Agency.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Additional data available on request. Please direct enquiries to Colin Wheeler (colin@southwoodresearch.com).
Collaborators: The participating investigators for the GO-COLITIS Study Group were: C Probert, Royal Liverpool Hospital, Liverpool, UK (PI); D Gaya, Glasgow Royal Infirmary, Glasgow, UK; S Sebastian, Hull Royal Infirmary, Hull, UK; A Hart, St Mark’s Hospital, London, UK; P Irving, St Thomas' Hospital, London, UK; T Ahmad, Royal Devon and Exeter, Exeter, Devon; UK; R Pollok, St George’s Hospital, London, UK; T Orchard, St Marys Hospital, London, UK, R Arasaradnam, University Hospital Coventry, Coventry, UK; T Iqbal, Queen Elizabeth Hospital, Birmingham, UK; M Johnson, Luton and Dunstable University Hospital, Luton, UK; A Kaser, Addenbrooke’s Hospital, Cambridge, UK; P Allen, The Ulster Hospital, Belfast, UK; J Gordon, Royal Hampshire County Hospital, Winchester, UK; C Preston, Bradford Royal Infirmary, Bradford, UK; R Shenderey, Airedale General Hospital, Keighley, UK; S Hoque, Whipps Cross University Hospital, London, UK; S Bloom, University College Hospital, London, UK; A Ansari, East Surrey Hospital, Redhill, UK; C Mowat, Medical Research Institute, Ninewells hospital, Dundee, UK; J Hamlin, St James’s Hospital, Leeds, UK; I Arnott, Western General Hospital, Edinburgh, UK; I Shaw, Gloucester Royal Hospital, Gloucester, UK; H Steed, Royal Wolverhampton hospital, Wolverhampton, UK; J Butterworth, Royal Shrewsbury Hospital, Shrewsbury, UK; A Robinson, Salford Royal NHS Foundation Trust, Salford, UK; J Mawdsley, West Middlesex University Hospital, Isleworth, UK; T Creed, Bristol Royal Infirmary, Bristol, UK; F Cummings, Southampton General Hospital, Southampton, UK.
Contributor Information
GO-COLITIS Study Group:
C Probert, D Gaya, S Sebastian, A Hart, P Irving, T Ahmad, R Pollok, T Orchard, R Arasaradnam, T Iqbal, M Johnson, A Kaser, P Allen, J Gordon, C Preston, R Shenderey, S Hoque, S Bloom, A Ansari, C Mowat, J Hamlin, I Arnott, I Shaw, H Steed, J Butterworth, A Robinson, J Mawdsley, T Creed, and F Cummings
Collaborators: GO-COLITIS Study Group
References
- 1. Peyrin-Biroulet L, Van Assche G, Sturm A, et al. Treatment satisfaction, preferences and perception gaps between patients and physicians in the ulcerative colitis CARES study: A real world-based study. Dig Liver Dis 2016;48:601–7. doi:10.1016/j.dld.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 2. Flamant M, Paul S, Roblin X. Golimumab for the treatment of ulcerative colitis. Expert Opin Biol Ther 2017;17:879–86. doi:10.1080/14712598.2017.1327576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferrante M, Vermeire S, Fidder H, et al. Long-term outcome after infliximab for refractory ulcerative colitis. J Crohns Colitis 2008;2:219–25. doi:10.1016/j.crohns.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 4. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:96–109. doi:10.1053/j.gastro.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 5. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:85–95. doi:10.1053/j.gastro.2013.05.048 [DOI] [PubMed] [Google Scholar]
- 6. European Medicines Agency. Simponi Summary of Product Characteristics,. 2015:1–205.
- 7. Irvine EJ, Feagan B, Rochon J, et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn’s Relapse Prevention Trial Study Group. Gastroenterology 1994;106:287–96. [DOI] [PubMed] [Google Scholar]
- 8. Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53–72. doi:10.1016/0168-8510(96)00822-6 [DOI] [PubMed] [Google Scholar]
- 9. Samaan MA, Pavlidis P, Johnston E, et al. Vedolizumab: early experience and medium-term outcomes from two UK tertiary IBD centres. Frontline Gastroenterol 2017;8:196–202. doi:10.1136/flgastro-2016-100720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taxonera C, Rodríguez C, Bertoletti F, et al. Clinical outcomes of golimumab as first, second or third Anti-TNF agent in patients with moderate-to-severe ulcerative colitis. Inflamm Bowel Dis 2017;23:1394–402. doi:10.1097/MIB.0000000000001144 [DOI] [PubMed] [Google Scholar]
- 11. Bressler B, Williamson MA, Camacho F, et al. Mo1902 Real world use and effectiveness of golimumab for ulcerative colitis in Canada. Gastroenterology 2016;150:Mo1902:S811 doi:10.1016/S0016-5085(16)32744-5 [Google Scholar]
- 12. Lewis JD, Chuai S, Nessel L, et al. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis 2008;14:1660–6. doi:10.1002/ibd.20520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reinisch W, Colombel JF, Feagan B, et al. Patient-reported outcomes can be used to monitor continuous clinical response in patients with moderately to severely active ulcerative colitis treated with golimumab: results from the PURSUIT maintenance study [P1614]. 23rd United European Gastroenterology Week 2015. [Google Scholar]
- 14. Dhanda AD, Creed TJ, Greenwood R, et al. Can endoscopy be avoided in the assessment of ulcerative colitis in clinical trials? Inflamm Bowel Dis 2012;18:2056–62. doi:10.1002/ibd.22879 [DOI] [PubMed] [Google Scholar]
- 15. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–38. doi:10.1038/ajg.2015.233 [DOI] [PubMed] [Google Scholar]
- 16. Manginot C, Baumann C, Peyrin-Biroulet L. An endoscopic Mayo score of 0 is associated with a lower risk of colectomy than a score of 1 in ulcerative colitis. Gut 2015;64:1181.2–2. doi:10.1136/gutjnl-2014-308839 [DOI] [PubMed] [Google Scholar]
- 17. Waljee AK, Joyce JC, Wren PA, et al. Patient reported symptoms during an ulcerative colitis flare: a Qualitative Focus Group Study. Eur J Gastroenterol Hepatol 2009;21:558–64. doi:10.1097/MEG.0b013e328326cacb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins PD, Schwartz M, Mapili J, et al. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut 2005;54:782–8. doi:10.1136/gut.2004.056358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colombel J-F, Reinisch W, Gibson PR, et al. Mo1794 Delayed response to golimumab therapy: UC patient characteristics and long-term clinical outcome: post-hoc analyses from the PURSUIT program. Gastroenterology 2016;150:S778 doi:10.1016/S0016-5085(16)32637-3 [Google Scholar]
- 20. Adedokun OJ, Xu Z, Marano CW, et al. Pharmacokinetics and exposure-response relationship of golimumab in patients with moderately-to-severely active ulcerative colitis: results from phase 2/3 PURSUIT induction and maintenance studies. J Crohns Colitis 2017;11:35–46. doi:10.1093/ecco-jcc/jjw133 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2018-000212supp001.docx (81.8KB, docx)