Abstract
Patients treated with conventional cancer chemotherapy suffer from side effects of the drugs due to non-selective action of chemotherapeutic drugs to normal cells. Active targeting nanoparticles that are conjugated to targeting ligands on the surface of nanoparticles play an important role in improving drug selectivity to the cancer cell. Several chemotherapeutic drugs and traditional/herbal medicines reported for anticancer activities have been investigated for their selective delivery to cancer cells by active targeting nanoparticles. This systematic review summarizes reports on this application. Literature search was conducted through PubMed database search up to March 2017 using the terms nanoparticle, chemotherapy, traditional medicine, herbal medicine, natural medicine, natural compound, cancer treatment, and active targeting. Out of 695 published articles, 61 articles were included in the analysis based on the predefined inclusion and exclusion criteria. The targeting ligands included proteins/peptides, hyaluronic acid, folic acid, antibodies/antibody fragments, aptamer, and carbohydrates/polysaccharides. In vitro and in vivo studies suggest that active targeting nanoparticles increase selectivity in cellular uptake and/or cytotoxicity over the conventional chemotherapeutic drugs and non-targeted nanoparticle platform, particularly enhancement of drug efficacy and safety. However, clinical studies are required to confirm these findings.
Keywords: active targeting, nanoparticles, ligands, chemotherapy, natural active compounds, cancer
Introduction
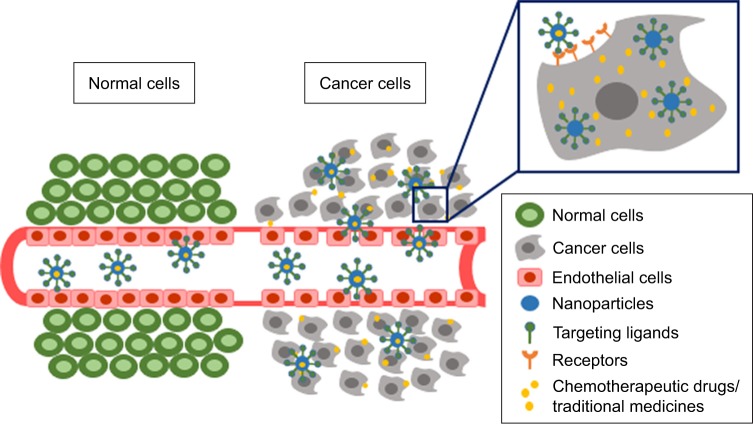
Cancer remains one of the major causes of deaths worldwide. In 2017, approximately 1.7 million new cases and 600 thousand deaths were estimated to occur in the USA.1 Most patients treated with conventional chemotherapy suffer from serious side effects due to non-selective action of chemotherapeutic drugs to normal cells. For a few decades, nanoparticles have been developed as a drug delivery system of various chemotherapeutic drugs to enhance drug efficacy and safety.2–4 Nanoparticles play an important role in increasing drug concentration in cancer cells by enhancing drug accumulation by passive and active targeting mechanisms as well as by decreasing drug efflux from cancer cells. The passive targeting nanoparticle is the mechanism by which the drugs leak from blood vessels supplying cancer cells and accumulate in the cells by enhanced permeability and retention (EPR) effect.5 The active targeting nanoparticles, on the other hand, target ligands conjugated on the surface of nanoparticles, resulting in increasing cellular uptake by receptor-mediated endocytosis and therefore increased drug accumulation in cancer cells. This mechanism relies on the interaction between tumor ligands conjugated on the surface of nanoparticles and cell-surface receptors or antigens on cancer cell surfaces (Figure 1).5 Nanoparticles acting via both mechanisms have been shown to increase drug concentration in cancer cells. Active targeting nanoparticles have been shown in various studies to be more efficient in increasing drug accumulation in cancer cells and therefore play important role not only in modern cancer chemotherapy, but also in cancer therapy with traditional/herbal medicines.6–11 A number of nanoparticle formulations derived from these active compounds have been developed for active targeting purpose to improve anticancer efficacy and to reduce side effects. The objective of this current review is to summarize the research articles relating to the application of active targeting nanoparticles delivering system for chemotherapeutic drugs derived from chemical synthesis as well as natural sources.
Figure 1.
Passive targeting and active targeting mechanisms of nanoparticles.
Materials and methods
Study selection and inclusion and exclusion criteria
This systematic review was conducted through the search from PubMed database up to March 2017. The following keywords were used: nanoparticle, chemotherapy, traditional medicine, herbal medicine, natural medicine, natural compound, cancer treatment, and active targeting. Inclusion criteria for selection of the searched articles were 1) articles in full text and written in English; 2) articles with in vitro or in vivo investigations of effects of nanoparticles delivering chemotherapeutic drugs or traditional/herbal medicines on efficacy and/or safety; and 3) articles with investigations of targeting and receptor/antigen. The articles with insufficient data for extraction or those with application for radiotherapy, gene therapy, photodynamic therapy, or for diagnostic purpose, or duplicate articles, or review articles were excluded from the analysis.
Data extraction and collection
The titles and abstracts of articles searched from PubMed database using the above mentioned keywords were initially screened to obtain relevant original research articles according to the eligibility criteria. Thereafter, the full texts of all relevant articles were carefully examined in details to confirm their compliance with the defined eligibility criteria. The studies of active targeting nanoparticles applied for both chemotherapeutic drugs and traditional/herbal medicines for cancer were classified according to the types of targeting ligands.
Results
Study description
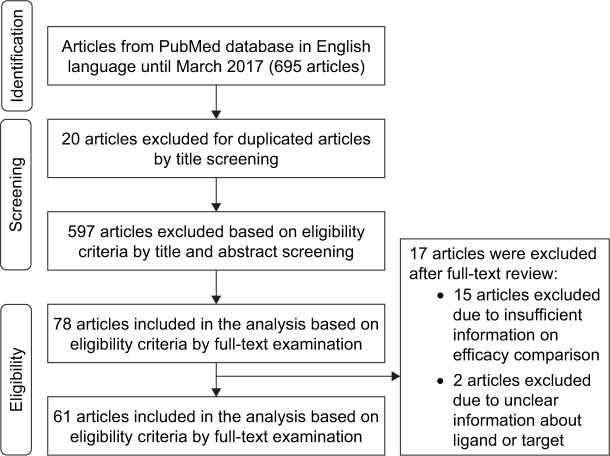
Twenty out of 695 research articles were initially excluded from the analysis during title screening for duplicate articles. The titles together with abstracts of the remaining articles were further checked for eligibility criteria and a total of 597 articles were excluded from the analysis. Finally, 61 out of 78 articles were included in the analysis, 17 articles being excluded due to unclear/inadequate information. The flow diagram of the search process is presented in Figure 2, and the effects of active targeting nanoparticles delivering modern chemotherapeutic drugs and traditional/herbal medicines for cancer are summarized in Tables 1 and 2.
Figure 2.
Flow diagram showing the different phases of the systematic review.
Table 1.
Summary of research articles that investigated active targeting NPs delivering chemotherapeutic drugs in cancer therapy
| Ligand | Receptor/antigen | Drug-NP platform | Types of study | Outcome
|
References | |
|---|---|---|---|---|---|---|
| Compared to non-targeted | Side effect | |||||
| Proteins/peptides | ||||||
| H2009.1 peptide | Integrin αvβ6 | Doxorubicin-liposome | In vivo: human non-small cell lung cancer cell lines (H2009) xenograft | No difference in tumor targeting and tumor growth inhibition rate | No significant change in body weight | 12 |
| IL-13 peptide | IL-13Rα2 receptor | Docetaxel-PEG-PCL | In vitro: human glioblastoma cell lines (U87) In vivo: cell lines U87 orthotopic xenograft |
Higher cellular uptake; 1.1-fold higher cellular apoptosis Higher tumor growth inhibition rate; 1.73-fold higher tumor targeting |
Not evaluated | 13 |
| AP-1 peptide | IL-4 receptor | Paclitaxel-cyclodextrin | In vivo: human breast adenocarcinoma cell lines MDA-MB-231 xenograft | Specifically targeting tumor site; higher tumor growth inhibition rate | Low nonspecific toxicity | 14 |
| Peptide CVKTPAQSC | CD133+ receptor | Docetaxel-PLA | In vitro: human lung cancer cell lines (A549) In vivo: cell lines A549 xenograft |
30.5% higher cellular uptake ratio Higher anti-metastatic efficacy |
No significant change in body weight | 15 |
| Transferrin | Transferrin receptor | Hydroxycamptothecin-PEG | In vivo: murine sarcoma cell lines (S180) xenograft | 9.03-Fold higher tumor accumulation; 1.85-fold higher tumor growth inhibition rate | No significant change in body weight | 16 |
| Transferrin | Transferrin receptor | Paclitaxel-PEG-chitosan | In vitro: non-small cell lung cancer cell lines (HOP-62) | Higher cellular uptake; 6.67-fold higher cytotoxicity | Not evaluated | 17 |
| cRGDyK | Integrin αvβ3 | Paclitaxel-PEG-PTMC | In vitro: human glioblastoma – astrocytoma, epithelial-like cell lines (U87MG) | 36.6% higher cellular uptake; 2.3-fold higher cytotoxicity; higher cellular apoptosis | Not evaluated | 18 |
| RGDS | Integrin αvβ3 | Doxorubicin-PEG-MIONP | In vitro: human cervical carcinoma cell lines (HeLa) | 11-Fold higher cellular uptake; higher cytotoxicity | Not evaluated | 19 |
| cRGDyK | Integrin αvβ3 | Paclitaxel-micelle | In vitro: human prostate cancer cell lines (PC-3) In vivo: cell lines PC-3 xenograft |
1.93-Fold higher cellular uptake; 1.26-fold higher cytotoxicity Higher tumor growth inhibition rate |
No significant change in body weight | 20 |
| RGD | Integrin αvβ3 receptor | Doxorubicin-dendritic poly-L-lysine-gelatin | In vitro: mouse mammary breast tumor cell lines (4T1) In vivo: cell lines 4T1 xenograft |
Higher cytotoxicity 1.18-Fold higher tumor accumulation; 10.6% higher tumor growth inhibition rate |
No significant change in body weight | 21 |
| Bombesin peptide | Gastrin-releasing peptide receptor | Docetaxel-PLGA | In vitro: human breast adenocarcinoma cell lines (MDA-MB-231) | 4-Fold higher cytotoxicity | Not evaluated | 22 |
| NR7 peptide | EGFR | Doxorubicin-PLGA-PEG | In vitro: human ovarian carcinoma cell lines (SKOV3) In vivo: cell lines SKOV3 xenograft |
3-Fold higher cellular uptake; 62.4-fold higher cytotoxicity 2.6-Fold higher tumor accumulation |
Low nonspecific toxicity | 23 |
| LHRH peptide | LHRHR | Methotrexate-HSA | In vitro: human breast carcinoma cell lines (T47D) | 71.5% higher cellular uptake; 8.5-fold higher cytotoxicity | Not evaluated | 24 |
| Angiopep-2 | LRP | Doxorubicin-dendritic poly-L-lysine-gelatin NP | In vitro: mouse mammary breast tumor cell lines (4T1) In vivo: cell lines 4T1 xenograft |
Higher cellular uptake; higher cellular apoptosis Higher accumulation of NP in tumor; higher tumor growth inhibition rate |
Low side effect to normal tissue | 25 |
| TbFGF peptide | FGFR | Paclitaxel-micelle | In vitro: murine Lewis lung carcinoma cell lines (LL/2), human hepatocellular liver carcinoma cell lines (HepG2), human lung cancer cell lines (A549), murine colorectal cancer cell lines (C26) | 18-Fold higher cytotoxicity to LL/2; higher cellular uptake by 6.6-fold for HepG2, 6.2-fold for A549, 2.9-fold for C26, and 2.7-fold for LL/2 | Not evaluated | 26 |
| Hyaluronic acid | ||||||
| Hyaluronic acid | CD44 receptor | Topotecan hydrochloride-dendrimer | In vitro: human colorectal cancer cell lines (HCT-116) In vivo: cell lines HCT-116 xenograft |
Higher cellular uptake; 3-fold higher cytotoxicity compared to free drug Higher tumor growth inhibition rate; 3.6-fold and 1.7-fold higher drug accumulation in tumor compared to kidney and liver | Not evaluated | 8 |
| Hyaluronic acid | CD44 receptor | Paclitaxel-micelle | In vitro: human breast adenocarcinoma cell lines (MCF-7) In vivo: murine hepatic carcinoma cell lines (Heps) xenograft |
4.1-Fold higher cellular uptake 2.80-Fold higher tumor accumulation; 31.89% higher tumor growth inhibition rate; higher median survival time |
No significant change in body weight | 27 |
| Hyaluronic acid | CD44 receptor | Cisplatin-chitosan | In vitro: human lung cancer cell lines (A549) | Higher cellular uptake; 8-fold higher cytotoxicity | Not evaluated | 28 |
| Hyaluronic acid | CD44 receptor | Rapamycin-LbL-LCNP | In vitro: human breast adenocarcinoma cell lines (MCF-7 and MDA-MB-231) | Higher cytotoxicity, 1.35-fold for MDA-MB-231, and 1.1-fold lower cytotoxicity to MCF-7 | No significant change in body weight | 29 |
| Hyaluronic acid | CD44 receptor | Doxorubicin-PBLG | In vivo: Ehrlich ascites tumor-bearing mice | Higher tumor growth inhibition rate; higher survival time | Not evaluated | 30 |
| Hyaluronic acid | CD44 receptor | Methotrexate-lipid-based NP | In vivo: murine melanoma cell lines (B16F10) xenograft | Higher tumor accumulation; higher tumor growth inhibition rate | Not evaluated | 31 |
| Hyaluronic acid | CD44 receptor | Doxorubicin hydroxylapatite | In vitro: human hepatocellular carcinoma cell lines (HepG2) In vivo: cell lines HepG2 xenograft |
Higher cellular uptake; 46.3% higher cytotoxicity compared to free drug Higher in tumor targeting; lower tumor volume | No significant change in body weight | 32 |
| Hyaluronic acid | CD44 receptor | Doxorubicin-HACE-PEG | In vitro: murine squamous cell carcinoma cell lines (SCC7) and mouse embryo fibroblast cell lines (NIH3T3) In vivo: cell lines SCC7 xenograft |
Higher cellular uptake in CD44 overexpressing (SCC7) compared to CD44 negative (NIH3T3); no difference in cellular uptake compared to free drug 30% higher tumor growth inhibition rate compared to free drug |
No significant change in body weight | 33 |
| Hyaluronic acid | CD44 receptor | Doxorubicin hyaluronic acid-Lys-LA10 | In vitro: doxorubicin-resistant human breast adenocarcinoma cell lines (MCF-7/ADR) In vivo: cell lines MCF-7/ADR xenograft |
Higher cellular uptake compared to free drug; no difference in cytotoxicity Lower relative tumor volume; higher median survival time |
No significant change in body weight and low nonspecific toxicity | 34 |
| Hyaluronic acid | CD44 receptor | Doxorubicin-PBLG-LA | In vitro: human breast adenocarcinoma cell lines (MCF-7) In vivo: cell lines MCF-7 xenograft |
10-Fold higher in cellular DOX level; higher cytotoxicity No difference in tumor growth inhibition rate; higher survival time |
No significant change in body weight and low nonspecific toxicity | 35 |
| Folate | ||||||
| Folic acid | Folate receptor | Docetaxel-PEG-PLGA | In vitro: human cervical carcinoma cell lines (HeLa) In vivo: cell lines HeLa xenograft |
26.7-Fold higher cellular uptake; 12-fold higher cytotoxicity compared to free drug Higher tumor targeting; higher tumor growth inhibition rate |
Not evaluated | 36 |
| Folic acid | Folate receptor | Doxorubicin-dendrimer | In vitro: human epidermal carcinoma cell lines (KB) | 1.4-Fold higher cellular uptake; 2.2-fold higher cytotoxicity | Not evaluated | 37 |
| Folic acid | Folate receptor | Gemcitabine-BSA | In vitro: human ovarian cancer cell lines (Ovcar-5) and human breast adenocarcinoma cell lines (MCF-7) In vivo: Ehrlich ascites carcinoma tumor cell-bearing mice |
2-Fold higher cellular uptake by MCF-7; higher cytotoxicity – 1.4-fold for MCF-7 and 1.6-fold for Ovcar-5; higher cellular apoptosis Higher tumor growth inhibition rate |
No significant change in body weight | 38 |
| Folic acid | Folate receptor | Carboplatin-PLGA-chitosan | In vitro: human cervical carcinoma cell lines (HeLa) | Higher cellular uptake in time-dependent manner; 1.67-fold higher cytotoxicity; higher cellular apoptosis | Not evaluated | 39 |
| Folic acid | Folate receptor | Doxorubicin-polymeric NP | In vivo: human epidermal carcinoma cell lines (KB) xenograft | 1.6-Fold higher tumor growth inhibition rate | Not evaluated | 7 |
| Folic acid | Folate receptor | Doxorubicin-PEG | In vitro: human epidermal carcinoma cell lines (KB), human lung cancer cell lines (A549) and human hepatocellular carcinoma cell lines (HepG2) In vivo: cell lines KB xenograft |
Higher cellular uptake by KB cell; higher cytotoxicity – 1.2-fold for A549, 3.5-fold for KB, and 2.1-fold for HepG2 Higher tumor targeting; higher tumor growth inhibition rate; higher survival time |
No significant change in body weight and less cardiotoxicity | 40 |
| Folic acid | Folate receptor | Cisplatin-PEG-MSN | In vitro: human cervical carcinoma cell lines (HeLa) | Higher cellular uptake | Not evaluated | 41 |
| Folic acid | Folate receptor | Doxorubicin-β-cyclodextrin | In vitro: human placenta choriocarcinoma cell lines (JAR), human colon adenocarcinoma cell lines (HT-29), human breast adenocarcinoma cell lines (MCF-7), and mouse fibroblast cell lines (3T3) | Higher cellular uptake – 2.09-fold by HT-29, 1.98-fold by MCF-7, and 7.31-fold by JAR; higher cytotoxicity – 12.39-fold for JAR, 6.73-fold for HT-29, and >1.5-fold for 3T3 | Not evaluated | 42 |
| Folic acid | Folate receptor | Paclitaxel-PEG-PLGA | In vitro: human endometrial carcinoma cell lines (HEC-1A) In vivo: cell lines HEC-1A xenograft |
Higher cellular uptake; 2.6-fold higher in cytotoxicity; 12% higher cellular apoptosis 16.48% higher tumor growth inhibition rate |
Not evaluated | 43 |
| Antibody | ||||||
| Anti-Fas mAb | Fas receptor | Camptothecin-PLGA | In vitro: human colorectal cancer cell lines (HCT116) | Higher cellular uptake; 58.9-fold higher cytotoxicity compared to free drug | Not evaluated | 44 |
| Anti-CD20 mAb | CD20 receptor | Doxorubicin-DSPE-PEG2000 | In vitro: human Burkitt’s lymphoma cell lines (Raji) | Selectively targeting CD-20-overexpressing cells (Raji) | Low nonspecific toxicity | 45 |
| Anti-CD47 mAb | CD47 receptor | Gemcitabine-MIONP | In vitro: human pancreatic ductal adenocarcinoma primary cells (Panc215 and Panc354) | Higher cellular uptake; higher cytotoxicity | Not evaluated | 46 |
| EGFR antibody | EGFR | Rapamycin-PLGA | In vitro: human breast adenocarcinoma cell lines (MCF-7) | 13-fold higher cellular uptake; higher cytotoxicity; 2.4-fold higher cellular apoptosis | Not evaluated | 47 |
| PR81 mAb | MUC1 receptor | 5-fluorouracil-BSA | In vitro: human breast adenocarcinoma cell lines (MCF-7) | Higher cytotoxicity | Not evaluated | 48 |
| Aptamer | ||||||
| Aptamer AS1411 | Nucleolin receptor | Doxorubicin-HPAEG | In vitro: human breast adenocarcinoma cell lines (MCF-7) | 2-fold higher cellular uptake; 1.7-fold higher cytotoxicity | Not evaluated | 6 |
| Aptamer AS1411 | Nucleolin receptor | Gemcitabine-PEG-PLGA | In vitro: human lung cancer cell lines (A549) | 36% higher cellular uptake; 5.9-fold higher cytotoxicity | Not evaluated | 49 |
| Aptamer AS1411 | Nucleolin receptor | Methotrexate-UnTHCPSi-PEI | In vitro: human breast adenocarcinoma cell lines (MDA-MB-231) | 1.6-fold and 4.7-fold higher cellular uptake for 3 h and 12 h, respectively; higher cytotoxicity | Not evaluated | 50 |
| Aptamer AS1411 | Nucleolin receptor | Docetaxel-mannitol-PLGA-TPGS | In vitro: human cervical carcinoma cell lines (HeLa) In vivo: cell lines HeLa xenograft |
Higher cellular uptake; 20-fold higher cytotoxicity 24.44% life time extended |
Not evaluated | 51 |
| Aptamer AS1411 | Nucleolin receptor | Doxorubicin-polymersome | In vitro: human breast adenocarcinoma cell lines (MCF-7) In vivo: cell lines MCF-7 xenograft |
1.73-fold higher cellular uptake compared to mutated aptamer conjugates; 1.75-fold higher cytotoxicity compared to mutated aptamer conjugates 1.75-fold higher tumor targeting; 21.8% higher tumor growth inhibition rate compared to mutated aptamer conjugated |
No significant change in body weight | 52 |
| Carbohydrates/polysaccharides | ||||||
| Lactose | ASGPR | Doxorubicin-lactose | In vitro: human hepatocellular carcinoma cell lines (SMMC-7721) In vivo: cell lines SMMC-7721 xenograft |
No difference in cytotoxicity and cellular apoptosis; higher cellular uptake in time-dependent manner Higher tumor targeting; no difference in tumor growth inhibition rate | Low nonspecific toxicity | 53 |
| Galactose | ASGPR | Doxorubicin-LPL | In vitro: human liver cancer cell lines (SK-HEP-1) In vivo: cell lines SK-HEP-1 orthotopic xenograft |
Higher cellular uptake; higher cytotoxicity in dose-dependent manner; higher cellular apoptosis Higher tumor growth inhibition rate |
No significant change in liver enzyme | 54 |
| Galactose | ASGPR | 5-Fluorouracil-pectin | In vitro: human hepatocellular carcinoma cell lines (HepG2) | Higher cellular uptake; 2.6-fold higher cytotoxicity compared to free drug | Not evaluated | 55 |
| Galactosamine | ASGPR | Paclitaxel-γ-PGA-PLA | In vitro: cell lines HepG2 | Higher cytotoxicity | Not evaluated | 56 |
| Galactose | Lecithin receptor | Doxorubicin solid lipid NP | In vitro: human lung cancer cell lines (A549) | 1.5-Fold higher cellular uptake; higher cytotoxicity | Not evaluated | 57 |
| Other molecules | ||||||
| EGF | EGFR | Gemcitabine-stearoyl | In vitro: human breast adenocarcinoma cell lines (MDA-MB-468, MDA-MB-231, and MCF-7) In vivo: cell lines MDA-MB-468 xenograft Ex vivo: MDA-MB-468 tumor |
Higher cellular uptake in MDA-MB-468; higher cytotoxicity Higher tumor growth inhibition rate; higher survival time Higher tumor accumulation |
Not evaluated | 58 |
| EGa1 | EGFR | Doxorubicin-micelle | In vitro: human mouth squamous cell carcinoma cell lines UM-SCC 14C In vivo: cell lines UM-SCC 14C xenograft |
Higher cellular uptake; higher cytotoxicity Higher tumor targeting; higher tumor growth inhibition rate; higher median survival time |
Not evaluated | 59 |
| CSA | CD44 receptor | Doxorubicin chondroitin sulfate A-deoxycholic acid | In vitro: human breast adenocarcinoma cell lines (MDA-MB-231) | Higher cellular uptake compared to free drug; 1.67-fold higher cytotoxicity compared to free drug | Not evaluated | 60 |
| Folic acid and bovine serum albumin | Folate receptor and SPARC | Paclitaxel-lipid | In vitro: human breast adenocarcinoma cell lines (MCF-7) | 1.9-Fold higher cellular uptake | No significant change in body weight | 61 |
| Hyaluronic acid and glycyrrhetinic acid | CD44 and glycyrrhetinic acid receptor | Paclitaxel glycyrrhetinic acid-graft-hyaluronic acid | In vitro: human hepatocellular carcinoma cell lines (HepG2) and murine melanoma cell lines (B16F10) | Higher cellular uptake compared to free drug; higher cytotoxicity to HepG2 | Not evaluated | 62 |
Abbreviations: ASGPR, asialoglycoprotein receptor; BSA, bovine serum albumin; cRGDyK, cyclic arginine-glycine-aspartic acid-tyrosine-lysine; DSPE-PEG2000, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy polyethylene glycol-2000; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; HACE, hyaluronic acid-ceramide; HSA, human serum albumin; HPAEG, hyperbranched poly(2-((2-(acryloyloxy) ethyl)disulfanyl)ethyl 4-cyano-4-(((propylthio)-carbonothioyl)-thio)-pentanoate-co-polyethylene glycol methacrylate; IL, interleukin; LbL-LCNP, layer-by-layer-liquid crystalline nanoparticle; LHRH, luteinizing hormone-releasing hormone; LHRHR, luteinizing hormone-releasing hormone receptor; LPL, lithocholic acid-polyethylene glycol-lactobionic acid; LRP, low density lipoprotein-receptor related protein; Lys-LA10, L-lysine methyl ester-lipoic acid; mAb, monoclonal antibody; MIONP, magnetic iron oxide nanoparticle; MSN, mesoporous silica nanoparticle; NP, nanoparticle; γ-PGA-PLA, poly(gamma-glutamic acid)-poly(lactic acid); PBLG, poly(γ-benzyl L-glutamate); PBLG-LA, G-poly(c-benzyl-L-glutamate)-lipoic acid; PCL, polyethylene glycol-poly(ε-caprolactone); PEG; polyethylene glycol; PEI, polyethylenimine; PLGA, poly(lactic-co-glycolic acid); PTMC, poly(trimethylene carbonate); RGD, arginine–glycine–aspartic acid peptide; RGDS, arginine–glycine–aspartic acid–serine peptide; SPARC, secreted protein, acidic and rich in cysteine; TbFGF, truncated basic fibroblast growth factor; TPGS, tocopheryl polyethylene glycol 1000 succinate; UnTHCPSi, undecylenic acid modified, thermally hydrocarbonized porous silicon.
Table 2.
Summary of research articles that investigated active targeting NP delivering traditional/herbal medicines in cancer therapy
| Ligand | Receptor/antigen | Drug-NP platform | Types of study | Outcome
|
References | |
|---|---|---|---|---|---|---|
| Compared to non-targeted | Side effect | |||||
| Proteins/peptides | ||||||
| cRGD | Integrin αvb3 | Tanshinone IIA-mPEG-PLGA-PLL | In vitro: human hepatocellular carcinoma cell lines (Hep G2) In vivo: cell lines Hep G2 bearing mice |
Higher cellular uptake; increase in cytotoxicity Higher tumor growth inhibition rate; higher accumulation of drug in tumor; 2.5-fold higher life-extended rate |
No significant change in body weight | 9 |
| RGD | Integrin αvb3 | Curcumin-lipid-shell-polymer-core hybrid | In vitro: murine melanoma cell lines (B16) In vivo: cell lines B16 xenograft |
No difference in cytotoxicity for B16; 19.6% higher cellular apoptosis compared to free drug Higher tumor growth inhibition rate |
No significant change in body weight | 10 |
| Hyaluronic acid | ||||||
| Hyaluronic acid | CD44 receptor | 3,4-difluorobenzylidene curcumin-styrene maleic acid | In vitro: human pancreatic cancer cell lines (MiaPaCa-2, AsPC-1) | Higher cellular uptake in time-dependent manner; higher cytotoxicity – 1.75-fold for MiaPaCa-2 and 2-fold for AsPC-1 | Not evaluated | 63 |
| Folate | ||||||
| Folic acid | Folate receptor | Honokiol-PCEC | In vitro: human nasopharynx carcinoma cell lines HNE-1 In vivo: cell lines HNE-1 tumor-bearing mice |
Higher cellular uptake; 2.1-fold higher cytotoxicity compared with free drug; 15.2% higher percent of cell apoptosis 1.3-fold delay in tumor growth compared with free drug; 1.7-fold higher median survival time |
Not evaluated | 11 |
| Antibody | ||||||
| Anti-annexin A2 antibody | Annexin A2 receptor | Curcumin-PLGA | In vitro: human breast adenocarcinoma cell lines (MDA-MB-231) | Higher cellular uptake | Not evaluated | 64 |
| Aptamer | ||||||
| EpCAM aptamer | EpCAM protein | Curcumin-lipid-PLGA-lecithin hybrid | In vitro: human colon adenocarcinoma cell lines (HT29) and human embryonic kidney cell lines (HEK293T) | 64-fold higher cellular uptake; higher cytotoxicity compared to EpCAM-negative HEK293T | Not evaluated | 65 |
| Other molecules | ||||||
| HACE and AMPB | CD44 receptor and salicylic acid | Manassantin B-AMPB-HACE | In vitro: human breast adenocarcinoma cell lines (MDA-MB-231) In vivo: cell lines MDA-MB-231 xenograft |
Higher cellular uptake compared to HACE conjugates alone; higher cytotoxicity compared to HACE conjugates alone 2.4-fold higher tumor targeting compared to HACE conjugates alone; higher tumor growth inhibition rate |
No significant change in body weight | 66 |
Abbreviations: AMPB, (3-aminomethylphenyl)boronic acid; cRGD, cyclic arginine–glycine–aspartic acid peptide; HACE, hyaluronic acid-ceramide; mPEG-PLGA-PLL, methoxy polyethylene glycol-poly(lactic-co-glycolic acid)-poly-L-lysine; NP, nanoparticle; PCEC, poly(ε-caprolactone)-polyethylene glycol-poly (ε-caprolactone); PLGA, poly(lactic-co-glycolic acid); RGD, arginine–glycine–aspartic acid peptide.
Of the 61 articles included in the analysis, 54 (88.5%) investigated nanoparticles delivering modern chemotherapeutic drugs; the majority was doxorubicin (40.7%), followed by paclitaxel (8.5%). Types of targeting ligand platforms used included proteins or small peptides (15 articles), hyaluronic acids (HAs; 10 articles), folic acids (9 articles), antibodies (5 articles), aptamers (5 articles), carbohydrates or polysaccharide (5 articles), and other molecules (5 articles). Seven articles (11.5%) investigated nanoparticles delivering traditional/herbal medicines; the majority was curcumin (42.9%). The ligand platforms used were proteins or small peptides (2 articles), HA (1 article), folic acid (1 article), antibody (1 article), aptamer (1 article), and other molecule (1 article).
Discussion
Ligands for nanoparticle platform
Proteins or small peptides
Various types of proteins or small peptides were used to conjugate on the surface of nanoparticles to improve selectivity of chemotherapeutic drugs or traditional/herbal medicines to cancer cells. Transferrin, a serum glycoprotein, was one of the widely used targeting ligands. It plays a role in transferring iron from blood stream into the cells by binding to transfer-rin receptor on the cell surface. Upregulation of transferrin receptor has been reported in metastatic and drug-resistant cancer cells.67 The transferrin-conjugating nanoparticles delivering chemotherapeutic drugs have been shown to improve cellular uptake of the drugs by cancer cells and enhance in vitro and in vivo cytotoxicity. For instance, the transferrin-conjugated polyethylene glycol (PEG) nano-particle delivering hydroxycamptothecin was shown to provide longer retention time of drug in blood circulation, higher drug accumulation in cancer cells, and higher in vivo growth inhibitory activity against S180 tumor compared with non-targeted nanoparticles.16 In the study of transferrin-conjugated chitosan-PEG nanoparticles delivering paclitaxel, the targeted nanoparticles also exhibited higher cytotoxic activity to transferrin-overexpressing human non-small cell lung cancer cells (HOP-62). The respective half-maximal inhibitory concentrations (ICs50) were 0.3 µM and 2.0 µM.17 Apart from transferrin, arginine–glycine–aspartic acid (RGD) peptide has been used as targeting ligand to conjugate on the surface of nanoparticles to specifically target integrin αvβ3 receptor. This receptor is expressed on the surface of tumor vessels and various types of cancer cells and plays important roles in tumor growth promotion, metastasis, and angiogenesis.18 A number of RGD-conjugated nanoparticles delivering chemotherapeutic drugs or traditional/herbal medicines have been developed and demonstrated to promote their delivery to the cancer cells. The cyclic arginine–glycine–aspartic acid–tyrosine–lysine c(RGDyK)-conjugated poly(trimethylene carbonate)-PEG micellar nanoparticle delivering paclitaxel was shown to enhance cytotoxic activity of the drug to integrin αvβ3-overexpressing human glioblastoma cells (U87MG) compared with non-targeted nanoparticles and free drugs (mean IC50: 0.022 µg/mL, 0.051 µg/mL, and 0.058 µg/mL, respectively). The targeted nanoparticles also exhibited greater activity on cell apoptosis (11.23% vs 8.31% and 8.03% vs 5.38% inhibition, for early and late apoptosis, respectively). The percentages (mean values) of free drug were 6.67% and 4.32%, respectively. In addition, cellular drug uptake by U87MG cells was significantly increased.18 Superior cytotoxic potency against integrin αvβ3-overexpressing human cervical carcinoma cells (HeLa) together with increased cellular uptake was also demonstrated with RGD-conjugated magnetic iron oxide nanoparticles (MIONPs)-PEG delivering doxorubicin compared with free drug and non-targeted MIONPs.19 In another study, improved cytotoxic activity by the cRGDyK-conjugated poly(2-ethyl-2-oxazoline)-poly(d,l-lactide) nanoparticles delivering paclitaxel over the non-targeted nanoparticles and free drug was reported (mean IC50: 51.16 ng/mL, 64.53 ng/mL, and 62.95 ng/mL, respectively). The enhanced activity was through direct targeting of the integrin αvβ3-overexpressing prostate cancer cells (PC-3), as well as increasing of cellular uptake by PC-3 cells. Moreover, the targeted nanoparticle was also shown to enhance in vivo tumor growth inhibition rate in PC-3 tumor-bearing mice.20
Other types of peptides that have been applied for conjugation on the surface of nanoparticles to increase selectivity of chemotherapeutic drugs to cancer cells include bombesin peptide-conjugated poly(lactic-co-glycolic acid) (PLGA) and NR7 peptide-conjugated PLGA-PEG nanoparticles. Bombesin-conjugated nanoparticles delivering docetaxel specifically bind to gastrin-releasing peptide receptor, which is overexpressed on cell surfaces of prostate, breast, ovarian, pancreatic, and colorectal cancers.22,68 This targeted nanoparticle was shown to enhance cytotoxic activity of the drug to the gastrin-releasing peptide receptor overexpressing human breast cancer cells (MDA-MB-231) compared with non-targeted nanoparticles (mean IC50: 35.53 ng/mL and 142.23 ng/mL, respectively).22 The NR7 peptide-conjugated PLGA-PEG nanoparticle delivering doxorubicin was used for specific drug binding to epidermal growth factor receptor (EGFR) on the cancer cell surface.23 The EGFR is a known receptor that is overexpressed on various types of cancer cell surfaces including head and neck, renal, ovarian, breast, and non-small-cell lung cancer.47,69 Activation of this receptor results in inhibition of cell apoptosis, promotion of cell proliferation, triggering of angiogenesis, and enhancement of tumor survival and metastasis. Therefore, inhibition of the function of this receptor would be expected to benefit cancer treatment. The NR7 peptide-conjugated PLGA-PEG nanoparticles exhibited higher cytotoxic activity against human ovarian carcinoma cells (SKOV3) compared with non-targeted nanoparticles (mean IC50: 0.05 µg/mL and 3.12 µg/mL, respectively).23 Although most studies demonstrated satisfactory outcomes of peptide- or protein-conjugated nanoparticles on targeting cancer cells, one study reported that H2009.1 peptide-conjugated liposome delivering doxorubicin to cancer cells expressing integrin αvβ6 receptor could not improve the efficacy of the drug. This was due to the liposome platform preventing the targeting ligand from binding to the receptor on the cancer cell surface, and resulted in relatively low drug accumulation in cancer cells.12
Hyaluronic acid
HA is a negatively charged linear glycosaminoglycan that consists of d-glucuronic acid and N-acetyl-d-glucosamine. It can specifically bind to CD44 receptor that is overexpressed on the cell surface of various types of cancer including lung, breast, colon, prostate, gastric, and head and neck cancers.70 HA is a widely used targeting ligand to conjugate on the surface of nanoparticles to improve selectivity of chemotherapeutic drugs to cancer cells and enhance drug efficacy and safety. In one study, HA with the two molecular weights, ie, 9.5 kDa and 35 kDa, was used to conjugate polymeric micelles delivering paclitaxel. The conjugate using 9.5 kDa HA was found to effectively increase drug cellular uptake by CD44-overexpressing human breast adenocarcinoma cells (MCF-7) compared with 35 kDa HA. In murine hepatic carcinoma (Heps), it also exhibited tumor growth inhibition at a higher rate and greater accumulation at the tumor site compared with other nanoparticle formulations.27 These results suggest that the molecular weight of HA directly influenced the efficacy of drug-loaded active targeting nanoparticles. The HA-conjugated chitosan nanoparticle delivering cisplatin was shown to increase drug cellular uptake by CD44-positive human lung cancer cells (A549) and effectively enhance cytotoxic activity of the drug, compared with non-targeted nanoparticles.28 The HA-conjugated liquid crystalline nanoparticle delivering rapamycin was reported to increase cytotoxic activity of the drug to CD44-overexpressing human breast adenocarcinoma cells (MDA-MB-231) compared with non-targeted nanoparticles (mean IC50: 18 µg/mL and 24.3 µg/mL, respectively). Moreover, the targeted nanoparticles also enhanced in vivo tumor growth inhibition rate in Ehrlich ascites tumor-bearing mice.29
For traditional/herbal medicines, HA has also been used for conjugation on the surface of nanoparticles delivering 3,4-difluorobenzylidene curcumin resulting in increased cellular uptake and cytotoxic activity of the drug against human pancreatic cancer cells (MiaPaCa-2 and AsPC-1) compared with non-targeted nanoparticles (mean IC50: 140 nM, 160 nM, and 245 nM, respectively). Interestingly, when the CD44 receptor was blocked by free soluble HA, the cytotoxic activity to MiaPaCa-2 cells was comparable between the targeted and non-targeted nanoparticles (mean IC50: 234 nM and 245 nM, respectively).63 These results confirm that targeting ligand-conjugated nanoparticles enhances drug efficacy by improving cellular uptake through receptor-mediated endocytosis mechanism.
Folate
Folate or vitamin B9 is a stable and poorly immunogenic water-soluble vitamin. It is essential for DNA synthesis and replication, methylation, cell division and growth, and cell survival, particularly in rapidly dividing cells or cancer cells.71 Folic acid receptor is overexpressed on several cancer cell surfaces including ovarian, cervical, breast, lung, kidney, colorectal, and brain cancers.71 Using folic acid as cancer cell targeting of chemotherapeutic drug nanocarriers has been demonstrated in various studies to improve drug efficacy and safety profiles. The folic acid-conjugated PEG-PLGA nanoparticle delivering docetaxel was shown to increase drug cellular uptake by human cervical carcinoma cells (HeLa) with enhanced cytotoxic activity compared with free drug (mean IC50: 0.69 µg/mL and 8.29 µg/mL, respectively). It also significantly inhibited tumor growth in HeLa tumor-bearing mice.36 The folic acid-conjugated albumin nanoparticle delivering gemcitabine was shown to enhance cytotoxic activity of the drug against folic acid receptor-overexpressing human breast adenocarcinoma cells (MCF-7) compared with non-targeted nanoparticles (mean IC50: 0.175 µM and 0.240 µM, respectively). Similarly, in folic acid receptor-overexpressing human ovarian cancer cells (Ovcar-5), the targeted nanoparticles exhibited superior cytotoxic activity over the non-targeted nanoparticles (mean IC50: 0.173 µM and 0.279 µM, respectively). On the other hand, activity of the targeted nanoparticles was found similar to that of non-targeted nanoparticles against folate receptor expressing human pancreatic cancer cells (MIAPaCa-2) (mean IC50: 0.166 µM and 0.169 µM, respectively).38 In one study, the folic acid-conjugated PEG-PLGA nanoparticle delivering paclitaxel was shown to increase drug cellular uptake by folic acid-overexpressing human endometrial carcinoma cells (HEC-1A) with superior cytotoxic activity over the non-targeted nanoparticle (mean IC50: 3.43 µg/mL and 8.81 µg/mL, respectively). Moreover, it also produced significantly higher cell apoptotic activity compared with non-targeted and free drug (35.94%, 23.97% and 19%, respectively). In vivo, it produced significant tumor growth inhibition in HEC-1A tumor-bearing mice.43 For traditional/herbal medicines, folic acid-conjugated poly(epsilon-caprolactone)-PEG-poly (epsilon-caprolactone) nanoparticle delivering honokiol, a traditional Chinese medicine, was shown to increase compound cellular uptake by folic acid-overexpressing human nasopharynx carcinoma cells (HNE-1) with enhanced cytotoxic activity compared with free drug (mean IC50: 18.41 µg/mL and 38.59 µg/mL, respectively). Furthermore, the targeted nanoparticles also resulted in significant cell apoptotic activity compared with non-targeted nanoparticles (86.07% and 70.89%, respectively) and prolongation of median survival time compared with non-targeted nanoparticles and free drug (median survival time: 57.5 days, 42.5 days, and 34 days, respectively).11
Antibodies or antibody fragments
Antibodies or antibody fragments are one of the first targeting ligands used for conjugation on the surface of nanoparticles to target cancer cells due to their potential to specifically bind to antigens or receptors on cancer cell surfaces with high affinity. Various types of antibodies or antibody fragments have been used as targeting agents including anti-CD20 monoclonal antibody, anti-CD47 monoclonal antibody, EGFR antibody, and anti-Fas monoclonal antibody. These targeted nanoparticles have been shown to improve cellular uptake by cancer cells and enhance cytotoxic activity of the drugs to cancer cells. For instance, anti-CD20 monoclonal antibody-conjugated 1,2-distearoyl-sn-glycero-3-phosphoe-thanolamine-N-methoxypolyethylene-glycol-2000 delivers doxorubicin active carbon nanoparticles to the target CD20 receptor. It exhibited higher cytotoxic activity against CD20-positive human Burkitt’s lymphoma cells (Raji) compared with non-targeted nanoparticles.45 The anti-CD47 monoclonal antibody-conjugated iron oxide magnetic nanoparticle delivering gemcitabine to the target CD47 receptor was shown to increase drug cellular uptake by human pancreatic ductal adenocarcinoma cells (Panc215 and Panc354). Their cytotoxic activity was also significantly enhanced.46 The EGFR antibody-conjugated PLGA nanoparticle delivering rapamycin was shown to exhibit higher cellular uptake by EGFR-overexpressing human breast adenocarcinoma cells (MCF-7) with enhanced cell apoptotic activity against all cell stages.47 For traditional/herbal medicines, the anti-annexin A2 antibody-conjugated PLGA nanoparticle delivering curcumin was shown to significantly increase compound cellular uptake by human breast adenocarcinoma cells (MDA-MB-231).64
Aptamers
Aptamers are short single-stranded DNA or RNA sequences that can fold to three-dimensional structure and bind to specific targets on the cancer cell surfaces that express specific targets for different aptamers. For instance, nucleolin receptor is specific for AS14111 aptamer and EpCAM protein is specific for EpCAM aptamer. The aptamer AS14111-conjugated PEG-PLGA nanoparticle delivering gemcitabine to target nucleolin receptor was shown to increase drug cellular uptake (36%) by nucleolin-overexpressing human lung cancer cells (A549) compared with non-targeted nanoparticles, with enhanced cytotoxic activity (IC50: 4.9 µg/mL and 28.9 µg/mL, respectively).49 The AS14111-conjugated undecylenic acid modified, thermally hydrocarbonized porous silicon (UnTH-CPSi) nanoparticle delivering methotrexate was shown to increase drug cellular uptake by nucleolin-overexpressing human breast adenocarcinoma cells (MDA-MB-231) with enhanced cytotoxic activity compared with non-targeted nanoparticles and nucleolin-negative fibroblasts cells (NIH 3T3).50 The aptamer AS14111-conjugated polymersome delivering doxorubicin was shown to increase drug cellular uptake by nucleolin-overexpressing human breast adenocarcinoma cells (MCF-7) with enhanced cytotoxic activity compared with mutated aptamer-conjugated nanoparticles (mean IC50: 210.9 ng/mL and 369.4 ng/mL, respectively). In addition, it also produced significant tumor growth inhibition in MCF-7 tumor-bearing mice compared with mutated aptamer-conjugated nanoparticles.52 For traditional/herbal medicines, the EpCAM aptamer-conjugated lipid-polymer-lecithin hybrid delivering curcumin was shown to increase compound cellular uptake by EpCAM-overexpressing human colon adenocarcinoma cells (HT29) compared with EpCAM-negative human embryonic kidney cells (HEK293T), (58.9%±2.6% and 72.4%±1.3%, respectively.65
Carbohydrates or polysaccharides
Galactose is one of targeting ligands in group of carbohydrates that is widely used as a targeting agent for nanoparticles. It is specifically recognized by the asialoglycoprotein receptor (ASGPR), which is overexpressed on liver cancer cell surface.54 The galactose-conjugated lithocholic acid-PEG-lactobionic acid nanoparticles delivering doxorubicin was shown to increase drug cellular uptake by human liver cancer cells (SK-HEP-1) leading to massive cell death and tumor growth inhibition compared with non-targeted nanoparticles.54 The galactose-conjugated pectin nanoparticle delivering 5-fluorouracil was shown to increase drug cellular uptake by human hepatocellular liver carcinoma cells (HepG2) with enhanced cytotoxic activity compared with free drug (mean IC50: 0.17×10−4 mol/L and 0.45×10−4 mol/L, respectively). Moreover, the targeted nanoparticle also improved pharmacokinetic profile of the drugs.55 On the other hand, the lactose-conjugated nanoparticle delivering doxorubicin was shown to improve drug efficacy, but not as good as galactose.53 The galactose conjugates not only specifically bind to ASGPR but also to lectin receptor, which is overexpressed on the alveolar macrophages, liver endothelial Kupffer cells, splenic macrophages, peritoneal macrophages, and macrophages of brain. The galactose-conjugated solid lipid nanoparticles delivering doxorubicin specifically targeted human lung cancer cells (A549) resulting in higher cellular uptake, enhanced cytotoxic activity, and improved pharmacokinetic profiles compared with non-targeted nanoparticles and free drug.57
Controlled drug release of active targeting nanoparticles
Controlled drug release is a property of drug delivery systems in cancer therapy. Drugs are delivered and released at specific location to avoid side effects to normal cells.72 Most studies included in this review showed biphasic characteristics of drugs released from both targeted and non-targeted nanoparticles, ie, initial burst release, followed by sustained release. For instance, about 48% and 46% of gemcitabine were released from folic acid-conjugated bovine serum albumin nanoparticles and non-targeted nanoparticles during the first 2 hours, respectively. Sustained release of up to 99% and 94% was observed at 36 hours and pH 7.4 after burst release of targeted and non-targeted nanoparticles, respectively.38 About 22% and 29% of doxorubicin were shown to release from galactose-conjugated solid lipid nanoparticles and non-targeted nanoparticles during the first 8 hours, respectively. After burst release, sustained released was observed up to 76% and 93% at 144 h and pH 7.4 for targeted and non-targeted nanoparticles, respectively.57 Moreover, in some cases, the amount of drug released from nanoparticles at endolysosomal environment (pH 5.5) or cancer cell environment (pH 6.8) was shown to be higher than that from physiological environment (pH 7.4). Up to 60% of doxorubicin was released from anti-CD20-conjugated active carbon nanoparticles and non-targeted nanoparticles at 12 hours and at pH 5.5. At pH 7.4, on the other hand, the release from nanoparticles was only 20%.45 Similarly, about 28% and 24% of gemcitabine burst were released during the first 24 hours from AS1411 aptamer-conjugated PEG-PLGA nanoparticles and non-targeted nanoparticles, respectively. After burst release, up to 44% and 41% sustained release were observed in both formulations at 120 hours and pH 5.5 for targeted and non-targeted nanoparticles, respectively. Only 30% release was observed at pH 7.4.49 In another study, doxorubicin released from chondroitin sulfate A-deoxycholic acid at day 6 was 92%, 53%, and 34% for pH 5.5, 6.8, and 7.4, respectively.60 These results suggested that conjugation of targeting ligands on the surface of nanoparticles did not affect drug release from nanoparticles. Furthermore, higher amount of drug released at acidic pH would benefit the delivery of cancer chemotherapeutic agents to cancer cells with lower side effects to normal cells.
Conclusion
Active targeting nanoparticles of chemotherapeutic drugs or traditional/herbal medicines have been demonstrated in various studies both in vitro and in vivo to improve selectivity of cellular uptake of drugs to cancer cells through receptor-mediated endocytosis and/or cytotoxicity. They provide several advantages over the conventional chemotherapeutic drugs and non-targeted nanoparticle platform, particularly in regard to enhancement of drug efficacy and safety. Active targeting nanoparticles possess several advantages in cancer therapy including enhancement of selectivity of drugs to cancer cells to avoid side effects to normal cells, enhancement of drug accumulation and anticancer activity in cancer cells, and efficiency in control of drug release. Nevertheless, some disadvantages of active targeting nanoparticles include their limitation of clinical uses in only certain types of cancer that express specific receptors on the cell surfaces. Moreover, manufacturing of nanoparticle platforms is costly and requires sophisticated technology. Selection of the types of targeting nanoparticles is determined by the types of target proteins or receptors expressed on cancer cell surfaces. Clinical studies are required to confirm their application in cancer patients.
Acknowledgments
The authors would like to thank the Center of Excellence in Pharmacology and Molecular Biology of Malaria and Cholangiocarcinoma, Chulabhorn International College of Medicine at Thammasat University, Rangsit Center, for providing all the necessary support in conducting this systematic review.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Kuppens IE, Bosch TM, van Maanen MJ, et al. Oral bioavailability of docetaxel in combination with OC144-093 (ONT-093) Cancer Chemother Pharmacol. 2005;55(1):72–78. doi: 10.1007/s00280-004-0864-4. [DOI] [PubMed] [Google Scholar]
- 3.Vandana M, Sahoo SK. Long circulation and cytotoxicity of PEGylated gemcitabine and its potential for the treatment of pancreatic cancer. Biomaterials. 2010;31(35):9340–9356. doi: 10.1016/j.biomaterials.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Yu H, Tang Z, Zhang D, et al. Pharmacokinetics, biodistribution and in vivo efficacy of cisplatin loaded poly(L-glutamic acid)-g-methoxy poly(ethylene glycol) complex nanoparticles for tumor therapy. J Control Release. 2015;205:89–97. doi: 10.1016/j.jconrel.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14(5):1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 6.Zhuang Y, Deng H, Su Y, et al. Aptamer-functionalized and backbone redox-responsive hyperbranched polymer for targeted drug delivery in cancer therapy. Biomacromolecules. 2016;17(6):2050–2062. doi: 10.1021/acs.biomac.6b00262. [DOI] [PubMed] [Google Scholar]
- 7.Wu G, Wang Z, Bian X, Du X, Wei C. Folate-modified doxorubicin-loaded nanoparticles for tumor-targeted therapy. Pharm Biol. 2014;52(8):978–982. doi: 10.3109/13880209.2013.874533. [DOI] [PubMed] [Google Scholar]
- 8.Qi X, Fan Y, He H, Wu Z. Hyaluronic acid-grafted polyamidoamine dendrimers enable long circulation and active tumor targeting simultaneously. Carbohydr Polym. 2015;126:231–239. doi: 10.1016/j.carbpol.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Song D, Costanza F, et al. Targeted delivery of tanshinone IIA-conjugated mPEG-PLGA-PLL-cRGD nanoparticles to hepatocellular carcinoma. J Biomed Nanotechnol. 2014;10(11):3244–3252. doi: 10.1166/jbn.2014.1982. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, Lin D, Wu F, et al. Discovery and in vivo evaluation of novel RGD-modified lipid-polymer hybrid nanoparticles for targeted drug delivery. Int J Mol Sci. 2014;15(10):17565–17576. doi: 10.3390/ijms151017565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang B, Ni X, Chen L, et al. Honokiol-loaded polymeric nanoparticles: an active targeting drug delivery system for the treatment of nasopharyngeal carcinoma. Drug Deliv. 2017;24(1):660–669. doi: 10.1080/10717544.2017.1303854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray BP, McGuire MJ, Brown KC. A liposomal drug platform overrides peptide ligand targeting to a cancer biomarker, irrespective of ligand affinity or density. PLoS One. 2013;8(8):e72938. doi: 10.1371/journal.pone.0072938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao H, Zhang S, Yang Z, Cao S, Jiang X, Pang Z. In vitro and in vivo intracellular distribution and anti-glioblastoma effects of docetaxel-loaded nanoparticles functioned with IL-13 peptide. Int J Pharm. 2014;466(1–2):8–17. doi: 10.1016/j.ijpharm.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Namgung R, Mi Lee Y, Kim J, et al. Poly-cyclodextrin and poly-paclitaxel nano-assembly for anticancer therapy. Nat Commun. 2014;5:3702. doi: 10.1038/ncomms4702. [DOI] [PubMed] [Google Scholar]
- 15.Yang N, Jiang Y, Zhang H, et al. Active targeting docetaxel-PLA nanoparticles eradicate circulating lung cancer stem-like cells and inhibit liver metastasis. Mol Pharm. 2015;12(1):232–239. doi: 10.1021/mp500568z. [DOI] [PubMed] [Google Scholar]
- 16.Hong M, Zhu S, Jiang Y, et al. Novel anti-tumor strategy: PEG-hydroxycamptothecin conjugate loaded transferrin-PEG-nanoparticles. J Control Release. 2010;141(1):22–29. doi: 10.1016/j.jconrel.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 17.Nag M, Gajbhiye V, Kesharwani P, Jain NK. Transferrin functionalized chitosan-PEG nanoparticles for targeted delivery of paclitaxel to cancer cells. Colloids Surf B Biointerfaces. 2016;148:363–370. doi: 10.1016/j.colsurfb.2016.08.059. [DOI] [PubMed] [Google Scholar]
- 18.Jiang X, Sha X, Xin H, et al. Self-aggregated pegylated poly (trim-ethylene carbonate) nanoparticles decorated with c(RGDyK) peptide for targeted paclitaxel delivery to integrin-rich tumors. Biomaterials. 2011;32(35):9457–9469. doi: 10.1016/j.biomaterials.2011.08.055. [DOI] [PubMed] [Google Scholar]
- 19.Nazli C, Demirer GS, Yar Y, Acar HY, Kizilel S. Targeted delivery of doxorubicin into tumor cells via MMP-sensitive PEG hydrogel-coated magnetic iron oxide nanoparticles (MIONPs) Colloids Surf B Biointerfaces. 2014;122:674–683. doi: 10.1016/j.colsurfb.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 20.Gao Y, Zhou Y, Zhao L, et al. Enhanced antitumor efficacy by cyclic RGDyK-conjugated and paclitaxel-loaded pH-responsive polymeric micelles. Acta Biomater. 2015;23:127–135. doi: 10.1016/j.actbio.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Hu G, Zhang H, Zhang L, Ruan S, He Q, Gao H. Integrin-mediated active tumor targeting and tumor microenvironment response dendrimer-gelatin nanoparticles for drug delivery and tumor treatment. Int J Pharm. 2015;496(2):1057–1068. doi: 10.1016/j.ijpharm.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Kulhari H, Pooja D, Shrivastava S, Naidu VGM, Sistla R. Peptide conjugated polymeric nanoparticles as a carrier for targeted delivery of docetaxel. Colloids Surf B Biointerfaces. 2014;117:166–173. doi: 10.1016/j.colsurfb.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 23.Liu CW, Lin WJ. Polymeric nanoparticles conjugate a novel heptapeptide as an epidermal growth factor receptor-active targeting ligand for doxorubicin. Int J Nanomedicine. 2012;7:4749–4767. doi: 10.2147/IJN.S32830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taheri A, Dinarvand R, Atyabi F, et al. Enhanced anti-tumoral activity of methotrexate-human serum albumin conjugated nanoparticles by targeting with Luteinizing Hormone-Releasing Hormone (LHRH) peptide. Int J Mol Sci. 2011;12(7):4591–4608. doi: 10.3390/ijms12074591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu G, Chun X, Wang Y, He Q, Gao H. Peptide mediated active targeting and intelligent particle size reduction-mediated enhanced penetrating of fabricated nanoparticles for triple-negative breast cancer treatment. Oncotarget. 2015;6(38):41258–41274. doi: 10.18632/oncotarget.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai L, Qiu N, Li X, et al. A novel truncated basic fibroblast growth factor fragment-conjugated poly (ethylene glycol)-cholesterol amphiphilic polymeric drug delivery system for targeting to the FGFR-overexpressing tumor cells. Int J Pharm. 2011;408(1–2):173–182. doi: 10.1016/j.ijpharm.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 27.Yin S, Huai J, Chen X, et al. Intracellular delivery and antitumor effects of a redox-responsive polymeric paclitaxel conjugate based on hyaluronic acid. Acta Biomater. 2015;26:274–285. doi: 10.1016/j.actbio.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 28.Suh MS, Shen J, Kuhn LT, Burgess DJ. Layer-by-layer nanoparticle platform for cancer active targeting. Int J Pharm. 2017;517(1–2):58–66. doi: 10.1016/j.ijpharm.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Freag MS, Elnaggar YS, Abdelmonsif DA, Abdallah OY. Layer-by-layer-coated lyotropic liquid crystalline nanoparticles for active tumor targeting of rapamycin. Nanomedicine (Lond) 2016;11(22):2975–2996. doi: 10.2217/nnm-2016-0236. [DOI] [PubMed] [Google Scholar]
- 30.Upadhyay KK, Mishra AK, Chuttani K, et al. The in vivo behavior and antitumor activity of doxorubicin-loaded poly (γ-benzyl l-glutamate)-block-hyaluronan polymersomes in Ehrlich ascites tumor-bearing BalB/c mice. Nanomedicine. 2012;8(1):71–80. doi: 10.1016/j.nano.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Mizrahy S, Goldsmith M, Leviatan-Ben-Arye S, et al. Tumor targeting profiling of hyaluronan-coated lipid based-nanoparticles. Nanoscale. 2014;6(7):3742–3752. doi: 10.1039/c3nr06102g. [DOI] [PubMed] [Google Scholar]
- 32.Xiong H, Du S, Ni J, Zhou J, Yao J. Mitochondria and nuclei dual-targeted heterogeneous hydroxyapatite nanoparticles for enhancing therapeutic efficacy of doxorubicin. Biomaterials. 2016;94:70–83. doi: 10.1016/j.biomaterials.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Cho HJ, Yoon IS, Yoon HY, et al. Polyethylene glycol-conjugated hyaluronic acid-ceramide self-assembled nanoparticles for targeted delivery of doxorubicin. Biomaterials. 2012;33(4):1190–1200. doi: 10.1016/j.biomaterials.2011.10.064. [DOI] [PubMed] [Google Scholar]
- 34.Zhong Y, Zhang J, Cheng R, et al. Reversibly crosslinked hyaluronic acid nanoparticles for active targeting and intelligent delivery of doxorubicin to drug resistant CD44+ human breast tumor xenografts. J Control Release. 2015;205:144–154. doi: 10.1016/j.jconrel.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Sun B, Deng C, Meng F, Zhang J, Zhong Z. Robust, active tumor-targeting and fast bioresponsive anticancer nanotherapeutics based on natural endogenous materials. Acta Biomater. 2016;45:223–233. doi: 10.1016/j.actbio.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 36.Tao W, Zhang J, Zeng X, et al. Blended nanoparticle system based on miscible structurally similar polymers: a safe, simple, targeted, and surprisingly high efficiency vehicle for cancer therapy. Adv Healthc Mater. 2015;4(8):1203–1214. doi: 10.1002/adhm.201400751. [DOI] [PubMed] [Google Scholar]
- 37.Cheng L, Hu Q, Cheng L, et al. Construction and evaluation of PAMAM-DOX conjugates with superior tumor recognition and intracellular acid-triggered drug release properties. Colloids Surf B Biointerfaces. 2015;136:37–45. doi: 10.1016/j.colsurfb.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Dubey RD, Alam N, Saneja A, et al. Development and evaluation of folate functionalized albumin nanoparticles for targeted delivery of gemcitabine. Int J Pharm. 2015;492(1–2):80–91. doi: 10.1016/j.ijpharm.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Ji J, Zuo P, Wang YL. Enhanced antiproliferative effect of carboplatin in cervical cancer cells utilizing folate-grafted polymeric nanoparticles. Nanoscale Res Lett. 2015;10(1):453. doi: 10.1186/s11671-015-1162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye WL, Du JB, Zhang BL, et al. Cellular uptake and antitumor activity of DOX-hyd-PEG-FA nanoparticles. PLoS One. 2014;9(5):e97358. doi: 10.1371/journal.pone.0097358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morelli C, Maris P, Sisci D, et al. PEG-templated mesoporous silica nanoparticles exclusively target cancer cells. Nanoscale. 2011;3(8):3198–3207. doi: 10.1039/c1nr10253b. [DOI] [PubMed] [Google Scholar]
- 42.Yin JJ, Sharma S, Shumyak SP, et al. Synthesis and biological evaluation of novel folic acid receptor-targeted, β-cyclodextrin-based drug complexes for cancer treatment. PLoS One. 2013;8(5):e62289. doi: 10.1371/journal.pone.0062289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang C, Yang Y, Ling Y, Huang Y, Li T, Li X. Improved therapeutic effect of folate-decorated PLGA-PEG nanoparticles for endometrial carcinoma. Bioorg Med Chem. 2011;19(13):4057–4066. doi: 10.1016/j.bmc.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 44.McCarron PA, Marouf WM, Quinn DJ, et al. Antibody targeting of camptothecin-loaded PLGA nanoparticles to tumor cells. Bioconjug Chem. 2008;19(8):1561–1569. doi: 10.1021/bc800057g. [DOI] [PubMed] [Google Scholar]
- 45.Jiang S, Wang X, Zhang Z, et al. CD20 monoclonal antibody targeted nanoscale drug delivery system for doxorubicin chemotherapy: an in vitro study of cell lysis of CD20-positive Raji cells. Int J Nanomedicine. 2016;11:5505–5518. doi: 10.2147/IJN.S115428. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trabulo S, Aires A, Aicher A, Heeschen C, Cortajarena AL. Multifunctionalized iron oxide nanoparticles for selective targeting of pancreatic cancer cells. Biochim Biophys Acta. 2017;1861(6):1597–1605. doi: 10.1016/j.bbagen.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 47.Acharya S, Dilnawaz F, Sahoo SK. Targeted epidermal growth factor receptor nanoparticle bioconjugates for breast cancer therapy. Biomaterials. 2009;30(29):5737–5750. doi: 10.1016/j.biomaterials.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 48.Kouchakzadeh H, Shojaosadati SA, Mohammadnejad J, Paknejad M, Rasaee MJ. Attachment of an anti-MUC1 monoclonal antibody to 5-FU loaded BSA nanoparticles for active targeting of breast cancer cells. Hum Antibodies. 2012;21(3–4):49–56. doi: 10.3233/HAB-2012-0261. [DOI] [PubMed] [Google Scholar]
- 49.Alibolandi M, Ramezani M, Abnous K, Hadizadeh F. AS1411 Aptamer-decorated biodegradable polyethylene glycol-poly(lactic-co-glycolic acid) nanopolymersomes for the targeted delivery of gemcitabine to non-small cell lung cancer in vitro. J Pharm Sci. 2016;105(5):1741–1750. doi: 10.1016/j.xphs.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 50.Zhang F, Correia A, Mäkilä E, et al. Receptor-mediated surface charge inversion platform based on porous silicon nanoparticles for efficient cancer cell recognition and combination therapy. ACS Appl Mater Interfaces. 2017;9(11):10034–10046. doi: 10.1021/acsami.7b02196. [DOI] [PubMed] [Google Scholar]
- 51.Xu G, Yu X, Zhang J, et al. Robust aptamer–polydopamine-functionalized M-PLGA–TPGS nanoparticles for targeted delivery of docetaxel and enhanced cervical cancer therapy. Int J Nanomedicine. 2016;11:2953–2965. doi: 10.2147/IJN.S103513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Zhu X, Qiu L. Constructing aptamer anchored nanovesicles for enhanced tumor penetration and cellular uptake of water soluble chemotherapeutics. Acta Biomater. 2016;35:269–279. doi: 10.1016/j.actbio.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 53.Mou Q, Ma Y, Zhu X, Yan D. A small molecule nanodrug consisting of amphiphilic targeting ligand-chemotherapy drug conjugate for targeted cancer therapy. J Control Release. 2016;230:34–44. doi: 10.1016/j.jconrel.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 54.Singh B, Jang Y, Maharjan S, et al. Combination therapy with doxorubicin-loaded galactosylated poly(ethyleneglycol)-lithocholic acid to suppress the tumor growth in an orthotopic mouse model of liver cancer. Biomaterials. 2017;116:130–144. doi: 10.1016/j.biomaterials.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 55.Yu CY, Wang YM, Li NM, et al. In vitro and in vivo evaluation of pectin-based nanoparticles for hepatocellular carcinoma drug chemotherapy. Mol Pharm. 2014;11(2):638–644. doi: 10.1021/mp400412c. [DOI] [PubMed] [Google Scholar]
- 56.Liang HF, Chen SC, Chen MC, Lee PW, Chen CT, Sung HW. Paclitaxel-loaded poly(gamma-glutamic acid)-poly(lactide) nanoparticles as a targeted drug delivery system against cultured HepG2 cells. Bioconjug Chem. 2006;17(2):291–299. doi: 10.1021/bc0502107. [DOI] [PubMed] [Google Scholar]
- 57.Jain A, Kesharwani P, Garg NK, et al. Galactose engineered solid lipid nanoparticles for targeted delivery of doxorubicin. Colloids Surf B Biointerfaces. 2015;134:47–58. doi: 10.1016/j.colsurfb.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 58.Sandoval MA, Sloat BR, Lansakara-P DS, et al. EGFR-targeted stearoyl gemcitabine nanoparticles show enhanced anti-tumor activity. J Control Release. 2012;157(2):287–296. doi: 10.1016/j.jconrel.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Talelli M, Oliveira S, Rijcken CJ, et al. Intrinsically active nanobody-modified polymeric micelles for tumor-targeted combination therapy. Biomaterials. 2013;34(4):1255–1260. doi: 10.1016/j.biomaterials.2012.09.064. [DOI] [PubMed] [Google Scholar]
- 60.Lee JY, Chung SJ, Cho HJ, Kim DD. Bile acid-conjugated chondroi-tin sulfate A-based nanoparticles for tumor-targeted anticancer drug delivery. Eur J Pharm Biopharm. 2015;94:532–541. doi: 10.1016/j.ejpb.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Chen C, Hu H, Qiao M, et al. Anti-tumor activity of paclitaxel through dual-targeting lipoprotein-mimicking nanocarrier. J Drug Target. 2015;23(4):311–322. doi: 10.3109/1061186X.2014.994182. [DOI] [PubMed] [Google Scholar]
- 62.Zhang L, Yao J, Zhou J, Wang T, Zhang Q. Glycyrrhetinic acid-graft-hyaluronic acid conjugate as a carrier for synergistic targeted delivery of antitumor drugs. Int J Pharm. 2013;441(1–2):654–664. doi: 10.1016/j.ijpharm.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 63.Kesharwani P, Banerjee S, Padhye S, Sarkar FH, Iyer AK. Hyaluronic acid engineered nanomicelles loaded with 3,4-difluorobenzylidene curcumin for targeted killing of CD44+ stem-like pancreatic cancer cells. Biomacromolecules. 2015;16(9):3042–3053. doi: 10.1021/acs.biomac.5b00941. [DOI] [PubMed] [Google Scholar]
- 64.Thamake SI, Raut SL, Ranjan AP, Gryczynski Z, Vishwanatha JK. Surface functionalization of PLGA nanoparticles by non-covalent insertion of a homo-bifunctional spacer for active targeting in cancer therapy. Nanotechnology. 2011;22(3):035101. doi: 10.1088/0957-4484/22/3/035101. [DOI] [PubMed] [Google Scholar]
- 65.Li L, Xiang D, Shigdar S, et al. Epithelial cell adhesion molecule aptamer functionalized PLGA-lecithin-curcumin-PEG nanoparticles for targeted drug delivery to human colorectal adenocarcinoma cells. Int J Nanomedicine. 2014;9:1083–1096. doi: 10.2147/IJN.S59779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jeong JY, Hong EH, Lee SY, et al. Boronic acid-tethered amphiphilic hyaluronic acid derivative-based nanoassemblies for tumor targeting and penetration. Acta Biomater. 2017;53:414–426. doi: 10.1016/j.actbio.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 67.Bazak R, Houri M, El Achy S, Kamel S, Refaat T. Cancer active targeting by nanoparticles: a comprehensive review of literature. J Cancer Res Clin Oncol. 2015;141(5):769–784. doi: 10.1007/s00432-014-1767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cornelio DB, Roesler R, Schwartsmann G. Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy. Ann Oncol. 2007;18(9):1457–1466. doi: 10.1093/annonc/mdm058. [DOI] [PubMed] [Google Scholar]
- 69.Master AM, Sen Gupta A. EGF receptor-targeted nanocarriers for enhanced cancer treatment. Nanomedicine (Lond) 2012;7(12):1895–1906. doi: 10.2217/nnm.12.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kapoor A, Kumar S. Cancer stem cell: a rogue responsible for tumor development and metastasis. Indian J Cancer. 2014;51(3):282–289. doi: 10.4103/0019-509X.146794. [DOI] [PubMed] [Google Scholar]
- 71.Zwicke GL, Mansoori GA, Jeffery CJ. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Reviews. 2012;3 doi: 10.3402/nano.v3403i3400.18496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodzinski A, Guduru R, Liang P, et al. Targeted and controlled anticancer drug delivery and release with magnetoelectric nanoparticles. Sci Rep. 2016;6:20867. doi: 10.1038/srep20867. [DOI] [PMC free article] [PubMed] [Google Scholar]