Abstract
Introduction
The prominent immune checkpoint molecule, programmed cell death ligand-1 (PD-L1), is the object of increasing attention. Here, we report a meta-analysis investigating the safety and efficacy of durvalumab (MEDI4736), an inhibitor of PD-L1, in various solid tumors.
Methods
A systematic search of PubMed, Embase, and related articles was performed. Safety data were analyzed using Comprehensive Meta-Analysis software program version 2. Ultimately, 17 studies with 1,529 patients were included in our analysis.
Results
The major adverse events associated with durvalumab were pruritus and fatigue, while pruritus, increased alanine transaminase, and increased aspartate aminotransferase were common among patients treated with a combination of durvalumab and tremelimumab. Higher PD-L1 expression was associated with a superior objective response rate.
Conclusion
Durvalumab is safe in patients with many solid cancers and, in combination with tremelimumab, it has a tolerable safety profile and is associated with improved prognosis. PD-L1 expression is a biomarker of the efficacy of durvalumab.
Keywords: durvalumab, solid cancers, adverse effects, efficacy, meta-analysis
Introduction
The American Cancer Society recently published data predicting that 1,688,780 new cancer cases and 600,920 cancer-related deaths would occur in the USA in 2017.1 Newly developing therapies for cancer are increasing and serve to complement traditional approaches, such as surgery, chemotherapy, and radiotherapy. Among emerging therapies, immunotherapy is particularly noteworthy, and there are several ongoing trials for this approach.2
An important feature of cancer cells is their ability to escape from immune surveillance by interacting with T-cell receptors.3 Such interactions can hinder T-cell immunity and help cancer cells escape from protective immune responses, referred to as immune checkpoints.4 Two vital immune checkpoint-associated molecules are programmed cell death ligand-1 (PD-L1 or CD274) and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4 or CD152). Programmed cell death-1 (PD-1 or CD279) is a receptor present on activated T cells, while PD-L1 is expressed, or overexpressed, on the surfaces of various cancer cells.4,5 On formation, the PD-L1 and PD-1 complex releases signals that have inhibitory effects on T cells. These inhibitory signals can suppress T-cell-mediated immunity and may lead to tumor progression.5,6 CTLA-4 interacts with B7, which is expressed on antigen-presenting cells,4 that also physically interacts with the costimulatory factor, CD28. Hence, the interaction of CTLA-4 and B7 can impede T-cell activation by blocking the contact between CD28 and B7.7
The blockade of immune checkpoints can facilitate the recognition of cancer cells by an organism, allowing it to enhance antitumor immunity accordingly. Inhibitors function in both the priming phase (CTLA-4/B7) and the effector phase (PD-1/PD-L1) of immune cell (IC) cycles.4 CTLA-4 inhibitors were approved for clinical use in 2011, the year when ipilimumab was first used for the treatment of unresectable or metastatic melanoma.8 Durvalumab, a human immunoglobulin G1κ monoclonal antibody with high selectivity and affinity, can block the binding of PD-L1 to PD-1 and CD80.9 The US Food and Drug Administration (FDA) granted breakthrough therapy designation to durvalumab in February 2016 for patients with inoperable or metastatic PD-L1-positive urothelial bladder cancer and cancer progression following chemotherapy.3 As a PD-L1 inhibitor, durvalumab can be used alone or in combination with other therapies, such as chemotherapy, radiotherapy, targeted therapy, or other immunotherapy. We conducted this meta-analysis to evaluate the safety and efficacy of durvalumab for the treatment of various cancers.
Materials and methods
Literature search
Articles were identified by searching PubMed and Embase using the keywords “MEDI4736”, “durvalumab”, or “Imfinzi” (publications from 1974 to September 24, 2017). Relevant articles were also obtained by searching the reference list of primary articles or via relevant clinical trial information in American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) Congress databases.
Inclusion and exclusion criteria
Studies eligible for inclusion met the following criteria: 1) clinical trials in any phase concerning durvalumab, durvalumab plus tremelimumab, or durvalumab plus other targeted drugs; 2) patients involved had pathologically confirmed cancer; and 3) adverse events (AEs) or efficacy data were reported.
Studies were excluded if they met one of the following conditions: 1) no raw data; 2) lack of adequate data to evaluate the efficacy or safety of durvalumab; and 3) reviews, editorials, cases, letters, errata, or nonhuman studies.
To avoid duplication of data, we chose articles with more useful data rather than the most recent publications or those including more patients.
Data extraction
Two authors (HY and KS) independently considered eligible articles and extracted data. Disagreements were resolved by discussing with a third reviewer. The following data were extracted from eligible articles: 1) the basic characteristics of studies, including first author, year of publication, clinical trial information, study phase, treatment, number of participants, and type of cancer; 2) AEs that appeared in at least two papers; and 3) efficacy data including median progression-free survival (mPFS), median overall survival (mOS), complete response, partial response (PR), stable disease, and objective response rate (ORR).
Statistical analysis
Safety data analysis was performed using the Comprehensive Meta-Analysis software program version 2 (CMA V2, Biostat, Englewood, NJ, USA). We calculated the percentage and derived 95% confidence interval (CI) for any grade AEs in each study. A random-effects model was applied, where I2 ≥50%.
Results
Eligible articles
A total of 109 articles and conference reports were assessed, of which 17 were eligible.10–26 Three articles had the same clinical trial number (NCT01693562); however, each focused on a different tumor type.11,14,15 Clinical trial NCT02141347 was designed to investigate the safety of combining durvalumab with tremelimumab; however, only the results for durvalumab were provided.19 Eight articles on durvalumab and nine articles on durvalumab along with another drug were eligible for the final analysis; the detailed process of study selection is presented in Figure 1. Eligible articles included two Phase II, nine Phase I, and five Phase I/II studies, and they were all published between 2015 and 2017. The basic data from the included studies are shown in Table 1.
Figure 1.
Flow chart illustrating the article searching process used for this study.
Table 1.
Basic characteristics of the included studies
| Author, year | Clinical trial information | Study title | Phase | Participants (N) | Disease |
|---|---|---|---|---|---|
| Iguchi et al, 201525 | NCT01938612 | A Phase I, open-label, multicenter study to evaluate the safety, tolerability, and pharmacokinetics of MEDI4736 in patients with advanced solid tumors. | I | 22 | Advanced solid tumors |
| Ribas et al, 201524 | NCT02027961 | A Phase I open-label study on safety and tolerability of MEDI4736 in subjects with metastatic or unresectable melanoma in combination with dabrafenib and trametinib or with trametinib alone. | I | 50 | Advanced melanoma |
| Takahashi et al, 201519 | NCT02141347 | A Phase I open-label multicenter study to assess safety, tolerability, pharmacokinetics, and antitumor activity of tremelimumab/tremelimumab with MEDI4736 in Japanese with advanced solid malignancies or tremelimumab in Japanese with malignant mesothelioma. | I | 8 | Advanced solid malignancies |
| Lee et al, 201723 | NCT02484404 | Phase I/II study of the antiprogrammed death ligand-1 antibody MEDI4736 in combination with olaparib and/or cediranib for advanced solid tumors and advanced or recurrent ovarian, triple-negative breast, lung, prostate, and colorectal cancers. | I | 26 | Recurrent women’s cancers |
| Garassino et al, 201716 | NCT02087423 | A Phase II, noncomparative, open-label, multicenter, international study of MEDI4736 in patients with locally advanced or metastatic non-small cell lung cancer (stage IIIB–IV) who have received at least two prior systemic treatment regimens, including one platinum-based chemotherapy regimen. | II | 333 | Advanced or metastatic stage IIIB–IV NSCLC |
| Ahn et al, 201720 | NCT02143466 | A multiarm, Phase Ib, open-label, multicenter study to assess the safety, tolerability, pharmacokinetics, and preliminary antitumor activity of AZD9291 in combination with ascending doses of novel therapeutics in patients with EGFR-mutant advanced NSCLC who have progressed following therapy with an EGFR TKI (TATTON). | Ib | 34 | EGFR-mutant NSCLC |
| Gibbons et al, 201622 | NCT02088112 | A Phase I, open-label, multicenter study to assess the safety, tolerability, pharmacokinetics, and preliminary antitumor activity of gefitinib in combination with MEDI4736 (anti-PD-L1) in subjects with NSCLC. | I | 20 | TKI-naive with EGFR mutant NSCLC |
| Powles et al, 201717 | NCT01693562 | A Phase I/II study to evaluate the safety, tolerability, and pharmacokinetics of MEDI4736 in subjects with advanced solid tumors. | I/II | 191 | Locally advanced or metastatic UCC |
| Santa-Maria et al, 201726 | NCT02536794 | A single-arm Phase II study evaluating the efficacy and safety of MEDI4736 in combination with tremelimumab in patients with metastatic Her2-negative breast cancer. | NM | 18 | Metastatic breast cancer |
| Kelley et al, 201710 | NCT02519348 | A study of safety, tolerability, and clinical activity of MEDI4736 and tremelimumab administered as monotherapy and in combination to subjects with unresectable hepatocellular carcinoma. | I/II | 40 | Unresectable HCC |
| Wainberg et al, 201711 | NCT01693562 | A Phase I/II study to evaluate the safety, tolerability, and pharmacokinetics of MEDI4736 in subjects with advanced solid tumors. | I/II | 40 | Advanced HCC |
| Callahan et al, 201718 | NCT01975831 | A Phase I study to evaluate the safety and tolerability of anti-PD-L1, MEDI4736, in combination with tremelimumab in subjects with advanced solid tumors. | I | 105 | Advanced solid tumors |
| Reardon et al, 201712 | NCT02336165 | Phase II study to evaluate the clinical efficacy and safety of MEDI4736 in patients with glioblastoma. | II | 154 | Glioblastoma |
| Lin et al, 201613 | NCT02572687 | An open-label, multicenter, Phase I study of ramucirumab plus MEDI4736 in patients with locally advanced and unresectable or metastatic gastrointestinal or thoracic malignancies. | Ia | 20 | Advanced gastrointestinal or thoracic malignancies |
| Antonia et al, 201614 | NCT01693562 | A Phase I/II study to evaluate the safety, tolerability, and pharmacokinetics of MEDI4736 in subjects with advanced solid tumors. | I/II | 304 | NSCLC |
| Antonia et al, 201621 | NCT02000947 | A Phase Ib open-label study to evaluate the safety and tolerability of MEDI4736 in combination with tremelimumab in subjects with advanced NSCLC. | Ib | 102 | Advanced NSCLC |
| Segal et al, 201615 | NCT01693562 | A Phase I/II study to evaluate the safety, tolerability, and pharmacokinetics of MEDI4736 in subjects with advanced solid tumors. | I/II | 62 | Recurrent and metastatic SCCHN |
Abbreviations: EGFR, epidermal growth factor receptor; HCC, hepatocellular carcinoma; Her2, human epidermal growth factor receptor 2; NM, not mentioned; NSCLC, non-small-cell lung cancer; PD-L1, programmed cell death ligand-1; SCCHN, squamous cell carcinoma of head and neck; TKI, tyrosine kinase inhibitor; UCC, urothelial carcinoma.
In total, 17 studies with 1,529 patients were included in our analysis. The major AEs associated with durvalumab were pruritus and fatigue. Pruritus, increased alanine transaminase (ALT), and increased aspartate aminotransferase (AST) were commonly recorded for patients treated with a combination of durvalumab and tremelimumab. According to our analysis, higher PD-L1 expression was associated with superior ORR.
Safety analysis
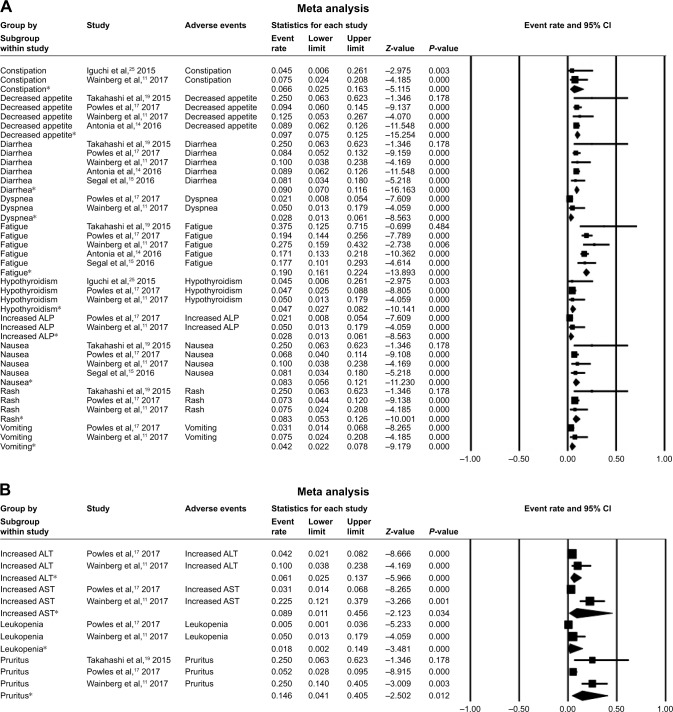
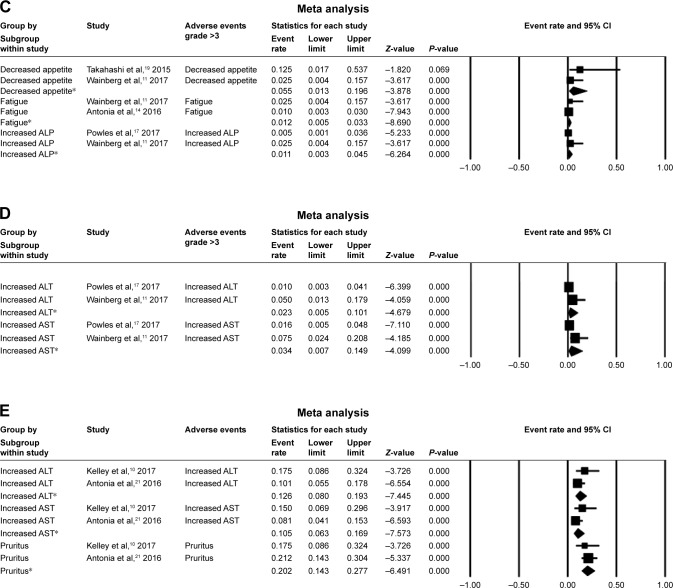
In our analysis, the major AEs associated with durvalumab were pruritus, fatigue, decreased appetite, diarrhea, increased AST, nausea, and rash. A random-effects model was used for analyses of increased ALT, increased AST, leukopenia, and pruritus. Pruritus had the highest overall event rate of 0.146 (95% CI: 0.041–0.405), followed by fatigue (0.190 [95% CI: 0.161–0.224]), and decreased appetite (0.097 [95% CI: 0.075–0.125]). Meanwhile, diarrhea and increased AST occurred at similar rates (0.090 vs 0.089). Common grade >3 AEs for durvalumab alone were decreased appetite (0.055 [95% CI: 0.013–0.196]) and increased AST (0.034 [95% CI: 0.007–0.149]). In trials combining durvalumab with tremelimumab, the most common AEs were pruritus (0.202 [95% CI: 0.143–0.277]), increased ALT (0.126 [95% CI: 0.080–0.193]), and increased AST (0.105 [95% CI: 0.63–0.169]) (Figure 2). Serious AEs that led to the discontinuation of the study drug were reported in 10 papers (Table 2).10,14,16,17,19–22,24,25 TATTON (NCT02143466) reported that grade 3–4 interstitial lung disease (ILD) occurred in 14.7% (5/34) of patients with epidermal growth factor receptor (EGFR)-positive advanced non-small-cell lung cancer (NSCLC) treated with a combination of durvalumab and AZD9291; this combination arm was terminated due to an increased incidence of ILD.20
Figure 2.
The rates and 95% CI of major AEs.
Notes: AE rates and 95% CI of (A) a fixed model for durvalumab alone and (B) a random model for durvalumab alone. Grade ≥3 AE rates and 95% CI of (C) a fixed model for durvalumab alone and (D) a random model for durvalumab alone. (E) AE rates and 95% CI of a fixed model for combination treatment with durvalumab and tremelimumab. *Analysis results of different trials for the adverse event.
Abbreviations: AE, adverse event; ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase.
Table 2.
Adverse events leading to therapy discontinuation
| Clinical trial information | Serious AEs | Incidence | Therapy | Cancer type |
|---|---|---|---|---|
| NCT01938612 | Grade 2 pneumonitis | 1/22 | Durvalumab | Solid tumors |
| NCT02141347 | NM | 1/8 | Durvalumab | Solid tumors |
| NCT02087423 | NM | 9/333 | Durvalumab | NSCLC |
| NCT01693562 | Autoimmune hepatitis Pneumonitis NM |
1/191 1/191 1/191 |
Durvalumab | UCC |
| NCT02519348 | Grade 3 pneumonitis Grade 3 colitis/diarrhea Asymptomatic grade 4 elevated AST and ALT |
1/40 1/40 1/40 |
Durvalumab | HCC |
| NCT01693562 | Grade 1–2 pneumonitis Grade 4 pneumonitis Grade 2–3 colitis Grade 4 colitis |
5/304 1/304 4/304 1/304 |
Durvalumab | NSCLC |
| NCT02027961 | Grade 3 thrombocytopenia Grade 3 choroidal effusion |
1/50 1/50 |
Durvalumab + trametinib ± dabrafenib | Melanoma |
| NCT02143466 | Grade 3–4 ILD | 5/34 | Durvalumab + AZD9291 | NSCLC |
| NCT02088112 | Grade 3–4 increased ALT and/or AST Grade 3–4 pneumonitis |
3/20 1/20 |
Durvalumab + gefitinib | NSCLC |
| NCT02000947 | Colitis Diarrhea Pneumonitis NM |
9/102 5/102 5/102 10/102 |
Durvalumab + tremelimumab | NSCLC |
Abbreviations: AEs, adverse events; ALT, alanine transaminase; AST, aspartate aminotransferase; HCC, hepatocellular carcinoma; ILD, interstitial lung disease; NM, not mentioned; NSCLC, non-small-cell lung cancer; UCC, urothelial carcinoma.
Efficacy analysis
There were five studies related to the efficacy of dur-valumab.11,12,15,16,25 Study NCT02087423 measured the expression of PD-L1 on tumor cell (TC) membranes in NSCLC patients and chose 25% as the cutoff value. The PD-L1-positive subgroup had higher ORR (16.4% vs 7.5%), mPFS (3.3 vs 1.9 months), and mOS (10.9 vs 9.3 months) values than the PD-L1-negative subgroup.16 When 90% was used as the cutoff value, ORR implausibly reached 30.9% (95% CI: 20.2–43.3) in the positive group, while mPFS was only 2.4 months (95% CI: 1.8–5.5).20 The other four studies, including 207 patients treated with durvalumab and tremelimumab, reported different ORRs ranging from 15% to 38% (Tables 3 and 4).10,18,21,26
Table 3.
Efficacy of treatment with durvalumab alone
| Clinical trial information | Subgroup | Evaluable patients (N) | PR (N) | CR (N) | SD (N) | ORR % (95% CI) | mPFS months (95% CI) | mOS months (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| NCT01938612 | Solid tumors | 22 | 1 | NM | 6 | NM | NM | NM | |
| NCT02087423 | Cohort 2 | PD-L1 ≥25% of TCs | 146 | NM | NM | NM | 16.4 (10.8–23.5) | 3.3 (1.9–3.7) | 10.9 (8.6–13.6) |
| PD-L1 <25% of TCs | 53 | NM | NM | NM | 7.5 (3.1–14.9) | 1.9 (1.8–1.9) | 9.3 (5.9–10.8) | ||
| Cohort 3 | PD-L1 ≥90% of TCs | 68 | NM | NM | NM | 30.9 (20.2–43.3) | 2.4 (1.8–5.5) | NR (5.9–NE) | |
| NCT01693562 | UCC | PD-L1 high | 98 | 23 | 4 | NM | 27.6 (19.0–37.5) | 2.1 (1.4–2.8) | 20.0 (11.6–NE) |
| PD-L1 low/negative | 79 | 2 | 2 | NM | 5.1 (1.4–12.5) | 1.4 (1.3–1.5) | 8.1 (3.1–NE) | ||
| NCT01693562 | HCC | 30 | 4 | NM | 14 | 10.0 (2.8–23.7) | 2.7 (1.4–5.3) | 13.2 (6.3–21.1) | |
| NCT02336165 | Glioblastoma | Cohort B | 30 | 4 | NM | 14 | NM | NM | NM |
Notes: PD-L1 high, ≥25% of either tumor cells or immune cells expressing PD-L1; PD-L1 low or negative, <25% of both tumor cells and immune cells expressing PD-L1.
Abbreviations: CR, complete response; HCC, hepatocellular carcinoma; m, median; mOS, median overall survival; mPFS, median progression-free survival; NE, not estimated; NM, not mentioned; NR, not reached; ORR, objective response rate; OS, overall survival; PD-L1, programmed cell death ligand-1; PFS, progression-free survival; PR, partial response; SD, stable disease; TCs, tumor cells; UCC, urothelial carcinoma.
Table 4.
The efficacy of durvalumab and tremelimumab
| Clinical trial information | Subgroup | Evaluable patient (N) | PR ORR % (N) (95% CI) |
|---|---|---|---|
| NCT02536794 | Breast cancer | 18 | 3 17 |
| NCT02519348 | HCC | 40 | NM 15 |
| NCT01975831 | Cervical cancer Colorectal cancer NTNBC Ovarian cancer RCC |
13 11 10 25 11 |
0 NM 1 NM 1 NM 2 NM 1 NM |
| NCT02000947 | PD-L1-positive NSCLC PD-L1-negative NSCLC PD-L1 0% NSCLC |
18 37 24 |
NM 33 (13–59) NM 30 (16–47) NM 38 (19–59) |
Notes: PD-L1 negative, <25% but <0% of tumor cells expressing PD-L1; PD-L1 positive, ≥25% of tumor cells expressing PD-L1.
Abbreviations: HCC, hepatocellular carcinoma; NM, not mentioned; NSCLC, non-small-cell lung cancer; NTNBC, non-triple-negative breast cancer; ORR, objective response rate; PD-L1, programmed cell death ligand-1; PR, partial response; RCC, renal cell carcinoma.
Discussion
Intravenous durvalumab (Imfinzi™; AstraZeneca, Cambridge, UK) received US FDA-accelerated approval for previously treated advanced bladder cancer in May 2017.27 It was reported to dramatically extend the mPFS of patients with stage III NSCLC after concurrent chemoradiotherapy compared with placebo (mPFS: 16.8% vs 5.6%) and is now recommended in the National Comprehensive Cancer Network (NCCN) guidelines.28 A large number of trials are currently ongoing, including the application of durvalumab in NSCLC, head and neck cancer, gastric cancer, hepato-cellular carcinoma, pancreatic cancer, mesothelioma, and hematologic cancers.4,5
Durvalumab attacks cancer cells via a complex mechanism. Under normal physiological conditions, PD-L1 is commonly expressed on ICs, epithelial cells, and endothelial cells, and its overexpression is observed in various cancers.5 The single-chain variable domain of durvalumab interacts with the immunoglobulin variable domain of PD-L1 on cancer cells, causing a steric clash that hinders the binding of PD-1 to PD-L1 and leads to T-cell activation and proliferation.29 Durvalumab has been demonstrated to inhibit tumor growth via a T-cell-associated mechanism in mouse xenograft models.30 The binding kinetics of durvalumab are similar to those of atezolizumab, which has been approved by the US FDA.5 Durvalumab does not bind with PD-L2 on macrophages and dendritic cells, avoiding potential toxic effects caused by PD-L2 inhibition.29
The recommended dosage of durvalumab is 10 mg/kg every 2 weeks via intravenous infusion until progression or unacceptable toxicity.27 Durvalumab exhibits a manageable safety profile and early-stage antitumor activity in various cancers, particularly those that are PD-L1-positive.
Our analysis indicates that the most common AEs associated with durvalumab treatment are pruritus and fatigue. This result is consistent with previous reports on other PD-1/PD-L1 inhibitors; pruritus, a dermatological AE, is observed in 34%–39% of patients and fatigue in 12%–37% of patients receiving PD-1/PD-L1 inhibitors.31 In our study, durvalumab was associated with a similar incidence of fatigue and a lower incidence of pruritus. Fortunately, the AEs mentioned above are generally mild and not dose-related.32
As a PD-L1 inhibitor, durvalumab not only helps to inhibit cancer development but also induces various immune responses. Such immune-related adverse events (irAEs) can influence many systems, causing diarrhea, colitis, skin rash, hyperthyroidism, hypothyroidism, thyroiditis, hepatic toxicities, pneumonitis, and other rare toxicities.33 Among these, skin rash is the most common, occurring in 34% patients receiving nivolumab and 39% of those administered pembrolizumab for melanoma.34,35 According to our study, the incidence of skin rash in patients receiving durvalumab is lower. Damage of the pulmonary system presents as pneumonitis of the lung parenchyma and occurs in <10% of patients treated with PD-1/PD-L1 inhibitors.33 Our analyses indicate that the occurrence of pneumonitis is rare among patients treated with durvalumab; therefore, compared with other PD-1/PD-L1 inhibitors, durvalumab is safer and its associated AEs are more tolerable.
To alleviate irAEs, immunosuppressive agents, including corticosteroids, antihistamines, and antitumor necrosis factor, can be used temporarily, without eliminating the antitumor response.36 Intravenous corticosteroids should be the first choice, while other immunosuppressive drugs, such as infliximab, could also be considered if corticosteroids are ineffective.36
The correlation between PD-L1 expression and efficacy remains a topic of discussion, and definitions of PD-L1 expression levels and the cutoff values used are controversial. Higher pretreatment PD-L1 expression (≥25%) and detectable interferon-gamma mRNA expression in tumor biopsies were reported as associated with higher ORR and better overall survival (OS) in NSCLC patients.37 Subsequently, Massard et al reported that PD-L1 status, defined by TC or IC (positive: TC or IC ≥25%; negative: TC and IC <25%), was not associated with durvalumab efficacy; rather, the combination of TC and IC had predictive value.38 Furthermore, agreement is yet to be reached on the standard antibody for testing PD-L1 expression by immunohistochemistry (IHC). The Blueprint PD-L1 IHC Assay Comparison Project compared four PD-L1 IHC assays (22C3, 28-8, SP263, and SP142) and concluded that three of them (22C3, 28-8, and SP263) generated similar TC staining; however, analysis of IC staining was lacking.39 PD-L1 expression varies among different tumor types and may alter following treatment;40 therefore, the expression of PD-L1 should be tested in samples obtained at several time points or from different locations.
PD-1/PD-L1 inhibitors are among the most effective immunotherapy methods; however, even for melanoma, the type of cancer most sensitive to immunotherapy, 60% patients continue to display primary resistance.5 Primary resistance is related to numerous factors, including both those that are tumor intrinsic and those that are extrinsic.41 Mutational burden is one of the most significant tumor intrinsic factors.42 Cancers with higher mutational loads, such as melanoma and NSCLC, exhibit superior responses to immunotherapy.43 For a given type of cancer, patients with higher nonsynonymous mutation burdens will have improved treatment responses and longer progression-free survival (PFS).44 EGFR is one of the most commonly mutated oncogenes in NSCLC. Patients with NSCLC with EGFR mutations or anaplastic lymphoma kinase (ALK) rearrangements exhibit limited responses to nivolumab, pembrolizumab, or atezolizumab.45 For NSCLC patients treated with PD-1/PD-L1 inhibitors, the objective responses were only 3.6% (1/28) in EGFR-mutant or ALK-positive patients vs 23.3% (7/30) in patients negative for these markers.45 Moreover, ~4 weeks after initial PR, these patients subsequently progressed. The latest update of ATLANTIC (NCT02087423) also demonstrated that the response was superior in EGFR−/ALK− NSCLC patients treated with durvalumab.46 Resistance may be associated with oncogenic signals in patients with EGFR mutations and ALK rearrangements, causing immunosuppression.47,48 Although EGFR mutations and ALK rearrangements lead to elevated PD-L1 expression, its levels are reduced after treatment with EGFR or ALK tyrosine kinase inhibitors (TKIs), which could cause resistance to PD-1/PD-L1 inhibitors.47–49 The major extrinsic factors influencing primary resistance are immunoregulatory factors within the tumor microenvironment, such as low PD-L1 expression levels, insufficient numbers of tumor-infiltrating lymphocytes, and severe exhaustion of T cells.41,42 The majority of primary responders eventually developed acquired resistance after treatment. The mechanism underlying such resistance may be associated with the loss of T-cell functional phenotype, as well as mutations of Janus kinase (JAK1/2), which limit the presentation of tumor antigens.42,50
To optimize the use of durvalumab and overcome resistance, combinations with other therapies have been designed. The combination of durvalumab with radiotherapy or chemotherapy resulted in IC death and the release of tumor antigens to promote immune responses.28,51 Inhibition of immunosuppressive factors can also enhance antitumor immunity.21,52 Among these, combination treatment with anti-CTLA-4 antibodies is particularly noteworthy because CTLA-4/B7 interaction has a separate function in T-cell immunity and could be used to target PD-L1-negative tumors. Patients with PD-L1-positive or -negative NSCLC who received durvalumab and tremelimumab combination therapy had similar ORRs because of the inclusion of the anti-CTLA-4 antibody.21 Our study indicates that the incidence of AEs increases but remains tolerable when combining durvalumab with other types of immunotherapy. Given tolerable AEs, the ORRs of combination immunotherapies are of great significance for cancer treatment.
Lung cancer was the major type of malignancy focused on among completed and ongoing trials of durvalumab, alone or as combination therapy with durvalumab and other treatments. A study on patients with NSCLC indicated that superior ORR, PFS, and OS were achieved using durvalumab to treat patients with high PD-L1 expression.16 In the trials discussed above, durvalumab was not only used alone but also combined with a CTLA-4 inhibitor (tremelimumab) for the treatment of advanced NSCLC or with EGFR-TKIs (gefitinib or AZD9291) for EGFR-positive NSCLC.21,22 In other ongoing or recruiting trials of NSCLC, durvalumab is reported to have multiple and noteworthy applications; for example, in maintenance, neoadjuvant/adjuvant, or first-line therapies.28,53–61 Several trials of the PD-L1 inhibitors, nivolumab and pembrolizumab, as neoadjuvant or adjuvant therapy are also in progress.62–65 Pembrolizumab was the first PD-L1 inhibitor approved by the US FDA as the first-line therapy for metastatic NSCLC patients with high PD-L1 expression (≥50%) and EGFR and ALK wild-type, or in combination with pemetrexed and carboplatin for metastatic nonsquamous NSCLC.66 Similar trials are investigating durvalumab, and another breakthrough for first-line therapy is anticipated in the future. There are two trials investigating the combination of durvalumab, tremelimumab, and radiation therapy (RT).67,68 Because of the complicated effects of RT on the immune system, such combinations may lead to improved efficacy. Nevertheless, there are concerns about the damage to the pulmonary system and the incidence of ILD. HUDSON (NCT03334617) is another important trial focusing on the treatment after resistance to durvalumab and may generate feasible suggestions for clinical applications (Table 5).69
Table 5.
Functions of durvalumab in ongoing trials of lung cancer
| Clinical trial information | Phase | Therapy | Disease |
|---|---|---|---|
| Maintenance therapy | |||
| NCT02117167 | II | Durvalumab | Metastatic NSCLC and SD/PR after four cycles of an induction platinum-based chemotherapy |
| NCT02125461 | III | Durvalumab | Stage III unresectable NSCLC and not progressed following definitive, platinum-based, concurrent chemoradiotherapy |
| Neoadjuvant and adjuvant therapy | |||
| NCT02273375 | III | Durvalumab | Completely resected NSCLC |
| NCT02572843 | II | Durvalumab | Primary resectable stage IIIA (N2) NSCLC |
| NCT03030131 | II | Durvalumab | Early stage (I–IIIA) NSCLC |
| First-line therapy | |||
| NCT02879617 | II | Durvalumab | Advanced NSCLC with ECOG performance status of 2 |
| NCT03003962 | III | Durvalumab | Advanced NSCLC with EGFR and ALK wild-type and high expression of PD-L1 |
| NCT03164616 | III | Durvalumab + chemotherapy with/without tremelimumab | Metastatic NSCLC with EGFR and ALK wild-type |
| NCT02453282 | III | Durvalumab with/without tremelimumab | NSCLC |
| NCT02542293 | III | Durvalumab + tremelimumab | NSCLC |
| With radiation therapy | |||
| NCT02888743 | II | Durvalumab + tremelimumab with/without RT | Stage IV NSCLC |
| NCT03275597 | I | Durvalumab + tremelimumab + SBRT | Stage IV oligometastatic NSCLC with EGFR and ALK wild-type |
| Others | |||
| NCT02669914 | II | Durvalumab | Lung cancer with refractory/recurrent brain metastases |
| NCT02352948 | III | Durvalumab with/without tremelimumab | Locally advanced or metastatic NSCLC (stage IIIB–IV) with EGFR and ALK wild-type |
| NCT02403271 | I/II | Durvalumab + ibrutinib | Relapsed or refractory NSCLC |
| NCT02503774 | I | Durvalumab + MEDI9447 | Advanced lung cancer |
| NCT02740985 | I | Durvalumab + AZD4635 | Advanced or metastatic NSCLC with EGFR and ALK wild-type |
| NCT02805660 | I/II | Durvalumab + mocetinostat | Advanced or metastatic NSCLC |
| NCT02898116 | I/II | Durvalumab + ensartinib | ALK-rearranged NSCLC |
| NCT02983578 | II | Durvalumab + AZD9150 | Advanced NSCLC |
| NCT03164772 | I/II | Durvalumab + mRNA vaccine with/without tremelimumab | Metastatic NSCLC with ALK wild-type |
| NCT03334617 | II | Durvalumab + olaparib/AZD9150/AZD6738/vistusertib | Metastatic or recurrent NSCLC with EGFR/ALK/ROS-1 wild-type and progressed on an anti-PD-1/PD-L1 containing therapy |
Abbreviations: ALK, anaplastic lymphoma kinase; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; NSCLC, non-small-cell lung cancer; PD-1, programmed cell death-1; PD-L1, programmed cell death ligand-1; PR, partial response; ROS-1, ROS proto-oncogene 1, receptor tyrosine kinase; RT, radiation therapy; SBRT, stereotactic body radiotherapy; SD, stable disease.
Overall, based on this meta-analysis of safety and efficacy, we conclude that durvalumab can be safely used for the treatment of many solid cancers and that its use in combination with tremelimumab deserves additional study. Combination treatment with durvalumab and tremelimumab leads to an increased incidence of AEs, including ALT, AST, and pruritus; therefore, patients receiving combination therapy should be closely followed up. High PD-L1 expression is a biomarker for better ORR, mPFS, and mOS for patients treated with durvalumab. Meanwhile, patients with high mutation burdens, EGFR wild-type, or ALK rearrangement-negative tumors will achieve greater benefit from durvalumab. The numerous ongoing or recruiting trials evaluating durvalumab and combination therapies for different solid tumors will provide additional data in the future.
Acknowledgments
We express our gratitude to Miss Sha Zhu who provided a great deal of assistance with manuscript editing, along with meaningful advice.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 3.Bellmunt J, Powles T, Vogelzang NJ. A review on the evolution of PD-1/PD-L1 immunotherapy for bladder cancer: the future is now. Cancer Treat Rev. 2017;54:58–67. doi: 10.1016/j.ctrv.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 5.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schildberg FA, Klein SR, Freeman GJ, Sharpe AH. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity. 2016;44(5):955–972. doi: 10.1016/j.immuni.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibrahim R, Stewart R, Shalabi A. PD-L1 blockade for cancer treatment: MEDI4736. Semin Oncol. 2015;42(3):474–483. doi: 10.1053/j.seminoncol.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. J Clin Oncol. 2017;35(15):4073. [Google Scholar]
- 11.Wainberg ZA, Segal NH, Jaeger D, et al. Safety and clinical activity of durvalumab monotherapy in patients with hepatocellular carcinoma (HCC) J Clin Oncol. 2017;35(15):4071. [Google Scholar]
- 12.Reardon DA, Kaley TJ, Dietrich J, et al. Phase 2 study to evaluate safety and efficacy of MEDI4736 (durvalumab [DUR]) in glioblastoma (GBM) patients: an update. J Clin Oncol. 2017;35(15):2042. [Google Scholar]
- 13.Lin CC, Golan T, Corral J, et al. Phase 1 study of ramucirumab (R) plus durvalumab (D) in patients (pts) with locally advanced and unresectable or metastatic gastrointestinal or thoracic malignancies ( NCT02572687); Phase 1a results. Ann Oncol. 2016;27(Suppl 8):viii1–viii17. [Google Scholar]
- 14.Antonia SJ, Kim SW, Spira AI, et al. Safety and clinical activity of durvalumab (MEDI4736), an anti-PD-LI antibody, in treatment-naive patients with advanced non-small-cell lung cancer. J Clin Oncol. 2016;34:9029. [Google Scholar]
- 15.Segal NH, Ou SHI, Balmanoukian AS, et al. Updated safety and efficacy of durvalumab (MEDI4736), an anti-PD-L 1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort. Ann Oncol. 2016;27(Suppl 6):vi328–vi350. [Google Scholar]
- 16.Garassino M, Vansteenkiste J, Kim JH, et al. PL04a.03: durvalumab in ≥3rd-line locally advanced or metastatic, EGFR/ALK wild-type NSCLC: results from the phase 2 ATLANTIC study. J Clin Oncol. 2017;12(1):S10–S11. [Google Scholar]
- 17.Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of Durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3(9):e172411. doi: 10.1001/jamaoncol.2017.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callahan MK, Odunsi K, Sznol M, et al. Phase 1 study to evaluate the safety and tolerability of MEDI4736 (durvalumab, DUR) + tremelimumab (TRE) in patients with advanced solid tumors. J Clin Oncol. 2017;35(15):3069. [Google Scholar]
- 19.Takahashi Y, Fujikawa K, Sagawa T, et al. A phase 1 study to assess the safety and tolerability of tremelimumab alone and in combination with MEDI4736 in Japanese patients with advanced solid malignancies. Eur J Cancer. 2015;5:S107. [Google Scholar]
- 20.Ahn MJ, Sun JM, Lee SH, Ahn JS, Park K. EGFR TKI combination with immunotherapy in non-small cell lung cancer. Expert Opin Drug Saf. 2017;16(4):465–469. doi: 10.1080/14740338.2017.1300656. [DOI] [PubMed] [Google Scholar]
- 21.Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicenter, phase 1b study. Lancet Oncol. 2016;17(3):299–308. doi: 10.1016/S1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibbons DL, Chow LQ, Kim DW, et al. 57O Efficacy, safety and tolerability of MEDI4736 (durvalumab [D]), a human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib (G): a phase I expansion in TKI-naïve patients (pts) with EGFR mutant NSCLC. J Thorac Oncol. 2016;11(Suppl 4):S79. [Google Scholar]
- 23.Lee JM, Cimino-Mathews A, Peer CJ, et al. Safety and clinical activity of the programmed death-ligand 1 inhibitor durvalumab in combination with poly (ADP-ribose) polymerase inhibitor olaparib or vascular endothelial growth factor receptor 1–3 inhibitor cediranib in women’s cancers: a dose-escalation, phase I study. J Clin Oncol. 2017;35(19):2193–2202. doi: 10.1200/JCO.2016.72.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribas A, Butler M, Lutzky J, et al. Phase I study combining anti-PD-L1 (MEDI4736) with BRAF (dabrafenib) and:or MEK (trametinib) inhibitors in advanced melanoma. J Clin Oncol. 2015;33(Suppl 15):3003. [Google Scholar]
- 25.Iguchi H, Nogami N, Kozuki T, et al. Phase I study to evaluate the safety and tolerability of MEDI4736, an anti-programmed cell death ligand-1 (PD-L1) antibody, in Japanese patients with advanced solid tumors. J Clin Oncol. 2015;33(Suppl 15):3039. [Google Scholar]
- 26.Santa-Maria CA, Jain S, Flaum L, et al. A phase II study of PD-L1 and CTLA-4 inhibition and immunopharmcogenomics in metastatic breast cancer. Cancer Res. 2017;77(Suppl 4) OT3-01-01. [Google Scholar]
- 27.Imfinzi™ (durvalumab): US prescribing information. 2017. [Accessed May 24, 2017]. Available from: https://www.fda.gov.
- 28.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 29.Zak KM, Kitel R, Przetocka S, et al. Structure of the complex of human programmed death 1, PD-1, and its ligand PD-L1. Structure. 2015;23(12):2341–2348. doi: 10.1016/j.str.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syed YY. Durvalumab: first global approval. Drugs. 2017;77(12):1369–1376. doi: 10.1007/s40265-017-0782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cousin S, Seneschal J, Italiano A. Toxicity profiles of immunotherapy. Pharmacol Ther. 2018;181:91–100. doi: 10.1016/j.pharmthera.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015;33(18):2092–2099. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber JS, Antonia SJ, Topalian SL, et al. Safety profile of nivolumab (NIVO) in patients (pts) with advanced melanoma (MEL): a pooled analysis. J Clin Oncol. 2015;33(Suppl 15):9018. [Google Scholar]
- 35.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 36.Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgs BW, Morehouse C, Streicher K, et al. Relationship of baseline tumoral IFNc mRNA and PD-L1 protein expression to overall survival in durvalumab-treated NSCLC patients. J Clin Oncol. 2016;34(Suppl):3036. [Google Scholar]
- 38.Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of Durvalumab(MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34(26):3119–3125. doi: 10.1200/JCO.2016.67.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12(2):208–222. doi: 10.1016/j.jtho.2016.11.2228. [DOI] [PubMed] [Google Scholar]
- 40.Madore J, Vilain RE, Menzies AM, et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res. 2015;28(3):245–253. doi: 10.1111/pcmr.12340. [DOI] [PubMed] [Google Scholar]
- 41.Pitt JM, Vétizou M, Daillère R, et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and-extrinsic factors. Immunity. 2016;44(6):1255–1269. doi: 10.1016/j.immuni.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Wang Q, Wu X. Primary and acquired resistance to PD-1/PD-L1 blockade in cancer treatment. Int Immunopharmacol. 2017;46:210–219. doi: 10.1016/j.intimp.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 43.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rizvi NA, Hellmann MD, Snyder KA, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garassino MC, Cho BC, Kim JH, et al. ATLANTIC Investigators Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521–536. doi: 10.1016/S1470-2045(18)30144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3(12):1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ota K, Azuma K, Kawahara A, et al. Induction of PD-L1 expression by the EML4-ALK oncoprotein and downstream signaling pathways in non-small cell lung cancer. Clin Cancer Res. 2015;21(17):4014–4021. doi: 10.1158/1078-0432.CCR-15-0016. [DOI] [PubMed] [Google Scholar]
- 49.Zhang M, Li G, Wang Y, et al. PD-L1 expression in lung cancer and its correlation with driver mutations: a meta-analysis. Sci Rep. 2017;7(1):10255. doi: 10.1038/s41598-017-10925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolchok JD, Saenger Y. The mechanism of anti-CTLA-4 activity and the negative regulation of T-cell activation. Oncologist. 2008;13(Suppl 4):2–9. doi: 10.1634/theoncologist.13-S4-2. [DOI] [PubMed] [Google Scholar]
- 53. [Accessed June 16, 2018];SAFIR02_Lung – Efficacy of targeted drugs guided by genomic profiles in metastatic NSCLC patients. Available from: https://ClinicalTrials.gov/show/NCT02117167. [Google Scholar]
- 54.Double blind placebo controlled study of adjuvant MEDI4736 In completely resected NSCLC. [Accessed June 16, 2018]. Available from: https://ClinicalTrials.gov/show/NCT02273375.
- 55.Anti-PD-L1 in stage IIIA(N2) non-small cell lung cancer (NSCLC) Available from: https://ClinicalTrials.gov/show/NCT02572843.
- 56.Immune neoadjuvant therapy study of durvalumab in early stage non-small cell lung cancer. [Accessed June 16, 2018]. Available from: https://ClinicalTrials.gov/show/NCT03030131.
- 57.A clinical trial of durvalumab (MEDI4736) as 1st line therapy in advanced non-small cell lung cancer patients. [Accessed June 16, 2018]. Available from: https://ClinicalTrials.gov/show/NCT02879617.
- 58.A study of durvalumab versus standard of care in advanced non small-cell lung cancer. [Accessed June 16, 2018]. Available from: https://ClinicalTrials.gov/show/NCT03003962.
- 59.Study of durvalumab + tremelimumab with chemotherapy or durvalumab with chemotherapy or chemotherapy alone for patients with lung cancer (POSEIDON) [Accessed June 16, 2018]. Available from: https://ClinicalTrials.gov/show/NCT03164616.
- 60.Phase III open label first line therapy study of MEDI 4736 (durvalumab) with or without tremelimumab versus SOC in non small-cell lung cancer (NSCLC) [Accessed June 16, 2018]. Available from: https://ClinicalTrials.gov/show/NCT02453282.
- 61.Study of 1st line therapy study of durvalumab with tremelimumab versus SoC in non small-cell lung cancer (NSCLC) (NEPTUNE) [Accessed June 16, 2018]. Available from: https://ClinicalTrials.gov/show/NCT02542293.
- 62.Study of induction checkpoint blockade for untreated stage I-IIIA non-small cell lung cancers amenable for surgical resection. [Accessed June 16, 2018]. Available from: https://ClinicalTrials.gov/show/NCT03158129.
- 63.Neoadjuvant anti PD-1 immunotherapy in resectable non-small cell lung cancer (NEOMUN) [Accessed June 16, 2018]. Available from: https://ClinicalTrials.gov/show/NCT03197467.
- 64.Adjuvant pembrolizumab in N2 positive non-small Cell lung Cancer Patients. [Accessed June 16, 2018]. Available from: https://ClinicalTrials.gov/show/NCT03053856.
- 65.Nivolumab after surgery and chemotherapy in treating patients with stage IB-IIIA non-small cell lung cancer (ANVIL) [Accessed June 16, 2018]. Available from: https://ClinicalTrials.gov/show/NCT02595944.
- 66.KEYTRUDA ®(pembrolizumab) Full Prescribing Information. Merck & Co., Inc; White-house Station, NJ, USA: 2017. [Google Scholar]
- 67.Durvalumab and tremelimumab with or without high or low-dose radiation therapy in treating patients with metastatic colorectal or non-small cell lung cancer. [Accessed June 16, 2018]. Available from: https://ClinicalTrials.gov/show/NCT02888743.
- 68.Phase Ib Study of Stereotactic Body Radiotherapy (SBRT) in Oligometastatic Non-small Lung Cancer (NSCLC) With Dual Immune Checkpoint Inhibition. [Accessed June 16, 2018]. Available from: https://ClinicalTrials.gov/show/NCT03275597.
- 69.Phase II umbrella study of novel anti-cancer agents in patients with NSCLC who progressed on an anti-PD-1/PD-L1 containing therapy. [Accessed June 16, 2018]. Available from: https://ClinicalTrials.gov/show/NCT03334617.