Abstract
Purpose
The aim of this study was to evaluate the clinical outcomes of a novel designed hydrophobic, acrylic, monofocal, fully preloaded intraocular lens (IOL; CT LUCIA 611P) 1 year after implantation. Scanning electron microscopic analysis regarding the optic–haptic junction and sharp edges of the IOL was performed.
Patients and methods
This is a noninterventional, observational prospective study of cataract patients who underwent implantation of the CT LUCIA 611P. Ninety-six eyes of 54 subjects were enrolled. Follow-up included visual acuity assessment, slit lamp examination with special focus on appearance of glistenings and evaluation of posterior capsule opacification (PCO). Scanning electron microscopic analysis of the new designed optic–haptic junction and edges of the IOL was performed.
Results
Best-corrected distance visual acuity increased from mean 0.48 logMAR (range 0.86–0.34) preoperatively to mean 0.02 logMAR (range 0.14 to −0.10) 1 year after surgery. Thirty-eight of 42 subjects’ eyes (90.5%), which underwent bilateral surgery with implantation of the IOL, never required glasses for distance again, while 4 (9.5%) required glasses only in rare cases (eg, driving at night). The spherical equivalent was within ±0.50 D in 88 of 96 subjects (91.7%) and within ±0.75 D in 96.9% of cases. Target refraction ±1.00 D was achieved in 100% of subject eyes. No glistenings were reported in any case. From the surgeons’ perspective, the wider, thicker optic–haptic transition of the IOL resulted in significantly increased stiffness, which enabled improved centering of the IOL and enhanced rotational stability and refractive predictability and stability and PCO prevention.
Conclusion
The results of this long-term observational study demonstrate the safety and efficacy of the IOL. Because of the completely new designed thicker and stiffer optic–haptic junction regarding improved characteristics of the IOL (stability in the capsular bag), some special attention has to be addressed to the slightly different behavior of the lens during implantation and unfolding process.
Keywords: intraocular lens, IOL, CT LUCIA 611P(Y), cataract surgery, quality of vision, aspheric design, glistenings, square edge, Achilles Heel of the IOL
Introduction
In recent years, more and more importance has been given to excellent visual and refractive outcomes following standard cataract surgery. The differentiation to refractive surgery gets negligible as every patient, regardless of age, is demanding optimal visual outcomes and reduced dependence on spectacle lenses postoperatively. Thus, cataract surgery is now considered a refractive procedure, not only in cases of clear lens extractions but also in standard procedures involving elderly patients. While there are number of manufacturers offering so-called “premium” intraocular lenses (IOLs) that aim to deliver on the promise of optimal visual outcomes post cataract, any novel IOL design must offer excellent optical performances (with minimal side effects and/or changes of refractive outcomes over time) as well as best possible user-friendliness for the surgeon and the scrub nurse.1 In general, in order to provide optimal vision following insertion, hydrophobic, acrylic IOLs must be made using a high-purity material designed to reduce the incidence of glistenings, possess optics that account for eyes requiring aspheric, spheric or neutral corrections and feature a lens design with an overall diameter that fits through small corneal incisions and is large enough to allow for optimal centration in the capsular bag for stable refractive results. Ideally, they should include square edge technology to prevent posterior capsule opacification (PCO).2,3 Moreover the lens should be made of a material that is consistent over time, meaning to be absolutely and verifiably glistening-free at any time. The visual significance of glistenings has been actively analyzed and extensively discussed for 25 years. Glistenings consist of multiple microvacuoles that cause retinal stray light and may affect quality of vision by having an impact on contrast sensitivity. The size, distribution and density of glistenings together with the index of IOL material contribute to the extinction coefficient, which is a parameter for the impact on contrast sensitivity.4–6
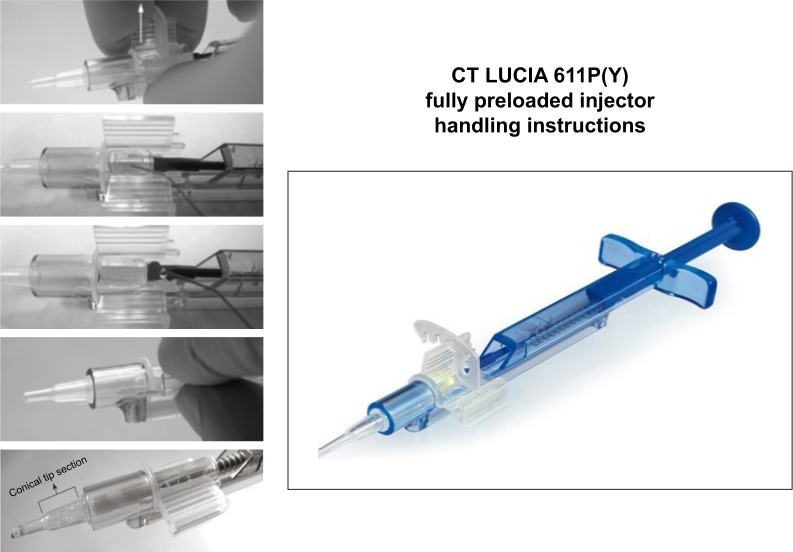
When a new monofocal IOL is placed on the market, it should fulfill all these properties. In this survey, we have not only clinically tested the refractive outcomes and stability but also evaluated the optical quality and reviewed if there is any kind of existence of glistenings at any time. The CT LUCIA 611P(Y) IOL by Zeiss Meditec (Jena, Germany) is a fully preloaded hydrophobic, acrylic, single-piece, heparin-coated IOL with an overall diameter of 13 mm and an optic diameter of 6 mm (Figure 1). The lens is made with ultrahigh-purity hydrophobic acrylic (copolymer of acrylates/methacrylates) and a proprietary cryo-lathing process. The material is Soxhlet-extracted for higher purity and has a water content of 0.3% and a refractive index of 1.49. The lens is available in clear UV-blocking (611P) and with blue light filtering (yellow tinted as 611Y) in a range of 4.00–34.00 D in 0.50 D increments. This lens comes completely preloaded in the Zeiss Blueject injector to facilitate a fast and easy lens preparation avoiding unwanted lens manipulation. The Blueject 2.0 tip injector can be used for the diopter range of 4.00–24.00 D, the 2.2 tip injector for the diopter range of 24.50–30.00 D and the 2.4 tip injector for the diopter range of 30.50–34.00 D. The incision size is recommended by the company to be 0.1–0.2 mm larger than the actual tip size (Figure 2).
Figure 1.
The design of the CT LUCIA 611P(Y) intraocular lens (Zeiss Meditec, Jena, Germany) and the specifications.
Figure 2.
The fully preloaded injector (Blueject 2.0) by Zeiss Meditec (Jena, Germany).
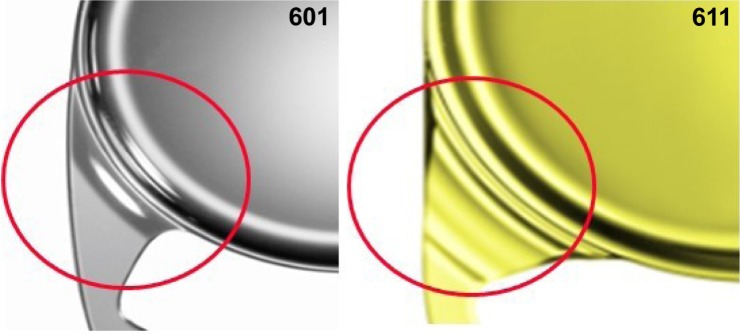
The 611P(Y) is equipped with a 360° square edge design (with a radius of <3 µm) on the entire IOL including the optic, the haptics and the optic–haptic transition (Figure 3) to prevent cell migration and PCO. In addition, the haptics are step-vaulted to translate the optic posteriorly for direct contact with the capsular bag. The optic of the lens has a special aspheric design, called ZO optic (patented by Zeiss Meditec), to compensate for the range of aberrations that arise from different corneal shapes and lens misalignments. The optic–haptic junction was completely redesigned and changed in comparison to the predecessor CT LUCIA 601 (Figure 4).7 In theory, the optic design should make the lens more insensitive to decentration and tilt, and given that the profile of the optic–haptic junction is thicker and more expanded, there should be more stiffness to the IOL, which would result in better centering and alignment properties in the capsular bag, thus improving refractive predictability and long-term results regarding refractive stability. For this study, we not only clinically tested the refractive outcomes and stability but also evaluated the optical quality (including the presence of glistenings postoperatively) of the CT LUCIA 611P(Y) IOL manufactured by Zeiss Meditec.
Figure 3.
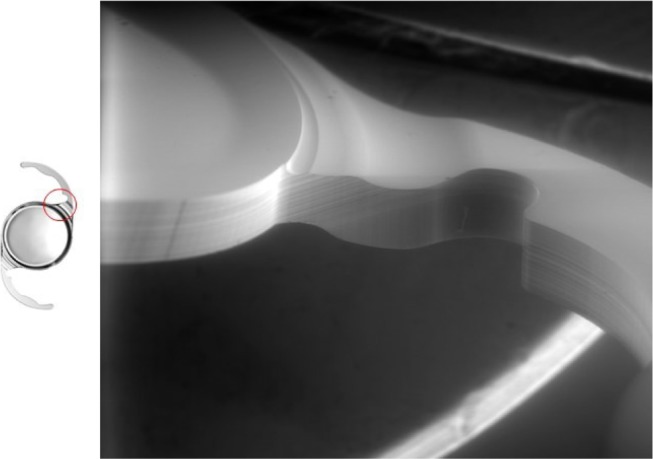
The optic–haptic junction (Achilles Heel) of the intraocular lens with 360° square edge technology. The shape of the CT LUCIA 611P(Y) was completely redesigned. It is thicker, wider and stiffer than the predecessor (601) providing more stability in the capsular bag; scanning electron microscopic analysis: Borkenstein EM, Borkenstein AF, Graz, Austria.
Figure 4.
Schematic illustration of the optic–haptic junction (601 and 611) in comparison. Courtesy of Zeiss Meditec (Jena, Germany).
Patients and methods
This noninterventional, observational prospective study was designed to evaluate the safety and efficacy of the CT LUCIA 611P(Y). The study was approved by the local ethics committee Ethikkommission der Medizinischen Universität Graz, Austria, and given a positive vote. As such, primary end points included visual and refractive outcomes and refractive stability. In addition, assessment for the presence of glistenings and PCO 1 year postoperatively was also considered an important metric for evaluation of the novel IOL’s capabilities. Written informed consent was obtained from all subjects prior to initiation, and the study was conducted in accordance with the local ethics committee. Subjects older than 50 years of age who had been diagnosed with cataracts and underwent standard phacoemulsification with implantation of the CT LUCIA 611P(Y) were considered for inclusion in the final analysis. The subjects also had to be willing and able to sign the informed consent documentation and complete all required postoperative visits. Moreover, all study subjects had to be clear of any intraocular media other than cloudy lens. Those with other ocular pathologies and degenerative visual disorders such as age-related macular degeneration, retinal disorders or glaucoma, amblyopia and preoperative astigmatism of >1.00 D were excluded from the final analysis, as were those who had undergone prior refractive surgery or who had any ocular inflammation or edema.
Eligible subjects who provided informed consent had a preoperative visit, an operative visit (Day 0) and 5 postoperative visits (visit 1, 1 day after surgery; visit 2, 1 week after surgery; visit 3, 4 weeks after surgery; visit 4, 3 months after surgery and visit 5, 1 year after surgery). The preoperative visit included a standard ophthalmic examination and manifest refraction, the evaluation of the best-corrected distance visual acuity (BCDVA), corneal topography, keratometry and pachymetry (Pentacam AXL; Oculus, Wetzlar, Germany), biometry and axial length measurement (IOL-Master; Zeiss Meditec) and optical coherence tomography examination of the retina. Lens status was evaluated by using the Lens Opacification Classification System III (LOCS III).8 IOL calculation aimed for target refraction of emmetropia using SRK/T formula or Haigis formula (eyes with axial length >26 mm). Postoperative assessments included evaluation of BCDVA, slit lamp evaluation for glistenings, Scheimpflug measurement and photo documentation, rating of PCO using the Evaluation of Posterior Capsule Opacification (EPCO) software system and monitoring for adverse events (AEs).9–11 With Scheimpflug measurement (Pentacam AXL; Oculus), the anterior chamber depth and the position of the IOL were analyzed. Postoperative measurements were compared, and images overlaid to recognize any myopic/hyperopic shifts of the IOL. Following every surgery, scrub nurses and the surgeon filled out a questionnaire designed to assess operating room workflow and the performance of the IOL’s fully preloaded injector system. In this questionnaire, handling of the injector and the lens, time for IOL preparation and overall satisfaction of the fully preloaded IOL were evaluated. In addition, the new IOL (CT LUCIA 611P) and the predecessor (CT LUCIA 601P) were analyzed by electron microscopy. The focus of attention was the optic–haptic junction which was completely new designed.
Results
A total of 96 eyes of 54 subjects were included in the final analysis. In 42 study subjects, cataract surgery was performed bilaterally within 3 weeks of the initial evaluation. In 12 subjects, surgery with implantation of the CT LUCIA 611P(Y) was performed unilaterally. The mean age of the study subjects was 75.6±12.3 years, and 33 were female (61.1%) and 21 were male (38.9%). In all cases, a clear corneal incision with 2.4 mm was performed. Phacoemulsification was uneventful in all cases. In 13 cases, a Malyugin ring 2.0 measuring 6.25 mm was used to expand the iris. In 6 cases with progressed cataracts, the colorant blue color cap was used to stain the anterior capsule prior performing rhexis. In 11 cases, a cohesive ophthalmic viscosurgical device (OVD) was used in addition to the standard OVD (methylcellulose 2%). In 5 ripe, mature cases of hard brunescent cataracts, the “Arshinoff soft-shell technique” were implemented to provide more endothelial protection as higher phaco energy dissipated in the eye. In these cases, 2 viscoelastic agents, a dispersive and a cohesive OVD, were used simultaneously.12,13 The cloudy lenses were graded with the LOCS III in 42 nuclear (8×NC4, 22×NC5, 12×NC6), 36 cortical (3×C2, 11×C3, 15×C4, 7×C5) and 18 subcapsular posterior (1×P3, 14×P4, 3×P5) lens opacities. The mean power of the implanted CT LUCIA 611P(Y) IOL was 22.50 D (range 6.00–32.50). IOL insertion was uneventful in all cases, except for 1 at the beginning of the study. In this subject, the scrub nurse filled the preloaded injector on the incorrect side of the OVD in error. After closing the lid of the IOL chamber and pushing the plunger forward, portions of the trailing haptic were trapped and broken. Fortunately, the error was recognized by the surgeon during the implantation process, with the IOL in corneal tunnel, and the broken lens was removed easily and replaced by a new, intact IOL.
Visual outcomes
In all study subjects, BCDVA increased from mean 0.48 logMAR (range 0.86–0.34) preoperatively to mean 0.06 logMAR (range 0.12 to −0.08) 3 months postoperatively and to mean 0.02 logMAR (range 0.14 to −0.10) 1 year after surgery. In all, 38 of 42 subjects (90.5%), who underwent bilateral surgery with implantation of the CT LUCIA 611P(Y), did not require glasses for distance vision 1 year postoperatively and 4 (9.5%) needed spectacles only in rare cases (eg, driving at night or in poor weather conditions). The postoperative spherical equivalent was within ±0.50 D in 88 of 96 subject eyes (91.7%) and within ±0.75 D in 96.9% of all subject eyes. The target refraction of ±1.00 D was achieved in all study subjects (100%). From the surgeon’s perspective, the novel IOL demonstrated visual and refractive stability over the entire follow-up period. No myopic shifts or changes in refraction were observed between 1 and 12 months following surgery. The enhancement of BCDVA between visit 3 and 5 (during which time refraction was stable) may be explained by neuroadaptation and customization in daily life. At postoperative visits 3, 4 and 5 (1 month, 3 months and 1 year after surgery), assessment for the presence of glistenings, using slit lamp, and their effect on study subjects’ visual function were performed. These assessments were photo documented for comparisons over time. However, absolutely no glistenings of any grade for any subject eyes were found at any study visit (0%).
The “Halo & Glare Simulator” showed inconspicuous results, and no complaints of halos or glare were documented. In all, 5 eyes (5.2%) had to undergo secondary interventions within the observational period. These included 2 cases of mature white cataracts with primary PCO, for which neodymium:YAG (Nd:YAG) capsulotomy was performed 8–10 weeks postoperatively. In addition, there was 1 case of higher myopia in which a retinal hole at the 2:00 o’clock position was detected~10 months after initial surgery. This was adequately and successfully rectified via laser treatment. In 1 case (1.04%), the subject eye’s posterior capsule became cloudy indicating the presence of PCÔ11 months following surgery. Two months later (13 months postoperatively), visual acuity degraded (2 lines) and symptoms of glare increased. Nd:YAG capsulotomy was performed, and visual performance fully recovered subsequently. In all other subject eyes (98.96%), no significant PCO that necessitates an intervention (laser capsulotomy) 1 year after surgery was detected at visit 5. (YAG capsulotomy of PCO was performed if BCDVA worsened >2 lines or in case of increase of subjective discomfort of glare.) No IOL explantations or other secondary procedures were performed during the observational period. In 4 subject eyes, secondary findings of dry eye syndrome (keratoconjunctivitis sicca) and related symptoms such as itchy and red eyes, blurred vision and photophobia were reported; these were treated with preservative-free artificial tears and symptoms resolved.
Surgical workflow with the Blueject injector
In results tabulated from the postoperative questionnaires submitted by the scrub nurse and the surgeon, overall satisfaction levels were high. On a scale of 1–10, average satisfaction score was 8 for nurses and 9 for the surgeon. Respondents confirmed easy, fast and secure handling of the IOL injector. The only negative finding among scrub nurses was difficulty in unbagging of the injector while taking it out of the box (92.7% of all cases). Surgeons reported slightly higher injection forces while inserting the IOL compared to other acrylic, hydrophobic lenses. When the injector preparation of the scrub nurse started too early, the IOL was dwelled to long in the bell section of the injector leading to higher injection forces. In some cases (7.3%), the trailing haptic was more difficult to position in the capsular bag because it rised up toward anterior chamber. All these facts may be attributed to the new, thicker and wider optic–haptic junction. As the length of the folded IOL is longer with the 611 than with the 601 (predecessor), the lens may roll to the left, the leading haptic may stretch the posterior capsule and the trailing haptic may be crumpled (35.4%). It seems to be essential to turn the injector a quarter clockwise to have the haptics delivered in a planar fashion. The unfolding process is smooth and slow without any sticking of the haptics to the optic.
Scanning electron microscopy
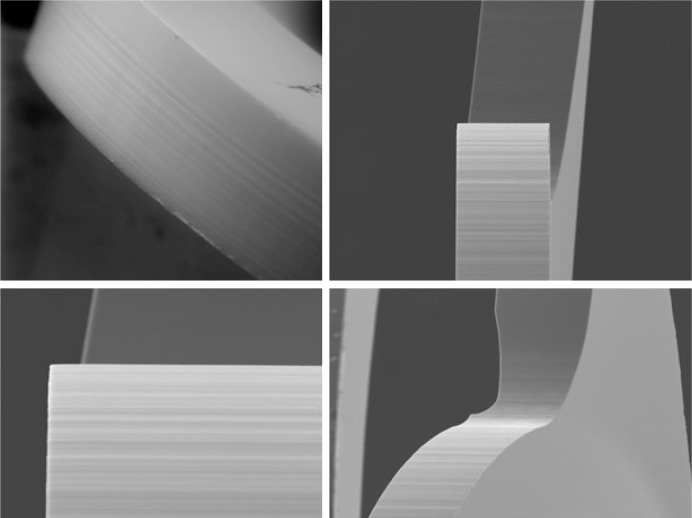
The analysis of the optic–haptic junction by scanning electron microscopy confirmed the clinical findings and the surgeons’ experience. The completely new designed optic–haptic transition (called Achilles Heel of the IOL) of the CT LUCIA 611P is thicker, wider and stiffer than the predecessor CT LUCIA 601P (Figure 4). This seems to be most important for good (better) IOL centration in the capsular bag and IOL stability. On the other side, the stiffness of the material and the optic–haptic junction is the reason why the lens may differ in the implantation and unfolding process from most other acrylic, hydrophobic, single-piece IOLs. Therefore, some special attention must be addressed to the slightly different behavior of the lens during implantation and unfolding process. Moreover, the analysis proved the 360° sharp edge technology, which is important for PCO prevention (Figure 5).
Figure 5.
Scanning electron microscopic analysis of the 360° sharp edge design of the CT LUCIA 611P.
Discussion
The results of our long-term observation demonstrate the safety and efficacy of the new hydrophobic, acrylic IOL (CT LUCIA 611P(Y)). One year after surgery, BCDVA reached an average of 0.02 logMAR, while 94.8% of all subject eyes achieved 0.10 logMAR or better. In addition, no intraoperative or postoperative AEs were observed.
Glistenings
Glistenings were detected in 0% of the subject eyes. Finally, no other disorders resulting in reduced contrast sensitivity or increased glare were reported. The findings in regard to glistenings with the CT LUCIA 611P(Y) are significant.
Glistenings, which are fluid-filled microvacuoles or tiny bubbles of liquid, can form within any IOL but are particularly common in those made from hydrophobic, acrylic material. First described by Nichamin and Apple in the 1990s, glistenings have been the focus of hundreds of studies designed to assess their significance.14–17 Apple et al postulated in their “Executive Summary” for the International Society for Intraocular Lens Safety (I.S.I.S.) that glistenings are much more common than believed and do indeed cause clinically relevant symptoms that may interfere with the purported IOL function. They reported that, in their unique database of 19,400 subjects with explanted IOLs, while >90% achieved BCDVA between 20/40 and 20/20 (Snellen), patients were dissatisfied with their IOLs because of poor visual quality due to the presence of glistenings.
More than 20 years later, opinion and clinical findings are still divided regarding the visual significance and effects of glistenings. Some authors have stated that visual effects of surface light scatter and decrease in contrast acuity change over time with IOL age (positive correlation). The number and/or size of glistenings may increase over time and worsen contrast visual acuity and retinal stray light. Today, glistenings are still a very important topic, especially regarding “premium” and multifocal lenses. A recently conducted experimental survey that studied the impact of loss in optical quality from glistenings using ray tracing in a model eye postulated that glistenings lead to a reduction of modulation transfer function (MTF). The MTF is a measurement of an IOL’s ability to transfer contrast at a specific resolution from the object to the image. In this study, the relative loss of MTF was even more significant in multifocal IOLs than in monofocal IOLs because of the design characteristics and nature of the lens.18 Recently, we have observed 3 “brand new” cases of glistenings in our practice. These 3 patients came to our facility for a second opinion, and had undergone cataract surgery at another clinic in mid-2017. In all 3 cases, glistenings were detected via slit lamp as visual acuity reached 20/20. The subjects, feeling miserable, reported major problems with quality of vision, including reduced contrast acuity and glare at night. Remarkable is the date of surgery! Therefore, glistenings have to cause concern nowadays. An important question is, why would surgeons still be implanting IOLs with proven glistening formation >20 years after this flaw was first identified in clinical studies?
One possible reason that glistenings are underdiagnosed and considered by some to be a rare complication is that many implantations are actually performed by active, high-volume cataract surgeons who ironically rarely see this condition. Many of these surgeons do not examine implanted patients’ eyes postoperatively. We believe, every surgeon should think about glistenings and the possible impact on quality of vision when choosing a monofocal or multifocal IOL (sometimes labeled as “premium”), and hopefully, economic aspects are never influencing the selection of an IOL.
Light transmission and quality of vision
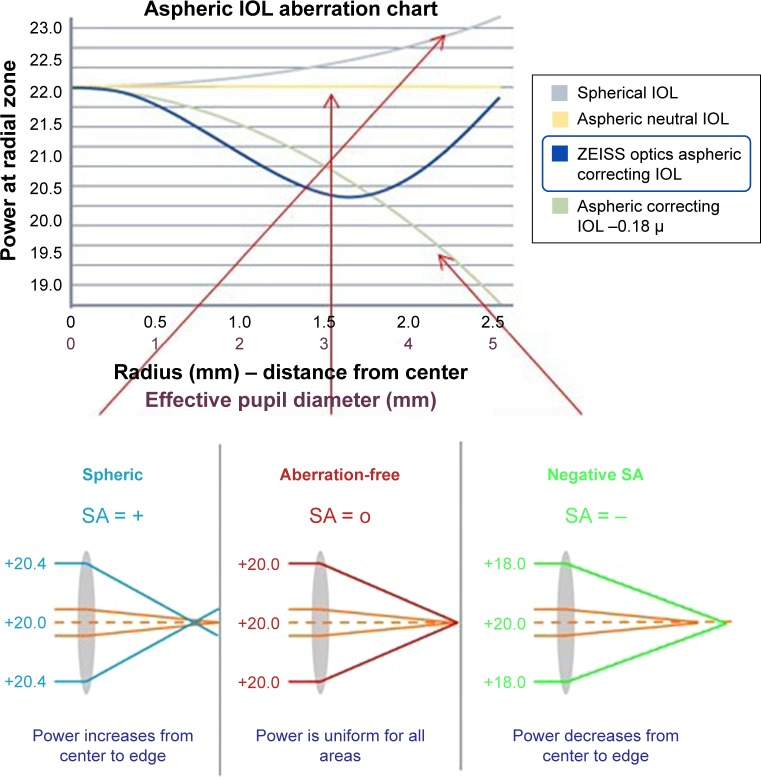
There are several other factors that are important to find the proper IOL for the particular case, summarized in the following as a decision guidance: The light transmission properties of IOLs are an important factor in determining postoperative visual quality. The tested CT LUCIA 611P(Y) is equipped with ZO(600) aspheric concept optic (Zeiss Meditec), which means that the power of the IOL is higher in the center and then varies toward the periphery (Figure 6). This results in a flatter lens surface at intermediate distance from the lens axis and a steepening at the peripheral region of the lens, components which may benefit patients with different corneal shapes and reduce incidence of higher-order aberrations (HOAs). The spherical aberration (SA) of the CT LUCIA 611P(Y) is −0.12 µm; it is optimized for corneal asphericity and designed to compensate a range of aberrations (Figure 4). The human eye is not optically symmetrical, and only very few IOLs are perfectly centered in the eye. Therefore, the tested lens is less prone for tilt and decentration while offering better contrast sensitivity in mesopic conditions and higher image quality with increased depth of focus, making it a viable option for eyes with pseudoexfoliation, weak zonules, pseudophacodonesis or postoperative complications (eg, posterior capsular tear) or trauma.19 On the contrary, IOLs with more negative SA (IOL power decreases from center to periphery) improve contrast sensitivity, but if the optic position is not well aligned, other HOAs, such as coma, could occur in these eyes. Moreover, it is well known that IOLs with a low refractive index and a high Abbe number have lower chromatic aberration. The tested CT LUCIA 611P has a relatively low refractive index of 1.49 and a high Abbe number of 51 providing improved quality of vision and less dispersion (Figure 1). When it comes to refractive outcomes and refractive stability, the lens design and size and shape of the optic and the haptics are fundamental.
Figure 6.
Zeiss asphericity concept ZO: combining the advantages from negative spherical aberration and aberration-neutral lenses. Courtesy of Zeiss Meditec (Jena, Germany).
Abbreviations: IOL, intraocular lens; SA, spherical aberration.
Optic–haptic junction and square edge technology
The CT LUCIA 611P(Y) has a step-vaulted haptic design to maximize the haptic contact angle under compression and enable good contact to the posterior capsule. This together with the overall diameter of the lens and the completely renewed thicker, stiffer and more rigid optic–haptic transition is to provide refractive stability and prevent myopic shifts. Regarding to numerous studies in the past, a square edge technology is most important to reduce incidence of PCO. Though a sharp-edged optic–haptic junction is especially important to prevent cells from migrating between posterior capsule and IOL toward the center (Figure 5). Apple called this part of the IOL (optic–haptic junction) the “Achilles Heel of the IOL” by reason of special significance.20,21 Therefore, in the additionally performed scanning electron microscopic analysis of the optic–haptic junction of the new CT LUCIA 611P(Y) (Figures 3 and 5), the results confirm a 360° square edge and a unique optic–haptic transition with much more stiffness than the predecessor CT LUCIA 601P. Our postoperative measurements with Pentacam and the photographic analysis with the slit lamp showed well-centered IOLs without any signs of rotation or tilt. In our opinion, this lens design would be eligible for a toric model platform too. In respect thereof, more studies should be conducted. Our findings suggest that the novel IOL is an excellent option for post-cataract patients seeking best visual quality. PCO results are encouraging, but we have to wait for long-term results.
Acknowledgments
The authors would like to acknowledge the work and effort put into this report by Miss Elisabeth Marie. We have been blessed by your daily attendance and your active support. We want to thank you for doing all the night shifts with tremendous energy and overwhelming enthusiasm.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wang SY, Stem MS, Oren G, Shtein R, Lichter PR. Patient-centered and visual quality outcomes of premium cataract surgery: a systematic review. Eur J Ophthalmol. 2017;27(4):387–401. doi: 10.5301/ejo.5000978. [DOI] [PubMed] [Google Scholar]
- 2.Vyas AV, Narendran R, Bacon PJ, Apple DJ. Three-hundred-sixty degree barrier effect of a square-edged and an enhanced-edge intraocular lens on centripetal lens epithelial cell migration two-year results. J Cataract Refract Surg. 2007;33(1):81–87. doi: 10.1016/j.jcrs.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 3.Apple DJ, Escobar-Gomez M, Zaugg B, Kleinmann G, Borkenstein AF. Modern cataract surgery: unfinished business and unanswered questions. Surv Ophthalmol. 2011;56(6 Suppl):S3–S53. doi: 10.1016/j.survophthal.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 4.van der Mooren M, Franssen L, Piers P. Effects of glistenings in intraocular lenses. Biomed Opt Express. 2013;4(8):1294–1304. doi: 10.1364/BOE.4.001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henriksen BS, Kinard K, Olson RJ. Effect of intraocular lens glistening size on visual quality. J Cataract Refract Surg. 2015;41(6):1190–1198. doi: 10.1016/j.jcrs.2014.09.051. [DOI] [PubMed] [Google Scholar]
- 6.DeHoog E, Doraiswamy A. Evaluation of the impact of light scatter from glistenings in pseudophakic eyes. J Cataract Refract Surg. 2014;40(1):95–103. doi: 10.1016/j.jcrs.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Borkenstein AF, Borkenstein EM. Patient and surgeon satisfaction levels after using an acrylic, hydrophobic, monofocal “premium” IOL and the Malyugin ring in pseudoexfoliation syndrome. J Ophthalmol. 2018;2018:3843098. doi: 10.1155/2018/3843098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davison JA, Chylack LT. Clinical application of the lens opacities classification system III in the performance of phacoemulsification. J Cataract Refract Surg. 2003;29(1):138–145. doi: 10.1016/s0886-3350(02)01839-4. [DOI] [PubMed] [Google Scholar]
- 9.Biwer H, Schuber E, Honig M, Spratte B, Baumeister M, Kohnen T. Objective classification of glistenings in implanted intraocular lenses using Scheimpflug tomography. J Cataract Refract Surg. 2015;41(12):2644–2651. doi: 10.1016/j.jcrs.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 10.Mönestam E, Behndig A. Impact on visual function from light scattering and glistenings in intraocular lenses, a long-term study. Acta Ophthalmol. 2011;89(8):724–728. doi: 10.1111/j.1755-3768.2009.01833.x. [DOI] [PubMed] [Google Scholar]
- 11.Chang A, Behndig A, Rønbeck M, Kugelberg M. Comparison of posterior capsule opacification and glistenings with 2 hydrophobic acrylic intraocular lenses: 5- to 7-year follow-up. J Cataract Refract Surg. 2013;39(5):694–698. doi: 10.1016/j.jcrs.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 12.Arshinoff SA. Dispersive-cohesive viscoelastic soft shell technique. J Cataract Refract Surg. 1999;25(2):167–173. doi: 10.1016/s0886-3350(99)80121-7. [DOI] [PubMed] [Google Scholar]
- 13.van den Bruel A, Gailly J, Devriese S, Welton NJ, Shortt AJ, Vrijens F. The protective effect of ophthalmic viscoelastic devices on endothelial cell loss during cataract surgery: a meta-analysis using mixed treatment comparisons. Br J Ophthalmol. 2011;95(1):5–10. doi: 10.1136/bjo.2009.158360. [DOI] [PubMed] [Google Scholar]
- 14.Schmidbauer JM, Werner L, Apple DJ, et al. Postoperative opacification of posterior chamber intraocular lenses – a review. Klin Monbl Augenheilkd. 2001;218(9):586–594. doi: 10.1055/s-2001-17635. German [with English abstract] [DOI] [PubMed] [Google Scholar]
- 15.Tognetto D, Toto L, Sanguinetti G, Ravalico G. Glistenings in foldable intraocular lenses. J Cataract Refract Surg. 2002;28(7):1211–1216. doi: 10.1016/s0886-3350(02)01353-6. [DOI] [PubMed] [Google Scholar]
- 16.Dhaliwal DK, Mamalis N, Olson RJ, et al. Visual significance of glistenings seen in the AcrySof intraocular lens. J Cataract Refract Surg. 1996;22(4):452–457. doi: 10.1016/s0886-3350(96)80041-1. [DOI] [PubMed] [Google Scholar]
- 17.Miyata A, Uchida N, Nakajima K, Yaguchi S. Clinical and experimental observation of glistening in acrylic intraocular lenses. Jpn J Ophthalmol. 2001;45(6):564–569. doi: 10.1016/s0021-5155(01)00429-4. [DOI] [PubMed] [Google Scholar]
- 18.DeHoog E, Doraiswamy A. Evaluation of loss in optical quality of multifocal intraocular lenses with glistenings. J Cataract Refract Surg. 2016;42(4):606–612. doi: 10.1016/j.jcrs.2015.10.071. [DOI] [PubMed] [Google Scholar]
- 19.Auffarth GU, Tsao K, Wesendahl TA, Sugita A, Apple DJ. Centration and fixation of posterior chamber intraocular lenses in eyes with pseudoexfoliation syndrome. An analysis of explanted autopsy eyes. Acta Ophthalmol Scand. 1996;74(5):463–467. doi: 10.1111/j.1600-0420.1996.tb00600.x. [DOI] [PubMed] [Google Scholar]
- 20.Nixon DR, Apple DJ. Evaluation of lens epithelial cell migration in vivo at the haptic-optic junction of a one-piece hydrophobic acrylic intraocular lens. Am J Ophthalmol. 2006;142(4):557–562. doi: 10.1016/j.ajo.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 21.Kleinmann G, Apple DJ. Capsular bend and PCO prevention. J Cataract Refract Surg. 2006;32(8):1242–1243. doi: 10.1016/j.jcrs.2006.02.071. [DOI] [PubMed] [Google Scholar]