Abstract
Purpose
Adjuvant tamoxifen treatment revolutionized the management of estrogen receptor (ER) positive breast cancers to prevent cancer recurrence; however drug resistance compromises its clinical efficacy. The mechanisms underlying tamoxifen resistance are not fully understood and no robust biomarker is available to reliably predict those who will be resistant. Here we study BQ323636.1, a novel splice variant of the NCOR2 gene and evaluate its efficacy in predicting tamoxifen resistance in breast cancer patients.
Experimental Design
A monoclonal anti-BQ323636.1 antibody that specifically recognizes the unique epitope of this splice variant was generated for in vitro mechanistic studies and for in vivo analysis by immunohistochemistry on tissue microarrays of two independent cohorts of 358 patients with more than 10 years clinical follow-up data, who had ER-positive primary breast cancer and received adjuvant tamoxifen treatment. Orthotopic mouse model was also used.
Results
Overexpression of BQ323636.1 conferred resistance to tamoxifen in both in vitro and in orthotopic mouse model. Mechanistically, co-immunoprecipitation showed BQ323636.1 could bind to NCOR2 and inhibit the formation of co-repressor complex for the suppression of ER signaling. Nuclear BQ3232636.1 overexpression in patients samples was significantly associated with tamoxifen resistance (p= 1.79 x 10-6, sensitivity 52.9%, specificity 72.0%). In tamoxifen-treated patients, nuclear BQ323636.1 overexpression was significantly correlated with cancer metastasis and disease relapse. Nuclear BQ323636.1 was also significantly associated with poorer overall survival (p=1.13 x 10-4) and disease-specific survival (p=4.02 x 10-5).
Conclusions
These findings demonstrate that BQ323636.1 can be a reliable biomarker to predict tamoxifen resistance in ER-positive breast cancer patients.
Keywords: NCOR2/SMRT, BQ323636.1, Tamoxifen-resistance, breast cancer
Introduction
Breast cancer is the most common female malignancy. The Estrogen Receptor (ER) signaling pathway is a fundamental pro-proliferative pathway in the context of breast cancer (1). Upon its activation by binding with estrogen, ER activates target gene transcription and cell growth either directly through its genomic pathway or indirectly through non-genomic pathway that involves the PI3K/AKT pathway. Most of the well-characterized ER-target genes are oncogenic that could promote cancer cell proliferation or apoptosis resistance; e.g. cyclin D1, anti-apoptotic Bcl2, and pS2 (2–4).
Around 70% of breast cancer patients are ER positive and can benefit from anti-estrogen therapy. Tamoxifen is a selective estrogen receptor modulator (SERM) that acts as an antagonist of estrogen in the context of breast and has been the most commonly prescribed anti-estrogen drug for preventing ER+ patients from cancer relapse or metastasis for over four decades (5). Binding of tamoxifen to ER triggers the recruitment of nuclear co-repressor 2 (NCOR2) as well as other co-repressors, such as GPS2, TBLR1, HDAC3, to suppress the pro-proliferative ER signaling pathway (6–8). In this co-repressive machinery, NCOR2 interacts with itself between regions encompassing amino acids 290-427 and 1788-1903 to form dimers in an anti-parallel fashion, serving as a central platform on which additional co-repressor proteins assemble (9). The NCOR2 protein consists of four known repression domains within the N-terminal portion (denoted RD1 to RD4) and a series of two C-terminal nuclear receptor interaction domains (denoted S1 and S2) (10, 11). Alternative splicing of NCOR2 at the C-terminal is commonly reported, generating multiple corepressor isoforms with conserved repression domains but with different affinities for different nuclear receptors (12, 13).
In the adjuvant setting, a large number of patients who have ER+ tumors are treated with tamoxifen for prevention of disease relapse and metastasis. Despite the success of tamoxifen, resistance is an outstanding problem with up to 50% of non-responding patients with many initial responders experiencing relapse (14). However, there is no clinically in-use biomarker for prediction of patients’ response to tamoxifen and the mechanisms for developing tamoxifen resistance are still not well understood.
By SpliceArray profiling, our group has reported a novel splice variant of NCOR2, named BQ323636.1 (BQ in short), with truncation at the C-terminus, retaining only the RD1 (15). In a cohort of 77 breast cancer patients, at the mRNA level, BQ323636.1 overexpression was significantly associated with tamoxifen resistance, poor overall and disease-free survival (15). The result led us to postulate that this splice variant could serve as a biomarker for prediction of tamoxifen response. BQ is capable of modulating transcription of ER by counteracting the transcription repressive activity of NCOR2 (15), suggesting that it could confer tamoxifen resistance and mediate cancer cell growth through competing and counteracting the repressive functions of its wild type.
Our previous study only focused on association of BQ32636.1 with tamoxifen resistance at the mRNA level because there was no commercially antibody available to distinguish this isoform from its wild-type and the molecular mechanism through which BQ could confer resistance to tamoxifen was unknown. In the present study, we generated a mouse monoclonal antibody specific for BQ323636.1, confirmed the role of BQ323636.1 in conferring tamoxifen resistance at protein level using both in vitro and in vivo models and characterized the molecular mechanism through which BQ323636.1 confers resistance to tamoxifen. Most importantly, we also confirmed in two independent breast cancer cohorts from Hong Kong and the United Kingdom that BQ, applied in immunohistochemical staining (IHC) on formalin fixed paraffin embedded primary breast cancer tissue, could be a reliable biomarker in predicting tamoxifen resistance.
Materials and Methods
Cell culture
Human breast cancer cell lines MCF-7 and ZR-75-1 were purchased from American Type Culture Collection (ATCC) and were re-authenticated by short tandem repeat profiling (15). MCF-7 was cultured in Dulbecco's Modified Eagle's Medium supplemented with 10% FBS and 1% penicillin/streptomycin. ZR-75-1 was cultured in Improved Minimum Essential Medium (IMEM) supplemented with 5% FBS and 1% penicillin/streptomycin. LCC2 and AK-47 are two tamoxifen resistant cell lines derived from MCF-7 and ZR-75-1 respectively, kindly provided by Dr. Robert Clarke (Georgetown University Medical School, Washington, D.C.) (16) and used in our previous study (15). LCC2 and AK-47 were both cultured in IMEM supplemented with 5% charcoal-stripped FBS and 1% penicillin/streptomycin. MCF-10A was purchased from ATCC and cultured in MEGM™ Mammary Epithelial Cell Growth Medium (Lonza) with 100 ng/ml cholera toxin, 0.5 mg/ml Hydrocortisone, 20 ng/ml EGF, 10 μg/ml Insulin, 5% FBS and 1% penicillin/streptomycin. All the cell lines used in this study have been passaged and kept fewer than 6 months after the re-authentication or thawing.
Total RNA extraction, reverse transcription and real-time quantitative PCR
TRIzol reagent (Invitrogen) was used for total RNA extraction following manufacturer’s protocol. Up to 2 µg total RNA were reverse transcribed into cDNA by SuperScript III reverse transcriptase (Invitrogen) following manufacturer’s protocol. Real-time PCR reaction was carried out with ABI 7900HT Fast Real-time PCR system. The primer sequences used were presented in Supplementary Table S1.
Western blot analysis
Cell lysates were prepared as previously described (17). The proteins were probed with antibody against BQ3236363.1 (Patent Cooperation Treaty filed), NCOR2 (ab24551, Abcam), ERα (HC-20, Santa Cruz), 6xHis-tag (9F2, Wako), β-tubulin (H-235, Santa Cruz), lamin B (C-20, Santa Cruz) and β-actin (AC-74, Sigma). Secondary antibodies were: HRP–conjugated anti-rabbit antibody (P0448, Dako), HRP-conjugated affiniPure anti-mouse antibody (Jackson Immnuno Research Laboratories), HRP-conjugated anti-goat antibody (SC2922, Santa Cruz). Protein A–HRP (ThermoFisher Scientific) was used to avoid detection of the heavy and light chains. The images were digitalized and, if necessary, processed by adjusting brightness or contrast only.
MTT assay
Cell viability was studied by MTT assay as previously described (15). 4-hydroxy tamoxifen (4-OH tamoxifen), an active metabolite of Tamoxifen was used. 4-OH tamoxifen and 17-β-estrodiol (E2) were purchased from Sigma and were dissolved in ethanol for in vitro assay.
Co-immunoprecipitation
Cells were lysed in IP lysis buffer and pre-cleared with 30 µl of Dynabeads Protein A/G by rotating at 4°C for 4 hours. After pre-clearing, protein concentration was measured and the lysate split into equal amount of proteins per tube and incubated with specific primary antibody or IgG negative control at 4°C for O/N with gentle rotating. On the second day, 40μl of beads were added to the mixture and incubated at 4°C for another 4 h. After incubation, the beads were washed 5 times with cold PBS and boiled at 100°C in 1X SDS loading dye for 5 min to elute the proteins. Proteins were separated by SDS–PAGE gel electrophoresis, transferred to nitrocellulose membrane and hybridized with the antibodies same as Western blotting.
Orthotopic mouse model
Female nude mice, aged 5 to 6 weeks were used for this study. On the day of inoculation, 1x106 ZR-75-1 cells or 5x106 MCF-7 cells were mixed with Matrigel (BD Bioscience) at a ratio of 1:1 and the 100µl cell mixture injected into the abdominal mammary fat pad of mice. When the tumors were palpable, mice were randomized into treatment and control groups where treatment group received 4-OH tamoxifen (Sigma) dissolved in ethanol and diluted in peanut oil (Sigma), given by subcutaneous injection 500 μg per day (500 μg/ml) for 6 consecutive days per week. The control group received solvent only. The tumor sizes were measured regularly using calipers and the tumor volume calculated as longest diameter x (shortest diameter)2/2. At the endpoint of experiment, mice were euthanized and tumors were harvested. All the procedures have been reviewed and approved by HKU Committee on the Use of Live Animals in Teaching and Research (CULATR No.:3259-14).
Pulse-chase assay
Before pulse labelling, the cells were incubated in plain DMEM without methionine and cysteine (DMEM –met/cys, Gibco) for one hour. While waiting, 35S-methoinine stock (43.3 mCi/ml, Perkin-Elmer) was diluted in DMEM –met/cys supplemented with 10% FBS (working concentration was 0.2 mCi per 100mm culture dish (hot medium). After cell starvation, hot medium was added and the cells labelled with 35S for 1 hour. After the pulse period, the hot medium was removed, cells were washed and harvested at indicated time points (chase period). Cell pellets were collected and lysed for immunoprecipitation (IP) using anti-NCOR2 antibody (Abcam) or anti-BQ323636.1 antibody (PCT filed) and the samples analyzed by SDS-PAGE.
Tissue Microarray
Two independent cohorts totaling 2095 breast cancer patients with pathological and clinical follow up data of over 20 years were used for this study. The cohort from Hong Kong comprised of 234 cases of breast cancer diagnosed between the years 1992 to 2008 retrieved from the records of the Department of Pathology, Queen Mary Hospital of Hong Kong, with approval by the Institutional Review Board of The University of Hong Kong (UW 08-147). The remaining 1861 cases were obtained from Nottingham University Hospital which consisted of a large cohort of patients comprising a well-characterized consecutive series of early stage sporadic primary operable invasive breast cancers from patients enrolled into the Nottingham Tenovus Primary Breast Carcinoma Series that presented at Nottingham City Hospital between 1989 and 1998. The study was approved by the Nottingham Research Ethics Committee 2 under the title ‘Development of a molecular genetic classification of breast cancer’.
All patients had early operable primary breast cancer undergoing surgery as their primary treatment. TMA sections were obtained from the surgical resection samples. Histological sections of all cases were reviewed by the pathologist, the representative paraffin tumor blocks were chosen as donor block for each case and the selected areas were marked for construction of TMA blocks. For the Hong Kong cohort, each case was constructed as duplicate in the TMA and average score of the duplicate was taken as the score.
There were from both cohorts, a total of 1271 cases that could be assessed and scored for BQ323636.1 staining. Of these 1271 primary breast cancer samples, 358 cases were ER positive, and had been given adjuvant tamoxifen treatment with available follow-up clinical data. Tamoxifen resistance is defined as patients who had been treated with tamoxifen in the adjuvant setting but subsequently developed disease relapse or distant metastases. Only cases with clear history of tamoxifen response were used for analysis.
Immunohistochemistry
The IHC was performed as previously described (17). BQ323636.1 antibody (clone D-12) was at diluted 1:50. Aperio ScanScope ® system (Aperio technology, USA) was used to assess BQ323636.1 expression. The intensities and percentages of the nuclear staining were scored by two independent individuals using H-scoring system (18). H-score = (1 × % of cells stained at intensity category 1) + (2 × % of cells stained at intensity category 2) + (3 × % of cells stained at intensity category 3). The cutoff was set as the median of H-score, which was 130.
Statistical analysis
Results were compared by two-tailed students’ t-test in Excel unless otherwise stated. Data from TMA were analyzed in SPSS (IBM, version 20). Where appropriate, the data were dichotomized into two groups including high or low expression using median expression level as cutoff. The correlations were analyzed by Chi-square tests. The expression levels of BQ were compared between different groups using Mann-Whiney U Rank test. Survival analyses were done by Kaplan–Meier estimates followed by Log-rank test, and Cox regression model. P values of less than 0.05 were considered statistically significant. In the figures, data was expressed as mean ± sd; ‘*’ indicates p <0.05, ‘**’ indicates p<0.01 and ‘***’ indicates p<0.001.
Methods for immunofluorescent staining, transient transfection, luciferase reporter assay, lentiviral transfection for generation of stable cell line and subcellular fractionation are described in supplementary material and methods.
Results
BQ323636.1 is overexpressed in tamoxifen resistant breast cancer cells
The monoclonal anti-BQ323636.1 antibody was generated by InVivo Biotech, Germany, and the protocol for antibody generation and quality control is described in supplementary material and methods. The epitope of this antibody maps to the last 11 amino acid residues on BQ323636.1 protein which distinguish the variant from its wildtype.
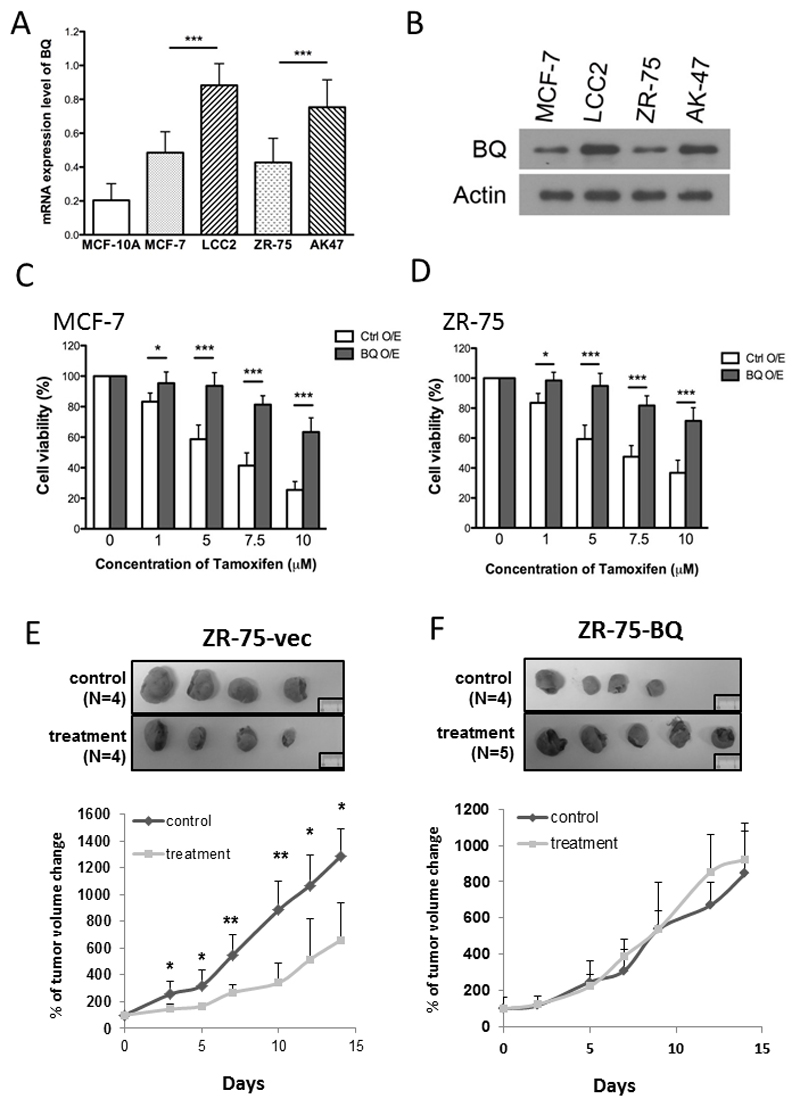
To confirm the correlation between BQ and tamoxifen resistance, we examined the expression level of BQ in two pairs of cell lines: MCF-7 (ER+ve, tamoxifen sensitive) vs LCC2 (ER+ve, tamoxifen resistant) and ZR75-1 (ER+ve, tamoxifen sensitive) vs AK47 (ER-ve, tamoxifen resistant). The expression level of BQ323636.1 was examined by western blot using our generated anti-BQ323636.1 specific antibody. Results showed that both mRNA and protein expression of BQ was higher in tamoxifen resistant cells (Figures 1A&B) compared with tamoxifen sensitive cells.
Figure 1. BQ323636.1 is overexpressed in tamoxifen resistant breast cancer cells.
A) mRNA expression of BQ323636.1 in different breast cell lines. qPCR was performed to determine the expression level of BQ in normal breast cell line (MCF-10A), Tamoxifen sensitive breast cancer cell lines (MCF-7 and ZR-75) and Tamoxifen resistant cell lines (LCC2 and AK-47). Actin was used as internal control. B) Protein expression of BQ323636.1 in Tamoxifen sensitive and resistant cell lines. Western blot was performed to determine the protein level of BQ323636.1. Actin was used as a loading control.
Overexpression of BQ323636.1 conferred Tamoxifen resistance in vitro and in vivo Overexpression of BQ323636.1 induced Tamoxifen resistance in the sensitive cell lines C) MCF-7 and D) ZR-75. The cells were treated with different concentration of Tamoxifen for 72 hours. MTT assay was performed to determine the cell viability. Samples were triplicated. E) Treatment of Tamoxifen could reduce xenograft tumor volume in control ZR-75 cells and as illustrated in the growth curve. The number of mice used in control group and treatment group were 4 and 4 respectively F) Overexpression of BQ323636.1 induced Tamoxifen resistance in vivo and as illustrated in the growth curve. The number of mice used in control group and treatment group were 4 and 5 respectively. % of tumor volume change was presented as mean ± s.d. Data was expressed as mean ± s.d from three independent experiments. * represents p<0.05; ** represents p<0.01; *** represent p<0.001. Statistical significance was determined by student T-Test.
BQ323636.1 overexpression induced tamoxifen resistance in vitro and in vivo
To confirm the effect of BQ on tamoxifen resistance, BQ was stably overexpressed using lentiviral transduction system in tamoxifen sensitive cell lines MCF-7 and ZR-75-1 which express low levels of BQ323636.1. Overexpression of BQ323636.1 was confirmed by RT-qPCR and western blot (Supplementary Figure 1). The responses of ZR75-1-BQ/ZR75-1-vec and MCF-7-BQ/MCF-7-vec to tamoxifen treatment were studied by MTT assay. Overexpression of BQ could significantly induce resistance to tamoxifen in both ZR-75-1 and MCF-7 cell lines (Figures 1C&D). This result was consistent with our previous finding using transient transfection (15) and strengthens the evidence supporting a role of BQ323636.1 in tamoxifen resistance.
The involvement of BQ323636.1 in tamoxifen resistance was further confirmed using an in vivo mouse model. As shown in Figure 1E, ZR75-1-vec tumors were sensitive to tamoxifen treatment as marked by significant reduction of tumor growth when compared with control. In comparison, BQ323636.1-overexpressed tumors did not respond to tamoxifen treatment (Figure 1F). Similar results were also observed using MCF-7 (Supplementary Figure 2), showing that the effects were not cell line specific. Taken together, these results provide solid proof to support the claim that BQ323636.1 overexpression was one of the factors contributing to tamoxifen resistance.
BQ323636.1 competed with NCOR2
Having established that BQ323636.1 overexpression could confer tamoxifen resistance, the next step was to understand the mechanism. It has been observed that this splice variant could modulate transcription of ERE-containing luciferase reporter in a way opposite to its wildtype (15). Since NCOR2 in response to tamoxifen functions as a co-repressor to suppress ER-target genes, therefore it was hypothesized that BQ323636.1 could compete with NCOR2 to restrict the suppressive functions of NCOR2, which in turn relieved the transcriptional suppression imposed by tamoxifen.
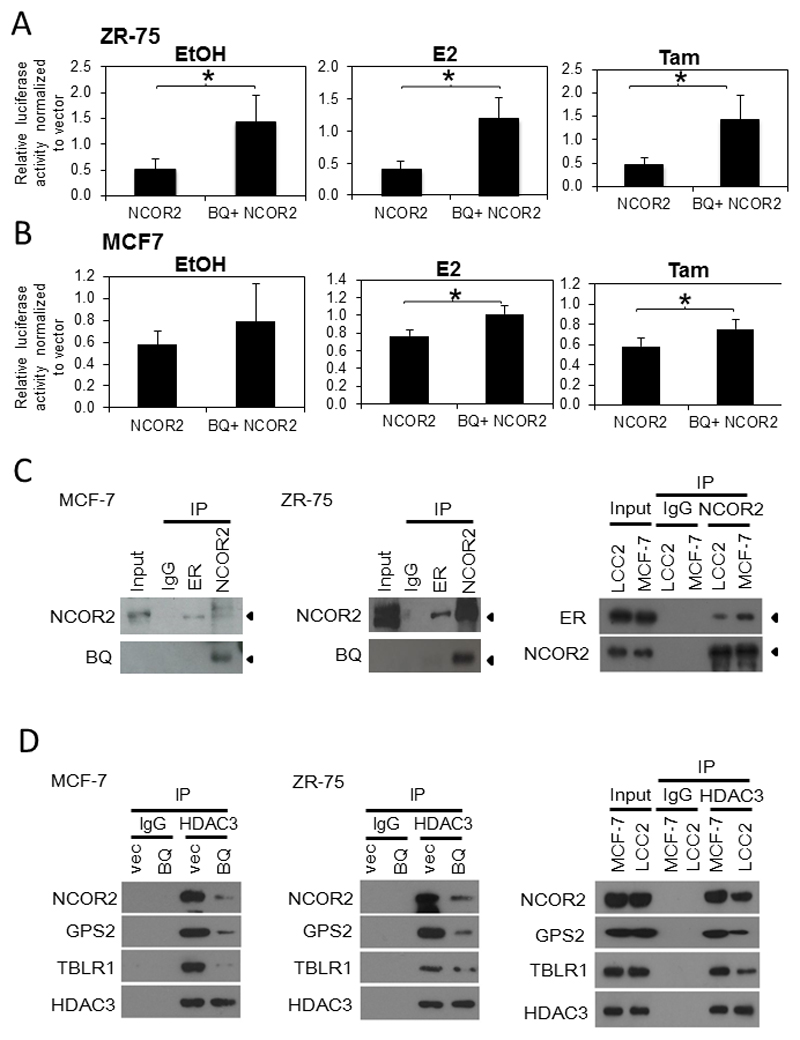
To test this, the effects of BQ323636.1 and/or NCOR2 overexpression on transcription of ERE-containing luciferase reporter (ERE-E1b-luc) were studied and the results were expressed as relative luciferase activity normalized against the vector control. Consistent to its co-repressor role in breast cancer, ectopic overexpression of NCOR2 was found to suppress luciferase activities in both ZR-75-1 and MCF-7 in all three conditions including non-treated (EtOH), treated with E2 or treated with tamoxifen (Figures 2A&B). However, when BQ323636.1 was overexpressed together with NCOR2, the luciferase activities were significantly higher when compared with NCOR2 overexpression alone (Figures 2A&B), indicating that the suppression on luciferase activity imposed by NCOR2 was, at least partially, rescued.
Figure 2. Overexpression of BQ323636.1 could rescue gene repressive function of NCOR2.
Luciferase reporter assay was performed on A) MCF-7 and B) ZR-75 cells were treated with either EtOH or 0.1 nM of E2. Cells were harvested after 48 hours of the treatment. E1b-ERE luciferase reporter was employed. Samples were prepared in triplicate. Renilla was used as internal control. Data was expressed as mean ± s.d from three independent experiments. *** represent p<0.001. Statistical significance was determined by student T-Test.
BQ323636.1 bound to NCOR2 and suppresses formation of co-repressor complex.
C) Co-IP demonstrated interaction between BQ323636.1 and NCOR2 in i) MCF-7 and ii) ZR-75 cells. iii) The interaction between ER and NCOR2 was compromised in tamoxifen resistant cells LCC2 compared with tamoxifen sensitive cells MCF-7. D) Overexpression BQ323636.1 suppressed the interaction between HDAC3 and NCOR2, GPS and TBLR1 in i) MCF-7 and ii) ZR-75 cells. The interaction of HDAC3 with NCOR2, GPS2 and TBLR1 was compromised in LCC2 compared with MCF-7 cells.
It was of great importance to understand how BQ could interfere with the functions of NCOR2. From these and previous results (15), BQ did not appear to exert a dominant negative effect on NCOR2, but instead, competed with NCOR2. It has been reported that NCOR2 functions as a dimer in the co-repressor complex by binding to itself in an anti-parallel fashion (aa290-427 binds to aa1788-1903) (9), serving as a central dock for the further recruitment of other co-repressor proteins. As BQ protein retains only the RD1 portion of NCOR2, theoretically, it would have lost the ability to interact with ER and with other co-repressor proteins such as HDACs. Therefore, we proposed that BQ323636.1 could bind to NCOR2, forming a defective platform to which other co-repressor proteins cannot be fully recruited.
To test this conjecture, co-immunoprecipitation was first performed to study the interactions among ER, NCOR2 and BQ. As shown in figures 2C, the interaction between NCOR2 and ER was confirmed in ZR-75-1 and MCF-7. More importantly, it was found that BQ323636.1 interacted with NCOR2 but not with ER, consistent with the prediction and supporting the notion that BQ323636.1 bound to NCOR2. Furthermore, tamoxifen resistant cells express more BQ which compete with NCOR2, hence it is expected that less NCOR2 be available for interaction with ER in LCC2 compared with tamoxifen sensitive cell MCF-7 as shown in Fig 2C(iii).
Next, the effects of BQ323636.1 overexpression on the interactions between NCOR2 and the co-repressor proteins HDAC3, TBLR1 and GPS2 were studied by Co-IP. To this end, the effects of BQ323636.1 on the expressions of NCOR2, HDAC3, TBLR1 and GPS2 were first examined by Western blot. It was observed that BQ323636.1 overexpression did not alter the expression levels of these proteins (Supplementary Figure 3). Co-IP further revealed that BQ323636.1 overexpression suppressed the interaction between HDAC3 and NCOR2, GPS2 and TLBR1 respectively for the tamoxifen sensitive cell lines MCF-7 and ZR-75 (Figures 2D(i) & (ii)). Likewise, tamoxifen resistant cells LCC2 which express more BQ that competes with NCOR2, suppress the interaction between HDAC3 and NCOR2, GPS2 and TLBR1 respectively when compared with MCF-7 (Fig 2D (iii)).
Under normal conditions, ERα binds to estrogen response element (ERE) of different genes such as GREB, LY6E, NRIP and pS2 to initiate the transcription (19). NCOR2 can bind to ERα on ERE to form a gene repressor complex which can thus repress the effect on ERα on transcription (20). ChIP assays performed showed that overexpression of BQ could reduce the amount of NCOR2 interacting with the ERE genes pS2, NRIP, LY6E and GREB (Supplementary Figure 4). Collectively, these data suggest that BQ323636.1 binds to NCOR2, to functionally interfere with the formation of NCOR2 gene co-repressor complex of ERα, thus confirming that the recruitment of the co-repressor complex to the endogenous gene promoters is indeed affected by BQ overexpression.
BQ323636.1 protein had a higher stability than NCOR2 protein
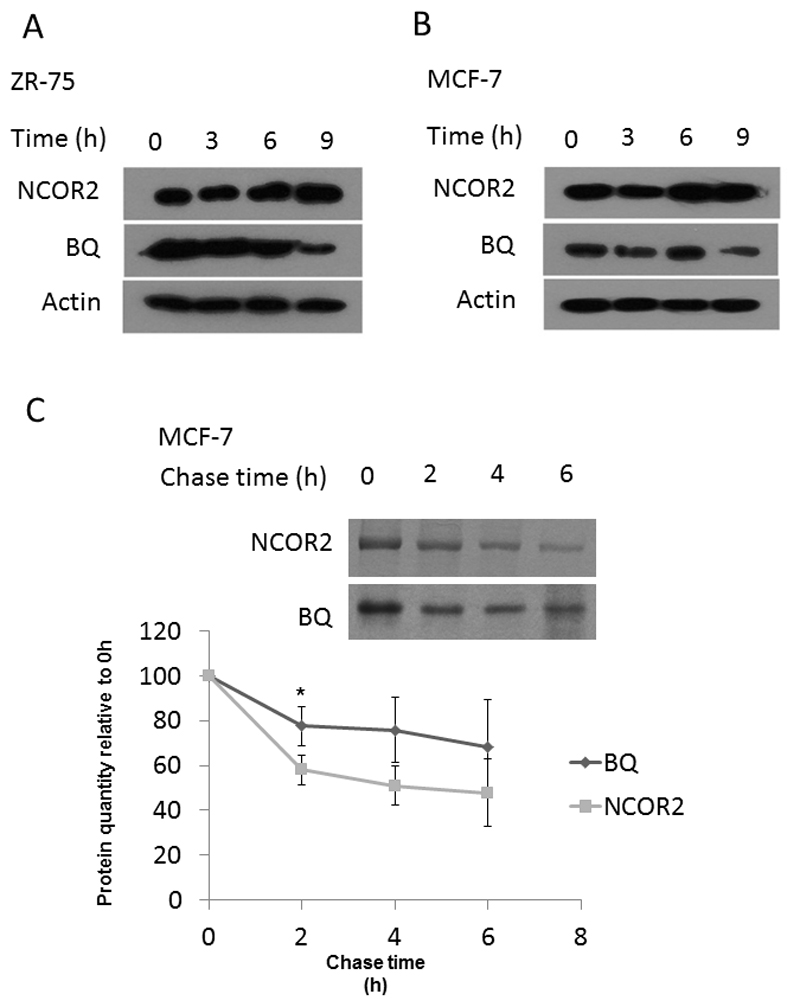
It had been previously observed that whilst protein levels of BQ323636.1 were high, there were very low expression levels of BQ323636.1 mRNA compared with the NCOR2 wild-type, suggesting there may be some post-transcriptional mechanism regulating BQ323636.1 expression (15). The phosphorylation site Ser2410 on NCOR2 reported to be responsible for proteasome-mediated degradation is deleted in BQ323636.1. Thus, compared with NCOR2, BQ323636.1 may be less susceptible to degradation. To study the proteasome-mediated degradation of BQ323636.1, MCF-7 and ZR-75-1 were treated with MG132, a potent proteasome inhibitor, for a time course as indicated in Figures 3A&B. It was found that MG132 treatment could cause the accumulation of NCOR2 but not BQ323636.1, suggesting that NCOR2 protein was actively degraded through the proteasome-mediated degradation pathway whilst BQ323636.1 was not. To gain definite knowledge of the turnover of these two proteins in the cells, pulse-chase analyses were performed to determine the half-life. It was found that NCOR2 degraded continuously along with time, while BQ323636.1 protein stayed relatively constant after an initial decrease from 0 h to 2 h (Figure 3C). The half-life of NCOR2 was around 4 h while that of BQ323636.1 was undetermined using the same time course (Figure 3C). Overall, our finding indicated that BQ323636.1 protein would have a higher stability than NCOR2 protein, probably because of the impaired proteasome-degradation pathway.
Figure 3. BQ323636.1 protein was more stable than NCOR2.
BQ323636.1 protein did not accumulate following MG132 treatment but NCOR2 did in A) MCF-7 and B) ZR-75 cells. 5µM of MG132 was employed. (C) BQ323636.1 degraded slower than NCOR2 and had a longer half-life in the cell. Band intensities were quantified by ImageJ and normalized against intensities of 0h. Data was expressed as mean ± s.d from three independent experiments. *, p<0.05. Statistical significance was determined by student T-Test.
BQ323636.1 nuclear overexpression is associated with tamoxifen resistance in breast cancer patient samples
Previously, in a small cohort of 77 breast cancer patients, we have shown that BQ323636.1 overexpression at mRNA level is significantly associated with tamoxifen resistance (15). As we have demonstrated, the BQ323636.1 protein binds to NCOR2 to suppress the formation of co-repressor complexes. Hence more informative results can be obtained by assessing BQ323636.1 protein expression in relation to tamoxifen resistance. With the use of the specific monoclonal anti-BQ323636.1 antibody, protein expression of BQ323636.1 was directly examined in breast cancer tumor tissue by immunohistochemistry as in vivo confirmation of the usefulness of BQ323636.1 as a predictive marker for tamoxifen resistance.
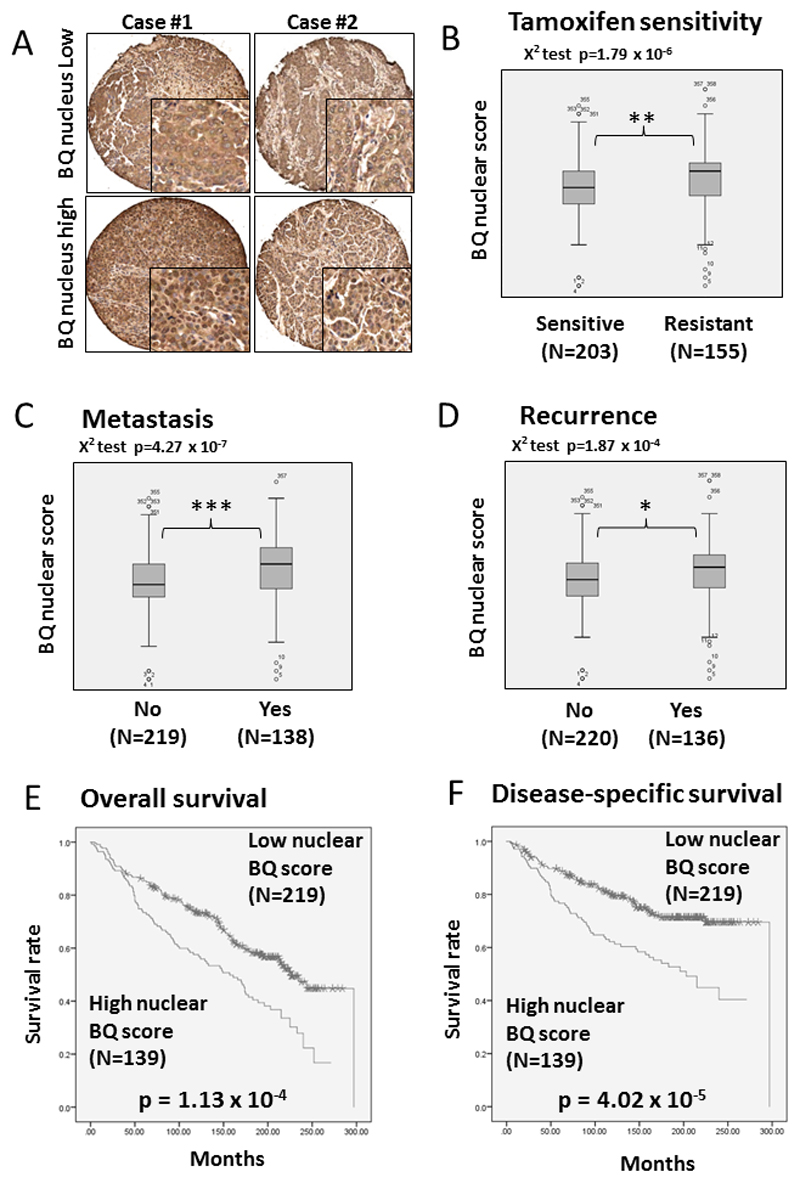
Representative images of IHC staining are shown in Figure 4A. It was observed that, whilst BQ323636.1 was expressed in the cytoplasm at a fairly similar level for all patients, the intensity and distribution of nuclear BQ323636.1 staining was heterogeneous and varied from patient to patient. Hence, semi-quantitative assessment of nuclear BQ323636.1 expression levels was performed using the H-score system which takes into account both the intensity and percentage of cells stained at the intensity category, which was named BQ nuclear score.
Figure 4. The clinical significance of nuclear expression level of BQ323636.1.
A) Representative images of BQ323636.1 IHC staining in primary breast cancer samples in TMA. Mann-Whitney test demonstrating BQ323636.1 nuclear score was associated with B) Tamoxifen resistance C) Metastasis and D) Recurrence *** p<0.001, ** p<0.01, * p<0.05. Nuclear BQ323636.1 scores were dichotomized at the median value and Chi square test performed for association with B) Tamoxifen resistance, C) Metastasis and D) Recurrence (Chi square test p<0.0001).
Kaplan-Meier estimate shows the patients with high nuclear BQ323636.1 expression were associated with poorer E) overall survival outcome (p < 0.001) and F) disease specific survival outcome (p<0.001) (Log-rank test). Nuclear BQ323636.1 scores were dichotomized at the median value.
Three hundred and fifty eight ER+ primary breast cancer samples recruited from two independent Hong Kong and United Kingdom cohorts were used for analysis. All patients had received adjuvant tamoxifen treatment with available follow-up clinical data and clear history of tamoxifen response. Tamoxifen resistance is defined as tamoxifen treated patients who subsequently developed disease relapse or distant metastases. The clinicopathological data of these 358 cases are shown in Supplementary Table 1, for which 78 cases were from Hong Kong and 280 cases from the United Kingdom. Both cohorts of patients when analyzed separately, each gave statistically significant results (Supplementary figures 5&6), and the pooled results are presented.
Statistical analysis revealed the BQ nuclear score was significantly associated with tamoxifen resistance by Chi-square test (p= 1.79 x 10-6), and significantly higher in patients found to be tamoxifen resistant (Mann-Whitney U-Rank test, p=0.001, figure 4B). In tamoxifen treated patients, BQ nuclear score was significantly correlated with cancer metastasis (Chi-square test, p= 4.27 x 10-7) and significantly higher in patients who developed metastasis (Mann-Whitney U-Rank test, p=0.00009, figure 4C). BQ nuclear score was also significantly associated with disease relapse (Chi-square test, p=1.87 x 10-4) and significantly higher in patients who developed disease relapse (Mann-Whitney U Rank test, p=0.018, figure 4D).
Consistent with its role in predicting tamoxifen resistance, BQ nuclear score was significantly associated with poorer survival by Kaplan-Meier estimate (Log-rank test, p=1.13 x 10-4 and p=4.02 x 10-5 for overall survival and disease-specific survival, respectively, figures 4E&F). By Cox-regression analysis (Table 1), it was also observed that BQ nuclear score was significantly associated with poorer overall survival RR=1.765, 95% CI 1.317 to 2.365; p=1.42 x 10-4) as well as with poorer disease-specific survival (RR=2.093, 95% CI 1.459 to 3.002; p=6.03 x 10-5). Multivariate cox-regression analyses showed that after adjustment for age, tumor stage, tumor grade and progesterone receptor (PR) status, BQ nuclear score remained to be significantly associated with higher risk of death; overall survival (RR=2.117, 95% CI 1.520 to 2.948; p=9.18 x 10-6) and for disease-specific survival (RR=2.604 , 95% CI 1.746 to 3.885; p=2.74 x 10-6).
Table 1. Survival analysis for tamoxifen-treated patients using Cox regression model.
Variables used in the Cox regression model were dichotomized as shown in Supplementary Table 1. A) Higher BQ nuclear score was significantly associated with higher risk of death even after adjustment for clinical parameters including age, tumor grade, PR and HER2 status. (RR=2.117; p <0.001. B) Higher BQ nuclear score was significantly associated with higher risk of dying from cancer even after adjustment for clinical parameters including tumor grade, PR and HER2 status. (RR=2.604; p<0.001). #Cut off = 130 which is the median BQ nuclear score.
A. Cox regression analysis of overall survival predictors for patients with breast cancer
| Clinical-pathological parameters | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| RR (95% Cl) | P value | RR (95% Cl) | P value | |
| Age (n=358) | 1.946 (1.36, 2.785) | 0.000273 | 2.019 (1.359, 2.998) | 0.001 |
| T-stage (n=358) | 1.123 (0.816, 1.546) | 0.476 | ||
| Lymph-node involvement | 1.077 (0.472, 2.455) | 0.861 | ||
| Tumor-Grade | 1.397 (1.035, 1.885) | 0.029 | 1.634 (1.168, 2.286) | 0.004 |
| PR status | 0.62 (0.448, 0.858) | 0.004 | 0.792 (0.555, 1.13) | 0.198 |
| HER2 status | 1.757 (1.136, 2.718) | 0.011 | 1.888 (1.185, 3.008) | 0.008 |
| BQ nucleus score # | 1.765 (1.317, 2.365) | <0.001 | 2.117 (1.520, 2.948) | <0.001 |
| B. Cox regression analysis of disease-free survival predictors for patients with breast cancer | ||||
| Clinical-pathological parameters | Univariate analysis | Multivariate analysis | ||
| RR (95% Cl) | P value | RR (95% Cl) | P value | |
| Age (n=358) | 1.326 (0.887, 1.982) | 0.169 | ||
| T-stage (n=358) | 1.326 (0.891, 1.973) | 0.165 | ||
| Lymph-node involvement | 1.52 (0.495, 4.671) | 0.465 | ||
| Tumor-Grade | 1.75 (1.197, 2.559) | 0.004 | 1.997 (1.315, 3.033) | 0.001 |
| PR status | 0.516 (0.352, 0.756) | 0.001 | 0.58 (0.383, 0.878) | 0.010 |
| HER2 status | 2.222 (1.377, 3.587) | 0.001 | 1.906 (1.147, 3.169) | 0.013 |
| BQ nucleus score # | 2.093 (1.459, 3.002) | <0.001 | 2.604 (1.746, 3.885) | <0.001 |
With the use of nuclear BQ score, this scoring method could predict tamoxifen resistance of the breast cancer patients with a sensitivity of 52.9%, specificity of 72.0%. Positive predictive value is 59.0% while the negative predictive value is 66.7%. The results indicate that BQ323636.1 overexpression is an independent prognostic marker for patients who have received tamoxifen treatment, consistent with its role in predicting tamoxifen resistance.
Discussion
The estrogen (ER) signaling pathway is an important pro-proliferative pathway in breast cancer, which upon binding with estrogen, activates target gene transcription and cell growth. More than two-thirds of breast cancer patients are ER+ and can be treated with endocrine therapy. Tamoxifen is most commonly prescribed to ER+ patients for the prevention of breast cancer relapse or metastasis, but despite significant successes in improving patients’ survival, tamoxifen resistance remains an outstanding issue (14). The binding of tamoxifen to ER triggers the recruitment of NCOR2 as well as other co-repressors such as GPS2, TBLR1, HDAC3, to suppress the ER-mediated cell proliferative signaling pathway (6–8). The mechanisms underlying tamoxifen resistance have been extensively studying but not yet well understood.
The pharmacological activity of tamoxifen is dependent upon its conversion by the metabolic enzyme CYP2D6 to its active metabolite, endoxifen. Patients with reduced CYP2D6 activity were considered to benefit less from tamoxifen treatment and CYP2D6 became a patented marker for prediction of tamoxifen response (21, 22). Subsequent clinical studies however have reported conflicting results, some supportive that patients with reduced CYP2D6 activity derive inferior therapeutic benefit from tamoxifen (23), whilst others finding the contrary (24). Therefore, it remains questionable whether CYP2D6 can serve as a robust biomarker for predicting tamoxifen resistance.
Therefore there is as yet no available robust biomarker to discriminate ER+ patients who will be sensitive to tamoxifen from those who will be resistant. Patients with ER+ disease will thus be prescribed with tamoxifen but clinicians can only document drug failure when cancer reoccurs and/or metastasizes.
BQ323636.1 is a splice variant of NCOR2 identified by our group with exon 11 skipping during mRNA splicing, resulting in an early translation stop codon and a truncated protein product retaining only the N-terminal fragment compared to its wild-type (15). Although BQ mRNA level is 2% to 5% of NCOR2, BQ323636.1 protein, on the other hand, was found to be expressed at similar levels to NCOR2 (15). This could be due in part to BQ323636.1 being not actively degraded through the proteasome-mediated pathway with a longer half-life in the cell compared with that of NCOR2, or that the translation efficiency of BQ323636.1 was higher than NCOR2. These mechanisms could help maintain protein expression of BQ323636.1 at a functionally significant level in the cell. The fact that NCOR2 functions as a dimer and that BQ323636.1 binds to NCOR2 could amplify the functional importance of the splice variant in the cells.
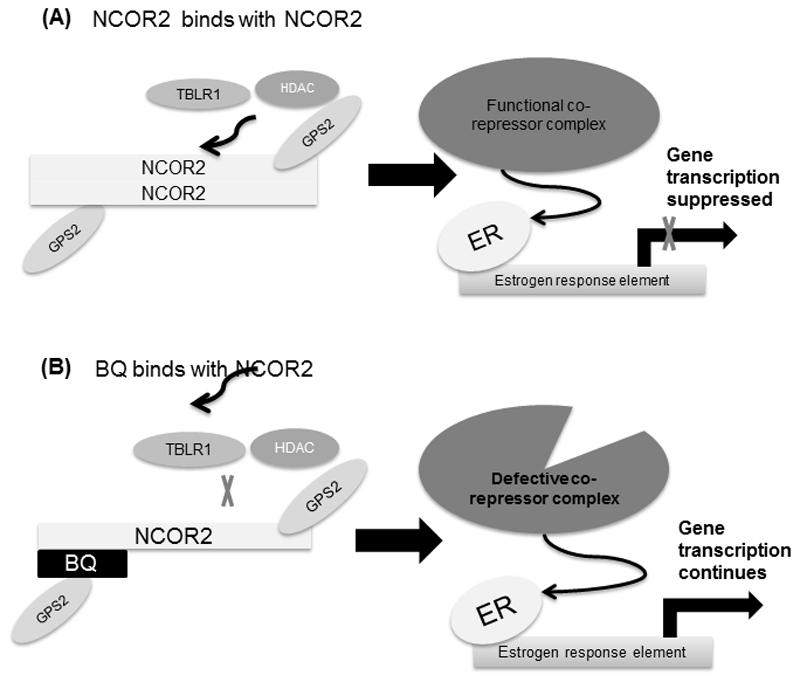
In this study, in vitro and in vivo evidence was presented to support that BQ323636.1 overexpression could confer tamoxifen resistance. Moreover, the functional roles of BQ323636.1 were studied. BQ323636.1 overexpression rescued the suppressive effect of NCOR2 overexpression exerted on the transcription of an ERE-containing luciferase reporter and compromised the inhibitory effects of tamoxifen on ER-target gene expression. Such effects can be explained by the fact that BQ323636.1 binds to NCOR2 but not to ER in the cells and that BQ323636.1 overexpression inhibits the interaction between HDAC3 and NCOR2, GPS2 and TBLR1 respectively, indicating the suppressed formation of co-repressor complex. Based on our findings, the mechanism by which BQ323636.1 overexpression contributes to tamoxifen resistance is proposed as follows (figure 6). In the absence of BQ323636.1, in response to tamoxifen treatment, NCOR2 homo-dimerizes in an anti-parallel fashion, serving as a dock for further recruitment of other co-repressor proteins, such as TBLR1, HDACs and GPS2, to form a complete and functional co-repressor complex which will bind to ER to suppress gene transcription and halt cell growth. In contrast, when BQ323636.1 is present, it binds to NCOR2, forming a faulty dock to which other co-repressor proteins cannot be fully recruited, resulting in a defective co-repressor complex which cannot bind to ER or is unable to fully suppress gene transcription (Figure 6). Hence the cells become inert to tamoxifen treatment.
Statistical analyses using TMA constructed from 358 primary breast cancer samples obtained from two independent cohorts of patients, recruited from Hong Kong and from the United Kingdom showed that, for patients who have been treated with tamoxifen, BQ323636.1 nuclear overexpression is significantly associated with tamoxifen resistance (Chi square test, p<0.001), which is defined as disease relapse or metastasis after being treated with tamoxifen. The correlation between nuclear BQ323636.1 enrichment and tamoxifen resistance is also observed in cell lines. It is also noted that, not only BQ323636.1 is expressed at higher levels in tamoxifen resistant cell lines (LCC2 and AK47) when compared with their parental tamoxifen sensitive cell lines (MCF-7 and ZR-75), but nuclear localization is observed more distinctly in tamoxifen resistant cell lines LCC2 and AK-47. Based on the existing knowledge, when present in the nucleus, BQ323636.1 is likely to be functionally active to mediate tamoxifen resistance. It is not yet clear why BQ323636.1 is expressed in both the cytoplasm and nucleus. Moreover, would be very interesting to further study what regulates the shuttling of BQ323636.1 between nucleus and cytoplasm.
Nuclear BQ323636.1 overexpression was significantly associated with tamoxifen resistance by Chi-square test, p= 1.79 x 10-6 with a sensitivity of 52.9% and specificity of 72.0%. The performance of BQ as a biomarker of tamoxifen response can be further improved using an expanded cohort. On the other hand, the cytoplasmic staining of BQ in IHC may have interfered with scorer’s interpretation of nuclear BQ expression. Unlike NCOR2, which is localized only in the nucleus, BQ was found to be expressed in both cytoplasm and nucleus. This raises a very interesting question as to what are the differences between cytoplasmic and nuclear BQ and what mechanisms, such as post-translational modifications, controls the shuttling of this protein between the cytoplasm and the nucleus. By investigating such questions, it may be possible to generate an antibody that specifically recognizes nuclear BQ, thus improving the accuracy of tamoxifen response prediction. Phosphorylation is a key regulatory step redirecting cellular localization between the cytoplasm and nucleus of many proteins. For example, nuclear iASSP, which is enriched in melanoma metastasis and associated with poor patient survival, is the phosphorylated form of iASSP and phosphorylation by cyclin B1/CDK1 on Ser-84 and Ser-113 triggers iASSP to enter the nucleus (25). Phosphorylation of FOXO3 on Ser-7 by p38 MAPK triggers the transition of FOXO3 from cytoplasm to nucleus upon doxorubicin treatment (26). Therfore, it is postulated that phosphorylation profile may distinguish nuclear from cytoplasmic BQ, and phosphorylation could signal BQ to enter the nucleus from the cytoplasm.
Aromatase inhibitors (AI) act by inhibiting the synthesis of estrogen from androgen by blocking the activity of the aromatase CYP19A1, resulting in reduced estrogen for induction of the ER-mediated signaling pathway (27). Our studies have confirmed that BQ activates ER-mediated signaling pathway in a ligand independent manner. Overexpression of BQ significantly enhanced the activity of ERE even in the absence of estrogen or tamoxifen (Figures 2 A&B), therefore it is likely that BQ may also be a predictive marker for AI resistance. This needs confirmation with a cohort of patients with available information of AI treatment and response.
To conclude, the current study has provided in vitro and in vivo evidence to support the notion that BQ overexpression could confer tamoxifen resistance and that BQ could be used as a predictive marker for patients’ response to tamoxifen treatment. The molecular mechanism through which BQ could induce tamoxifen resistance has also been studied and proposed. More studies are required using patient samples from different ethnic backgrounds to further verify the reliability of BQ in predicting tamoxifen resistance. Furthermore, it would be interesting to investigate the significance of BQ overexpression in relation to treatment with other novel anti-estrogens.
Supplementary Material
Translational Relevance.
More than two-thirds of all breast cancers are ER-positive. However, almost half of these treated with tamoxifen eventually develop resistance. By the time drug resistance is established, the cancer has already progressed and sometimes metastasized. The development of reliable biomarker, such as BQ323636.1, which could be assessed by immunohistochemistry on formalin fixed paraffin embedded tissue sections of newly diagnosed primary breast cancer would enable appropriate alternative therapy to be given at an early stage, thus saving the patient from the side effects as well as the risk of inappropriate treatment by tamoxifen.
Figure 5. The proposed mechanism of how BQ323636.1 could induce tamoxifen resistance in breast cancer.
When BQ323636.1 interacts with NCOR2, this prevents other co-repressor proteins such as TBLR1 and HDAC3 from binding to NCOR2, which renders NCOR2 unable to form functional repressor complexes to suppress ER-mediated gene transcription.
Acknowledgements
This project was kindly supported by the Innovative Technology Fund, HKSAR (ITF Ref. ITS/015/13), the Committee on Research and Conference Grants from the University of Hong Kong Project number 201309176148, the Medical Research Council, UK (MR/N012097/1) and Cancer Research UK (A12011). We would like to thank Dr. Robert Clarke (Georgetown University Medical School, Washington, D.C., USA) for kindly providing us LCC2 and AK-47 cell lines and Dr. Carolyn Smith (Baylor College of Medicine, Houston, TX) for providing the luciferase reporter ERE-TK-Luc. We also thank Faculty Core Facility of LKS Faculty of Medicine, HKU, for providing technical support in confocal microscopy. We thank the Nottingham Health Science Biobank and Breast Cancer Now Tissue Bank for the provision of tissue samples.
Financial support: This study was supported by grants from the Innovative Technology Fund, HKSAR (ITF Ref. ITS/015/13), the Committee on Research and Conference Grants from the University of Hong Kong Project number 201309176148, the Medical Research Council, UK (MR/N012097/1) and Cancer Research UK (A12011).
Footnotes
Conflicts of interest: Authors of this article declare there is no conflict of interest.
References
- 1.Jensen EV, Jordan VC. The estrogen receptor: a model for molecular medicine. Clin Cancer Res. 2003;9(6):1980–9. [PubMed] [Google Scholar]
- 2.Yamaga R, Ikeda K, Horie-Inoue K, Ouchi Y, Suzuki Y, Inoue S. RNA sequencing of MCF-7 breast cancer cells identifies novel estrogen-responsive genes with functional estrogen receptor-binding sites in the vicinity of their transcription start sites. Horm Cancer. 2013;4(4):222–32. doi: 10.1007/s12672-013-0140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rio MC, Chambon P. The pS2 gene, mRNA, and protein: a potential marker for human breast cancer. Cancer Cells. 1990;2(8–9):269–74. [PubMed] [Google Scholar]
- 4.Pelden S, Insawang T, Thuwajit C, Thuwajit P. The trefoil factor 1 (TFF1) protein involved in doxorubicininduced apoptosis resistance is upregulated by estrogen in breast cancer cells. Oncology reports. 2013;30(3):1518–26. doi: 10.3892/or.2013.2593. [DOI] [PubMed] [Google Scholar]
- 5.Jordan VC. Tamoxifen: catalyst for the change to targeted therapy. Eur J Cancer. 2008;44(1):30–8. doi: 10.1016/j.ejca.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng X, Kao HY. G protein pathway suppressor 2 (GPS2) is a transcriptional corepressor important for estrogen receptor alpha-mediated transcriptional regulation. J Biol Chem. 2009;284(52):36395–404. doi: 10.1074/jbc.M109.062109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang XM, Chang Q, Zeng L, Gu J, Brown S, Basch RS. TBLR1 regulates the expression of nuclear hormone receptor co-repressors. BMC Cell Biol. 2006;7:31. doi: 10.1186/1471-2121-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma D, Saxena NK, Davidson NE, Vertino PM. Restoration of tamoxifen sensitivity in estrogen receptor-negative breast cancer cells: tamoxifen-bound reactivated ER recruits distinctive corepressor complexes. Cancer Res. 2006;66(12):6370–8. doi: 10.1158/0008-5472.CAN-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varlakhanova N, Hahm JB, Privalsky ML. Regulation of SMRT corepressor dimerization and composition by MAP kinase phosphorylation. Mol Cell Endocrinol. 2011;332(1–2):180–8. doi: 10.1016/j.mce.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perissi V, Staszewski LM, McInerney EM, Kurokawa R, Krones A, Rose DW, et al. Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev. 1999;13(24):3198–208. doi: 10.1101/gad.13.24.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagy L, Kao HY, Love JD, Li C, Banayo E, Gooch JT, et al. Mechanism of corepressor binding and release from nuclear hormone receptors. Genes Dev. 1999;13(24):3209–16. doi: 10.1101/gad.13.24.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodson ML, Jonas BA, Privalsky ML. Alternative mRNA splicing of SMRT creates functional diversity by generating corepressor isoforms with different affinities for different nuclear receptors. J Biol Chem. 2005;280(9):7493–503. doi: 10.1074/jbc.M411514200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malartre M, Short S, Sharpe C. Alternative splicing generates multiple SMRT transcripts encoding conserved repressor domains linked to variable transcription factor interaction domains. Nucleic Acids Res. 2004;32(15):4676–86. doi: 10.1093/nar/gkh786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr Relat Cancer. 2004;11(4):643–58. doi: 10.1677/erc.1.00776. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Gong C, Lau SL, Yang N, Wong OG, Cheung AN, et al. SpliceArray profiling of breast cancer reveals a novel variant of NCOR2/SMRT that is associated with tamoxifen resistance and control of ERalpha transcriptional activity. Cancer Res. 2013;73(1):246–55. doi: 10.1158/0008-5472.CAN-12-2241. [DOI] [PubMed] [Google Scholar]
- 16.Wong LJ, Dai P, Lu JF, Lou MA, Clarke R, Nazarov V. AIB1 gene amplification and the instability of polyQ encoding sequence in breast cancer cell lines. BMC Cancer. 2006;6:111. doi: 10.1186/1471-2407-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Gomes AR, Monteiro LJ, Wong SY, Wu LH, Ng TT, et al. Constitutively nuclear FOXO3a localization predicts poor survival and promotes Akt phosphorylation in breast cancer. PLoS One. 2010;5(8):e12293. doi: 10.1371/journal.pone.0012293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Detre S, Saclani Jotti G, Dowsett M. A "quickscore" method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48(9):876–8. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labhart P, Karmakar S, Salicru EM, Egan BS, Alexiadis V, O'Malley BW, et al. Identification of target genes in breast cancer cells directly regulated by the SRC-3/AIB1 coactivator. Proc Natl Acad Sci U S A. 2005;102(5):1339–44. doi: 10.1073/pnas.0409578102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong MM, Guo C, Zhang J. Nuclear receptor corepressor complexes in cancer: mechanism, function and regulation. Am J Clin Exp Urol. 2014;2(3):169–87. [PMC free article] [PubMed] [Google Scholar]
- 21.de Souza JA, Olopade OI. CYP2D6 genotyping and tamoxifen: an unfinished story in the quest for personalized medicine. Semin Oncol. 2011;38(2):263–73. doi: 10.1053/j.seminoncol.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dezentje VO, Guchelaar HJ, Nortier JW, van de Velde CJ, Gelderblom H. Clinical implications of CYP2D6 genotyping in tamoxifen treatment for breast cancer. Clin Cancer Res. 2009;15(1):15–21. doi: 10.1158/1078-0432.CCR-08-2006. [DOI] [PubMed] [Google Scholar]
- 23.Schroth W, Goetz MP, Hamann U, Fasching PA, Schmidt M, Winter S, et al. Association Between CYP2D6 Polymorphisms and Outcomes Among Women With Early Stage Breast Cancer Treated With Tamoxifen. JAMA : the journal of the American Medical Association. 2009;302(13):1429–36. doi: 10.1001/jama.2009.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goetz M, Berry D, Klein T. Adjuvant Tamoxifen Treatment Outcome According to Cytochrome P450 2D6 (CYP2D6) Phenotype in Early Stage Breast Cancer: Findings from the International Tamoxifen Pharmacogenomics Consortium. Cancer Research. 2009;69(24 Supplement):33. [Google Scholar]
- 25.Lu M, Breyssens H, Salter V, Zhong S, Hu Y, Baer C, et al. Restoring p53 function in human melanoma cells by inhibiting MDM2 and cyclin B1/CDK1-phosphorylated nuclear iASPP. Cancer cell. 2013;23(5):618–33. doi: 10.1016/j.ccr.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Ho KK, McGuire VA, Koo CY, Muir KW, de Olano N, Maifoshie E, et al. Phosphorylation of FOXO3a on Ser-7 by p38 promotes its nuclear localization in response to doxorubicin. J Biol Chem. 2012;287(2):1545–55. doi: 10.1074/jbc.M111.284224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma CX, Reinert T, Chmielewska I, Ellis MJ. Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer. 2015;15(5):261–75. doi: 10.1038/nrc3920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.