Abstract
The capacity to regenerate entire body parts, tissues, and organs had generally been thought to be lost in evolution with very few exceptions (eg. the liver) surviving in mammals. The discovery of the MRL mouse and the elucidation of the underlying molecular pathway centering around hypoxia inducible factor, HIF-1α, has allowed a drug and materials approach to regeneration in mice and hopefully humans. The HIF-1α pathway is ancient and permitted the transition from unicellular to multicellular organisms. Furthermore, HIF-1α and its regulation by PHDs, important oxygen sensors in the cell, provides a perfect drug target. We review the historical background of regeneration biology, the discovery of the MRL mouse, and its underlying biology, and novel approaches to drugs, targets, and delivery systems.
Keywords: Accumulation Blastema, Aerobic Glycolysis, HIF-1α, MRL mouse, PEG-hydrogels, PHDs, Pluripotency Markers
Graphic Abstract
“If there were no regeneration, there could be no life. If everything regenerated, there would be no death.”
RJ Goss, 1969. Principles of Regeneration. Academic Press, New York. (1)
Regeneration – A Background (2)
The ability to regenerate has many meanings. To some, it means that a stem cell can divide and will be capable of maturing into a long-lived functional cell that is able to replace a non-functional or missing cell. To some, it can mean that a progenitor cell can replace a cell that has a high turn over rate. And it can mean that cells en masse can replace missing tissue.
However, it can also mean that an appendage or an organ with particular architecture and function containing multiple tissue types can be replaced perfectly and then function perfectly. This is known as epimorphic regeneration and is observed in the newt, for example, where a severed limb is completely replaced. Here, there is rapid epithelial covering of the wound and the formation of a highly cellular tissue structure at the wound site known as the accumulation blastema where cells collect, de-differentiate, divide and then re-differentiate to produce mature cells of different lineages (3).
To review the regenerative phenotype, we have to look back to the 1600’s.
Very early interest in the study of regeneration was shown in 1686 where lizard tail regeneration was demonstrated at the Paris Academy of Science (2) and was followed soon after by observations of human fingertip amputation with nail regrowth, crayfish appendage regeneration, and polyps or hydra regeneration by Abraham Trembley in 1744 (4) who showed that these animals could regrow their head and feet.
In 1768, Lazzaro Spallanzani published his extensive studies on regeneration of many organisms such as earthworms, slugs and snails, tadpoles, salamanders, and young toads. (5). This was a period of active studies in both developmental and regenerative biology. Only a few decades after Robert Hooke and Antonie van Leeuwenhoek first described eukaryotic cells, Spallanzani first described the blastema. However, there was certainly no consensus as to where the cells came from that made up this structure. Ultimately, there were four proposed derivations of these cells, the epidermis, mesenchyme, cells from the blood, and reserve cells or cells coming from the remains of missing tissue (3,6). It is probably true that there is a contribution from all of the above.
Beyond descriptive studies, a focus on examining patterning of genes and positional information in the field of developmental biology resulted in a comparison to regenerative biology with evidence of many similarities between the two (7).
More recent and popular studies have continued in animals such as the hydra, which as noted above, can regrow its head and feet and also seems to bud continuously with evidence of immortality (8–10). Sea cucumbers can eviscerate and regrow their intestines as a defense strategy against predators (11). Planaria can be cut into 279 parts and from each of these can regrow into a new organism within weeks through their pluripotent stem cells or neoblasts (12–15). Newts and axolotls can regrow limbs, tails, spinal cords and other parts of the nervous system including the eye and optic nerve (3, 16–18) Also, some of their genomes have been sequenced and genetically engineered animals have been produced (19–22). All of these organisms can be said to have a “full parts and labor plan” but mammals seem to be conspicuously absent from the list.
In organisms that show epimorphic regeneration, this means that a process of de-differentiation with the formation of an accumulation blastema contributed by the migration of cell populations and a massive remodeling response, leads to a regrowth of missing parts (3,17).
In mammals, there are, however, several examples of epimorphic regeneration. Seasonal antler regrowth appears to display epimorphic regeneration (23). Also, ear hole wounds in rabbits and holes in bat wings close over the open space (24–26). In both cases, a circular blastema filled with cells forms a “donut” at the wound margin and progressively fills the space without scar tissue leading to normal architecture with the formation of cartilage, new hair follicles and glands.
The mouse, of course, is a most desirable animal to study with its wealth of genetic analysis and extensive biological and genomic manipulation. In 1998, the MRL mouse, used mainly for studying autoimmune SLE (27,28), was shown to close ear-holes in a regenerative manner similar to rabbits (29). Subsequent studies by multiple groups showed that this mouse not only displayed regenerative ear hole closure but regeneration of multiple organ tissue types (30,31) including heart myocardium (32–34), digit (35, 36), articular cartilage (37–39), tendon (40,41), cornea (42), retina (43), peripheral nerves (44) and CNS (45,46), myometrium (47), transplanted skin (48) and muscle regeneration in a muscular dystrophy model leading to much reduced symptoms (49).
The Metabolic Status of a Regenerative Response
There have been several reports of super regenerating vertebrates using a glycolytic metabolic state during a regenerative response as opposed to one that is more focused on OXPHOS. They include studies in newts (50), axolotls (51) and zebrafish (52).
The MRL mouse can be added to this list. This mouse was bred from a mixture of mouse strains to retain the gene “cn” for achondroplagia found in AKR mice. These AKR mice were then bred to C57BL/6 (B6) and C3H and finally to LG mice which constitutes 75% of the MRL genome (53) and has been shown to be the main contributor to the MRL regenerative phenotype (54–56). These LG/J, MRL/MpJ, and MRL/lpr mice continue to grow with age and could be twice the size of normal mouse strains and put on excess weight. This suggested a metabolic difference between normal mice and these regenerating mice.
To examine such metabolic differences, adult MRL ear pinna-derived cells in culture and tissues from untreated or injured mice showed that their mitochondria had reduced activity with low mitochondrial membrane potentials and low levels of reactive oxygen species (ROS), a byproduct of oxygen metabolism in mitochondria, compared to non-regenerative C57BL/6 (B6) mouse cells. MRL cells, on the other hand, had high levels of lactate, suggesting that instead of using oxidative phosphorylation (OXPHOS) like B6, MRL mice were employing aerobic glycolysis (57–58), the same metabolic state used by stem cells, embryos, and cancer cells (59,60).
One molecule known to be responsible for such an unusual basal adult metabolic state is HIF-1α (61). HIF-1α is constitutively made by cells, found in the cytoplasm, and rapidly degraded under normoxic conditions. Since its protein expression is regulated by oxygen levels, molecules sensitive to such oxygen levels, mainly EGLNs or PHDs, recognize and hydroxylate proline residues in HIFαs, which are then recognized by pVHL and its E3-ligase complex which provides a ubiquination signal leading to proteosomal degradation. Thus, the stability of HIF-1α protein is clearly reduced. If HIF-1α survives, it moves from the cytoplasm to the nucleus, binds to HIF-1β and functions as a transcription factor, binding to HIF-responsive elements (HREs) found in the promoters of a very large number of genes related to energy metabolism, angiogenesis, vasculogenesis, cell migration and survival (86).
Since previous data had shown that the MRL mouse used a glycolytic metabolism, we examined HIF-1α protein levels in this mouse during healing using western blot analysis and immunohistochemistry of ear-hole tissue. MRL mice did express higher levels of HIF-1α compared to non-regenerating C57BL/6 mice. Si-RNA to HIF-1α (siHif1α) in vivo could completely block the regenerative response, indicating the necessary involvement of HIF-1α in epimorphic regeneration (62). Furthermore, this elevation of HIF-1α led to increased nuclear transcriptional activation and elevation of genes associated with aerobic glycolysis, vasculogenesis, tissue remodeling, and migration (62).
HIF-1α: A Gatekeeper of Regeneration
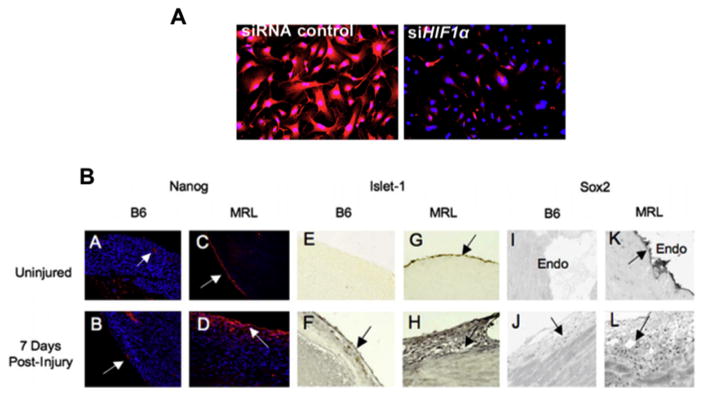
Since HIF-1α is required for ear hole closure, what are the potential functions it affects? One important function of HIF-1α is its role in de-differentiation (62–68), a major characteristic of the regeneration blastema (3,17). Cultured regenerating MRL cells express high levels of NANOG (Fig 2A) and many other embryonic stem cell markers, likely due to chromatin remodeling and a de-differentiative state (60). However, we had seen this previously (57) where high NANOG mRNA and protein expression levels as well as SOX2 and ISLET1 were found in MRL but not B6 cardiac tissue pre-injury (MRL: B6 levels = approx.50:1) and post-injury (MRL:B6 = approx. 420:10) (Fig 2B), consistent with the regeneration phenotype (57). The role of HIF-1α in NANOG expression levels was tested in cultured MRL ear fibroblasts by treating those cells with siHif-1α. Immunostaining for NANOG showed that siHif1α led to the subsequent elimination of NANOG staining (Fig 2A) (62).
Fig. 2.
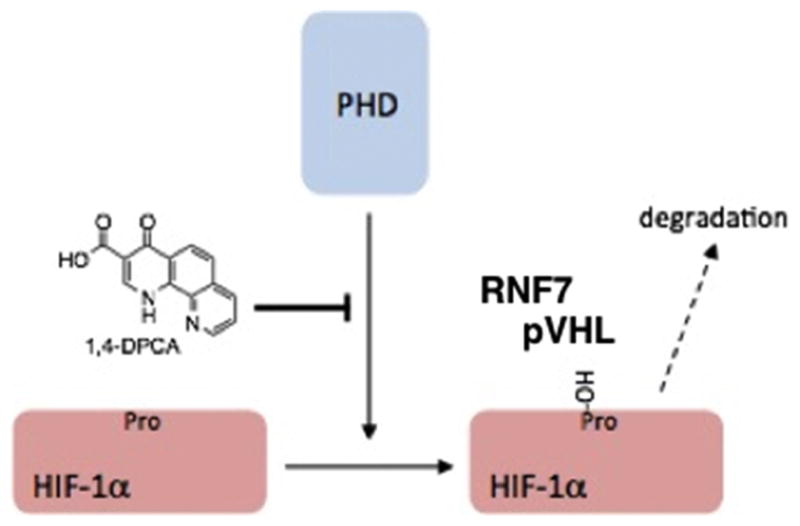
Diagram showing that PHDs hydroxylate the prolines in HIF-1α, which are then bound by pVHL followed by RNF7 and their respective E3-ligase complexes, ubiquinated, and then degraded. 1,4-DPCA acts as an inhibitor of PHDs and slows down or eliminates hydroxylation and degradation of HIF-1α. (from Zhang (62) Fig 2A)
Punched ear holes in the MRL mouse displayed a biphasic HIF-1α expression pattern in which HIF-1α protein levels rose after injury over a 2-week period and this phase was associated with the expression of de-differentiation markers in-vitro and in-vivo (62). After those two weeks, as HIF-1α levels declined, wound site tissues underwent a re-differentiation process with characteristic mature cell markers (62). What might be causing this HIF-1α response? Are the oxygen levels more pronounced in these mice? In studies to map genes involved in the regenerative MRL (LG) response (55,56), one candidate gene associated with regenerative responses provided another major clue to what might be happening in these mice. This molecule is RNF7, part of an E3-ligase complex necessary for HIF-1α degradation (69), which functions along with the pVHL-containing E3-ligase complex. The MRL(LG)-derived RNF7 shows non-coding sequence differences, with both MRL(LG) RNF7 mRNA and protein being poorly expressed compared to a non-regenerative mouse (56) in both normal and injured mice. Thus, it is possibly not an issue of oxygen, per se, rather it may be that HIF-1α is stabilized in MRL mice due to a defective degradation pathway via RNF7, at least in part, and the HIF-1α/1β complex transcription factor then goes on to activate the genes necessary for the regenerative program.
PHDs: A target for HIF-1α Regulation
Prolyl hydroxylase domain proteins (PHDs) are molecules that appeared early in complex organisms and could sense the level of oxygen and regulate effective cellular oxygen levels through the degradation of HIF-1α’s, among other targets. PHDs regulate HIF-1α degradation by hydroxylating prolines in the ODD region of HIF-1α which can then be recognized by pVHL, an E3 ligase subunit. A second E3 ligase containing RNF7 must also bind (69). HIF-1α is then ubiquinated and subsequently proteolyzed (Fig 3). Three PHD isoforms have been identified and are distinguished by their ability to hydroxylate HIF-α’s differentially (70). Much work has been carried out identifying PHD inhibitors leading to stabilization of HIF-α’s with the potential of regulating EPO, a HIF target (71), for example. The obvious question is whether we could induce regeneration by the simple modulation of the key oxygen regulator/sensor PHD using the known PHD inhibitor, 1,4-dihydrophenonthrolin-4-one-3-carboxylic acid (1,4-DPCA) (73).
Fig. 3.
A) SiRNA blocks NANOG expression. MRL cells were treated with either siRNA control (left panel) or siHif1 (right panel) for 48 hours. The cells were immunostained with anti-NANOG antibody. (from Zhang (62) Fig 5). B) Stem Cell Markers in the Adult MRL Heart. Panels A–D are sections are stained for NANOG. Arrows indicate areas of expression. NANOG expression was confined to vessel endothelium and endocardium in uninjured B6 (A,B). Robust expression was observed in epicardium of uninjured MRL heart (C), with increased expression and migration into the myocardium in cryo-injured MRL heart (32) (D). Panels E–H are stained for ISLET-1. Panels I–L are stained for SOX2. The epicardium is shown in all sections except in Panels I and K, in which endocardium is shown. Normal tissues before injury are seen in panels A, C, E, G, I, and K. Injured tissues, 7 days after RV cryoinjury, are shown in panels B, D, F, H, J, and L. (from Naviaux (57) Fig 3).
The Delivery of a PHD Inhibitor to Induce an Epimorphic Regenerative Response
Development of a Hydrogel Delivery System for 1,4-DPCA
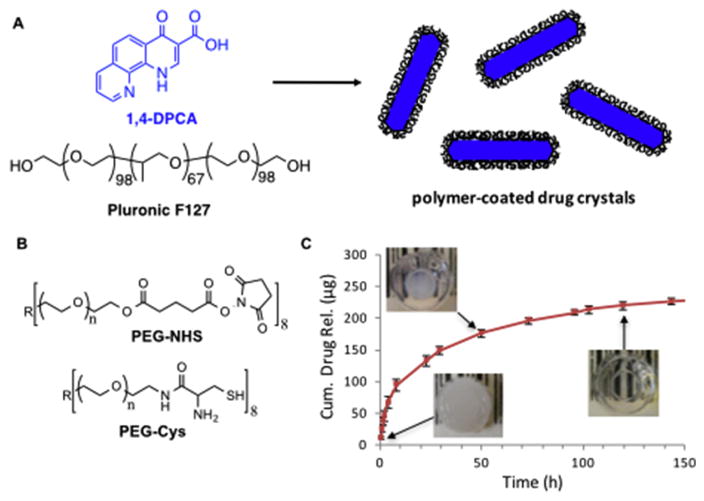
1,4-DPCA is a poorly soluble drug and presented some challenges for delivery. We ultimately achieved successful injectable in-vivo delivery of 1,4-DPCA by embedding polymer-coated 1,4-DPCA crystals (Fig 4A) in a polymer hydrogel system composed of branched PEG precursors containing N-hydroxysuccinimide (NHS) activated ester and N-terminal cysteine (N-Cys) endgroups (Fig 4B) (72). This hydrogel system exhibited rapid post-injection gelation by native chemical ligation (NCL) under physiological conditions, good biocompatibility, and other favorable properties for in-vivo use (72). Drug-loaded hydrogels were formed by suspending polymer-stabilized 1,4-DPCA microcrystals in an aqueous mixture of PEG precursors, which solidified in less than one minute to entrap the drug microcrystals within the hydrogel. In-vitro drug release studies demonstrated the delivery of 1,4-DPCA from the NCL hydrogel over several days (Fig 4C).
Fig. 4.
Components of a polymer hydrogel system used to deliver 1,4-DPCA. A) Poorly soluble 1,4-DPCA was crystallized in the presence of Pluronic F-127 to yield polymer-coated 1,4-DPCA microcrystals. B) Drug microcrystals were entrapped within an in-situ forming hydrogel formed by a rapid cross-linking reaction between PEG-NHS and PEG-Cys. C) The resulting hydrogels initially appear white due to the scattering of light by the microcrystals but become transparent over the course of ~5 days due to the gradual dissolution of 1,4-DPCA
1,4-DPCA/hydrogel delivered to non-regenerative mice leads to a regenerative response
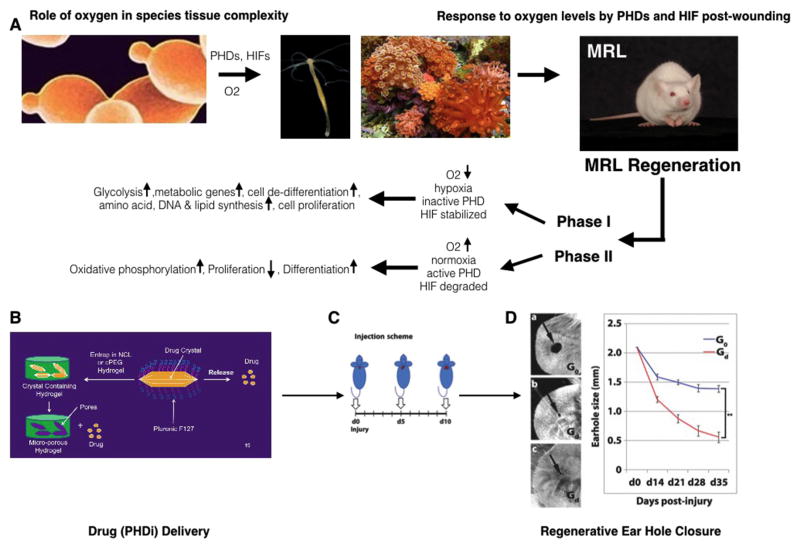
Although there are many known stabilizers of HIF-1α, the drug 1,4-DPCA was chosen due to an earlier report of in-vitro inhibitory activity on PHDs (73). The 1,4-DPCA containing hydrogel described in Figure 4 proved to be a convenient delivery vehicle for 1,4-DPCA because it exhibits a brief liquid state that facilitated injection of the material into tissue by syringe and needle. The drug delivery system was first tested in-vitro in a cell assay and shown to stabilize HIF-1α followed by an in-vivo test. In this regard, non-regenerating Swiss Webster (SW) mice, which do not close ear holes, were injected with a single subcutaneous dose of 1,4-DPCA/hydrogel, resulting in high HIF-1α levels that subsided by day 5. To mimic MRL HIF-1α levels, a single injection was given once every 5 days (day 0, 5, and 10). A complete ear-hole closure response was seen in these mice (see Fig 1). Animals were injected subcutaneously once with 0.1ml at each time point into adjacent sites at the back of the neck. Controls (Go) were given hydrogel alone, and experimentals (Gd) were given hydrogel + 0.2mg of crystallized drug. Under these conditions, ear-hole closure was achieved in SW mice. These data strongly support the central role of HIF-1α in the regenerative capacity of MRL mice and, moreover, demonstrated that the 1,4-DPCA/hydrogel can be used as a means to confer a similar regenerative ability to non-regenerating mice.
Fig. 1.
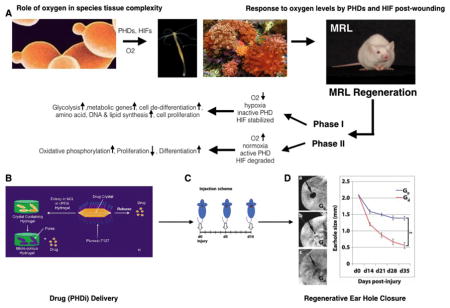
A) Eukaryotic single cell organisms with mitochondria (ie yeast, far left panel) prospered using plant-derived glucose and atmospheric oxygen to efficiently generate ATP. This allowed further evolution to metazoans or multicellular life (ie hydra and sponges, middle panels). High oxygen levels present problems for cells and therefore must be regulated. The toxic properties of oxygen are regulated by PHDs and HIFα’s where with high O2 levels, PHDs are active and hydroxylate HIFα prolines leading to HIFα degradation with a concomitant oxidative phosphorylation metabolic state, increased differentiation, and reduced proliferation (87). With low levels of O2, leading to hypoxic conditions, PHDs are inactive, HIFα prolines are not hydroxylated and HIFα is not degraded and shows increased protein levels (stable HIFα). HIFα now can move into the nucleus, and together with HIF1β, acts as a transcription factor activating genes specific for the glycolytic metabolic state, cell de-differentiation, amino acid, DNA, and lipid synthesis and enhanced cell proliferation. The regenerative MRL mouse (far right panel) during regeneration displays a biphasic response. Phase I (da 0–14 post injury) shows characteristics of a low level O2 state with high levels of HIFα, increased de-differentiation and proliferation. This is followed by Phase II (da 15–30 post injury) in which a higher O2-type response with decreased HIFα, re-differentiation, and reduced proliferation are seen. Both Phases appear to be necessary to achieve a full regenerative healing response, first breaking down tissue and then rebuilding it (62, 86). B) To recreate regeneration in non-regenerating mice, a delivery system using crystallized PHD inhibitor (1,4-DPCA) encapsulated in a PEG hydrogel (Gd) slowly releases drug C) which is given at multiple timepoints (da 0, 5, and 10) to induce a Phase I response. As drug levels decline by day 15, Phase II ensues, D) resulting in a 30 day regenerative complete ear hole closure response identical to that observed in the spontaneously regenerating MRL mouse. Gel without drug shows little healing (G0). (from Zhang (62) Fig 4).
1,4-DPCA/hydrogel induces de-differentiation as shown by markers in-vitro and in-vivo, which could be blocked by siHif1α
As mentioned above, it has been shown that a glycolytic metabolic state is maintained by pluripotent embryonic stem cells in the embryo that, upon differentiation, switch to oxidative phosphorylation (59). Adult quiescent mesenchymal and hematopoietic stem cells also use a glycolytic metabolism (59,60) like that used by adult MRL mice (62), other animal models of regeneration (50,51), and surgical wounds (74). The finding that de-differentiation of mature cells occurs under a hypoxic environment and elevated HIF-1α has been previously reported (65–68).
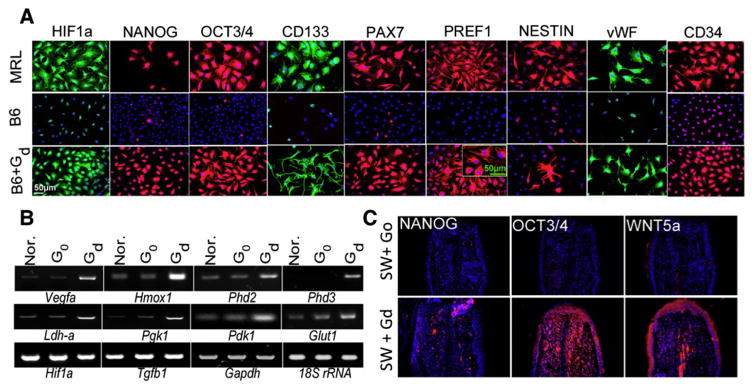
We found that untreated MRL mouse ear tissue and ear tissue-derived cells, under standard culture conditions, show an unusual expression of a range of diverse stem cell markers both in vitro and in vivo, including NANOG, SOX2, OCT3/4, CD34, and CD133, all pluripotency markers (Fig 5). It should be noted that these markers were not observed in ear tissue or cells from non-regenerating B6 or SW mice. However, HIF-1α stabilization by the 1,4-DPCA hydrogel in B6 and SW ear-derived cells led to an increase in all of these differentiation markers, although only transiently.
Fig. 5.
De-differentiation markers. In A, MRL ear fibroblasts in-vitro show the expression of HIF-1α, NANOG, OCT3/4, CD133, PAX7, PREF1, NESTIN, vWF, and CD34, all stem cell or progenitor cell markers by immunofluorescence whereas untreated B6 cells do not. B6 cells treated with 1,4-DPCA/hydrogel (Gd) show the same de-differentiation cell markers as MRL (from Zhang (62) (Fig 5)). In B, mRNA from B6 cells from A post G0 and Gd treatment showed the activation of HIF-1α target gene transcription (from Zhang (62) (Fig 3)). In C, expression of NANOG, OCT3/4, and WNT5A in ear holes from Swiss Webster (SW) mice treated with hydrogel, G0 (upper panels) or 1,4-DPCA/hydrogel, Gd (lower panels) show in-vivo de-differentiation effects by 1,4-DPCA (62).
Future opportunities for biomaterials in drug-induced tissue regeneration
A key ‘part’ of the ‘full parts and labor plan’ for tissue regeneration is a delivery system capable of sustained delivery of a HIF-1α agonist/PHD antagonist. Hydrogels have many attractive properties for drug delivery, however achieving high loading, homogeneity and controlled release of drugs can be difficult (75). The poor solubility of drugs like 1,4-DPCA in aqueous media represents a further challenge for controlled delivery from a hydrogel because of limited solubility within the hydrogel. The system described above is an example of a hydrogel drug delivery system that shows significant promise despite a simple approach to entrapment of drug microcrystals within a hydrogel. Future improvements in performance of both the ear hole as well as other tissue regeneration may be realized through the implementation of novel concepts in drug delivery systems. For example, tailoring of drug release kinetics may be afforded by new molecular designs that integrate both delivery system and drug in a unified way, such as through the use of polymer pro-drugs. In polymer pro-drugs, the drug is integrated into the molecular design of the delivery system and linked to the polymer delivery vehicle via a chemical linker capable of hydrolytic cleavage to release the drug.
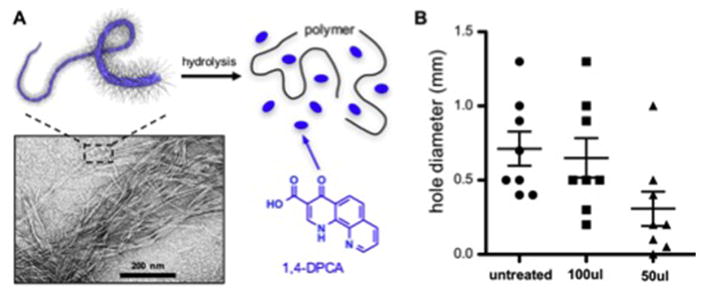
In some cases, manipulation and extension of drug release times can be accomplished by utilizing coulombic or weak intermolecular interactions between drug components in polymer prodrug systems (75,76). Such noncovalent interactions can be used to drive gel formation, disruption of the gel under shear as the gel passes through a needle during injection into a tissue, and dynamic re-association of the disrupted gel by the same intermolecular interactions. Only a handful of reports describe prodrugs that self-assemble into nanofibrillar gels, most of which are for cancer drug delivery (75–80). In the case of 1,4-DPCA specifically, our own efforts are providing early evidence that polymer prodrugs can be used in this way. We are developing a drug-polymer conjugate that spontaneously forms drug-filled nanofibrils due to the disparate polarities of the drug (nonpolar) and polymer (polar) (Fig 6). Our early studies show that an aqueous suspension of nanofibrils injected subcutaneously produces a regenerative response in non-healing mice that is reminiscent of the MRL mouse. Further refinement of this and similar systems may lead to new clinically relevant regeneration therapies.
Fig. 6.
Example of self-assembling polymer prodrug approach to 1,4-DPCA delivery. A. 1,4-DPCA is chemically conjugated to a biocompatible polymer via a hydrolysable ester, and self-assembles into a nanofibril gel that can be injected and provides a depot for drug release by hydrolysis. B. Preliminary data obtained with the nanofiber gel shows evidence of enhanced earhole closure at 30 d in Swiss Webster mice (n=8, 2 exp). The x-axis shows untreated mice, and mice injected with 100 microliters (100ul) or 50 ul of nanofiber gel.
Other Targets, Other Systems
Besides HIF-1α, we previously showed that the cell cycle checkpoint regulator CDKN1 or p21cip/waf when genetically eliminated from mice induces regeneration (81, 82). These p21knockout (p21KO) mice show many characteristics similar to HIF-elevated mice and we are currently examining the interactions between HIF and p21 during regeneration. A second study (83) has also shown that p21KO mice can regenerate ear holes. This is accompanied by a reduction in the normal expression of SDF, a molecule known to interact with CXCR4, and a reduction in CXCR4-positive cells at the wound site. Using the drug AMD3100 (84), a CXCR4 antagonist which can block the interaction of SDF and CXCR4, ear hole closure is significantly though partially enhanced showing the importance of such interactions. A very recent study using a KO mouse for the gene ASK1 or apoptosis signal regulating kinase1 has also shown ear hole closure (85). Furthermore, NQDI-1, a drug inhibitor of ASK1, can be applied to the ear hole topically and will lead to partial ear hole closure. Interestingly, p21 is down-regulated in the ASK1KO mouse. Thus, the underlying mechanisms in ASK1 down-regulation may be related to both p21 loss and HIF up-regulation. This nascent field with multiple drug targets is only the beginning of drug-based regulation of regeneration in mammals.
Conclusion
The new understanding of the central role of HIF-1α as a gatekeeper of mammalian regeneration opens up many possibilities for a drug approach to treat loss or damage of tissues and organs. It points to downstream targets as well. New and improved drug moieties and biomaterials may insure that future patients will have a “full parts and labor plan”.
Table 1.
List and Definition of Terms
| Accumulation Blastema. After an amputation wound, there is rapid epithelial covering of the wound and the formation of a highly cellular tissue structure at the wound site known as the accumulation blastema where cells collect, de-differentiate, divide and then re-differentiate to produce mature cells of different lineages. |
| Aerobic Glycolysis. This is a metabolic state where there is a conversion of glucose to lactate generating low amounts of ATP even in the presence of oxygen. This is also known as the Warburg effect. The use of aerobic glycolysis results in increased lipid, DNA, and protein synthesis for increased cell proliferation. Otto Warburg in 1924 showed that tumor cells showed an increased dependence on glycolysis instead of oxidative phosphorylation which uses mitochondria to produce large amount of ATP. |
| ASK1. This molecule is an apoptosis signal-regulating kinase, thus its activation leads to apoptosis. It is involved in innate immune responses and mediates responses to oxidative stress and inflammatory mediators such as TNF and LPS. |
| HIF-1α. The heterodimeric transcription factor HIF1 is made up of two subunits, HIF-1α and HIF-1β. HIF-1α is a basic helix-loop-helix PAS domain-containing protein and is regulated through an interaction with prolyl-hydroxylating PHDs. |
| NANOG. This is a transcription factor found in embryonic stem cells and considered to be a major factor in maintaining pluripotency and self renewal of undifferentiated cells. |
| MRL. The MRL mouse strain is a cross between AKR, C57BL/6, C3H and Large (LG), the latter contributing 75% of its genome to the MRL. This mouse was named after Murphy and Roth (two researchers involved in breeding and using these mice for autoimmunity studies) and the Large (LG) mouse. |
| PHDs. HIF prolyl hydroxylases, also known as EGLNs or prolyl hydroxylase domain-containing proteins are molecules that recognize proline residues in HIF-1α, can then hydroxylate those residues making them targets of E3 ligases, ubiquination, and proteosomal degradation. PHDs have a high affinity for iron (II) and 2 oxoglutarate, forming a long lived complex. |
| RNF-7. This protein is a ring box protein 2 which is an essential subunit of the SKP1-cullin/CDC53F box protein ubiquitin ligase. It binds to the pVHL- HIF-1α complex and is necessary for HIF-1α degradation. |
| SDF/CXCR4. Stromal derived factor 1 (SDF1) is also known as the chemokine CXCL12, it is produced by many tissue and induced by inflammatory signals. It plays a role in angiogenesis by directing endothelial precursors from the bone marrow to the blood vessels. CXCR4 is a receptor for SDF-1. It is involved in mobilization of stem cells from the bone marrow into the circulation. Thus together, they are involved in migration, angiogenesis, and cell adhesion. |
| SOX2. This is a transcription factor essential for maintaining self-renewal of undifferentiated embryonic stem cells and pluripotency. |
| pVHL. The protein von Hippel-Lindau tumor suppressor was identified by mutations in this protein leading to a dominantly inherited cancer syndrome. Its major activity is to act as an E3 ubiquitin ligase along with elongin B and C and cullin-2. It recognizes hydroxylated prolines in HIF-1α and ensures its polyubiquination leading to proteosomal degradation. |
Acknowledgments
This work was supported by grants from the National Institutes of Health Grants (DE021104, DE021215, CA180070) and from DOD (DARPA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goss RJ. Principles of Regeneration. Academic Press; New York: 1969. [Google Scholar]
- 2.Dinsmore Charles E., editor. Milestones in the evolution of a science. New York: Cambridge University Press; 1991. A History of Regeneration Research; p. 228. [Google Scholar]
- 3.Stocum DL, Cameron JA. Looking Proximally and Distally: 100 Years of Limb Regeneration and Beyond. Developmental Dynamics. 2011;240:943–968. doi: 10.1002/dvdy.22553. [DOI] [PubMed] [Google Scholar]
- 4.Trembley A. Observations and experiments upon the fresh-water polypus. Philos Trans R Soc London. 1744;42:283–291. [Google Scholar]
- 5.Spallanzani L. In: An Essay on Animal Reproduction. Maty M, translator. London: 1765. p. 1769. T Becket and DeHondt. [Google Scholar]
- 6.Liversage RA. Origin of the blastema cells in epimorphic regeneration of urodele appendages: a history of ideas in A History of Regeneration Research. In: Dinsmore Charles E., editor. Milestones in the evolution of a science. New York: Cambridge University Press; 1991. pp. 179–199. [Google Scholar]
- 7.Wolpert L. Positional information and the spatial pattern of cellular differentiation. Theoretical Biology. 1969;25:1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- 8.Holstein TW, Hobmayer E, David CN. Pattern of epithelial cell cycling in hydra. Dev Biol. 1991;148:602–611. doi: 10.1016/0012-1606(91)90277-a. [DOI] [PubMed] [Google Scholar]
- 9.Martínez DE, Bridge D. Hydra, the everlasting embryo, confronts aging. Int J Dev Biol. 2012;56:479–87. doi: 10.1387/ijdb.113461dm. [DOI] [PubMed] [Google Scholar]
- 10.Dańko MJ, Kozłowski J, Schaible R. Unraveling the non-senescence phenomenon in Hydra. J Theor Biol. 2015;382:137–49. doi: 10.1016/j.jtbi.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 11.Mashanov VS, Zueva OR, García-Arrarás JE. Expression of pluripotency factors in echinoderm regeneration. Cell Tissue Res. 2015;359:521–36. doi: 10.1007/s00441-014-2040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Handberg-Thorsager M, Fernandez E, Salo E. Stem cells and regeneration in planarians. Frontiers in Bioscience: A Journal and Virtual Library. 2008;13:6374–6394. doi: 10.2741/3160. [DOI] [PubMed] [Google Scholar]
- 13.Aziz Aboobaker A. Planarian stem cells: a simple paradigm for regeneration. Trends in Cell Biology. 2011;21:304–311. doi: 10.1016/j.tcb.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Newmark PA, Alvarado AS. Not Your Father’s Planarian: A Classic Model Enters The Era Of Functional Genomics. Nature Reviews Genetics. 2002;3:210–219. doi: 10.1038/nrg759. [DOI] [PubMed] [Google Scholar]
- 15.Aoki R, Wake H, Sasaki H, Agata K. Recording and spectrum analysis of the planarian electroencephalogram. Neuroscience. 2009;159:908–914. doi: 10.1016/j.neuroscience.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Gardiner DM, Bryant SV. Molecular mechanisms in the control of limb regeneration: the role of homeobox genes. Int J Dev Biol. 1996;40:797–805. [PubMed] [Google Scholar]
- 17.Brockes JP, Kumar A. Appendage Regeneration in Adult Vertebrates and Implications for Regenerative Medicine. Science. 2005;310:1919–1923. doi: 10.1126/science.1115200. [DOI] [PubMed] [Google Scholar]
- 18.Sousounis K, Qi F, Yadav MC, Millán JL, Toyama F, Chiba C, Eguchi Y, Eguchi G, Tsonis PA. A robust transcriptional program in newts undergoing multiple events of lens regeneration throughout their lifespan. Elife. 2015 Nov 2;:4. doi: 10.7554/eLife.09594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith JJ, Putta S, Zhu W, Pao GM, Verma IM, Hunter T, Bryant SV, Gardiner DM, Harkins TT, Voss SR. Genic regions of a large salamander genome contain long introns and novel genes. BMC Genomics. 2009;10:19. doi: 10.1186/1471-2164-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wittlieb J, Khalturin K, Lohmann JU, Anton-Erxleben F, Bosch TC. Transgenic Hydra allow in vivo tracking of individual stem cells during morphogenesis. Proc Natl Acad Sci. 2006;103:6208–11. doi: 10.1073/pnas.0510163103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarado AS, Tsonis P. Bridging the regeneration GAP: Genetics insights from diverse animal models. Nature Reiews. 7Z:873. doi: 10.1038/nrg1923. [DOI] [PubMed] [Google Scholar]
- 22.Wasik K, Gurtowski J, Zhou X, Ramos OM, Delás MJ, Battistoni G, El Demerdash O, Falciatori I, Vizoso DB, Smith AD, Ladurner P, Schärer L, McCombie WR, Hannon GJ, Schatz M. Genome and transcriptome of the regeneration-competent flatworm, Macrostomum lignano. PNAS. 2015;112:12462–7. doi: 10.1073/pnas.1516718112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price J, Faucheux C, Allen S. Deer antlers as a model of Mammalian regeneration. Curr Top Dev Biol. 2005;67:1–48. doi: 10.1016/S0070-2153(05)67001-9. [DOI] [PubMed] [Google Scholar]
- 24.Joseph J, Dyson M. Tissue replacement in the rabbit’s ear. Br J Surg. 1966;53:372–38. doi: 10.1002/bjs.1800530415. [DOI] [PubMed] [Google Scholar]
- 25.Goss RJ, Grimes LN. Tissue Interactions in Regeneration of Rabbit Ear Holes. Am Zool. 1972;12:151–157. [Google Scholar]
- 26.Goss RJ. Prospects of regeneration in man. Clin Orthop Relat Res. 1980;151:270–82. [PubMed] [Google Scholar]
- 27.Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 28.Kono D, Theofilopoulos AN. Genes and genetics of murine lupus. In: Theofilopoulos AN, Bona CA, editors. The Molecular Pathology of Autoimmune Diseases. New York: Taylor and Francis; 2002. pp. 353–375. [Google Scholar]
- 29.Clark LD, Clark RK, Heber-Katz EE. A new murine model for mammalian wound repair and regeneration. Clin Immunol Immunopathol. 1998;88:35–45. doi: 10.1006/clin.1998.4519. [DOI] [PubMed] [Google Scholar]
- 30.Heydemann A. The super super-healing MRL mouse strain. Front Biol. 2012;7:522–538. doi: 10.1007/s11515-012-1192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards RG. From embryonic stem cells to blastema and MRL mice. Reprod BioMed Online. 2008;16:425–461. doi: 10.1016/s1472-6483(10)60605-0. [DOI] [PubMed] [Google Scholar]
- 32.Leferovich JM, Bedelbaeva K, Samulewicz S, Zhang XM, Zwas D, Lankford EB, Heber-Katz E. Heart regeneration in adult MRL mice. Proc Natl Acad Sci USA. 2001;98:9830–9835. doi: 10.1073/pnas.181329398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naseem RH, Meeson AP, Michael DiMaio J, White MD, Kallhoff J, Humphries C, Goetsch SC, De Windt LJ, Williams MA, Garry MG, Garry DJ. Reparative myocardial mechanisms in adult C57BL/6 and MRL mice following injury. Physiol Genomics. 2007;30:44–52. doi: 10.1152/physiolgenomics.00070.2006. [DOI] [PubMed] [Google Scholar]
- 34.Alfaro MP, Pagni M, Vincent A, Atkinson J, Hill MF, Cates J, Davidson JM, Rottman J, Lee E, Young PP. The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci USA. 2008;105:18366–18371. doi: 10.1073/pnas.0803437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gourevitch DL, Clark L, Bedelbaeva K, Leferovich J, Heber-Katz E. Dynamic changes after murine digit amputation: The MRL mouse digit shows waves of tissue remodeling, growth, and apoptosis. Wound Repair and Regeneration. 2009;17:447–455. doi: 10.1111/j.1524-475X.2009.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chadwick RB, Bu L, Yu H, Hu Y, Wergedal JE, Mohan S, Baylink DJ. Digit tip regrowth and differential gene expression in MRL/Mpj, DBA/2, and C57BL/6 mice. Wound Repair and Regeneration. 2007;15:275–284. doi: 10.1111/j.1524-475X.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- 37.Fitzgerald J, Rich C, Burkhardt D, Allen J, Herzka AS, Little CB. Evidence for articular cartilage regeneration in MRL/MpJ mice. Osteoarthritis and Cartilage. 2008;16:1319–1326. doi: 10.1016/j.joca.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 38.Rai MF, Hashimoto S, Johnson EE, Janiszak KL, Fitzgerald J, Heber-Katz E, Cheverud JM, Sandell LJ. Heritability of articular cartilage regeneration and its association with ear wound healing in mice. Arthritis Rheum. 2012;64:2300–2310. doi: 10.1002/art.34396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward BD, Furman BD, Huebner JL, Kraus VB, Guilak F, Olson SA. Absence of posttraumatic arthritis following intraarticular fracture in the MRL/MpJ mouse. Arthritis Rheum. 2008;58:744–53. doi: 10.1002/art.23288. [DOI] [PubMed] [Google Scholar]
- 40.Sereysky JB, Flatow EL, Andarawis-Puri N. Musculoskeletal regeneration and its implications for the treatment of tendinopathy. International Journal of Experimental Pathology. 2013;94:293–303. doi: 10.1111/iep.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lalley AL, Dyment NA, Kazemi N, et al. Improved Biomechanical and Biological Outcomes in the MRL/MpJ Murine Strain Following a Full-Length Patellar Tendon Injury. Journal of orthopaedic research. 2015;33:1693–1703. doi: 10.1002/jor.22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ueno Ueno M, Lyons BL, Burzenski LM, Gott B, Shaffer DJ, Roopenian DC, Shultz LD. Accelerated Wound Healing of Alkali-Burned Corneas in MRL Mice Is Associated with a Reduced Inflammatory Signature. Investigative Ophthalmology & Visual Science. 2005;46:4097–4106. doi: 10.1167/iovs.05-0548. [DOI] [PubMed] [Google Scholar]
- 43.Xia H, Krebs MP, Kaushal S, Scott EW. Enhanced retinal pigment epithelium regeneration after injury in MRL/MpJ mice. Experimental Eye Research. 2011;9:862–72. doi: 10.1016/j.exer.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buckley G, Metcalfe AD, Ferguson MWJ. Peripheral nerve regeneration in the MRL/MpJ ear wound model. Journal of Anatomy. 2011;218:163–72. doi: 10.1111/j.1469-7580.2010.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thuret S, Thallmair M, Horky LL, Gage FH. Enhanced Functional Recovery in MRL/MpJ Mice after Spinal Cord Dorsal Hemisection. PLoS ONE. 7:1–15. doi: 10.1371/journal.pone.0030904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balu DT, Hodes GE, Hill TE, Ho N, Rahman Z, Bender CN, Ring RH, Dwyer JM, Rosenzweig-Lipson S, Hughes ZA, Schechter LE, Lucki I. Flow Cytometric Analysis of BrdU Incorporation as a High-Throughput Method for Measuring Adult Neurogenesis in the Mouse. Journal of Pharmacological and Toxicological Methods. 2008;59:100–7. doi: 10.1016/j.vascn.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buhimschi CS, Zha G, Sora N, Madri JA, Irina A, Buhimschi IA. Myometrial Wound Healing Post-Cesarean Delivery in the MRL/MpJ Mouse Model of Uterine Scarring. The American Journal of Pathology. 2010;177:197–207. doi: 10.2353/ajpath.2010.091209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tolba RH, Schildberg FA, Decker D, Abdullah Z, Büttner R, Minor T, Von Ruecker A. Mechanisms of improved wound healing in Murphy Roths Large (MRL) mice after skin transplantation. Wound Repair and Regeneration. 2010;18(6):662–70. doi: 10.1111/j.1524-475X.2010.00631.x. [DOI] [PubMed] [Google Scholar]
- 49.Heydemann A, Swaggart KA, Kim GH, Holley-Cuthrell J, Hadhazy M, McNally EM. The superhealing MRL background improves muscular dystrophy. Skelet Muscle. 2012;2:26. doi: 10.1186/2044-5040-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt A. Metabolites and Metabolism in Repair and Regeneration. In: Schmidt A, editor. Cellular Biology of Vertebrate Regeneration and Repair. Chicago: University of Chicago Press; 1968. pp. 121–240. [Google Scholar]
- 51.Rao N, Jhamb D, Milner D, Li B, Song F, Wang M, Voss SR, Palakal M, King M, Saranjami B, Nye H, Cameron J, Stocum D. Proteomic analysis of blastema formation in regenerating axolotl limbs. BMC Biology. 2009;7:83. doi: 10.1186/1741-7007-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elks PM, Renshaw SA, Meijer AH, Walmsley SR, van Eeden FJ. Exploring the HIFs, buts and maybes of hypoxia signalling in disease: lessons from zebrafish models. Dis Model Mech. 2015;8:1349–60. doi: 10.1242/dmm.021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy ED. Lymphoproliferation (lpr) and other single-locus models for murine lupus. In: Gershwin ME, Merchant B, editors. Immunologic Defects in Laboratory Animals. New York: Plenum Press; 1981. pp. 143–173. [Google Scholar]
- 54.Kench JA, Russell DM, Fadok VA, Young SK, Worthen GS, Jones-Carson J, Henson JE, Nemazee D. Aberrant wound healing and TGF-beta production in the autoimmune-prone MRL/+ mouse. Clinical Immunology. 1999;92:300–310. doi: 10.1006/clim.1999.4754. [DOI] [PubMed] [Google Scholar]
- 55.Blankenhorn EP, Bryan G, Kossenkov AV, Clark LD, Zhang XM, Chang C, Horng W, Pletscher LS, Cheverud JM, Showe LC, Heber-Katz E. Genetic loci that regulate healing and regeneration in LG/J and SM/J mice. Mamm Genome. 2009;20:720–733. doi: 10.1007/s00335-009-9216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheverud JM, Lawson HA, Bouckaert K, Kossenkov A, Showe L, Cort L, Blankenhorn EP, Bedelbaeva K, Gourevitch D, Arthur LM, Heber-Katz E. Fine-mapping quantitative trait loci affecting murine external ear tissue regeneration in the LG/J by SM/J advanced intercross line. Heredity. 2014;112:508–18. doi: 10.1038/hdy.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naviaux RK, Le TP, Bedelbaeva K, Leferovich J, Gourevitch D, Sachadyn P, Zhang XM, Clark L, Heber-Katz E. Retained features of embryonic metabolism in the adult MRL mouse. Molecular Genetics and Metabolism. 2009;96:133–144. doi: 10.1016/j.ymgme.2008.11.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heber-Katz E, Naviaux R. The MRL Mouse: A Model of Regeneration and Cancer. In: Berger NA, editor. Murine Models, Energy Balance, and Cancer. Springer; 2015. pp. 47–64. [Google Scholar]
- 59.Shyh-Chang N, Daley GQ, Cantley LC. Stem cell metabolism in tissue development and aging. Development. 2013;140:2535–2547. doi: 10.1242/dev.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, Sadek HA. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Semenza GL. Hypoxia-inducible factor 1: Regulator of mitochondrial metabolism and mediator of ischemic conditioning. BBA. 2011;1813:1263–1268. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, Bedelbaeva K, Strehin I, Gourevitch D, Messersmith PB, Heber-Katz E. Drug-induced Regeneration in Adult Mice. Science Transl Med. 2015;7:290. doi: 10.1126/scitranslmed.3010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shyh-Chang N, Daley GQ, Cantley LC. Stem cell metabolism in tissue development and aging. Development. 2013;140:2535–2547. doi: 10.1242/dev.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, Sadek HA. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dunwoodie SL. The role of hypoxia in development of the mammalian embryo. Dev Cell. 2009;17:755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 66.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 67.Mathieu J, Zhang Z, Zhou W, et al. HIF induces human embryonic stem cell markers in cancer cells. Cancer Res. 2011;71:4640–4652. doi: 10.1158/0008-5472.CAN-10-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sperber H, Mathieu J, Wang Y, et al. The metabolome regulates the epigenetic landscape during naive-to-primed human embryonic stem cell transition. Nat Cell Biol. 2015;17:1523–35. doi: 10.1038/ncb3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan M, Gu Q, He H, Pamarthy D, Semenza GL, Sun Y. SAG/ROC2/RBX2 is a HIF-1 target gene that promotes HIF-1a ubiquitination and degradation. Oncogene. 2008;27:1404–1411. doi: 10.1038/sj.onc.1210780. [DOI] [PubMed] [Google Scholar]
- 70.Applehoff RJ, et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–65. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- 71.Heber-Katz E. Oxygen, Metabolism, and Regeneration – Lessons from Mice. Trends in Molecular Medicine. 2017 doi: 10.1016/j.molmed.2017.08.008. online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strehin I, Gourevitch D, Zhang Y, Heber-Katz E, Messersmith PB. Hydrogels Formed by Oxo-ester Mediated Native Chemical Ligation. Biomater Sci. 2013;6:603–613. doi: 10.1039/C3BM00201B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Franklin TJ, Morris WP, Edwards PN, Large MS, Stephenson R. Inhibition of prolyl 4-hydroxylase in vitro and in vivo by members of a novel series of phenanthrolinones. Biochem J. 2001;353(Pt 2):333–8. doi: 10.1042/0264-6021:3530333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hunt TK, Aslam RS, Beckert S, Wagner S, Ghani QP, Hussain MZ, Roy S, Sen CK. Aerobically derived lactate stimulates revascularization and tissue repair via redox mechanisms. Antioxid Redox Signal. 2007;9:1115–1124. doi: 10.1089/ars.2007.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoare TR, Kohane DS. Hydrogels in drug delivery: Progress and challenges. Polymer. 2008;49(8):1993–2007. [Google Scholar]
- 76.Vemula PK, et al. Prodrugs as self-assembled hydrogels: a new paradigm for biomaterials. Curr Opin in Biotech. 2013;24:1174–1182. doi: 10.1016/j.copbio.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 77.Li J, et al. Dephosphorylation of d-Peptide Derivatives to Form Biofunctional, Supramolecular Nanofibers/Hydrogels and Their Potential Applications for Intracellular Imaging and Intratumoral Chemotherapy. J Amer Chem Soc. 2013;135:9907–9914. doi: 10.1021/ja404215g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ling Y, et al. Using a peptide segment to covalently conjugate doxorubicin and taxol for the study of drug combination effect. RSC Adv. 2015;5:101475–101479. [Google Scholar]
- 79.Mao L, et al. Conjugation of two complementary anti-cancer drugs confers molecular hydrogels as a co-delivery system. Chem Comm. 2012;48:395–397. doi: 10.1039/c1cc16250k. [DOI] [PubMed] [Google Scholar]
- 80.Matson JB, Stupp SI. Drug release from hydrazone-containing peptide amphiphiles. Chem Comm. 2011;47:7962–7964. doi: 10.1039/c1cc12570b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bedelbaeva K, Snyder A, Gourevitch D, Clark L, Zhang XM, Leferovich J, Cheverud JM, Lieberman P, Heber-Katz E. Lack of p21 expression links cell cycle control and appendage regeneration in mice. Proc Natl Acad Sci U S A. 2010;107:5845–50. doi: 10.1073/pnas.1000830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heber-Katz E, Zhang Y, Bedelbaeva K, Song F, Chen X, Stocum DL. Cell Cycle Regulation and Regeneration. Curr Top Microbiol Immunol. 2013;367:253–276. doi: 10.1007/82_2012_294. [DOI] [PubMed] [Google Scholar]
- 83.Leung TH, Snyder ER, Liu Y, Wang J, Kim SK. A cellular, molecular, and pharmacological basis for appendage regeneration in mice. Genes Dev. 2015;29:2097–107. doi: 10.1101/gad.267724.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hendrix CW, Collier AC, Lederman MM, Schols D, Pollard RB, Brown S, Jackson JB, Coombs RW, Glesby MJ, Flexner CW, et al. Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV- 1 infection. J Acquir Immune Defic Syndr. 2004;37:1253–1262. doi: 10.1097/01.qai.0000137371.80695.ef. [DOI] [PubMed] [Google Scholar]
- 85.Zhang QS, Kurpad DS, Mahoney MG, Steinbeck MJ, Freeman TA. Inhibition of apoptosis signal-regulating kinase 1 alters the wound epidermis and enhances auricular cartilage regeneration. PLoS One. 2017;12:e0185803. doi: 10.1371/journal.pone.0185803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heber-Katz E. Oxygen, Metabolism, and Regeneration: Lessons from Mice. Trends Mol Med. 2017;23:1024–1036. doi: 10.1016/j.molmed.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Semenza GL. Life with Oxygen. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]