Abstract
Epidemiological and laboratory data support the protective effects of bioactive nutrients in our diets for various diseases. Along with various factors, such as genetic history, alcohol, smoking, exercise, and dietary choices play a vital role in affecting an individual’s immune responses towards a transforming cell, by either preventing or accelerating a neoplastic transformation. Ample evidence suggests that dietary nutrients control the inflammatory and pro-tumorigenic responses in immune cells. Immunoprevention is usually associated with the modulation of immune responses that help in resolving the inflammation, thus improving clinical outcome. Various metabolic pathway-related nutrients, including glutamine, arginine, vitamins, minerals, and long-chain fatty acids, are important components of immunonutrient mixes. Epidemiological studies related to these substances have reported different results, with no or minimal effects. However, several studies suggest that these nutrients may have immune-modulating effects that may lower cancer risk. Preclinical studies submit that most of these components may provide beneficial effects. The present review discusses the available data, the immune-modulating functions of these nutrients, and how these substances could be used to study immune modulation in a neoplastic environment. Further research will help to determine whether the mechanistic signaling pathways in immune cells altered by nutrients can be exploited for cancer prevention and treatment.
Graphic abstract
mnfr.201500884 - Prevention and Treatment of Cancers by Immune Modulating Nutrients
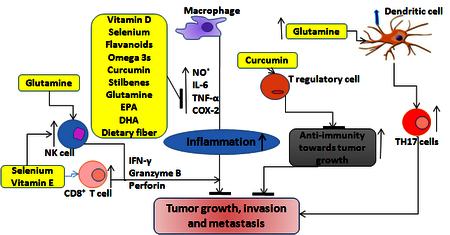
Immune modulatory effects of dietary nutrients during tumor growth: Several bioactive nutrients enhance innate immune responses by increasing natural killer and CD8 cell cytotoxicity towards inhibition of tumor growth. Nutrients levels are to be balanced to achieve immune responses to modulate the tumor growth. Bioactive components of dietary nutrients enhance anti-inflammatory cytokines and decrease T regulatory cells and enhance anti-tumor immunity. Overall dietary nutrients inhibit tumor growth, invasion and metastases.
Introduction
Bioactive nutrient-induced immune system involvement in defense against cancer has been explored for decades. Recent evidence suggests that nutrition plays an important role in cancer development and progression. Animal data clearly demonstrated that the use of bioactive agents isolated from foods modulated the immune system, where the nutrient(s) can identify and eradicate tumors. These findings are supported by epidemiological human data on the consumption of various foods and reduced risk for inflammation and cancer. Although technology has developed to a point that we are able to study each individual cytokine or immune cell’s function, it is difficult to demonstrate the powerful role of the immune system in cancer prevention or treatment, due to tumor complexity or heterogeneity in different patient populations. However, as research throws more light on interactions in immune responses and cancer, novel prevention and therapeutic strategies that involve modulation through bioactive agents can and will be developed.
Many bioactive components of food play an important role in immune functions [1–3]. Immune functions are indispensible for their protective roles against antigens or transforming or transformed neoplastic cells. Specific bioactive agents affect cell-mediated immune responses; this is evident from preclinical and clinical studies related to dietary deficiencies of specific bioactive nutrients altering cell-mediated immune responses [4]. Tumor resistance depends upon the host’s innate immune responses, directed towards the tumor-induced immunologic defense mechanisms [5]. In this review, we will discuss studies detailing the tumor-induced immune evasion mechanisms involving macrophages, T-cells and NK cells, and how bioactive agents, modulated these immune cells in reversing the defense mechanisms developed by tumor cells. This review may stimulate future research seeking novel bioactive agents from various natural sources. These agents may possess immune-modulating properties, which may help to reverse tumor-promoting immune checkpoint functions.
We will discuss macronutrients that provide protection against tumors. These substances include amino acids, lipids, the novel sea cucumber mixture frondanol A5, common antioxidant vitamins, and minerals. Phytochemicals or isothiocyanates are also potent immunomodulators. These macronutrients have a wide spectrum of impacts on the immune system.
Altered metabolic pathways and their contribution to tumor cell survival or inhibition
Arginine:
The amino acid L-arginine is a substrate of two enzymes, arginase and nitric oxide synthase, which eventually produce nitric oxide (NO). Catabolism of L-arginine (Arg) by arginases, which are overexpressed in cancer cells, results in ornithine. Ornithine, in turn, aids in the formation of polyamines after it is conversion by ornithine decarboxylase (ODC). Polyamines and arginine in particular are well known requirements for cancer cell proliferation, especially when endogenous arginine synthesis is blocked by deficient argininosuccinate synthetase expression [6–9]. Early studies showed that human lung and colon carcinomas are usually positive for argininosuccinate synthetase [10]. Low concentrations of arginine and its metabolite citrulline in the sera of patients with colorectal cancer and higher concentrations of arginine and citrulline in the cancer tissues were recently reported, indicating that arginine metabolism is higher in colon cancer tissues [11]. High arginine levels, either in the serum or in the tumor tissue, has been reported in patients with various malignancies, including gastric, colon, breast, prostate, renal, and lung cancers [12–15] (Fig. 1A).
Fig 1A:
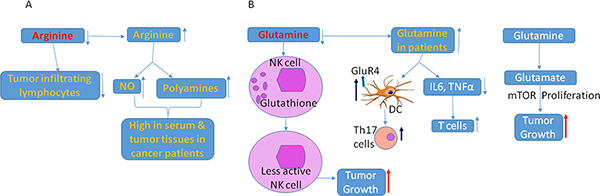
Arginine and glutamine levels are to be balanced to achieve its tumor inhibiting functions. Both low levels and high levels of arginine is having negative effects on immune responses and tumor tissues. Low levels of arginine will lead to reduced infiltration of lymphocytes in tumor microenvironment. However, increased levels of arginine will eventually lead to sustained continuous production of NO and high levels of polyamines which help in tumor cell proliferation and development. Hence, a balanced concentrations of arginine is necessary for achieving best tumor inhibiting functions of arginine. B. A similar functions of glutamine are being discussed in this review. Lower glutamine levels results in reducing cytotoxic functions of NK cells leading to immune evasion of tumor cells. Increased levels of glutamine were reported in patients, resulting in increased T cells, and activation of DC in promoting TH17 immune cell responses, also resulting in tumor cell proliferation and tumor growth. Balancing the concentrations of glutamine is very important to achieve its beneficial effects on tumor inhibition.
As a precursor of NO, arginine plays a significant role in the signaling mechanisms related to cancer biology. Tumor-associated macrophages promote tumor growth and suppress immune cell functions by producing large amounts of arginase 1 and iNOS, resulting in increased superoxide and NO production, inhibiting lymphocyte responses (Fig. 1A). Accumulating evidence suggests that there are two facets of NO: it may act as both a tumor promoter and a tumor cell inhibitor [16]. The dual role of NO in cancer depends on its concentration and other associated factors. Although it is reported that physiologically high concentrations of NO will suppress proliferation and differentiation of TH1, TH2, and TH17 cells, the physiologically low levels of NO in cancer patients are suggested to induce the TH17 immune response that is mostly produced by myeloid-derived suppressor cells (MDSCs) or by IL-1β/IL-6/IL-23. MDSCs and/or these cytokines supported the development of RORγt(Rorc)+IL-23R+IL-17+ Th17 cells in patients with cancer, suggesting the role of NO in stabilizing and inducing TH17 responses [17]. Recently, we have extensively reviewed the immune modulatory functions of NO in tumor progression [18].
l-Arginine is known to possess strong immune-enhancing properties. Arginine (Arg) deficiency is also reported to affect immune cell functions, mainly of tumor-infiltrating lymphocytes, such as cytotoxic T-cells, macrophages, natural killer (NK) cells, and dendritic cells (DC) (Fig. 1A). Due to low Arg availability in the tumor microenvironment, these cells are found to be functionally deficient in cancer tissues [19–22] (Table 1). As this amino acid appears to have dual roles as an immune function enhancer and through indirect involvement in tumor promotion through NO production, as evidenced in colon and other cancers, it is critical to determine the concentrations that are necessary to inhibit tumor growth.
Table 1:
Dietary nutrients and their effects on immune cells
| Dietary ingredient | Cell Type and cytokines | Proposed function | Reference |
|---|---|---|---|
| Arginine | Cytotoxic T-cells, macrophages, NK cells, and DCs | Immune-enhancing properties | [19–22] |
| Glutamine | Th17 cell | Influence cell response by activating the GluR4 receptor on DCs | [28, 29] |
| NK cells | Gln deficiency leads to impeding NK cells ability to protect cells and promote immune function | [30, 31] | |
| Naïve CD4T cells | Gln deprivation leads to conversion of naïve CD4 T cells to Foxp3 expressing T regulatory cells | [33] | |
| Macrophages | Suppressed phagocytic properties | [37] | |
| Tryptophan | GM-CSF, G-CSF, IFN-γ, TNF-α, IL-6, and MCP-1/CCL2 | Inducing effect on inflammatory cytokines | [44] |
| T cells | Tryptophan depletion leads to T cell apoptosis | [45] | |
| DCs, T cells, T regs | Tryptophan alters immune signaling pathways causing T cell tolerance | [46] | |
| Vitamin C | IL-1α, IL-2, IL-8, TNF-α, chemokine eotaxin, and CRP | Reduction in pro-inflammatory cytokines | [62] |
| Vitamin D | DCs | Morphology and functions are altered | [64–66] |
| TH2 cytokines (IL-3, IL-4, IL-5, IL-10) | Induce anti-inflammatory effect by increase in TH2 cytokines | [67, 68] | |
| Th1 cytokines (IL-2, IFN-γ, TNF-α) | Inhibit pro-inflammatory Th1 cytokines | [67, 68] | |
| Vitamin E | CD4, CD8 cells | Increased CD4:CD8 ratios | [100] |
| Th 1 cells | enhanced production of T helper 1 cytokines IL2 and IFN-γ | [100] | |
| Zinc | monocytes, polymorpho nuclear-, natural killer-, T-, and B-cells | Development and functions | [102] |
| Selenium | NK cells | Enhance cytotoxicity | [118–120] |
| T-cells and CD4 cells | Increase in number | [118] | |
| IL-6 and IFN-γ | Activation of IL-6 and IFN-γ pathways | [121] | |
| Curcumin | NK cells | Reduced IFN-g production | [143] |
| “ | NK cell activation by enhancing the ubiquitin-proteasome system | [150, 151] | |
| T-cell | Prevent the T-cell loss, expand central and effector memory T-cells popoulations | [135] | |
| Lymphocytes | Induced cell death through caspase-3 activation | [148] | |
| CD4+ T-cells and B-cells | Increase the cell frequency leading to tumor suppression | [137] | |
| EGCG | Tregs | Increase number | [159, 160] |
| Resveratrol | Macrophages | prevented pro-inflammatory and pro-angiogenic cytokines | [172] |
| PUFAs | T cells | inhibition of T-cell signaling responses | [202] |
| Monocytes | Increased IL10, IL3 and IL5 | [209] | |
| Fiber | Immune cells | inhibit TNF-α, IL-8, −10 and −12 cytokines through SCFA | [214] |
| NK cell | Increased activity | [215] | |
| CD8+ T cells and CD4+ T cells | Increased proportion | [215] |
Glutamine:
Glutamine (Gln) is a versatile nutrient that participates in energy formation, macromolecular synthesis, redox homeostasis, and signaling in cancer cells. Gln helps growing tumor cells by providing its two nitrogen atoms to synthesize nucleotides, hexosamines, and amino acids. The mutations in a cancer cell promote Gln metabolism, which supports the production of NADPH or Gln. Gln is converted to glutamate, which becomes a part of the Tri Carboxylic Acid (TCA) cycle as metabolic intermediates oxaloacetic acid and α-ketoglutarate. In rapidly growing tumor cells, pyruvate is converted to lactic acid and the metabolites upstream of pyruvate are converted to other biosynthetic pathways, such as amino acid synthesis and nucleotide synthesis. Therefore, to speed up the TCA cycle, the dividing tumor cell replenishes the TCA cycle with alternate glutamate anaplerosis. Gln is usually converted to glutamate by glutaminases (GLS). These enzymes are highly expressed in experimental tumors from rats and mice, with high enzyme activity correlating with tumor growth rate and malignancy. We have observed high glutaminase activity in pancreatic tumors compared with normal pancreas. Pancreatic ductal adenocarcinoma (PDAC) cells have been shown to use glutamine to support anabolic processes that fuel cancer cell proliferation. PDAC is sensitive to Gln deprivation. Cancer cell sensitivity increases if glutamine is associated with increased Myc expression in these cells [23, 24]. Son et al. showed that oncogenic KRAS mediates reprogramming of glutamine metabolism for its survival [25]. A positive role of Gln was demonstrated by silencing GLS activity, which resulted in tumor inhibition [26]. Gln was also shown to activate the mTOR pathway, which is involved in cell proliferation [27] (Fig. 1B).
High rates of Gln utilization take place in lymphocytes, due to the need for these cells to respond to immune challenge at any time. Gln is reported to influence Th17 cell responses by activating the GluR4 receptor on dendritic cells [28, 29] (Fig. 1B) (Table 1). Most of the glutamine is concentrated at the tumor cells site, with low glutamine in other cells. This also leads to a net deficit in available GSH [30]. This leads to low glutathione in NK cells, impeding their ability to protect cells and promote immune function, which is critical for patients with cancer [30, 31] (Table 1). Patients with cancer suffer from a negative balance of nitrogen, due to high levels of Gln at tumor sites, leading to cachexia. Although Gln is positively helping in tumor growth, supplementation of glutamine in these patients could counter the deficit [32]. A recent report by Klysz et al. (2015) stated that Gln deprivation leads to the conversion of naïve CD4 T cells to Foxp3-expressing T regulatory cells [33] (Table 1). Tregs promote tumor growth. Less Gln in the tumor microenvironment, accompanied by the mostly high concentrations of glutamine that are found within tumor cells, aids in increasing Foxp3-expressing Tregs, which accelerate tumor growth by suppressing immune responses against tumor formation.
Gln is reported to maintain mucosal integrity and protect the intestinal epithelial tight junctions to preserve the gut barrier function, which may help prevent infections and control neoplastic transformation and spread [34, 35]. Gln administration (25 mg/kg) for 7 days in rats reduced 2,4,6, trinitrobenzene sulphonic acid-induced colitis by inhibiting oxidative damage and pro-inflammatory cytokines, TNF-α and IFN-γ [36]. Reduced Gln levels can lead to suppressed T lymphocytes and phagocytic properties of macrophages [37] (Fig.1B). Glutamine added to total parenteral nutrition for patients with post-operative colorectal cancer resulted in elevation of T lymphocytes, with no effect on IL6 and TNF [38] (Fig.1B). Therefore, Gln is necessary for optimal immune functioning.
Tryptophan:
This substance is one of the essential amino acids that are required in minute concentrations for healthy nutrition [39, 40]. Tryptophan is involved in protein synthesis and is a precursor of two metabolic pathways: kynurenine synthesis and serotonin synthesis. It is also involved niacin, melatonin, and NAD/NADP synthesis. Thus, tryptophan plays an important role in neurotransmission, modulation, immune responses, and immune tolerance [41, 42]. Tryptophan is a known powerful mediator of both the innate and adaptive immune responses. The three dioxygenase enzymes, tryptophan 2,3-dioxygenase (TDO), indoleamine 2,3-dioxygenase 1 (IDO1), and indoleamine 2,3-dioxygenase 2 (IDO2), are involved in tryptophan-to-kynurenine synthesis [43]. These enzymes promote immune tolerance towards tumor growth and immune dysfunction, helping tumor cells evade the host’s immune responses. IDO2 is necessary for inducing inflammatory cytokines, such as granulocyte-macrophage colony-stimulating factor (GM-CSF), G-CSF, IFN-γ, TNF-α, IL-6, and monocyte chemoattractant protein-1 (MCP-1/CCL2) [44]. Tryptophan depletion results in modulation of immune responses, leading to cell cycle arrest and apoptosis of T cells [45]. Tryptophan levels also play a role in complex interacting signals between DCs, T cells, and T regs, altering immune signaling pathways and causing T cell tolerance [46].
IDO1 activity is a significant factor in preventing immune function, tumor recognition, tumor cell proliferation, and metastases [47]. Human pancreatic cancer cells express high levels of IDO1, which result in NK cell dysfunction due to reduced secretion of cytokines (TNF-α and IFN-γ) and cytotoxic granules (Granzyme B and perforin) [48]. A phase I/II study is recruiting patients with metastatic adenocarcinoma to study an IDO Inhibitor (Indoximod) in combination with gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer (ClinicalTrials.gov Identifier: NCT02077881). A high kynurenine:tryptophan ratio was observed in patients with CRC compared with normal controls [49]. High IDO1 levels in the neoplastic epithelium were linked to poor survival in patients with CRC [50]. IDO1 expression has been associated with increased liver metastases in patients with CRC [51]. High density of IDO1-expressing cells in the lymph nodes is linked to reduced 5-year survival in patients with CRC [52]. INCB024360 is a selective inhibitor of human IDO1. INCB024360 inhibition of IDO1 promoted T cell and NK cell growth and cytotoxicity by increasing IFN- γ, reduced the conversion of T cells to T regs, and increased CD86-expressing DCs [49]. A dose-dependent decrease in IDO1-expressing PAN02 pancreatic carcinomas was observed after treatment with INCB024360 in C57BL/6 mice, with no effect in immunodeficient mice bearing IDO1-expressing PAN02 pancreatic carcinomas: this finding suggests that INCB024360 anti-tumor activity occurs through the lymphocytes [49]. Another study reported lower quality of life for CRC patients, in whose serum samples decreased IFN-γ mediated tryptophan levels were found [53].
Vitamins and their role in preventing cancer.
Vitamin C:
Ascorbate (Vitamin C) can be pro-oxidant or anti-oxidant, depending on the presence of catalytic metals [54]. The main proposed vitamin C mechanism consists of an ascorbate radical that produces H2O2, which acts like the cytotoxic effector species. Vitamin C is also reported to increase calcium influx into the endoplasmic reticulum and to induce apoptosis by increasing Bad translocation and Bax expression in human colon cancer cells [55]. Pharmacological levels of vitamin C, that is, the doses achievable in humans, killed pancreatic cancer cells [56–58]. Further administration of pharmacologic vitamin C in combination with the standard chemotherapy drug gemcitabine resulted in 50% growth inhibition in a non-responsive tumor compared with treatment with gemcitabine alone, in a study of mouse PANC1 xenografts [59]. The reduction was significant with a lower gemcitabine dose, thus reducing the toxic effects of gemcitabine. When a combination of Vitamin C and gemcitabine was tested in Phase 1 clinical trials in patients with stage IV pancreatic adenocarcinoma, the treatment was well tolerated and safe, and improved progression-free survival [60]. This study involved a small number of patients: only three patient’s experienced improvement.
Vitamin C is not particularly linked to any immune cell mechanisms, and may have non-specific effects on diverse parts of immune system [61]. High doses of Vitamin C (7.5 g to 50 g) in cancer patients after standard treatments resulted in a significant reduction in pro-inflammatory cytokines, including IL-1α, IL-2, IL-8, TNF-α, chemokine eotaxin, and CRP, which correlated with tumor marker levels [62] (table 1). Though the exact immune modulation mechanism has not been studied for this agent, reports suggest that Vitamin C affects immune cells. Further studies are necessary to pinpoint what concentrations modulate inflammatory cytokines and how, and which immune cells are regulated by vitamin C in cancers.
Vitamin D:
This vitamin is generally associated with mineral metabolism. It was recently reported that vitamin D-metabolizing enzymes and their receptors are expressed in many cell types of the intestines, pancreas, and immune system [63]. Calcitriol is a hormonally active metabolite of Vitamin D. Calcitriol alters the morphology and functions of DCs, and inhibits T cell cytokines, IL-2, IL-17, IL-6, and toll-like receptors on monocytes [64–66] (Table 1). Calcitriol treatment mostly influences the anti-inflammatory profiles of cytokines by increasing TH2 cytokines (IL-3, IL-4, IL-5, IL-10) and inhibiting pro-inflammatory Th1 cytokines (IL-2, interferon-γ, tumor necrosis factor α) [67, 68] (Table 1).
The prospective, case-control, observational studies suggest an inverse relationship between Vitamin D active metabolite 1α,25-dihydroxyvitamin D3 (25(OH)D) and CRC [69–73]. These data reiterate the positive effects of Vitamin D on inhibition of CRC. However, sufficiently large prospective clinical trials are needed to assure this inverse association. An ongoing Phase III randomized clinical study is analyzing the effects of the Vitamin D and OmegA-3 TriaL (VITAL) supplements on primary prevention of cancers and cardiovascular disease (ClinicalTrials.gov Identifier: NCT01169259). Another large Phase II and III randomized study, the Vitamin D/Calcium Polyp Prevention Study, is investigating the chemopreventive effects of vitamin D and calcium, and whether calcium with vitamin D is more effective than calcium alone in reducing colorectal neoplasia (ClinicalTrials.gov Identifier: NCT00153816). A Western-style diet with decreased vitamin D and calcium fed to C57BL/6 mice resulted in developing colon tumors, whereas supplementation with Vitamin D and calcium resulted in less tumor formation and fewer visible colon tumors by end of 2 years. These data clearly suggest the inhibitory effects of Vitamin D and calcium in CRC [74]. Clear evidence of Vitamin D’s inhibitory effects on CRC was demonstrated in the ApcMin/+ model, in which Vdr−/+ mice were bred with ApcMin/+ mice to study the effects of loss of Vitamin D receptors on colon tumor development in Apc-deficient conditions. These studies showed increased tumor burden in Vdr−/−ApcMin/+ mice compared with Vdr+/+ ApcMin/+ mice, indicating an important role of Vitamin D in preventing CRC [75, 76].
Results from epidemiological studies reporting on the role of Vitamin D and pancreatic cancer (PC) are inconsistent. Some suggest that Vitamin D levels are associated with PC prognosis, while others suggest no association between circulating 25(OH)D and prognosis in patients with PC [77, 78]. A recent study pooled analysis of Vitamin D intake (dietary, supplementary, and total) and PC risk using data from case-control studies participating in the International Pancreatic Cancer Case-Control Consortium (PanC4). The researchers concluded that high intake of Vitamin D is associated with increased risk for PC. To further confirm this analysis and address this issue, a large supplementation trial is needed.
The exact mechanisms by which Vitamin D modulates immune responses during tumor initiation or development are not yet fully understood. Studies on how the innate and adaptive immune responses altered by Vitamin D are modified during tumor cell initiation and development are warranted, to exploit the beneficial effects of Vitamin D in cancer prevention settings.
Vitamin E:
This vitamin consists of tocopherols and tocotrienols that act as anti-oxidants. There are conflicting reports on the role of vitamin E in cancer. Many studies strongly suggest than vitamin E has protective effects. Epidemiological studies on vitamin E intake and risk of colon and other cancers are inconsistent; results showed either no association or reduced risk of cancers [79–86]. Previous studies examining the effects of α-tocopherol on colon carcinogenesis were unfavorable [87]. Out of ten preclinical studies using 1, 2 dimethylhydrazine (DMH) /azoxymethane (AOM) modes of colon cancer, only one study showed that vitamin E reduced cancer risk [88]. Eight other studies showed no effect [89–96], while one study showed increased colon carcinogenesis with Vitamin E administration [97]. However, Newmark et al. (2006) reported that dietary administration of mixed tocopherols (0.1% in AIN76A diet) resulted in inhibition of Aberrant Crypt Foci (ACF) in rats [98]. Another study demonstrated that a gamma-tocopherol rich mixture of tocopherols (0.3 and 0.17% in AIN93M diet) produced increased apoptosis and significant inhibition of inflammation and colon tumors in AOM/DSS mice [87]. These studies in preclinical models suggest that different concentrations of vitamin E have different effects on colon carcinogenesis.
A study of the impact of vitamin E supplementation (200 mg/day) in smokers and non-smokers revealed increased lymphocyte proliferation and more B-cells in non-smokers compared with those receiving placebo, suggesting that Vitamin E has immune protective effects [99]. Supplementation with vitamin E (750 mg/kg) for two weeks in patients with advanced colorectal cancer increased CD4:CD8 ratios and enhanced production of T helper 1 cytokines, IL2, and IFN-γ [100] (Table 1). α-tocopherol was also reported to stimulate cAMP production, mediated by prostaglandin EP2 and EP4 receptors in human peripheral mononuclear cells, thereby altering their immune functions. This may be an immune modulatory mechanism that is worthy of further study [101] (Table 1).
Minerals that modulate the immune response, leading to cancer prevention.
Zinc:
This mineral is largely available in animal proteins. Zinc is involved in cellular functions that are mainly related to cell cycle, apoptosis, and immune cell functioning. Zinc deficiency affects the development of immune cells (monocytes, polymorphonuclear-, natural killer-, T-, and B-cells), as well as their functions [102]. Intake of dietary zinc is associated with a decreased risk of both proximal and distal colon cancer in postmenopausal women [103]. Epidemiologic studies gave conflicting reports on the association of zinc intake and CRC, but a recent meta-analysis of the prospective studies suggested a significant reduced dose–response association of zinc intake [104]. Due to its immune-modulating properties, zinc might be critical in the host defense against the initiation and progression of cancer. It is important to note that zinc intake was found to be negatively associated with gastric, esophageal, and colorectal cancer risk [105].
Dani et al. 2007 reported that zinc inhibited the histological changes and antioxidant status in rat colons during the initiation and promotion phases of experimentally induced colon carcinogenesis. Results from these studies clearly indicated that the administration of 227 mg/L zinc to rats in drinking water in the presence of DMH significantly reduced ACF, tumor incidence, and tumor number, with noted alterations in the antioxidant status, such as lipid peroxidation, glutathione-S-transferase, superoxide dismutase (SOD), and catalase, with restoration of normal colonic histoarchitecture [106, 107]. The regulatory role of zinc was studied in the membrane fluidity parameters and surface abnormalities following DMH-induced colon carcinogenesis in rats [108]. Zinc was observed to possess profound membrane stabilizing properties in this CRC model.
Further, zinc has demonstrated a broad impact on immunity mediators, enzymes, peptides, and cytokines affecting lymphoid cell proliferation, activation, and apoptosis. Beach et al. reported that zinc deficiency in mice carried on for generations reflecting in offspring’s. These data suggest the importance of zinc in regular functioning of the immune system. In addition, higher concentrations of zinc inhibit immune cell functions. The plasma concentration of zinc should not exceed 30 μm, and supplementation should be applied based on the levels present in a patient, to avoid immune-suppressive effects [109, 110]. The role of zinc in immune regulation in general and during tumor growth in particular is poorly understood. The literature suggests that zinc might affect immune cell functions by altering membrane structure and receptor expressions, thereby enhancing or decreasing signaling between immune cells and neoplastic cells.
Selenium:
Selenium is a trace element involved in different bodily functions, mostly acting as a co-factor for anti-oxidant selenoprotein enzymes [111, 112]. Epidemiological data suggest that selenium deficiency increases cancer risk. Selenium supplementation resulted in lower cancer risk [113, 114]. A meta-analysis of nine randomized controlled clinical trials showed that supplementation of selenium reduced risk for all cancers by 24%. Low baseline selenium levels increased cancer preventive effects by 36% [115]. Along with its anti-oxidant properties, selenium is reported to have anti-inflammatory properties, inhibit DNA alterations, inactivate cancer-promoting factors, hinder cell cycle replication in cancer cells, inhibit tumor invasion and metastases, and enhance immune system activity. A recent nested case-control design (966 CRC cases; 966 matched controls) within the European Prospective Investigation into Cancer and Nutrition study involving 520,000 participants across 10 Western European countries reported that higher levels of selenium are associated with reduced risk of colorectal cancer. As long as it is administered in suboptimal doses, selenium shows preventive effects, whereas higher levels of selenium are associated with toxicity [116]. Selenium is reported to affect both the innate and adaptive immune systems. Selenium deficiency results in a reduced number of lymphocytes in both the thymus and bursa.
Interestingly, doses of selenium up to 400 ng/ml did not inhibit the activity of NK cells, while inhibiting tumor cells. This finding indicates that a given concentration of selenium can show differential activity against various cells, which can be exploited to enhance immune responses to prevent cancer growth [117]. Six months of selenium intake in healthy humans increased T-cells and CD4 cells by 65%, and was accompanied by a 58% increase in NK cell cytotoxicity [118] (Fig.2) (Table 1). Mice with reduced selenium levels showed reduced NK cell activity [119, 120] (Table 1). A recent report demonstrated that six weeks of dietary sodium selenium supplementation resulted in the activation of IL-6 and interferon-γ pathways in mice [121] (Table 1). Selenium is necessary for the production of selenoproteins and is usually transported by selenoprotein P (SEPP1). Mutations and/or haplo insufficiency in this SEPP1 transporter protein increased genomic instability and colitis-associated colon carcinogenesis. Reduced function of the SEPP1 transporter gene resulted in polarization of macrophages, specifically the M2 type, indicating the role of selenoproteins in immune responses [122].
Fig 2.
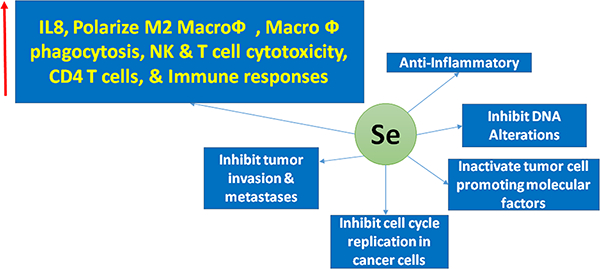
Tumor inhibitory functions of selenium: Selenium posses anti-inflammatory properties, along with other potent tumor inhibiting functions such as, inactivation of tumor cell promoting factors, blocking cell cycle regulation their by inhibit tumor invasion and metastases. Selenium is able to inhibit tumor-promoting functions by modulating various immune cell responses as listed above.
Further, selenium deficiency led to low GSHPx activity, resulting in loss of phagocytic activity of neutrophils and macrophages towards infectious agents or transformed cells [123, 124]. We reported that dietary administration of Se-PBIT improved phagocytic activity and increased macrophages in treated animal spleens compared with untreated spleens (Fig.2). In an iNOS-targeting molecule, a novel isosteric analog of S,S’−1,4-phenylenebis(1,2-ethanediyl)bis-isothiourea (PBIT), PBI-Se, was developed in which sulfur was replaced with selenium. Se-PBIT caused a significant dose-dependent inhibition of ACF in AOM-induced colon cancer in F344 rats. Se-PBIT did not decrease IL8 cytokine production, as this cytokine acts as a chemo-attractant for neutrophils, basophils, and a subset of T-cells that aid in tumor eradication [125]. Hence, these studies suggest that selenium has protective functions against tumors, not only due to its anti-oxidant properties, but also by modulating immune cells and cytokines. Future studies are needed to determine the type of selenium and the required doses for prevention or treatment strategies.
Polyphenols and their immune-modulating and protective functions in cancer.
Polyphenols are a large family of natural compounds that are characterized by the presence of several phenol groups (i.e., aromatic rings with hydroxyl groups). These compounds are secondary metabolites of plants and are widely distributed in plant foods (fruits, vegetables, nuts, flowers, bark, and seeds) and beverages (tea, coffee, juice, wine, and beer). Polyphenolic compounds have been shown to delay, inhibit, or prevent the oxidation of oxidizable materials by scavenging free radicals and diminishing oxidative stress. The antioxidant properties are due to their structural chemistry, that is, phenolic hydroxyl groups that are prone to donate a hydrogen atom or an electron to a free radical and/or extended conjugated aromatic system to delocalize an unpaired electron [126–128] .
Previous studies showed no risk, slightly reduced risk, or significantly decreased risk of colorectal cancer with coffee consumption [129–131]. However, recent data from a community-based case-control study in Japan addressed the association of polyphenol consumption, mainly in the form of tea and coffee, and risk of colorectal cancer. In this study, 816 cases of colorectal cancer and 815 community-based controls were analyzed for polyphenol intake and their colorectal cancer risk. The findings suggested that a decreased risk of colorectal cancer is associated with consumption of coffee. The polyphenolic compounds present in coffee include ferulic, caffeic, chlorogenic and diterpenes (cafestol and kahweol), and cumaric acids [132]. Polyphenols may be categorized as phenolic acids (e.g., curcumin), flavonoids (e.g., EGCG), stilbenes (e.g., resveratrol), and lignans (e.g., secoisolariciresinol). Polyphenols are shown to have a potential role in the protection of human health from chronic diseases like cancer and cardiovascular disease through multiple mechanisms of action [126]. One such mechanism is modulation of the immune system and functioning.
Curcumin (diferuloylmethane) is the bioactive component of turmeric (Curcuma longa). It has been widely used in Indian medicine to treat hepatic disorders, anorexia, cough, diabetic wounds, rheumatoid arthritis, and sinusitis. Curcumin is now recognized as a promising anti-tumor drug due to its anti-inflammatory, anti-oxidant, and anti-carcinogenic activities [133, 134] (Fig.3A). Results from in vitro and in vivo studies show that curcumin can inhibit tumor initiation, promotion, invasion, and metastasis through a variety of mechanisms [135–139]. Due to a vast number of biological targets and virtually no side effects, curcumin has generated interest for its cancer prevention potential [140]. Supplementation with 0.5% curcumin for six weeks increased lactobacillales in colons of IL10 mice on a 129/SvEv background with AOM-induced colitis, resulting in complete loss of tumor burden and increased survival. This finding suggests that curcumin is able to modulate microbiota that confer protection and restore or repair mucosal damage, reducing colitis and tumor formation [141]. Further, 0.02% curcumin reversed the tumor-enhancing properties of a high protein diet (50% casein) studied in azoxymethane (AOM)-DSS-induced colorectal tumors in female Balb/c mice. This preventive effect was attributed to curcurmin’s anti-inflammatory properties by reducing COX-2, iNOS in colonic mucosa, and short- and branched-chain fatty acid levels, decreasing cell proliferation, and inhibiting toxic metabolite production (Fig.3A). These data suggest that curcumin can neutralize negative effects of tumor-promoting foods when it is consumed at the same time [142].
Fig 3A:
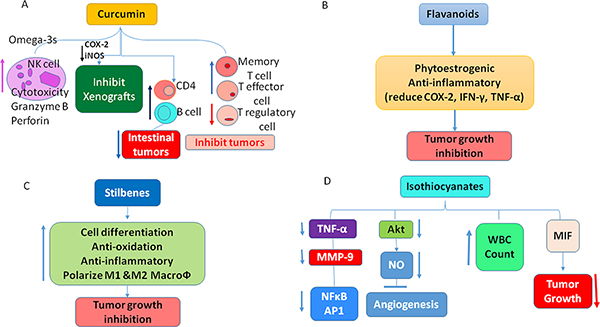
Anti-inflammatory and immune modulating effects of Phtochemicals. Curcumin enhances cytotoxicity of NK cells, and increase T effector, T memory cells and reduce T regulatory cells helping in inhibiting tumor growth. B. Flavanoids posses anti-inflammatory functions and also inhibit human pancreatic cancer stem cells. C. Stilbenes along with anti-inflammatory, anti-oxidant functions, it can polarize M1 and M2 macrophages helping in inhibition of tumors. D. Isothiocyanates increase proliferation of WBC cells. It can interact with Macrophage Inhibitory Factor (MIF) enhancing immune responses towards inhibition of tumor formation. Further, it was demonstrated to posses’ angiogenesis inhibitor properties.
Curcumin was reported to reduce IFN-γ production in NK cells. Whereas, a recent report on the combination of curcumin with omega-3s demonstrated that the treatment significantly potentiated NK cell cytotoxicity against pancreatic cancer cells [143] (Table 1). This report suggests that the use of curcumin enhances innate immune responses. Detailed studies with combinations of other bioactive foods will delineate the exact immune functions of curcumin in preventing inflammation and cancer.
We reported the therapeutic potential of curcumin in combination with omega-3s against pancreatic cancer. BxPC3 pancreatic tumor xenografts were established in nude mice (Fig.3A). When tumors reached a size of 190–200mm3, the mice were treated with diets with or without 2,000 ppm curcumin in 18% corn oil or 15% fish oil + 3% corn oil for six weeks, before the tumor growth and volume were assessed. The combination of curcumin with omega-3s resulted in inhibition of inflammatory COX-2 and 5-LOX, and decreased proliferation in mouse xenografts [144]. A novel nanomicelle of hyaluronic acid conjugate copoly(styrene maleic acid) (HA-SMA) with 3,4-difluorobenzylidene curcumin enhanced internalization of HA-engineered nanomicelles with effective killing of triple-marker positive (CD44+/CD133+/EpCAM+) pancreatic cancer stem-like cells (CSLCs) [145]. These data suggest the use of nanomicelles for increasing bioavailability, which is an issue and hurdle with curcumin use in cancer treatment.
Overwhelming reports have suggested that the modulation of immune responses by curcumin plays a dominant role in the treatment of inflammation and metabolic diseases and cancer prevention [146]. Studies have shown that tumor-bearing mice treated with curcumin experienced retarded tumor growth and longer survival attributed to a T-cell-mediated adaptive immune response [135, 137]. Immune dysfunction during tumor progression contributes to tumor immune evasion. Tumors often target and inhibit T-cell function to escape from immune surveillance. This dysfunction includes loss of effector and memory T-cells, bias towards type 2 cytokines, and expansion of T-regulatory (Treg) cells (Fig.3A). Curcumin was found to prevent this loss of T-cells, expand central memory T-cell and effector memory T-cell populations, reverse the type 2 immune bias, and attenuate the tumor-induced inhibition of T-cell proliferation in tumor-bearing hosts, leading to an enhanced ability of effector T-cells to kill cancer cells [135].
Curcumin can modulate the activation of T-cells, B-cells, splenic lymphocytes, cytotoxic T lymphocytes, macrophages, neutrophils, NK cells, and dendritic cells, as well as the secretion of immune cytokines in the normal body [147]. Curcumin induced cell death in normal quiescent and proliferating human lymphocytes through caspase-3 activation [148]. Dietary curcumin was shown to enhance the frequency of CD4+ T-cells and B-cells with significant suppression of intestinal tumors [137] (Fig.3A). Curcumin is shown to prevent the tumor-induced apoptosis of T-cells and restore progenitor, effector, and circulating T-cells by modulating Jak-3/Stat-5 activity [149]. NK cells directly participate in the killing of tumor cells after the recognition of stress-inducible ligands; killing involves the induction of cell death by perforin and granzyme B (Fig.3A). Enhanced activation of NK cells was observed after curcumin treatment; this was correlated with the effect of curcumin on the tumor in vivo [150] (Table 1). Tumors secrete exosomes, multivesicular bodies containing a distinct set of proteins that can fuse with cells of the circulating immune system. Curcumin was found to reverse tumor exosome-mediated inhibition of natural killer cell activation by enhancing the ubiquitin-proteasome system [151]. However, the in vivo effects of curcumin are highly dependent on the bioavailable concentration of curcumin. Due to its insolubility in water, curcumin has very poor bioavailability. Its cellular uptake is slow and it is metabolized rapidly once inside the cell [152]. Therefore, oral doses are required to achieve significant concentrations inside the cells for any physiological effects to occur. To address these limitations, numerous curcumin analogs, nanoparticle-based drug delivery vehicles, and co-administration with agents that enhance bioavailability have been prepared. These methods have demonstrated improved uptake, metabolism, and activity [152].
EGCG, stilbenes, and lignans in modulating immune responses for cancer prevention.
Flavonoid (−)-epigallocatechin gallate (EGCG) is present in green tea and is reported to possess primary and tertiary prevention properties for colon cancer. EGCG has also been shown to have anti-inflammatory and anti-oxidant effects in arthritis and autoimmune diseases. Most of these anti-inflammatory properties occur through inhibition of COX-2, IFN-γ, and TNF-α [153, 154] (Fig.3B). A 2008 prospective cohort study report suggested that consumption of green tea delayed cancer onset [155]. Supplementation with green tea extract also prevented recurrence of colorectal adenoma [156]. EGCG was found to be effective in killing human pancreatic cancer stem cells, alone and in combination with quercetin [157]. Further, EGCG was found to be synergistic in combination with NSAIDs like sulindac in an Apc min model of colon cancer [158]. Results from in vitro and in vivo studies indicated that EGCG increases Tregs [159, 160] (Table 1). Though our data show that Tregs are highly expressed in cancer, and help tumors evade the immune response, most EGCG data come from studies of arthritis or other autoimmune diseases. How EGCG might modulate Tregs or immune cells is yet to be determined in cancer models.
Stilbenes are naturally occurring phytochemicals. One such stilbene, resveratrol, is known to induce cell differentiation, mediate anti-inflammatory action, and provide anti-oxidant properties [161–164] (Fig.3C). Pterostilbene, a naturally occurring stilbene in blueberries, was reported to have potential chemopreventive effects, mediated by inhibition of iNOS in ACF, in AOM-induced ACF in rats [165]. Many studies have demonstrated that stilbenes have profound preventive effects on colon cancer in well-established animal models [166–170]. A study of rats supplemented with resveratrol and a high protein diet during radiation suggested that this combination significantly minimized the damage caused by ionizing radiation. Most importantly, the high protein and resveratrol diets were reported to decrease regulatory T-cells in rats treated with abdominal radiation [171]. Further, resveratrol prevented pro-inflammatory and pro-angiogenic cytokines in both M1 and M2 type macrophages [172] (Table 1) (Fig.3C). This report suggests that resveratrol may function differently in different types of immune cells under different conditions. This type of functional difference is yet to be investigated in cancers.
Lignans are abundantly found in whole grains, mostly in wheat and rye. Most of the cancer preventive functions of lignin are attributed to its anti-oxidant, anti-estrogenic properties. Epidemiological studies reported that a substantial reduction in colon cancer risk was associated with high levels of lignan metabolites due to high lignin intake [173]. Another population case control study reported a similar reduction in colon cancer risk with high dietary lignin intake [174]. Preclinical rat models of colon cancer showed consistently similar protective functions of lignans on ACF [175]. Dietary administration of 2.5/5% flaxseed or defatted flaxseed containing secoisolariciresinol diglucoside for 100 days significantly reduced AOM-induced ACF formation in male Sprague-Dawley rats [176]. Lignans and their metabolites are reported to have minor effects on immune cells [177].
Isothiocyanates (ITC) are abundant in cruciferous vegetables, such as broccoli, watercress, and Brussels sprouts. Cruciferous vegetables have been widely accepted as foods that may decrease the risk of cancer. Phenethyl isothiocyanate treatment reduced the number of polyps in Apc(Min/+) mice [178]. Benzyl isothiocyanate also inhibited TNF-α-induced MMP-9 secretion by down-regulating NF-κB and AP-1 [179] (Fig.3D). Isothiocyanates have been shown to inhibit tumor-specific angiogenesis by inactivating AKT and down-regulating nitric oxide, TNF-α, and pro-inflammatory cytokine production [180–182] (Fig.3D). Administration of allyl isothiocyanate (AITC) and phenyl isothiocyanate (PITC) was found to enhance the total WBC count in mice on the 9th and 12th days after treatment [183]. Brown et al. reported that ITCs are capable of modifying of the N-terminal proline residue of macrophage migration inhibitory factor (MIF), which is known to be associated with many cancers, including colon cancer [184] (Fig.3D). Further, it was observed that two subsites are available for binding on MIFs by ITC inhibitors [185]. Therefore, these data will facilitate the development of site-specific inhibitors for increased efficacy in treatments using these agents. Different isothiocyanates possessing an aromatic moiety were demonstrated to inhibit MIF. These studies clearly suggest that ITCs possess immune-modulating abilities that can be exploited in cancer prevention or treatment.
Polyunsaturated fatty acids, eicosapentaenoic acid (EPA; 20:5n-3), and docosahexaenoic acid (DHA; 22:6n-3) in immune modulation and cancer prevention
It has been well documented in experimental models of colorectal cancer (CRC) that fish oil supplements or consumption of fish provides anti-neoplastic benefits, such as apoptosis of cancer cells, anti-proliferation, and anti-angiogenesis [186–189]. Results from epidemiological studies of the impact of fish and fish oil consumption on risk of colon cancer are inconsistent [154, 190–192]. Recent human data on fish and fish oil consumption suggested that three or more years of fish oil supplementation reduced CRC risk by 49% [193]. Recent scientific evidence suggests that EPA and DHA omega-3 fatty acids found in fish oil help in regulating body’s immune responses, when these responses are triggered by stress, inflammation, or injury [194]. These long chain fatty acids are usually incorporated in cell membrane phospholipids, affecting the fluidity of the membranes, producing signal transduction, and functioning as substrates for other chemical mediators. The presence of DHA results in retroconversion to EPA forming less potent eicosanoids, such as 3-series prostaglandins (PGE3s), thromboxanes, and 5-series leukotrienes (Fig. 4). The reason for the less active eicosanoid products from EPA and DHA is that the receptors for these mediators have less affinity for eicosanoids produced from EPA and DHA than they do for those produced from arachidonic acid.
Fig 4.
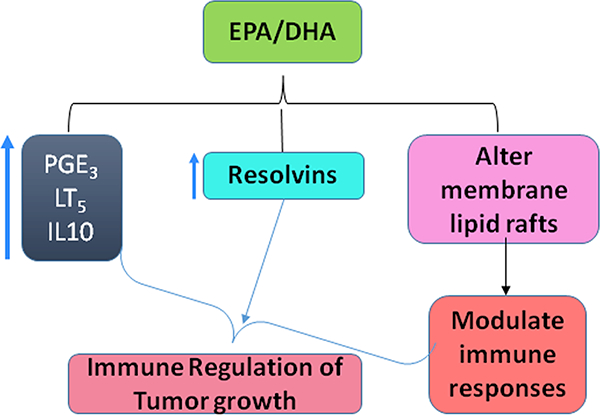
Polyunsaturated omega 3 fatty acids in inhibiting tumors. EPA and DHA interactions with lipid rafts in altering the immune cell responses and generate less potent 3 series and 5 series eicosanoid metabolites which will not promote tumor development
EPA and DHA primarily resolve inflammation. Failure to resolve inflammation may result in a transition to a disease state. Further, DHA and EPA will also lead to formation of anti-inflammatory resolvins [195]. Increased resolvins were produced by fish oil diets, and increased resolvin production was observed in colitis-induced Fat1 mice [196, 197]. We have reported decreased PanIN lesions and PDAC in a K-ras mouse model of pancreatic cancer, due to changes in eicosanoid profiles [198, 199].
Supplementation with EPA and DHA often results in recognizable changes in immune responses and cytokine production levels, which also depend on the ratio of omega-6 to omega-3 fatty acids [200, 201]. EPA was demonstrated to inhibit anchoring of T-cells to cell membranes by inhibiting a protein called linker, which explains the inhibition of T-cell signaling responses in an in vitro system [202]. EPA interrupts immune cell responses through alterations in lipid rafts. Both in vitro and in vivo studies demonstrated how n-3 fatty acids disrupt the lipid rafts, thereby altering the immune cell signaling mechanism in these cells [202–207] (Fig.4). A report by Gurzell et al. suggested that DHA enhanced the B-cell immune responses against infection and helped in clearing pathogens by producing antibodies and dampening inflammation, both ex vivo and in vivo using a SMAD−/− colitis-prone mouse model [208]. In this study, the mechanism by which EPA increased B-cell immune responses was also shown to be related to changes in lipid rafts. A recent report by Jaudszus et al. suggested that EPA and DHA promote proresolving mediators. EPA and DHA increased IL10 and 3 series and 5 series eicosanoids in monocytes without affecting IL6 and TNF-α levels [209] (Fig. 4) (Table 1). This finding suggests that although lipid raft disruption is involved in both the cases, n-3 fatty acids function differently in T- and B-cells. The functions of n-3 polyunsaturated fatty acids should be explored under neoplastic conditions in order to analyze how these changes in lipid rafts alter the signaling events in the tumor-associated microenvironment.
We have reported the cancer preventive functions of the sea cucumber extract Frondanol A5. Frondanol A5 contains a mixture of various bioactive ingredients, including eicosapentaenoic acid, monosulfated triterpenoid glycoside Frondoside A, disulfated glycoside Frondoside B, trisulfated glycoside Frondoside C, 12-methyltetradecanoic acid, fucosylated chondroitin sulfate, and small amounts of canthaxanthin/astaxanthin. This extract prompted a dose-dependent inhibition of ACF in a rat model of AOM-induced colon cancer. Further, this agent was tested in an Apc min mice model, in which its immune-modulating functions were demonstrated. Macrophage polarization was observed with increased GLT expression, which might have helped decrease intestinal tumor burden in a Min mice model of colon carcinogenesis. The alterations in immune cell functions may be due to the presence of EPA and other novel glycosides. A detailed understanding of the effects of individual components is needed to pinpoint the mechanistic effects of those compounds in altering immune cell responses and changing the production of various cytokines.
Dietary Fiber:
Fiber is an important part of our nutrition, which plays a vital role in defining the composition of gut microbiota. Fiber plays a multifaceted role in modulating tissue immune responses, inflammation in the intestine, and systemic inflammation: this nutrient consists of complex carbohydrate components that are neither digestible nor absorbed by the intestinal cells [210]. In a large-scale dietary intervention study, high fiber consumption was shown to promote/increase growth of Bifidobacterium, with an increase in the concentrations of short-chain fatty acids (SCFA) [211]. The amount of fiber intake inversely affected the secretion of IL-6 and C-reactive protein in a clinical trial [212]. SCFAs, such as propionate and butyrate, act as ligands for G protein-coupled receptor (GPR43) [213]. The inflammatory responses are signaled through GPR43 in the presence of SCFA in the intestinal cells. These receptors are widely expressed in intestinal cells, neutrophils, eosinophils, and macrophages [213]. Among SCFAs, butyrate and propionate are reported to exhibit strong anti-inflammatory properties by inhibiting TNF-α, IL-8, IL-10, and IL-12 cytokines in immune and colonic cells [214] (Table 1). Consumption of high fiber content also increased the proportion of CD8+ T cells and CD4+ T cells and increased NK cell activity in the lamina propria and peripheral blood [215] (Table 1). Findings from a large prospective study involving more than 55000 participants suggested that dietary fiber intake reduced the risk of distal colon cancer and incidence of distal colorectal adenomas [216]. These results clearly suggest that dietary fiber will alter the immune cells and their innate and adoptive immune signaling within the localized region and systemically. A large colonoscopy-based case-control study reported a 38% reduced risk of high-risk adenomatous polyps for those consuming high fiber: this association was much more significant in smokers [217]. A meta-analysis of case-control and cohort studies suggested that high dietary fiber consumption is associated with lower risk for colorectal adenomas [218].
In BALB/c mice, treatment with five injections of AOM and three cycles of DSS resulted in high tumor multiplicity and exposure to B. fibrisolvens. A high-fiber diet had a protective effect in combination with B. fibrisolvens (3 tumors/mouse), whereas this protective effect was not observed when agents were fed alone (8–11 tumors/mouse) [219]. A grape anti-oxidant dietary fiber treatment, consisting of soluble phenolics, insoluble phenolic acids, and other components of the dietary fiber fraction, including polysaccharides and lignins, prevented polyp formation by ~81% in ApcMin/+ mice by modifying cell cycle genes. DNA microarray analysis of colonic mucosa revealed downregulation of immune response genes (CXCR4 signaling via second messenger, T-cell receptor, and CD28 co-stimulation in activation of nuclear factor-κB, inducible T-cell co-stimulator pathway in T-helper cells, T-cell receptor signaling pathway, nuclear factor of activated T cells in immune response) in the colonic mucosa of treated animals compared with untreated animals [220]. Several dietary fiber studies conducted by our group suggest that a high-fiber diet protects against colon and other cancers; the lipid fraction of wheat bran fiber in particular provides maximal protection against AOM-induced colon cancer in rats [221, 222]. Future detailed studies examining fiber content and its product SCFAs, types of SCFAs, and fiber’s influence on type of microbial flora using an in vivo model of colon cancer will help us to better understand the role of dietary fiber in CRC.
In a recent meta-analysis involving case-control and cohort studies with different types of fiber intake, high fiber intake was reported to result in significant risk reduction in case-control groups, with no reduction in cohort groups [223]. In another study, vegetable and fruit fiber consumption had similar results [224]. Another case-control study that enrolled incident PC patients reported that fruit fiber intake was inversely related to risk of PC, compared with grain fiber intake [225]. Overall, these data suggest that the selected fiber contents from various sources may have different effects on PC risk. Although the mechanistic basis and immune regulations have not been fully evaluated in relation to different fiber components, alone or in combination, and the development of PC, the clinical and epidemiological data suggest that these components possess anti-cancer properties.
Conclusions and Future Directions
Several dietary ingredients are involved in metabolic, physiological, and cellular signaling mechanisms that influence immune functions and, thus, affect tumor growth and progression. Many diet-derived factors have been found to possess cancer risk-reduction capabilities. Their anti-tumorigenic properties occur in part through immune-modulating properties. The evidence is supported by both experimental and clinical studies. New findings regarding novel dietary ingredients and their effects on and regulation of various immune cells are slowly emerging. The mechanisms of each diet-derived immunomodulatory agent in cancer prevention are yet to be understood. Therefore, great opportunities exist to explore precise mechanisms. An improved understanding of the mechanistic basis of the immune-modulating effects of dietary factors could be used to better design and optimize a combination of dietary immunomodulators for cancer prevention and treatment. Carefully designed randomized controlled trails are needed to establish whether dietary ingredients can modulate inflammatory genes by interacting with immune cells. The exploration of the extent to which these dietary ingredients alter human genome-wide changes is critical to determining their tumor-inhibiting and/or cancer risk reduction effects. A combination of an immunomodulatory gene profile derived from newly developed database genome-wide profiling with dietary immunomodulatory agents may provide unique opportunities for stratification of response and non-response populations. Furthermore, identifying immune biomarkers of specific dietary factors during immunomodulation leading to tumor inhibition will aid in diagnosis and treatment.
Acknowledgments
The authors thank the University of Oklahoma Health Sciences Center staff editor, Ms. Kathy Kyler, for help in editing the manuscript.
Abbreviations
- NO
Nitric oxide
- ODC
ornithine decarboxylase
- Arg
Arginine
- Gln
Glutamine
- TCA
tri carboxylic acid
- GLS
glutaminases
- IDO2
indoleamine 2,3-dioxygenase 2
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- MCP-1/CCL2
monocyte chemoattractant protein-1
- SEPP1
selenoprotein P
- PBIT
S,S’−1,4-phenylenebis(1,2-ethanediyl)bis-isothiourea
- AOM
azoxymethane
- Treg
T-regulatory
- EGCG
epigallocatechin gallate
- ACF
aberrant crypt foci
- ITC
Isothiocyanates
- AITC
allyl isothiocyanate
- PITC
phenyl isothiocyanate
- MIF
macrophage migration inhibitory factor
- CRC
colorectal cancer
- PanINs
pancreatic intraepithelial neoplastic lesions
- SCFA
short-chain fatty acids
REFERENCE
- [1].Percival SS, Nutrition and Immunity: Balancing Diet and Immune Function. Nutrition Today 2011, 46, 12–17. [Google Scholar]
- [2].Liu RH, Dietary bioactive compounds and their health implications. Journal of food science 2013, 78, A18–A25. [DOI] [PubMed] [Google Scholar]
- [3].Manhart N, Stehle P, Nutritive amino acids--effective modulators of the immune response. Forum of nutrition 2002, 56, 151–154. [PubMed] [Google Scholar]
- [4].McMurray D, Cell-mediated immunity in nutritional deficiency. Progress in food & nutrition science 1983, 8, 193–228. [PubMed] [Google Scholar]
- [5].Gajewski TF, Schreiber H, Fu Y-X, Innate and adaptive immune cells in the tumor microenvironment. Nature immunology 2013, 14, 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cheng PN-M, Lam T-L, Lam W-M, Tsui S-M , et al. , Pegylated recombinant human arginase (rhArg-peg5, 000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer research 2007, 67, 309–317. [DOI] [PubMed] [Google Scholar]
- [7].Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA, Pegylated arginine deiminase (ADI-SS PEG20, 000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer research 2002, 62, 5443–5450. [PubMed] [Google Scholar]
- [8].Kim RH, Coates JM, Bowles TL, McNerney GP , et al. , Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspase-independent apoptosis. Cancer research 2009, 69, 700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yoon CY, Shim YJ, Kim EH, Lee JH , et al. , Renal cell carcinoma does not express argininosuccinate synthetase and is highly sensitive to arginine deprivation via arginine deiminase. International journal of cancer 2007, 120, 897–905. [DOI] [PubMed] [Google Scholar]
- [10].Dillon BJ, Prieto VG, Curley SA, Ensor CM , et al. , Incidence and distribution of argininosuccinate synthetase deficiency in human cancers. Cancer 2004, 100, 826–833. [DOI] [PubMed] [Google Scholar]
- [11].Lu Y, Wang W, Wang J, Yang C , et al. , Overexpression of Arginine Transporter CAT-1 Is Associated with Accumulation of L-Arginine and Cell Growth in Human Colorectal Cancer Tissue. PloS one 2013, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chrzanowska A, Graboń W, Mielczarek-Puta M, Barańczyk-Kuźma A, Significance of arginase determination in body fluids of patients with hepatocellular carcinoma and liver cirrhosis before and after surgical treatment. Clinical biochemistry 2014, 47, 1056–1059. [DOI] [PubMed] [Google Scholar]
- [13].Gannon PO, Godin-Ethier J, Hassler M, Delvoye N , et al. , Androgen-regulated expression of arginase 1, arginase 2 and interleukin-8 in human prostate cancer. PloS one 2010, 5, e12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wu C-W, Kao H-L, Lui W-Y, P’eng F-K , et al. , Immunohistochemical study of arginase in cancer of the stomach. Virchows Archiv 1996, 428, 325–331. [DOI] [PubMed] [Google Scholar]
- [15].Zea AH, Rodriguez PC, Atkins MB, Hernandez C , et al. , Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer research 2005, 65, 3044–3048. [DOI] [PubMed] [Google Scholar]
- [16].Ochoa AC, Zea AH, Hernandez C, Rodriguez PC, Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clinical Cancer Research 2007, 13, 721s–726s. [DOI] [PubMed] [Google Scholar]
- [17].Obermajer N, Wong JL, Edwards RP, Chen K , et al. , Induction and stability of human Th17 cells require endogenous NOS2 and cGMP-dependent NO signaling. The Journal of experimental medicine 2013, 210, 1433–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Janakiram NB, Rao CV, iNOS-selective inhibitors for cancer prevention: promise and progress. Future medicinal chemistry 2012, 4, 2193–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lamas B, Vergnaud-Gauduchon J, Goncalves-Mendes N, Perche O , et al. , Altered functions of natural killer cells in response to L-Arginine availability. Cellular immunology 2012, 280, 182–190. [DOI] [PubMed] [Google Scholar]
- [20].Norian LA, Rodriguez PC, O’Mara LA, Zabaleta J , et al. , Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via L-arginine metabolism. Cancer research 2009, 69, 3086–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Oberlies J, Watzl C, Giese T, Luckner C , et al. , Regulation of NK cell function by human granulocyte arginase. The Journal of Immunology 2009, 182, 5259–5267. [DOI] [PubMed] [Google Scholar]
- [22].Rodriguez PC, Quiceno DG, Ochoa AC, L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 2007, 109, 1568–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gao P, Tchernyshyov I, Chang T-C, Lee Y-S , et al. , c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 2009, 458, 762–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wise DR, DeBerardinis RJ, Mancuso A, Sayed N , et al. , Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proceedings of the National Academy of Sciences 2008, 105, 18782–18787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Son J, Lyssiotis CA, Ying H, Wang X , et al. , Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013, 496, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang J-B, Erickson JW, Fuji R, Ramachandran S , et al. , Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer cell 2010, 18, 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nicklin P, Bergman P, Zhang B, Triantafellow E , et al. , Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 2009, 136, 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hansen AM, Caspi RR, Glutamate joins the ranks of immunomodulators. Nature medicine 2010, 16, 856–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fallarino F, Volpi C, Fazio F, Notartomaso S , et al. , Metabotropic glutamate receptor-4 modulates adaptive immunity and restrains neuroinflammation. Nature medicine 2010, 16, 897–902. [DOI] [PubMed] [Google Scholar]
- [30].Klimberg VS, Is glutamine effective in enhancing host immune response to tumors? The Journal of nutrition 2005, 135, 2920S–2920S. [Google Scholar]
- [31].Viora M, Quaranta MG, Straface E, Masella R, Malorni W, Redox imbalance and immune functions: opposite effects of oxidized low‐density lipoproteins and N‐acetylcysteine. Immunology 2001, 104, 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kuhn KS, Muscaritoli M, Wischmeyer P, Stehle P, Glutamine as indispensable nutrient in oncology: experimental and clinical evidence. European journal of nutrition 2010, 49, 197–210. [DOI] [PubMed] [Google Scholar]
- [33].Klysz D, Tai X, Robert PA, Craveiro M , et al. , Glutamine-dependent α-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Sci. Signal. 2015, 8, ra97–ra97. [DOI] [PubMed] [Google Scholar]
- [34].Quan Z-F, Yang C, Li N, Li J-S, Effect of glutamine on change in early postoperative intestinal permeability and its relation to systemic inflammatory response. World Journal of Gastroenterology 2004, 10, 1992–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rao R, Samak G, Role of glutamine in protection of intestinal epithelial tight junctions. Journal of epithelial biology & pharmacology 2012, 5, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kretzmann NA, Fillmann H, Mauriz JL, Marroni CA , et al. , Effects of glutamine on proinflammatory gene expression and activation of nuclear factor kappa B and signal transducers and activators of transcription in TNBS‐induced colitis. Inflammatory bowel diseases 2008, 14, 1504–1513. [DOI] [PubMed] [Google Scholar]
- [37].Cetinbas F, Yelken B, Gulbas Z, Role of glutamine administration on cellular immunity after total parenteral nutrition enriched with glutamine in patients with systemic inflammatory response syndrome. Journal of critical care 2010, 25, 661 e661–661 e666. [DOI] [PubMed] [Google Scholar]
- [38].O’Riordain MG, Fearon K, Ross JA, Rogers P , et al. , Glutamine-supplemented total parenteral nutrition enhances T-lymphocyte response in surgical patients undergoing colorectal resection. Annals of surgery 1994, 220, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hopkins FG, Cole SW, A contribution to the chemistry of proteids. The Journal of physiology 1901, 27, 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sainio E-L, Pulkki K, Young S, L-Tryptophan: Biochemical, nutritional and pharmacological aspects. Amino Acids 1996, 10, 21–47. [DOI] [PubMed] [Google Scholar]
- [41].Fallarino F, Grohmann U, You S, McGrath BC , et al. , Tryptophan catabolism generates autoimmune-preventive regulatory T cells. Transplant immunology 2006, 17, 58–60. [DOI] [PubMed] [Google Scholar]
- [42].Szczepanik M, Melatonin and its influence on immune system. Journal of physiology and pharmacology 2007, 58, 115–124. [PubMed] [Google Scholar]
- [43].Yamamoto S, Hayaishi O, Tryptophan pyrrolase of rabbit intestine d-and l-tryptophan-cleaving enzyme or enzymes. Journal of Biological Chemistry 1967, 242, 5260–5266. [PubMed] [Google Scholar]
- [44].Metz R, Smith C, DuHadaway JB, Chandler P , et al. , IDO2 is critical for IDO1-mediated T-cell regulation and exerts a non-redundant function in inflammation. International immunology 2014, 26, 357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Munn DH, Sharma MD, Baban B, Harding HP , et al. , GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2, 3-dioxygenase. Immunity 2005, 22, 633–642. [DOI] [PubMed] [Google Scholar]
- [46].Manlapat AK, Kahler DJ, Chandler PR, Munn DH, Mellor AL, Cell‐autonomous control of interferon type I expression by indoleamine 2, 3‐dioxygenase in regulatory CD19+ dendritic cells. European journal of immunology 2007, 37, 1064–1071. [DOI] [PubMed] [Google Scholar]
- [47].Prendergast G, Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene 2008, 27, 3889–3900. [DOI] [PubMed] [Google Scholar]
- [48].Peng Y-P, Zhu Y, Zhang J-J, Liang W. b. , et al. , Elevation of MMP-9 and IDO induced by pancreatic cancer cells mediates natural killer cell dysfunction. BMC cancer 2014, 14, 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Liu X, Shin N, Koblish HK, Yang G , et al. , Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood 2010, 115, 3520–3530. [DOI] [PubMed] [Google Scholar]
- [50].Ferdinande L, Decaestecker C, Verset L, Mathieu A , et al. , Clinicopathological significance of indoleamine 2, 3-dioxygenase 1 expression in colorectal cancer. British journal of cancer 2012, 106, 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Brandacher G, Perathoner A, Ladurner R, Schneeberger S , et al. , Prognostic value of indoleamine 2, 3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clinical Cancer Research 2006, 12, 1144–1151. [DOI] [PubMed] [Google Scholar]
- [52].Gao Y-F, Peng R-Q, Li J, Ding Y , et al. , The paradoxical patterns of expression of indoleamine 2, 3-dioxygenase in colon cancer. Journal of translational medicine 2009, 7, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Huang A, Fuchs D, Widner B, Glover C , et al. , Serum tryptophan decrease correlates with immune activation and impaired quality of life in colorectal cancer. British journal of cancer 2002, 86, 1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Carr A, Frei B, Does vitamin C act as a pro-oxidant under physiological conditions? The FASEB Journal 1999, 13, 1007–1024. [DOI] [PubMed] [Google Scholar]
- [55].Kim JE, Kang JS, Lee WJ, Vitamin C induces apoptosis in human colon cancer cell line, HCT-8 via the modulation of calcium influx in endoplasmic reticulum and the dissociation of bad from 14–3-3β. Immune network 2012, 12, 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Chen Q, Espey MG, Krishna MC, Mitchell JB , et al. , Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proceedings of the national academy of sciences of the United States of America 2005, 102, 13604–13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chen Q, Espey MG, Sun AY, Lee J-H , et al. , Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proceedings of the National Academy of Sciences 2007, 104, 8749–8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Du J, Martin SM, Levine M, Wagner BA , et al. , Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clinical Cancer Research 2010, 16, 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Espey MG, Chen P, Chalmers B, Drisko J , et al. , Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radical Biology and Medicine 2011, 50, 1610–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Welsh J, Wagner B, Van’t Erve T, Zehr P , et al. , Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer chemotherapy and pharmacology 2013, 71, 765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hemilä H, in: Packer L FJ, eds (Ed.), Vitamin C in Health and Disease, NY: Marcel Dekker; 1997a, pp. 471–503. [Google Scholar]
- [62].Mikirova N, Casciari J, Rogers A, Taylor P, Effect of high-dose intravenous vitamin C on inflammation in cancer patients Journal of translational medicine 2012, 10, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Battault S, Whiting S, Peltier S, Sadrin S , et al. , Vitamin D metabolism, functions and needs: from science to health claims. European journal of nutrition 2013, 52, 429–441. [DOI] [PubMed] [Google Scholar]
- [64].Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C, Vitamin D: modulator of the immune system. Current opinion in pharmacology 2010, 10, 482–496. [DOI] [PubMed] [Google Scholar]
- [65].Ferreira GB, van Etten E, Verstuyf A, Waer M , et al. , 1, 25‐Dihydroxyvitamin D3 alters murine dendritic cell behaviour in vitro and in vivo. Diabetes/metabolism research and reviews 2011, 27, 933–941. [DOI] [PubMed] [Google Scholar]
- [66].Müller K, Diamant M, Bendtzen K, Inhibition of production and function of interleukin-6 by 1, 25-dihydroxyvitamin D 3. Immunology letters 1991, 28, 115–120. [DOI] [PubMed] [Google Scholar]
- [67].Lemire JM, Archer C, ߣCK L, SPIEGELBERGÃŊ MHL, Immunosuppressive Actions of l, 25-Dihydroxyvitamin D3: Preferential Inhibition of Th! Functions1 2. transplantation 1995, 125, 17045–17085. [DOI] [PubMed] [Google Scholar]
- [68].Boonstra A, Barrat FJ, Crain C, Heath VL , et al. , 1α, 25-Dihydroxyvitamin D3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. The Journal of Immunology 2001, 167, 4974–4980. [DOI] [PubMed] [Google Scholar]
- [69].Freedman DM, Looker AC, Chang S-C, Graubard BI, Prospective study of serum vitamin D and cancer mortality in the United States. Journal of the National Cancer Institute 2007, 99, 1594–1602. [DOI] [PubMed] [Google Scholar]
- [70].Gandini S, Boniol M, Haukka J, Byrnes G , et al. , Meta‐analysis of observational studies of serum 25‐hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. International Journal of Cancer 2011, 128, 1414–1424. [DOI] [PubMed] [Google Scholar]
- [71].Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ , et al. , Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: a nested case-control study. Bmj 2010, 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ma Y, Zhang P, Wang F, Yang J , et al. , Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. Journal of Clinical Oncology 2011, 29, 3775–3782. [DOI] [PubMed] [Google Scholar]
- [73].Woolcott CG, Wilkens LR, Nomura AM, Horst RL , et al. , Plasma 25-hydroxyvitamin D levels and the risk of colorectal cancer: the multiethnic cohort study. Cancer Epidemiology Biomarkers & Prevention 2010, 19, 130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Newmark HL, Yang K, Kurihara N, Fan K , et al. , Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis 2009, 30, 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zheng W, Wong KE, Zhang Z, Dougherty U , et al. , Inactivation of the vitamin D receptor in APCmin/+ mice reveals a critical role for the vitamin D receptor in intestinal tumor growth. International Journal of Cancer 2012, 130, 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Larriba MJ, Ordóñez-Morán P, Chicote I, Martín-Fernández G , et al. , Vitamin D receptor deficiency enhances Wnt/β-catenin signaling and tumor burden in colon cancer. PloS one 2011, 6, e23524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Van Loon K, Owzar K, Jiang C, Kindler HL , et al. , 25-Hydroxyvitamin D levels and survival in advanced pancreatic cancer: findings from CALGB 80303 (Alliance). Journal of the National Cancer Institute 2014, 106, dju185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wang K, Dong M, Sheng W, Liu Q , et al. , Expression of vitamin D receptor as a potential prognostic factor and therapeutic target in pancreatic cancer. Histopathology 2015, 67, 386–397. [DOI] [PubMed] [Google Scholar]
- [79].Bostick RM, Potter JD, McKenzie DR, Sellers TA , et al. , Reduced risk of colon cancer with high intake of vitamin E: the Iowa Women’s Health Study. Cancer research 1993, 53, 4230–4237. [PubMed] [Google Scholar]
- [80].Ghadirian P, Lacroix A, Maisonneuve P, Perret C , et al. , Nutritional factors and colon carcinoma: a case-control study involving French Canadians in Montreal, Quebec, Canada. Cancer 1997, 80, 858–864. [DOI] [PubMed] [Google Scholar]
- [81].Ingles SA, Bird CL, Shikany JM, Frankl HD , et al. , Plasma tocopherol and prevalence of colorectal adenomas in a multiethnic population. Cancer research 1998, 58, 661–666. [PubMed] [Google Scholar]
- [82].Longnecker MP, Martin-Moreno JM, Knekt P, Nomura AM , et al. , Serum alpha-tocopherol concentration in relation to subsequent colorectal cancer: pooled data from five cohorts. Journal of the National Cancer Institute 1992, 84, 430–435. [DOI] [PubMed] [Google Scholar]
- [83].Malila N, Virtamo J, Virtanen M, Pietinen P , et al. , Dietary and serum alpha-tocopherol, beta-carotene and retinol, and risk for colorectal cancer in male smokers. European journal of clinical nutrition 2002, 56, 615–621. [DOI] [PubMed] [Google Scholar]
- [84].White E, Shannon JS, Patterson RE, Relationship between vitamin and calcium supplement use and colon cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research , cosponsored by the American Society of Preventive Oncology 1997, 6, 769–774. [PubMed] [Google Scholar]
- [85].Wu K, Willett WC, Chan JM, Fuchs CS , et al. , A prospective study on supplemental vitamin e intake and risk of colon cancer in women and men. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research , cosponsored by the American Society of Preventive Oncology 2002, 11, 1298–1304. [PubMed] [Google Scholar]
- [86].Slattery ML, Edwards SL, Anderson K, Caan B, Vitamin E and colon cancer: is there an association? Nutrition and cancer 1998, 30, 201–206. [DOI] [PubMed] [Google Scholar]
- [87].Ju J, Hao X, Lee MJ, Lambert JD , et al. , A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice. Cancer prevention research 2009, 2, 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Cook MG, McNamara P, Effect of dietary vitamin E on dimethylhydrazine-induced colonic tumors in mice. Cancer research 1980, 40, 1329–1331. [PubMed] [Google Scholar]
- [89].Chester JF, Gaissert HA, Ross JS, Malt RA, Weitzman SA, Augmentation of 1,2-dimethylhydrazine-induced colon cancer by experimental colitis in mice: role of dietary vitamin E. Journal of the National Cancer Institute 1986, 76, 939–942. [PubMed] [Google Scholar]
- [90].Chung H, Wu D, Han SN, Gay R , et al. , Vitamin E supplementation does not alter azoxymethane-induced colonic aberrant crypt foci formation in young or old mice. The Journal of nutrition 2003, 133, 528–532. [DOI] [PubMed] [Google Scholar]
- [91].Exon JH, South EH, Taruscio TG, Clifton GD, Fariss MW, Chemopreventive effect of dietary d-alpha-tocopheryl succinate supplementation on precancer colon aberrant crypt formation and vitamin E analogue levels in young and old rats. Nutrition and cancer 2004, 49, 72–80. [DOI] [PubMed] [Google Scholar]
- [92].Hagiwara A, Boonyaphiphat P, Tanaka H, Kawabe M , et al. , Organ-dependent modifying effects of caffeine, and two naturally occurring antioxidants alpha-tocopherol and n-tritriacontane-16,18-dione, on 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-induced mammary and colonic carcinogenesis in female F344 rats. Japanese journal of cancer research : Gann 1999, 90, 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Maziere S, Meflah K, Tavan E, Champ M , et al. , Effect of resistant starch and/or fat-soluble vitamins A and E on the initiation stage of aberrant crypts in rat colon. Nutrition and cancer 1998, 31, 168–177. [DOI] [PubMed] [Google Scholar]
- [94].Reddy BS, Tanaka T, Interactions of selenium deficiency, vitamin E, polyunsaturated fat, and saturated fat on azoxymethane-induced colon carcinogenesis in male F344 rats. Journal of the National Cancer Institute 1986, 76, 1157–1162. [PubMed] [Google Scholar]
- [95].Temple NJ, el-Khatib SM, Cabbage and vitamin E: their effect on colon tumor formation in mice. Cancer letters 1987, 35, 71–77. [DOI] [PubMed] [Google Scholar]
- [96].Yao K, Latta M, Bird RP, Modulation of colonic aberrant crypt foci and proliferative indexes in colon and prostate glands of rats by vitamin E. Nutrition and cancer 1996, 26, 99–109. [DOI] [PubMed] [Google Scholar]
- [97].Toth B, Patil K, Enhancing effect of vitamin E on murine intestinal tumorigenesis by 1,2-dimethylhydrazine dihydrochloride. Journal of the National Cancer Institute 1983, 70, 1107–1111. [PubMed] [Google Scholar]
- [98].Newmark HL, Huang M-T, Reddy BS, Mixed tocopherols inhibit azoxymethane-induced aberrant crypt foci in rats. Nutrition and cancer 2006, 56, 82–85. [DOI] [PubMed] [Google Scholar]
- [99].Jubri Z, Latif AA, Top AG, Ngah WZ, Perturbation of cellular immune functions in cigarette smokers and protection by palm oil vitamin E supplementation. Nutrition journal 2013, 12, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Malmberg KJ, Lenkei R, Petersson M, Ohlum T , et al. , A short-term dietary supplementation of high doses of vitamin E increases T helper 1 cytokine production in patients with advanced colorectal cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2002, 8, 1772–1778. [PubMed] [Google Scholar]
- [101].Salinthone S, Kerns AR, Tsang V, Carr DW, alpha-Tocopherol (vitamin E) stimulates cyclic AMP production in human peripheral mononuclear cells and alters immune function. Molecular immunology 2013, 53, 173–178. [DOI] [PubMed] [Google Scholar]
- [102].Bonaventura P, Benedetti G, Albarède F, Miossec P, Zinc and its role in immunity and inflammation. Autoimmunity reviews 2015, 14, 277–285. [DOI] [PubMed] [Google Scholar]
- [103].Lee D-H, Anderson KE, Harnack LJ, Folsom AR, Jacobs DR, Heme iron, zinc, alcohol consumption, and colon cancer: Iowa Women’s Health Study. Journal of the National Cancer Institute 2004, 96, 403–407. [DOI] [PubMed] [Google Scholar]
- [104].Qiao L, Feng Y, Intakes of heme iron and zinc and colorectal cancer incidence: a meta-analysis of prospective studies. Cancer Causes & Control 2013, 24, 1175–1183. [DOI] [PubMed] [Google Scholar]
- [105].Li P, Xu J, Shi Y, Ye Y , et al. , Association between zinc intake and risk of digestive tract cancers: A systematic review and meta-analysis. Clinical Nutrition 2014, 33, 415–420. [DOI] [PubMed] [Google Scholar]
- [106].Dani V, Goel A, Vaiphei K, Dhawan D, Chemopreventive potential of zinc in experimentally induced colon carcinogenesis. Toxicology letters 2007, 171, 10–18. [DOI] [PubMed] [Google Scholar]
- [107].Chadha VD, Vaiphei K, Dhawan D, Zinc mediated normalization of histoarchitecture and antioxidant status offers protection against initiation of experimental carcinogenesis. Molecular and cellular biochemistry 2007, 304, 101–108. [DOI] [PubMed] [Google Scholar]
- [108].Chadha VD, Dhawan D, Membrane fluidity and surface changes during initiation of 1, 2 dimethylhydrazine-induced colon carcinogenesis: protection by zinc. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics 2009, 18, 17–23. [DOI] [PubMed] [Google Scholar]
- [109].Chandra RK, Immunodeficiency in undernutrition and overnutrition. Nutrition reviews 1981, 39, 225–231. [DOI] [PubMed] [Google Scholar]
- [110].Chandra RK, Dayton DH, Trace element regulation of immunity and infection. Nutrition Research 1982, 2, 721–733. [Google Scholar]
- [111].Brozmanova J, [Selenium and cancer: from prevention to treatment]. Klinicka onkologie : casopis Ceske a Slovenske onkologicke spolecnosti 2011, 24, 171–179. [PubMed] [Google Scholar]
- [112].Naithani R, Organoselenium compounds in cancer chemoprevention. Mini reviews in medicinal chemistry 2008, 8, 657–668. [DOI] [PubMed] [Google Scholar]
- [113].Fleet JC, Dietary selenium repletion may reduce cancer incidence in people at high risk who live in areas with low soil selenium. Nutrition reviews 1997, 55, 277–279. [DOI] [PubMed] [Google Scholar]
- [114].Peters U, Takata Y, Selenium and the prevention of prostate and colorectal cancer. Molecular nutrition & food research 2008, 52, 1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Lee EH, Myung SK, Jeon YJ, Kim Y , et al. , Effects of selenium supplements on cancer prevention: meta-analysis of randomized controlled trials. Nutrition and cancer 2011, 63, 1185–1195. [DOI] [PubMed] [Google Scholar]
- [116].Hughes DJ, Fedirko V, Jenab M, Schomburg L , et al. , Selenium status is associated with colorectal cancer risk in the European prospective investigation of cancer and nutrition cohort. International journal of cancer . Journal international du cancer 2015, 136, 1149–1161. [DOI] [PubMed] [Google Scholar]
- [117].Kay HD, Petrie H, Klassen LW, In vitro effects of selenium and t-cell growth-factor (tcgf) on natural-killer (nk) cell-function of lymphocytes from human peripheral-blood. IRCS Medical Science 1986, 14, 691–692. [Google Scholar]
- [118].Wood SM, Beckham C, Yosioka A, Darban H, Watson RR, beta-Carotene and selenium supplementation enhances immune response in aged humans. Integrative medicine : integrating conventional and alternative medicine 2000, 2, 85–92. [DOI] [PubMed] [Google Scholar]
- [119].Meeker H, Eskew M, Scheuchenzuber W, Scholz R, Zarkower A, Antioxidant effects on cell-mediated immunity. Journal of leukocyte biology 1985, 38, 451–458. [DOI] [PubMed] [Google Scholar]
- [120].Roy M, Kiremidjian-Schumacher L, Wishe H, Cohen M, Stotzky G, Selenium and immune cell functions. II. Effect on lymphocyte-mediated cytotoxicity. Experimental biology and medicine 1990, 193, 143–148. [DOI] [PubMed] [Google Scholar]
- [121].Tsuji PA, Carlson BA, Anderson CB, Seifried HE , et al. , Dietary Selenium Levels Affect Selenoprotein Expression and Support the Interferon-gamma and IL-6 Immune Response Pathways in Mice. Nutrients 2015, 7, 6529–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Barrett CW, Reddy VK, Short SP, Motley AK , et al. , Selenoprotein P influences colitis-induced tumorigenesis by mediating stemness and oxidative damage. The Journal of clinical investigation 2015, 125, 2646–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Boyne R, Arthur J, The response of selenium-deficient mice to Candida albicans infection. The Journal of nutrition 1986, 116, 816–822. [DOI] [PubMed] [Google Scholar]
- [124].Turner R, Finch J, Immunological malfunctions associated with low selenium-vitamin E diets in lambs. Journal of comparative pathology 1990, 102, 99–109. [DOI] [PubMed] [Google Scholar]
- [125].Janakiram NB, Mohammed A, Ravillah D, Choi CI , et al. , Chemopreventive effects of PBI-Se, a selenium-containing analog of PBIT, on AOM-induced aberrant crypt foci in F344 rats. Oncology reports 2013, 30, 952–960. [DOI] [PubMed] [Google Scholar]
- [126].Maru GB, Kumar G, Ghantasala S, Tajpara P, in: Watson RR, Preedy VR, Zibadi S (Eds.), Polyphenols in Health and Diseases, Elsevier; 2013, pp. 1141–1179. [Google Scholar]
- [127].Kumar G, Dange P, Kailaje V, Vaidya MM , et al. , Polymeric black tea polyphenols modulate the localization and activity of 12-O-tetradecanoylphorbol-13-acetate-mediated kinases in mouse skin: mechanisms of their anti-tumor-promoting action. Free radical biology & medicine 2012, 53, 1358–1370. [DOI] [PubMed] [Google Scholar]
- [128].Kumar G, Pillare SP, Maru GB, Black tea polyphenols-mediated in vivo cellular responses during carcinogenesis. Mini reviews in medicinal chemistry 2010, 10, 492–505. [DOI] [PubMed] [Google Scholar]
- [129].Galeone C, Turati F, La Vecchia C, Tavani A, Coffee consumption and risk of colorectal cancer: a meta-analysis of case–control studies. Cancer Causes & Control 2010, 21, 1949–1959. [DOI] [PubMed] [Google Scholar]
- [130].Je Y, Liu W, Giovannucci E, Coffee consumption and risk of colorectal cancer: A systematic review and meta‐analysis of prospective cohort studies. International Journal of Cancer 2009, 124, 1662–1668. [DOI] [PubMed] [Google Scholar]
- [131].Sinha R, Cross AJ, Daniel CR, Graubard BI , et al. , Caffeinated and decaffeinated coffee and tea intakes and risk of colorectal cancer in a large prospective study. The American journal of clinical nutrition 2012, ajcn. 031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Wang Z-J, Ohnaka K, Morita M, Toyomura K , et al. , Dietary polyphenols and colorectal cancer risk: the Fukuoka colorectal cancer study. World journal of gastroenterology: WJG 2013, 19, 2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Jurenka JS, Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev 2009, 14, 277. [PubMed] [Google Scholar]
- [134].Kumar G, Tajpara P, Bukhari AB, Ramchandani AG , et al. , Dietary curcumin post-treatment enhances the disappearance of B(a)P-derived DNA adducts in mouse liver and lungs. Toxicology Reports 2014, 1, 1181–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Bhattacharyya S, Hossain DMS, Mohanty S, Sen GS , et al. , Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cellular & molecular immunology 2010, 7, 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Li Y, Zhang T, Targeting cancer stem cells by curcumin and clinical applications. Cancer letters 2014, 346, 197–205. [DOI] [PubMed] [Google Scholar]
- [137].Churchill M, Chadburn A, Bilinski RT, Bertagnolli MM, Inhibition of intestinal tumors by curcumin is associated with changes in the intestinal immune cell profile. Journal of Surgical Research 2000, 89, 169–175. [DOI] [PubMed] [Google Scholar]
- [138].Maru GB, Ramchandani AG, Kumar G, Garg R, in: Watson RR, Preedy VR (Eds.), Bioactive Foods and Extracts 2010, pp. 181–203. [Google Scholar]
- [139].Kumar G, Tajpara P, Maru G, Dietary turmeric post-treatment decreases DMBA-induced hamster buccal pouch tumor growth by altering cell proliferation and apoptosis-related markers. Journal of environmental pathology , toxicology and oncology : official organ of the International Society for Environmental Toxicology and Cancer 2012, 31, 295–312. [DOI] [PubMed] [Google Scholar]
- [140].Singh S, Khar A, Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents) 2006, 6, 259–270. [DOI] [PubMed] [Google Scholar]
- [141].McFadden RM, Larmonier CB, Shehab KW, Midura-Kiela M , et al. , The Role of Curcumin in Modulating Colonic Microbiota During Colitis and Colon Cancer Prevention. Inflammatory bowel diseases 2015, 21, 2483–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Byun S-Y, Kim D-B, Kim E, Curcumin ameliorates the tumor-enhancing effects of a high-protein diet in an azoxymethane-induced mouse model of colon carcinogenesis. Nutrition Research 2015, 35, 726–735. [DOI] [PubMed] [Google Scholar]
- [143].Fiala M, Curcumin and Omega-3 Fatty Acids Enhance NK Cell-Induced Apoptosis of Pancreatic Cancer Cells but Curcumin Inhibits Interferon-γ Production: Benefits of Omega-3 with Curcumin against Cancer. Molecules 2015, 20, 3020–3026.25685909 [Google Scholar]
- [144].Swamy MV, Citineni B, Patlolla JM, Mohammed A , et al. , Prevention and treatment of pancreatic cancer by curcumin in combination with omega-3 fatty acids. Nutrition and cancer 2008, 60, 81–89. [DOI] [PubMed] [Google Scholar]
- [145].Kesharwani P, Banerjee S, Padhye S, Sarkar FH, Iyer AK, Hyaluronic Acid Engineered Nanomicelles Loaded with 3, 4-Difluorobenzylidene Curcumin for Targeted Killing of CD44+ Stem-Like Pancreatic Cancer Cells. Biomacromolecules 2015, 16, 3042–3053. [DOI] [PubMed] [Google Scholar]
- [146].Sunagawa Y, Katanasaka Y, Hasegawa K, Morimoto T, Clinical applications of curcumin. PharmaNutrition 2015, 3, 131–135. [Google Scholar]
- [147].Sikora E, Bielak-Zmijewska A, Piwocka K, Janusz S, Radziszewska E, Inhibition of proliferation and apoptosis of human and rat T lymphocytes by curcumin, a curry pigment. Biochemical pharmacology 1997, 54, 899–907. [DOI] [PubMed] [Google Scholar]
- [148].Magalska A, Brzezinska A, Bielak-Zmijewska A, Piwocka K , et al. , Curcumin induces cell death without oligonucleosomal DNA fragmentation in quiescent and proliferating human CD8+ cells. Acta Biochimica Polonica 2006, 53, 531. [PubMed] [Google Scholar]
- [149].Bhattacharyya S, Mandal D, Saha B, Sen GS , et al. , Curcumin prevents tumor-induced T cell apoptosis through Stat-5a-mediated Bcl-2 induction. Journal of Biological Chemistry 2007, 282, 15954–15964. [DOI] [PubMed] [Google Scholar]
- [150].Bhaumik S, Jyothi MD, Khar A, Differential modulation of nitric oxide production by curcumin in host macrophages and NK cells. FEBS letters 2000, 483, 78–82. [DOI] [PubMed] [Google Scholar]
- [151].Zhang H-G, Kim H, Liu C, Yu S , et al. , Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 2007, 1773, 1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Siviero A, Gallo E, Maggini V, Gori L , et al. , Curcumin, a golden spice with a low bioavailability. Journal of Herbal Medicine 2015, 5, 57–70. [Google Scholar]
- [153].Haqqi TM, Anthony DD, Gupta S, Ahmad N , et al. , Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proceedings of the National Academy of Sciences 1999, 96, 4524–4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Wu D, Wang J, Pae M, Meydani SN, Green tea EGCG T cells, and T cell-mediated autoimmune diseases. Molecular aspects of medicine 2012, 33, 107–118. [DOI] [PubMed] [Google Scholar]
- [155].Nakachi K, Matsuyama S, Miyake S, Suganuma M, Imai K, Preventive effects of drinking green tea on cancer and cardiovascular disease: epidemiological evidence for multiple targeting prevention. Biofactors 2000, 13, 49–54. [DOI] [PubMed] [Google Scholar]
- [156].Shimizu M, Fukutomi Y, Ninomiya M, Nagura K , et al. , Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiology Biomarkers & Prevention 2008, 17, 3020–3025. [DOI] [PubMed] [Google Scholar]
- [157].Tang SN, Fu J, Nall D, Rodova M , et al. , Inhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristics. International Journal of Cancer 2012, 131, 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Suganuma M, Ohkura Y, Okabe S, Fujiki H, Combination cancer chemoprevention with green tea extract and sulindac shown in intestinal tumor formation in Min mice. Journal of cancer research and clinical oncology 2001, 127, 69–72. [DOI] [PubMed] [Google Scholar]
- [159].Wang J, Ren Z, Xu Y, Xiao S , et al. , Epigallocatechin-3-gallate ameliorates experimental autoimmune encephalomyelitis by altering balance among CD4+ T-cell subsets. The American journal of pathology 2012, 180, 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Wong CP, Nguyen LP, Noh SK, Bray TM , et al. , Induction of regulatory T cells by green tea polyphenol EGCG. Immunology letters 2011, 139, 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP , et al. , Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer research 2004, 24, 2783–2840. [PubMed] [Google Scholar]
- [162].Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G, Inflammation and cancer: how hot is the link? Biochemical pharmacology 2006, 72, 1605–1621. [DOI] [PubMed] [Google Scholar]
- [163].Baur JA, Pearson KJ, Price NL, Jamieson HA , et al. , Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Baur JA, Sinclair DA, Therapeutic potential of resveratrol: the in vivo evidence. Nature reviews Drug discovery 2006, 5, 493–506. [DOI] [PubMed] [Google Scholar]
- [165].Suh N, Paul S, Hao X, Simi B , et al. , Pterostilbene, an active constituent of blueberries, suppresses aberrant crypt foci formation in the azoxymethane-induced colon carcinogenesis model in rats. Clinical Cancer Research 2007, 13, 350–355. [DOI] [PubMed] [Google Scholar]
- [166].Sale S, Tunstall RG, Ruparelia KC, Potter GA , et al. , Comparison of the effects of the chemopreventive agent resveratrol and its synthetic analog trans 3, 4, 5, 4′‐tetramethoxystilbene (DMU‐212) on adenoma development in the ApcMin+ mouse and cyclooxygenase‐2 in human‐derived colon cancer cells. International journal of cancer 2005, 115, 194–201. [DOI] [PubMed] [Google Scholar]
- [167].Schneider Y, Duranton B, Goss F, Schleiffer R , et al. , Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutrition and cancer 2001, 39, 102–107. [DOI] [PubMed] [Google Scholar]
- [168].Sengottuvelan M, Viswanathan P, Nalini N, Chemopreventive effect of trans-resveratrol-a phytoalexin against colonic aberrant crypt foci and cell proliferation in 1, 2-dimethylhydrazine induced colon carcinogenesis. Carcinogenesis 2006, 27, 1038–1046. [DOI] [PubMed] [Google Scholar]
- [169].Tessitore L, Davit A, Sarotto I, Caderni G, Resveratrol depresses the growth of colorectal aberrant crypt foci by affecting bax and p21CIP expression. Carcinogenesis 2000, 21, 1619–1622. [PubMed] [Google Scholar]
- [170].Ziegler CC, Rainwater L, Whelan J, McEntee MF, Dietary resveratrol does not affect intestinal tumorigenesis in ApcMin/+ mice. The Journal of nutrition 2004, 134, 5–10. [DOI] [PubMed] [Google Scholar]
- [171].Kim K-O, Park H, Chun M, Kim H-S, Immunomodulatory Effects of High-Protein Diet with Resveratrol Supplementation on Radiation-Induced Acute-Phase Inflammation in Rats. Journal of medicinal food 2014, 17, 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Buttari B, Profumo E, Segoni L, D’Arcangelo D , et al. , Resveratrol counteracts inflammation in human M1 and M2 macrophages upon challenge with 7-oxo-cholesterol: potential therapeutic implications in atherosclerosis. Oxidative medicine and cellular longevity 2014, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Kuijsten A, Arts IC, Hollman PC, van’t Veer P, Kampman E, Plasma enterolignans are associated with lower colorectal adenoma risk. Cancer Epidemiology Biomarkers & Prevention 2006, 15, 1132–1136. [DOI] [PubMed] [Google Scholar]
- [174].Thompson LU, Boucher BA, Liu Z, Cotterchio M, Kreiger N, Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutrition and cancer 2006, 54, 184–201. [DOI] [PubMed] [Google Scholar]
- [175].Wang W, Ayella A, Jiang Y, Ouyang P, Qu H , Wheat lignans: promising cancer preventive agents Wheat Antioxidants. Hoboken , New Jersey: John Wiley & Sons, Ltd; 2008, 264–272. [Google Scholar]
- [176].Jenab M, Thompson LU, The influence of flaxseed and lignans on colon carcinogenesis and β-glucuronidase activity. Carcinogenesis 1996, 17, 1343–1348. [DOI] [PubMed] [Google Scholar]
- [177].Gredel S, Grad C, Rechkemmer G, Watzl B, Phytoestrogens and phytoestrogen metabolites differentially modulate immune parameters in human leukocytes. Food and Chemical toxicology 2008, 46, 3691–3696. [DOI] [PubMed] [Google Scholar]
- [178].Khor TO, Cheung WKL, Prawan A, Reddy BS, Kong ANT, Chemoprevention of familial adenomatous polyposis in ApcMin/+ mice by phenethyl isothiocyanate (PEITC). Molecular carcinogenesis 2008, 47, 321–325. [DOI] [PubMed] [Google Scholar]
- [179].Nakamura Y, Miyoshi N, Takabayashi S, Osawa T, Benzyl isothiocyanate inhibits oxidative stress in mouse skin: involvement of attenuation of leukocyte infiltration. Biofactors 2004, 21, 255–257. [DOI] [PubMed] [Google Scholar]
- [180].Xiao D, Singh SV, Phenethyl isothiocyanate inhibits angiogenesis in vitro and ex vivo. Cancer research 2007, 67, 2239–2246. [DOI] [PubMed] [Google Scholar]
- [181].Thejass P, Kuttan G, Inhibition of endothelial cell differentiation and proinflammatory cytokine production during angiogenesis by allyl isothiocyanate and phenyl isothiocyanate. Integrative cancer therapies 2007, 6, 389–399. [DOI] [PubMed] [Google Scholar]
- [182].Thejass P, Kuttan G, Allyl isothiocyanate (AITC) and phenyl isothiocyanate (PITC) inhibit tumour-specific angiogenesis by downregulating nitric oxide (NO) and tumour necrosis factor-α (TNF-α) production. Nitric Oxide 2007, 16, 247–257. [DOI] [PubMed] [Google Scholar]
- [183].Manesh C, Kuttan G, Effect of naturally occurring isothiocyanates on the immune system. Immunopharmacology and immunotoxicology 2003, 25, 451–459. [DOI] [PubMed] [Google Scholar]
- [184].Brown KK, Blaikie FH, Smith RA, Tyndall JD , et al. , Direct modification of the proinflammatory cytokine macrophage migration inhibitory factor by dietary isothiocyanates. Journal of Biological Chemistry 2009, 284, 32425–32433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Spencer ES, Dale EJ, Gommans AL, Rutledge MT , et al. , Multiple binding modes of isothiocyanates that inhibit macrophage migration inhibitory factor. European journal of medicinal chemistry 2015, 93, 501–510. [DOI] [PubMed] [Google Scholar]
- [186].Calviello G, Di Nicuolo F, Gragnoli S, Piccioni E , et al. , n-3 PUFAs reduce VEGF expression in human colon cancer cells modulating the COX-2/PGE2 induced ERK-1 and-2 and HIF-1α induction pathway. Carcinogenesis 2004, 25, 2303–2310. [DOI] [PubMed] [Google Scholar]
- [187].Clarke RG, Lund EK, Latham P, Pinder AC, Johnson IT, Effect of eicosapentaenoic acid on the proliferation and incidence of apoptosis in the colorectal cell line HT29. Lipids 1999, 34, 1287–1295. [DOI] [PubMed] [Google Scholar]
- [188].Courtney E, Matthews S, Finlayson C, Di Pierro D , et al. , Eicosapentaenoic acid (EPA) reduces crypt cell proliferation and increases apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas. International journal of colorectal disease 2007, 22, 765–776. [DOI] [PubMed] [Google Scholar]
- [189].Latham P, Lund EK, Johnson IT, Dietary n-3 PUFA increases the apoptotic response to 1, 2-dimethylhydrazine, reduces mitosis and suppresses the induction of carcinogenesis in the rat colon. Carcinogenesis 1999, 20, 645–650. [DOI] [PubMed] [Google Scholar]
- [190].Geelen A, Schouten JM, Kamphuis C, Stam BE , et al. , Fish consumption, n-3 fatty acids, and colorectal cancer: a meta-analysis of prospective cohort studies. American Journal of Epidemiology 2007, 166, 1116–1125. [DOI] [PubMed] [Google Scholar]
- [191].Gerber M, Omega-3 fatty acids and cancers: a systematic update review of epidemiological studies. British Journal of Nutrition 2012, 107, S228–S239. [DOI] [PubMed] [Google Scholar]
- [192].MacLean CH, Newberry SJ, Mojica WA, Khanna P , et al. , Effects of omega-3 fatty acids on cancer risk: a systematic review. Jama 2006, 295, 403–415. [DOI] [PubMed] [Google Scholar]
- [193].Kantor ED, Lampe JW, Peters U, Vaughan TL, White E, Long-chain omega-3 polyunsaturated fatty acid intake and risk of colorectal cancer. Nutrition and cancer 2014, 66, 716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Calder PC, n− 3 Polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids 2003, 38, 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Serhan CN, Arita M, Hong S, Gotlinger K, Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids 2004, 39, 1125–1132. [DOI] [PubMed] [Google Scholar]
- [196].Hong S, Gronert K, Devchand PR, Moussignac R-L, Serhan CN, Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells autacoids in anti-inflammation. Journal of Biological Chemistry 2003, 278, 14677–14687. [DOI] [PubMed] [Google Scholar]
- [197].Hudert CA, Weylandt KH, Lu Y, Wang J , et al. , Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proceedings of the National Academy of Sciences 2006, 103, 11276–11281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Mohammed A, Janakiram N, Madka V, Brewer M , et al. , Targeting pancreatitis blocks tumor-initiating stem cells and pancreatic cancer progression. Oncotarget 2015, 6, 15524–15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Mohammed A, Janakiram NB, Brewer M, Vedala K , et al. , Multitargeted low-dose GLAD combination chemoprevention: a novel and promising approach to combat colon carcinogenesis. Neoplasia 2013, 15, 481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Field CJ, Thomson CA, Van Aerde JE, Parrott A , et al. , Lower proportion of CD45R0+ cells and deficient interleukin-10 production by formula-fed infants, compared with human-fed, is corrected with supplementation of long-chain polyunsaturated fatty acids. Journal of pediatric gastroenterology and nutrition 2000, 31, 291–299. [DOI] [PubMed] [Google Scholar]
- [201].Kelley DS, Modulation of human immune and inflammatory responses by dietary fatty acids. Nutrition 2001, 17, 669–673. [DOI] [PubMed] [Google Scholar]
- [202].Zeyda M, Staffler G, Hořejší V, Waldhäusl W, Stulnig TM, LAT displacement from lipid rafts as a molecular mechanism for the inhibition of T cell signaling by polyunsaturated fatty acids. Journal of Biological Chemistry 2002, 277, 28418–28423. [DOI] [PubMed] [Google Scholar]
- [203].Stulnig TM, Berger M, Sigmund T, Raederstorff D , et al. , Polyunsaturated fatty acids inhibit T cell signal transduction by modification of detergent-insoluble membrane domains. The Journal of cell biology 1998, 143, 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Stulnig TM, Huber J, Leitinger N, Imre E-M , et al. , Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. Journal of Biological Chemistry 2001, 276, 37335–37340. [DOI] [PubMed] [Google Scholar]
- [205].Stulnig TM, Zeyda M, Immunomodulation by polyunsaturated fatty acids: impact on T-cell signaling. Lipids 2004, 39, 1171–1175. [DOI] [PubMed] [Google Scholar]
- [206].Zeyda M, Stulnig TM, Lipid Rafts & Co.: an integrated model of membrane organization in T cell activation. Progress in lipid research 2006, 45, 187–202. [DOI] [PubMed] [Google Scholar]
- [207].Zeyda M, Szekeres AB, Säemann MD, Geyeregger R , et al. , Suppression of T cell signaling by polyunsaturated fatty acids: selectivity in inhibition of mitogen-activated protein kinase and nuclear factor activation. The Journal of Immunology 2003, 170, 6033–6039. [DOI] [PubMed] [Google Scholar]
- [208].Gurzell EA, Teague H, Harris M, Clinthorne J , et al. , DHA-enriched fish oil targets B cell lipid microdomains and enhances ex vivo and in vivo B cell function. Journal of leukocyte biology 2013, 93, 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Jaudszus A, Gruen M, Watzl B, Ness C , et al. , Evaluation of suppressive and pro-resolving effects of EPA and DHA in human primary monocytes and T-helper cells. Journal of lipid research 2013, 54, 923–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Brownawell AM, Caers W, Gibson GR, Kendall CW , et al. , Prebiotics and the health benefits of fiber: current regulatory status, future research, and goals. The Journal of nutrition 2012, 142, 962–974. [DOI] [PubMed] [Google Scholar]
- [211].Fava F, Gitau R, Griffin B, Gibson G , et al. , The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’population. International Journal of Obesity 2013, 37, 216–223. [DOI] [PubMed] [Google Scholar]
- [212].Herder C, Peltonen M, Koenig W, Sütfels K , et al. , Anti-inflammatory effect of lifestyle changes in the Finnish Diabetes Prevention Study. Diabetologia 2009, 52, 433–442. [DOI] [PubMed] [Google Scholar]
- [213].Maslowski KM, Vieira AT, Ng A, Kranich J , et al. , Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Bailón E, Cueto-Sola M, Utrilla P, Rodríguez-Cabezas ME , et al. , Butyrate in vitro immune-modulatory effects might be mediated through a proliferation-related induction of apoptosis. Immunobiology 2010, 215, 863–873. [DOI] [PubMed] [Google Scholar]
- [215].Field CJ, McBurney MI, Massimino S, Hayek MG, Sunvold GD, The fermentable fiber content of the diet alters the function and composition of canine gut associated lymphoid tissue. Veterinary Immunology and Immunopathology 1999, 72, 325–341. [DOI] [PubMed] [Google Scholar]
- [216].Kunzmann AT, Coleman HG, Huang W-Y, Kitahara CM , et al. , Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. The American journal of clinical nutrition 2015, 102, 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Fu Z, Shrubsole MJ, Smalley WE, Ness RM, Zheng W, Associations between dietary fiber and colorectal polyp risk differ by polyp type and smoking status. The Journal of nutrition 2014, 144, 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Ben Q, Sun Y, Chai R, Qian A , et al. , Dietary fiber intake reduces risk for colorectal adenoma: a meta-analysis. Gastroenterology 2014, 146, 689–699. e686. [DOI] [PubMed] [Google Scholar]
- [219].Donohoe DR, Holley D, Collins LB, Montgomery SA , et al. , A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota-and butyrate-dependent manner. Cancer discovery 2014, 4, 1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Sánchez-Tena S, Lizárraga D, Miranda A, Vinardell MP , et al. , Grape antioxidant dietary fiber inhibits intestinal polyposis in ApcMin/+ mice: relation to cell cycle and immune response. Carcinogenesis 2013, 34, 1881–1888. [DOI] [PubMed] [Google Scholar]
- [221].Reddy BS, Mori H, Effect of dietary wheat bran and dehydrated citrus fiber on 3, 2′-dimethyl-4-aminobiphenyl-induced intestinal carcinogenesis in F344 rats. Carcinogenesis 1981, 2, 21–25. [DOI] [PubMed] [Google Scholar]
- [222].Reddy BS, Mori H, Nicolais M, Effect of dietary wheat bran and dehydrated citrus fiber on azoxymethane-induced intestinal carcinogenesis in Fischer 344 rats. Journal of the National Cancer Institute 1981, 66, 553–557. [PubMed] [Google Scholar]
- [223].Wang C-H, Qiao C, Wang R-C, Zhou W-P, Dietary fiber intake and pancreatic cancer risk: a meta-analysis of epidemiologic studies. Scientific reports 2015, 5, 10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Jansen RJ, Robinson DP, Stolzenberg-Solomon RZ, Bamlet WR , et al. , Fruit and vegetable consumption is inversely associated with having pancreatic cancer. Cancer Causes & Control 2011, 22, 1613–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Bidoli E, Pelucchi C, Zucchetto A, Negri E , et al. , Fiber intake and pancreatic cancer risk: a case–control study. Annals of oncology 2012, 23, 264–268. [DOI] [PubMed] [Google Scholar]


