Abstract
Over the past few decades, clinical and preclinical studies have clearly demonstrated the role of mucins in tumor development. It is well established that mucins form a barrier impeding drug access to target sites, leading to cancer chemoresistance. Recently gained knowledge regarding core enzyme synthesis has opened avenues to explore the possibility of disrupting mucin synthesis to improve drug efficacy. Cancer cells exploit aberrant mucin synthesis to efficiently mask the epithelial cells and ensure survival under hostile tumor microenvironment conditions. However, O-glycan synthesis enzyme core 2 beta 1,6 N-acetylglucosaminyltransferase (GCNT3/C2GnT-2) is overexpressed in Kras-driven mouse and human cancer, and inhibition of GCNT3 has been shown to disrupt mucin synthesis. This previously unrecognized developmental pathway might be responsible for aberrant mucin biosynthesis and chemoresistance. In this molecular pathways article, we briefly discuss the potential role of mucin synthesis in cancers, ways to improve drug delivery and disrupt mucin mesh to overcome chemoresistance by targeting mucin synthesis, and the unique opportunity to target the GCNT3 pathway for the prevention and treatment of cancers.
Keywords: molecular pathways, mucins, drug delivery, cancer, GCNT3, talniflumate, prevention, treatment
Background
Mucins
Mucin biology, synthesis, and functions are complex. Mucins form a mucous gel barrier and protect the epithelia of most organs from physical and chemical damage and infection (1). In 1835, Nicolas Theodore de Saussure first used the term “mucin” to describe these substances (2). Two years later, Eichwald identified that mucin is a combination of carbohydrates and proteins (3). Mucins are comprised of amino- and carboxy-terminal protein regions with a large central region formed by amino acid tandem repeats rich in proline, threonine, and serine residues with O-linked oligosaccharides or N-linked oligosaccharides (1, Fig 1A). Most mucin core glycans are O-linked oligosaccharides composed of N-acetyl galactosamine (GalNAc), N-acetyl glucosamine (GlcNAc), galactose (Gal), fucose (Fuc), and neuraminic acid (sialic acid, NeuNAc) linked to serine or threonine.
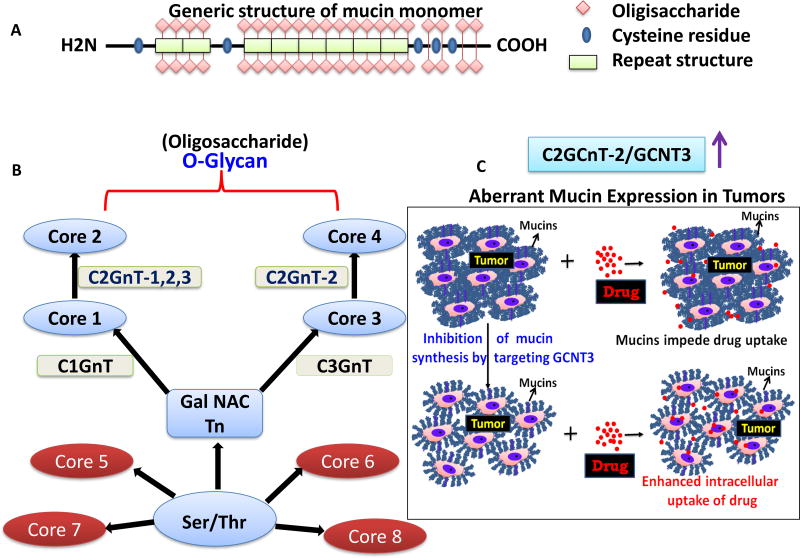
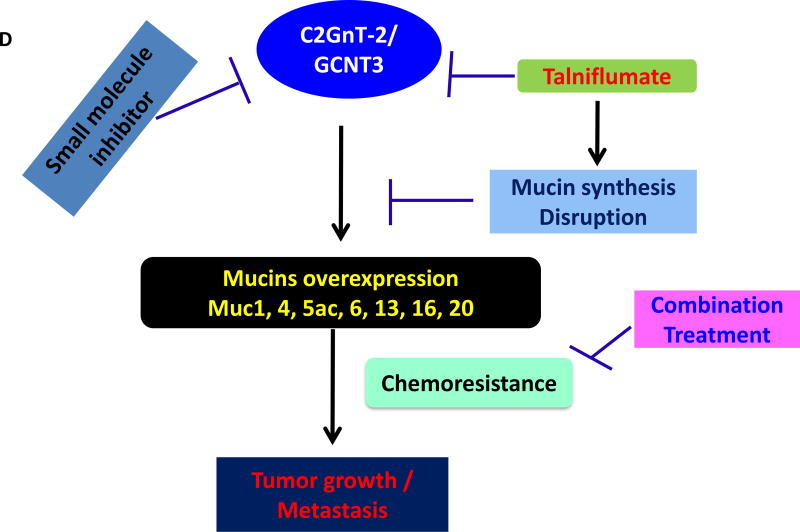
Figure 1.
A. General structure of mucin, B. mucin glycan synthesis, C. aberrant expression of GCNT3 leading to mucin mesh and drug resistance in tumors. Inhibition of mucin synthesis by targeting GCNT3 enhances drug uptake, D. schematic representation showing the GCNT3 pathway inhibition by small molecules and combination treatments to overcome chemoresistance caused by mucins.
There are eight O-GalNAc glycan core structures, designated cores 1– 8: core T antigen, Galβ1–3GalNAcαSer/Thr (core 1), GlcNAcβ1–6(Galβ1–3)GalNAcαSer/Thr (core 2), GlcNAcβ1–3GalNAcαSer/Thr (core 3), (GlcNAcβ1–6(GlcNAcβ1–3)GalNAcαSer/Thr (core 4), GalNAcα1–3GalNAcαSer/Thr (core 5), GlcNAcβ1–6GalNAcαSer/Thr (core 6), GalNAcα1–6GalNAcαSer/Thr (core 7), and Galα1–3GalNAcαSer/Thr (core 8; Fig. 1B). Starting from GalNAc on serine or threonine residues in a polypeptide, Core 1 synthase (C1GnT) transfers galactose to make the Core 1 structure, and Core 3 synthase (C3GnT) transfers GlcNAc to form a Core 3 structure. Core 1 is converted to Core 2 by C2GnT-1, C2GnT-2, and C2GnT-3, whereas Core 3 is converted to Core 4 by C2GnT-2. Mannose, fucose, glucose, and GlcNAc are directly linked to Ser/Thr; they form core 5–8 structures. Many sugars modified by acetylation or sulfation on the mucin O-glycans are antigenic. Mucin O-glycans are involved in biological processes, including embryonic development, immune responses, protein folding, cell signaling, and malignancies. These compounds are often heterogeneous.
Over 20 mucins have been identified; they are classified as either membrane-bound mucins or secreted mucins (1,4). MUC2, MUC5AC, MUC5B, MUC6, MUC7, MUC9, and MUC19 are secreted mucins. Their primary function is to participate in mucus formation to protect the underlying epithelia against injuries related to inflammation and infection. Membrane-bound mucins include MUC1, MUC3A/B, MUC4, MUC12, MUC13, MUC15, MUC16, MUC17, MUC20, and MUC21. They are thought to play important roles in cellular interactions, molecular cell signaling, and biological processes. Recent studies of secretory and transmembrane mucins focused on their role in malignancies. Glycosylation in mucins may restrict their cellular and tissue-level expression. However, information regarding which cores are attached to mucins of known MUC backbones is limited. O-glycans purified from normal and diseased tissues have different core types. Whether the complexity of O-glycan structures reflects altered levels of specific mucins or alterations in glycosylation of specific MUC proteins in disease or inflammation is unknown. Multiple core type O-glycans may be attached to a single MUC backbone, especially in mucins that have more than one TR-type domain.
Differential roles of mucins in cancer
Mucin deregulation is observed, and MUC1 is overexpressed, in pancreatic, lung, breast, colon, ovarian, and prostate cancers (5). MUC4 is overexpressed in colon adenocarcinoma and pancreatic cancer. MUC16 is elevated in ovarian and pancreatic cancers. Mucins have been identified as significant components of the glycocalyx in various tumors (6,7). Large glycoproteins are abundantly expressed in tumor cells, the microenvironment, and on circulating tumor cells from patients with advanced disease (7). Because of their specific pattern of expression during tumor progression, mucins remain under intense investigation as biomarkers and therapeutic targets. For example, full-length MUC13 expressed in MUC13-null pancreatic cancer cell lines significantly increases cell motility, invasion, proliferation, and clonogenicity. Exogenous MUC13 expression significantly enhanced pancreatic tumor growth and reduced survival in a xenograft mouse model. These characteristics were correlated with the upregulation/phosphorylation of HER2, p21-activated kinase 1 (PAK1), extracellular signal-regulated kinase (ERK), Akt and metastasin (S100A4), and p53 suppression (8). Similarly, esophageal cancer cells lacking MUC1 proliferated, migrated, and invaded less. Subcutaneous xenografts were significantly smaller when cells did not express MUC1 (9). Thus, mucin subtypes play a key role in cancer cell proliferation, migration, invasion, and tumor growth.
The modified forms of glycosylated tumor-associated mucins promote tumor cell invasion, migration, intravasation, and extravasation which help in immunosuppression or immune evasion (10–15). Galectin-3 binding to Major histocompatibility complex class I-related chain A carrying core2 O-glycans (MICAC2) through poly-N-acetyllactosamine impair NK cell activation by reducing IFN-g and granzyme B secreation (10–14). Studies are warranted to identify if these molecules are immunogenic under these situations. MUC16 and mesothelin were co-expressed in infiltrating components, promoting pancreatic cancer invasion (16). The presence of MUC-1-specific CD8 cytotoxic lymphocytes in breast and ovarian cancer and PDAC prompted the design of a MUC1-targeting vaccine (17,18). Preclinical studies with MUC1 transgenic animals demonstrated its efficacy in stimulating immune responses against this antigen (19).
The mucin cores are deregulated during tumor cell transformation, leading to aberrant expression. MUC1, MUC4, MUC5AC, and MUC16 are strongly up-regulated in patients with PDAC, pancreatic intraepithelial neoplasia (PanINs), and intrapapillary mucinous neoplasia (IPMNs; 20). However, some mucins play controversial roles. While most mucins are involved in tumor progression, MUC2 suppresses tumors. In colon cancer, MUC2 helps protect the barrier functions of normal colonic crypts. Loss of MUC2 leads to colonization of the intestinal tract with different microbial flora, which may have pro-carcinogenic or bystander effects (21). Similarly, MUC17 is highly expressed on the intestinal epithelial surface and helps in epithelial restitution and protection against E. Coli infection (22). Mucins vary depending on the glycan moieties present on the peptide backbone.
Targeting mucin synthesis
Clinical and preclinical studies elucidated the tumor-promoting roles of mucins in cancer. Based on these findings, several mucins were individually targeted. Little knowledge exists about potential intervention approaches involving mucin-glycan synthesizing genes. All mucins contain one or more of the core glycans. C1GNT is involved in forming core 1, and C3GNT forms core 3. C2GNT is involved in forming core 2 from core 1 and core 4 from core 3. The core 2 beta 1,6 N-acetylglucosaminyltransferase (GCNT3/ C2GNT) plays a significant role in mucin glycan biosynthesis. Aberrant GCNT3 expression leads to mucin overexpression (23–26). GCNT3 activity plays an important role in physiological processes, including inflammatory and immune responses. Core 3-derived glycans, a major type of O-glycan expressed by normal gastrointestinal epithelial cells, are downregulated during malignancy, due to loss of functional β3-N-acetylglucosaminyltransferase-6 (C3GnT, core 3 synthase) expression. Expression of core 3-derived O-glycans in pancreatic cancer cells suppressed tumor growth and metastasis through modulation of mucin glycosylation and other cell surface and extracellular matrix proteins (27).
CLINICAL-TRANSLATIONAL ADVANCES
For several decades, researchers focused on the protective role of mucins in epithelial cells and their role in cancer progression. Less attention was given to glycan synthesis, which comprises more than 50% of the mucin structure, and to enzymes or genes involved in core glycan synthesis. Recent studies have explored the aberrant expression of mucin-glycan synthesis genes, suggesting the possibility of evading chemoresistance caused by mucins and improving existing therapies, or developing novel glycan synthesis enzyme inhibitors or combined strategies to simultaneously disrupt mucin synthesis and inhibit tumor growth (Fig. 1C,D). In this section, we discuss how to improve drug delivery and disrupt mucin mesh to overcome chemoresistance by targeting mucin synthesis.
Drug Delivery and Disruption of Mucin Barrier
Targeting mucinous cancer cells with drugs is challenging. Although several drugs inhibit tumor cells in vitro, the effect is seldom replicated in vivo. There are several reasons for treatment failure. One such factor for chemoresistance and poor prognosis is the mucin mesh barrier (28–38). Aberrant Muc1 expression is associated with poor disease-free and overall survival in non-small-cell lung cancer (39–41). Although the involvement of mucins is unclear, evidence supports the involvement of a physical barrier, resistance to apoptosis, drug metabolism, cell stemness, and EMT as factors responsible for chemoresistance (42).
Most mucins carry an electronegative potential that may create electrostatic interactions with positively charged drugs, thereby decreasing their diffusion (42). Understanding the mechanisms of resistance involving mucins will contribute to the development of next-generation targeted therapy molecules. For example, in vitro and in vivo MUC1 knockdown reduced tumor cell growth, cell proliferation, MAPK, cell migration, and invasion via MMP13, and cell survival and apoptosis via Akt and Bcl2 (43). Researchers have suggested a correlation between mucin overexpression and cancer, and demonstrated a link between the aberrant and differential overexpression of mucin glycoproteins and disease initiation, progression, and poor prognosis (35–38, 44–46).
Previous research has demonstrated aberrant expression of membrane-bound and secreted mucins in ductal adenocarcinomas, pancreatic intraepithelial neoplasia, IPMNs, and mucinous cystic neoplasms (35–38, 44–46). The extent to which the dense mucin mesh influences the antiproliferative activity of 5-fluorouracil (5-FU) was investigated in human pancreatic cancer cells (5, 47). MUC1 vaccine in vivo and a small molecule inhibitor drug (GO-201) that inhibits MUC1-cytoplasmic tail oligomerization have shown promising results (48–54). Administration of GO-201 to nude mice bearing human breast tumor xenografts was associated with loss of tumorigenicity and extensive necrosis, resulting in prolonged tumor growth regression.
Most reports focus on mucin peptide gene expression or aspects of the glycosylated mucins, with little mention of the enzymes that catalyze mucin biosynthesis. Little knowledge exists regarding potential intervention approaches involving mucin-glycan-synthesizing genes. Core mucin genes were recently evaluated as targets to disrupt mucin mesh formation, leading to targeted drug delivery for pancreatic cancer. Using survival data from human patients with pancreatic cancer, next-generation sequencing of genetically engineered Kras-driven mouse pancreatic tumors, and human pancreatic cancer cells, a novel core mucin-synthesizing enzyme, GCNT3 (core 2 beta 1,6 N-acetylglucosaminyltransferase), was identified. In mouse pancreatic tumors, GCNT3 upregulation was correlated with increased expression of mucins. GCNT3 was significantly overexpressed in human pancreatic cancer and reduced patient survival by 7 months. High GCNT3 expression was also associated with patients’ drinking, smoking habits, and diabetes diagnosis (26). Aberrant GCNT3 expression was linked with increased mucin production, aggressive tumorigenesis, and reduced patient survival; CRISPR-mediated GCNT3 knockout in pancreatic cancer cells reduced proliferation and spheroid formation (26). A small molecule inhibitor that selectively binds to GCNT3, talniflumate, was identified via in silico small molecular docking simulation approaches (26). Talniflumate enhanced drug delivery and the antitumor effects of EGFR inhibitor (26). Docking predictions suggested that three hydrogen bonds between talniflumate and GCNT3 contribute to a docking affinity of −8.3 kcal/mol. Furthermore, talniflumate alone and in combination with low-dose gefitinib reduced GCNT3 expression, leading to disrupted mucin production in vivo and in vitro (26). Thus, targeting mucin biosynthesis through GCNT3 may improve drug responsiveness (Fig. 1C). Further development of agents and their mechanisms targeting mucin synthesis and permitting access of drugs to epithelial cells is needed.
Developmental state of the pathway
Many data suggest the role of mucins in the acquisition of chemoresistance. Thus, there is the possibility of interfering with the pathway responsible for mucin production as a treatment strategy. Current strategies, however, focus on individual mucins. One promising approach is targeting the key pathway leading to core glycan synthesis. However, there are no reports evaluating the mucin-glycan-synthesizing genes as targets. C2GNT-1, 2, and 3 are important core glycan-synthesizing enzymes. These core enzymes form basic structure of most mucins. C2GNT2/GCNT3 have been studied in vitro and in vivo and are reported as attractive novel targets for pancreatic cancer treatment (26). GCNT3 activity plays an important role in physiological processes, including inflammatory and immune responses. Furthermore, the expression of distinct oligosaccharide structures, together with differential glycosylation of mucin core proteins, confers on tumor cells an enormous range of potential ligands for interaction with other cell surface receptors. The process of natural selection within growing tumor cell populations creates cells that express novel combinations and forms of mucins, which contribute to the survival of these tumor cells during invasion and metastasis. Mucin core enzymes might promote biological properties, like inflammation and immune suppression, leading to enhanced tumor growth.
Drug development, strategies to overcome resistance, and opportunities for treatment
Since mucin glycan synthesis and signaling is involved in cancer cell immunosuppression and growth and metastasis, there is significant interest in developing therapies targeted against this glycan synthesis pathway. Applying novel technologies and utilizing crystal structures of core enzymes, specific inhibitors for GCNT3 or other core enzymes can be designed and evaluated. GCNT3’s crystal structure has been reported, enabling the development of small molecule inhibitor talniflumate. However, the development of direct small-molecule GCNT3 inhibitors is underway. Development of glycan core enzyme inhibitors will enable chemoprevention and pancreatic cancer treatment. GCNT3 core glycan enzyme synthesis increases in correlation with the stepwise progression of PanIN lesions to ductal adenocarcinoma. Hence, using GCNT3 inhibitors blocks mucin synthesis and might help in delaying PanIN progression (Fig 1D). During the adenocarcinoma stage, it appears possible to disrupt the mucin mesh with specific core enzyme inhibitors, thereby potentially preventing tumor progression into metastasis and helping with delivery of standard drugs to target sites (Fig. 1C,D). GCNT3 inhibitors may act independently to inhibit tumor growth or in combination with other standard drugs, like gemcitabine, to synergistically inhibit tumors. However, the mechanisms underlying GCNT3 inhibitor activity remain poorly defined. Genome-editing technologies, such as CRISPR/CAS9, should enable precise and efficient mutation of core enzymes to study mechanisms that are induced by the tumor microenvironment. In proof-of-concept experiments, studies using GCNT3 KO mice aim to demonstrate its role in mucins and tumor progression. Cutting-edge genetic methods for targeting GCNT3 signaling may enhance the efficacy of current pancreatic cancer therapies. GCNT3 overexpression was also seen in hepatocellular carcinoma cell lines and orthotopic xenograft tumors (55). However, in colon cancer like MUC2 subtype, GCNT3 expression is low (56). As per human protein atlas, most cancer tissues displayed weak to strong granular cytoplasmic GCNT3 staining. Strong staining was seen in pancreatic, stomach and ovarian cancers. Gliomas, lymphomas and skin cancers were negative (57).
Mucins have been investigated as therapeutic targets for pancreatic and other cancers. MUC1 vaccine was well tolerated, with no adverse effects. It was effective in animal models, alone and in combination with COX-2 inhibitors and gemcitabine (58). Several clinical trials (Clinical trials.gov, NCT00669734, NCT00008099, NCT00597129, NCT00603863) involving MUC1 vaccines for pancreatic cancer are underway or have been completed. A Phase I/II Trial in patients with relapsed or refractory acute myeloid leukemia is studying the targeted MUC1 inhibitor, GO-203-2C, alone and in combination with gemcitabine. GO-203-2c targets cancer cells, while leaving healthy cells unaffected. MUC2 vaccine, combined with QS21, was used to treat patients with prostate cancer. A preclinical study showed that a peptide vaccine of MUC1 inhibited tumor growth, and significantly regressed breast cancer tumors (48). One-third of patients with pancreatic and biliary cancer in a Phase I/II clinical trial of a MUC1 peptide-loaded DC vaccine administered post-resection survived, without evidence of recurrence (54). Many formulations of MUC1 vaccine were tested in patients with pancreatic cancer showing increased disease-free survival with mucin-specific humoral and T-cell responses (50, 51). A follow-up of a phase III study with patients with stage II breast cancer showed that those who were using oxidized mannan-MUC1 (M-FP) vaccine survived longer, without evidence of toxicity or autoimmunity (59). A phase III START study with tecemotide, a MUC1-antigen-specific cancer immunotherapy, reported a notable survival benefit for drug-treated patients with unresectable stage III non-small-cell lung cancer versus those treated with placebo (60). A 21-mer peptide MUC1 vaccine induced T and B cell MUC1-specific immunity in patients with multiple myeloma (61).
These findings suggest that mucins play a significant role in tumor growth and metastases, and blocking mucins with vaccines can control tumor growth and spread. Immunological Targeting core enzymes for decreased mucin production or disrupting mucin synthesis are attractive strategies.
Conclusions and Perspectives
The GCNT3 branch of core glycan synthesis is a novel and ill-characterized pathway with significant therapeutic relevance in human cancer. This molecular pathway controls unique biologic processes in mucin synthesis and tumor-infiltrating immune cells to promote tumor progression. Although mucin synthesis signaling can be targeted through various methods (Fig 1C, D), potent small-molecule inhibitors are an attractive strategy to disrupt mucin synthesis, permitting drug access to target sites, thereby overcoming chemoresistance.
Acknowledgments
Grant Support: This work was supported by the College of Medicine Alumni Association Grant and Stephenson Cancer Center Research Support Fund to A.M. and the Kerley-Cade Endowed Chair Fund and National Cancer Institute NCI-N01-CN53300 to C.V.R.
We thank Ms. Agata Bien and Ms. Kathy Kyler for editing this review.
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
References
- 1.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 2.Gottschalk A. In: The Chemistry and Biology of Sialic Acids and Related Substances. Gottschalk A, editor. Cambridge University Press; New York: 1960. pp. 1–11. [Google Scholar]
- 3.Montreuil J, Vliegenthart JFG, Schachter H. Glycoproteins and Disease. Vol. 30 Elsevier; Amsterdam: 1995. [Google Scholar]
- 4.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–85. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonckheere N, Skrypek N, Van Seuningen I. Mucins and tumor resistance to chemotherapeutic drugs. Biochim Biophys Acta. 2014;1846(1):142–51. doi: 10.1016/j.bbcan.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Kalra AV, Campbell RB. Mucin overexpression limits the effectiveness of 5-FU by reducing intracellular drug uptake and antineoplastic drug effects in pancreatic tumours. European Journal of Cancer. 2009;45:164–73. doi: 10.1016/j.ejca.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Paszek MJ, DuFort CC, Rossier O, Bainer R, Mouw JK, Godula K, et al. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature. 2014;511:319–25. doi: 10.1038/nature13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chauhan SC, Ebeling MC, Maher DM, Koch MD, Watanabe A, Aburatani H, et al. MUC13 mucin augments pancreatic tumorigenesis. Mol Cancer Ther. 2012;11(1):24–33. doi: 10.1158/1535-7163.MCT-11-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gronnier C, Bruyère E, Lahdaoui F, Jonckheere N, Perrais M, Leteurtre E, et al. The MUC1 mucin regulates the tumorigenic properties of human esophageal adenocarcinomatous cells. Biochim Biophys Acta. 2014;1843(11):2432–7. doi: 10.1016/j.bbamcr.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Komatsu M, Tatum L, Altman NH, Carothers Carraway CA, Carraway KL. Potentiation of metastasis by cell surface sialomucin complex (rat MUC4), a multifunctional anti-adhesive glycoprotein. Int J Cancer. 2000;87(4):480–6. doi: 10.1002/1097-0215(20000815)87:4<480::aid-ijc4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Komatsu M, Yee L, Carraway KL. Overexpression of sialomucin complex, a rat homologue of MUC4, inhibits tumor killing by lymphokine-activated killer cells. Cancer Res. 1999;59(9):2229–36. [PubMed] [Google Scholar]
- 12.Tinder TL, Subramani DB, Basu GD, Bradley JM, Schettini J, Million A, et al. MUC1 enhances tumor progression and contributes toward immunosuppression in a mouse model of spontaneous pancreatic adenocarcinoma. J Immunol. 2008;181(5):3116–25. doi: 10.4049/jimmunol.181.5.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuboi S, Sutoh M, Hatakeyama S, Hiraoka N, Habuchi T, Horikawa Y, et al. A novel strategy for evasion of NK cell immunity by tumours expressing core2 O-glycans. EMBO J. 30(15):3173–85. doi: 10.1038/emboj.2011.215. 20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senapati S, Chaturvedi P, Chaney WG, Chakraborty S, Gnanapragassam VS, Sasson AR, et al. Novel INTeraction of MUC4 and galectin: potential pathobiological implications for metastasis in lethal pancreatic cancer. Clin Cancer Res. 2011;17(2):267–74. doi: 10.1158/1078-0432.CCR-10-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swanson BJ, McDermott KM, Singh PK, Eggers JP, Crocker PR, Hollingsworth MA. MUC1 is a counter-receptor for myelin-associated glycoprotein (Siglec-4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Cancer Res. 2007;67(21):10222–9. doi: 10.1158/0008-5472.CAN-06-2483. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu A, Hirono S, Tani M, Kawai M, Okada K, Miyazawa M, et al. Coexpression of MUC16 and mesothelin is related to the invasion process in pancreatic ductal adenocarcinoma. Cancer Sci. 2012;103(4):739–46. doi: 10.1111/j.1349-7006.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karanikas V, Hwang LA, Pearson J, Ong CS, Apostolopoulos V, Vaughan H, et al. Antibody and T cell responses of patients with adenocarcinoma immunized with mannan-MUC1 fusion protein. J Clin Invest. 1997;100:2783–92. doi: 10.1172/JCI119825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ioannides CG, Fisk B, Jerome KR, Irimura T, Wharton JT, Finn OJ. Cytotoxic T cells from ovarian malignant tumors can recognize polymorphic epithelial mucin core peptides. J Immunol. 1993;151:3693–703. [PubMed] [Google Scholar]
- 19.Soares MM, Mehta V, Finn OJ. Three different vaccines based on the 140-amino acid MUC1 peptide with seven tandemly repeated tumor-specific epitopes elicit distinct immune effector mechanisms in wild-type versus MUC1-transgenic mice with different potential for tumor rejection. J Immunol. 2001;166(11):6555–63. doi: 10.4049/jimmunol.166.11.6555. [DOI] [PubMed] [Google Scholar]
- 20.Nissim S, Idos GE, Wu B. Genetic markers of malignant transformation in intraductal papillary mucinous neoplasm of the pancreas: a meta-analysis. Pancreas. 2012;41:1195–1205. doi: 10.1097/MPA.0b013e3182580fb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–29. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 22.Resta-Lenert S, Das S, Batra SK, Ho SB. Muc17 protects intestinal epithelial cells from enteroinvasive E. coli infection by promoting epithelial barrier integrity. Am J Physiol Gastrointest Liver Physiol. 2011;300:1144–55. doi: 10.1152/ajpgi.00138.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yonezawa S, Nakamura A, Horinouchi M, Sato E. The expression of several types of mucin is related to the biological behavior of pancreatic neoplasms. J Hepatobiliary Pancreat Surg. 2002;9:328–41. doi: 10.1007/s005340200037. [DOI] [PubMed] [Google Scholar]
- 24.Kim GE, Bae HI, Park HU, Kuan SF, Crawley SC, Ho JJ, et al. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology. 2002;123:1052–60. doi: 10.1053/gast.2002.36018. [DOI] [PubMed] [Google Scholar]
- 25.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core Signaling Pathways in Human Pancreatic Cancers Revealed by Global Genomic Analyses. Science. 2008;321:1801–06. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao CV, Janakiram NB, Madka V, Kumar G, Scott EJ, Pathuri G, et al. Small-Molecule Inhibition of GCNT3 Disrupts Mucin Biosynthesis and Malignant Cellular Behaviors in Pancreatic Cancer. Cancer Res. 2016;76:1965–74. doi: 10.1158/0008-5472.CAN-15-2820. [DOI] [PubMed] [Google Scholar]
- 27.Radhakrishnan P, Grandgenett PM, Mohr AM, Bunt SK, Yu F, Chowdhury S, Hollingsworth MA. Expression of core 3 synthase in human pancreatic cancer cells suppresses tumor growth and metastasis. Int J Cancer. 2013;133:2824–33. doi: 10.1002/ijc.28322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonckheere N, Skrypek N, Seuningen IV. Mucins and Pancreatic Cancer. Cancers. 2010;2:1794–1812. doi: 10.3390/cancers2041794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skrypek N, Duchêne B, Hebbar M, Leteurtre E, Seuningen IV, Jonckheere N. The MUC4 mucin mediates gemcitabine resistance of human pancreatic cancer cells via the Concentrative Nucleoside Transporter family. Oncogene. 2013;32:1714–23. doi: 10.1038/onc.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonckheere N, Skrypek N, Seuningen IV. Mucins and tumor resistance to chemotherapeutic drugs. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2014;1846:142–51. doi: 10.1016/j.bbcan.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Mimeault M, Johansson SL, Senapati S, Momi N, Chakraborty S, Batra SK. MUC4 down-regulation reverses chemoresistance of pancreatic cancer stem/progenitor cells and their progenies. Cancer Lett. 2010;295:69–84. doi: 10.1016/j.canlet.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wissniowski TT, Meister S, Hahn EG, Kalden JR, Voll R, Ocker M. Mucin production determines sensitivity to bortezomib and gemcitabine in pancreatic cancer cells. Int J Oncol. 2012;40:1581–89. doi: 10.3892/ijo.2012.1337. [DOI] [PubMed] [Google Scholar]
- 33.Nath S, Daneshvar K, Roy LD, Grover P, Kidiyoor A, Mosley L, et al. MUC1 induces drug resistance in pancreatic cancer cells via upregulation of multidrug resistance genes. Oncogenesis. 2013;2:e51. doi: 10.1038/oncsis.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mekenkamp LJ, Heesterbeek KJ, Koopman M, Tol J, Teerenstra S, Venderbosch S, et al. Mucinous adenocarcinomas: poor prognosis in metastatic colorectal cancer. Eur J Cancer. 2012;48:501–09. doi: 10.1016/j.ejca.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Nagao T, Kinoshita T, Hojo T, Tsuda H, Tamura K, Fujiwara Y. The differences in the histological types of breast cancer and the response to neoadjuvant chemotherapy: the relationship between the outcome and the clinicopathological characteristics. Breast. 2012;21:289–95. doi: 10.1016/j.breast.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Oberholzer K, Menig M, Kreft A, Schneider A, Junginger T, Heintz A, et al. Rectal cancer: mucinous carcinoma on magnetic resonance imaging indicates poor response to neoadjuvant chemoradiation. Int J Radiat Oncol Biol Phys. 2012;82:842–48. doi: 10.1016/j.ijrobp.2010.08.057. [DOI] [PubMed] [Google Scholar]
- 37.Poujade O, Morice P, Rouzier R, Madelenat P, Lecuru F, Muray JM, et al. Pathologic response rate after concomitant neo-adjuvant radiotherapy and chemotherapy for adenocarcinoma of the uterine cervix: a retrospective multicentric study. Int J Gynecol Cancer. 2010;20:815–20. doi: 10.1111/IGC.0b013e3181df7406. [DOI] [PubMed] [Google Scholar]
- 38.Messager M, Lefevre JH, Pichot-Delahaye V, Souadka A, Piessen G, Mariette C. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative study. Ann Surg. 2011;254:684–93. doi: 10.1097/SLA.0b013e3182352647. [DOI] [PubMed] [Google Scholar]
- 39.Situ D, Wang J, Ma Y, Zhu Z, Hu Y, Long H, et al. Expression and prognostic relevance of MUC1 in stage IB non-small cell lung cancer. Med Oncol. 2010;28:596–604. doi: 10.1007/s12032-010-9752-4. [DOI] [PubMed] [Google Scholar]
- 40.Khodarev N, Pitroda S, Beckett M, MacDermed D, Huang L, Kufe D, et al. MUC1-induced transcriptional programs associated with tumorigenesis predict outcome in breast and lung cancer. Cancer Res. 2009;69:2833–7. doi: 10.1158/0008-5472.CAN-08-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacDermed DM, Khodarev NN, Pitroda SP, Edwards DC, Pelizzari CA, Huang L, et al. MUC1-associated proliferation signature predicts outcomes in lung adenocarcinoma patients. BMC Medical Genomics. 2010;3:16. doi: 10.1186/1755-8794-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khanvilkar K, Donovan MD, Flanagan DR. Drug transfer through mucus. Adv Drug Deliv Rev. 2001;48:173–93. doi: 10.1016/s0169-409x(01)00115-6. [DOI] [PubMed] [Google Scholar]
- 43.Tréhoux S, Duchêne B, Jonckheere N, Van Seuningen I. The MUC1 oncomucin regulates pancreatic cancer cell biological properties and chemoresistance. Implication of p42–44 MAPK, Akt, Bcl-2 and MMP13 pathways. Biochem Biophys Res Commun. 2015;456(3):757–62. doi: 10.1016/j.bbrc.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 44.Nagata K, Horinouchi M, Saitou M, Higashi M, Nomoto M, Goto M, et al. Mucin expression profile in pancreatic cancer and the precursor lesions. Journal of hepatobiliarypancreatic surgery. 2007;14:243–54. doi: 10.1007/s00534-006-1169-2. [DOI] [PubMed] [Google Scholar]
- 45.Moniaux N, Andrianifahanana M, Brand RE, Batra SK. Multiple roles of mucins in pancreatic cancer, a lethal and challenging malignancy. Br J Cancer. 2004;91:1633–8. doi: 10.1038/sj.bjc.6602163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torres MP, Chakraborty S, Souchek J, Batra SK. Mucin-based targeted pancreatic cancer therapy. Curr Pharm Des. 2012;18:2472–81. doi: 10.2174/13816128112092472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalr AV, Campbell RB. Mucin impedes cytotoxic effect of 5-FU against growth of human pancreatic cancer cells: overcoming cellular barriers for therapeutic gain. Br J Cancer. 2007;8:910–18. doi: 10.1038/sj.bjc.6603972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bitler BG, Menzl I, Huerta CL, Sands B, Knowlton W, Chang A, Schroeder JA. Intracellular MUC1 peptides inhibit cancer progression. Clin Cancer Res. 2009;15:100–09. doi: 10.1158/1078-0432.CCR-08-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The Prioritization of Cancer Antigens: A National Cancer Institute Pilot Project for the Acceleration of Translational Research. Clin Cancer Res. 2009;15:5323–37. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramanathan RK, Lee KM, McKolanis J, Hitbold E, Schraut W, Moser AJ, et al. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunotherapy. 2005;54:254–64. doi: 10.1007/s00262-004-0581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamamoto K, Ueno T, Kawaoka T, Hazama S, Fukui M, Suehiro Y, et al. MUC1 Peptide Vaccination in Patients with Advanced Pancreas or Biliary Tract Cancer. Anticancer Res. 2005;25:3575–80. [PubMed] [Google Scholar]
- 52.Soares MM, Mehta V, Finn OJ. Three Different Vaccines Based on the 140-Amino Acid MUC1 Peptide with Seven Tandemly Repeated Tumor-Specific Epitopes Elicit Distinct Immune Effector Mechanisms in Wild-Type Versus MUC1-Transgenic Mice with Different Potential for Tumor Rejection. Journal of Immunology. 2001;166:6555–63. doi: 10.4049/jimmunol.166.11.6555. [DOI] [PubMed] [Google Scholar]
- 53.Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ. Tolerance and Immunity to MUC1 in a Human MUC1 Transgenic Murine Model. Cancer Research. 1998;58:315–21. [PubMed] [Google Scholar]
- 54.Lepisto AJ, Moser AJ, Zeh H, Lee K, Bartlett D, McKolanis JR, et al. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Therapy. 2008;6:955–64. [PMC free article] [PubMed] [Google Scholar]
- 55.Liu T, Zhang S, Chen J, Jiang K, Zhang Q, Guo K, et al. The transcriptional profiling of glycogenes associated with hepatocellular carcinoma metastasis. PLoS One. 2014;9(9):e107941. doi: 10.1371/journal.pone.0107941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.González-Vallinas M, Vargas T, Moreno-Rubio J, Molina S, Herranz J, Cejas P, et al. Clinical relevance of the differential expression of the glycosyltransferase gene GCNT3 in colon cancer. Eur J Cancer. 2015;51(1):1–8. doi: 10.1016/j.ejca.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 57.http://www.proteinatlas.org/ENSG00000140297-GCNT3/cancer
- 58.Mukherjee P, Basu GD, Tinder TL, Subramani DB, Bradley JM, Arefayene M, et al. Progression of Pancreatic Adenocarcinoma Is Significantly Impeded with a Combination of Vaccine and COX-2 Inhibition. Journal of Immunology. 2009;182:216–24. [PMC free article] [PubMed] [Google Scholar]
- 59.Vassilaros S, Tsibanis A, Tsikkinis A, Pietersz GA, McKenzie IF, Apostolopoulos V. Up to 15-year clinical follow-up of a pilot Phase III immunotherapy study in stage II breast cancer patients using oxidized mannan-MUC1. Immunotherapy. 2013;5(11):1177–82. doi: 10.2217/imt.13.126. [DOI] [PubMed] [Google Scholar]
- 60.Carmon L, Avivi I, Kovjazin R, Zuckerman T, Dray L, Gatt ME, et al. Phase I/II study exploring ImMucin, a pan-major histocompatibility complex, anti-MUC1 signal peptide vaccine, in multiple myeloma patients. Br J Haematol. 2015;169(1):44–56. doi: 10.1111/bjh.13245. [DOI] [PubMed] [Google Scholar]
- 61.Mitchell P, Thatcher N, Socinski MA, Wasilewska-Tesluk E, Horwood K, Szczesna A, et al. Tecemotide in unresectable stage III non-small-cell lung cancer in the phase III START study: updated overall survival and biomarker analyses. Ann Oncol. 2015;26(6):1134–42. doi: 10.1093/annonc/mdv104. [DOI] [PubMed] [Google Scholar]