Abstract
Background
Hepatitis C virus (HCV) infection is the major cause of end-stage liver disease (LD) worldwide. The aim of this study was to assess sustained virological response (SVR) rates in a real-world cohort of patients with HCV infection treated with interferon-free direct antiviral agents (DAA).
Patients and methods
All patients with genotypes 1, 2 or 3 HCV infection who started interferon-free treatment at a university hospital from December 2015 through July 2017 were included. The primary outcome was SVR at post-treatment week 12 by intention-to-treat (ITT) and modified ITT (mITT) analysis.
Results
Five hundred twenty seven patients were enrolled, 51.6% with cirrhosis. Most patients received sofosbuvir + daclatasvir + ribavirin (60.7%) and sofosbuvir + simeprevir (25.6%). Overall SVR rates were 90.5% for ITT and 96% for mITT. SVR rates were higher in non-cirrhotic (94.2% in ITT and 96.8% in mITT) versus cirrhotic patients (87.1% in ITT and 95.2% in mITT). In ITT and mITT assessments, SVR rates were higher in patients with Child-Pugh A (n = 222, 88.7% and 95.7%, respectively) versus Child-Pugh B or C (n = 40, 80% and 90%, respectively); SVR rates were higher in patients with genotype 1 (n = 405, 92.1% and 98.2%), followed by genotype 2 (n = 13, 84.6% and 92.7%) and genotype 3 (n = 109, 84.4% and 88.4%). Lower comorbidity index (p = 0.0014) and absence of cirrhosis (p = 0.0071) were associated with SVR. Among cirrhotic patients, lower Model for End-Stage Liver Disease (p = 0.0258), higher albumin (p = 0.0015), and higher glomerular filtration rate (p = 0.0366) were related to SVR. Twenty-two cirrhotic patients (8%) had clinical liver decompensation during treatment. Complications of advanced LD were responsible for discontinuation of treatment and death in 12 and 7 patients, respectively.
Conclusion
Treatment with all-oral DAA achieved high SVR rates, particularly in patients without cirrhosis and few comorbidities. Advanced LD is associated to poor outcome, such as treatment failure and death.
Introduction
Hepatitis C virus (HCV) chronic infection affects 1.1% of the global population and is the leading cause of end-stage liver disease, hepatocellular carcinoma (HCC) and liver-related mortality in the Western world [1–3]. A sustained virologic response (SVR) after effective antiviral treatment is associated with decreased risk in liver disease progression and its complications, such as portal hypertension, hepatic decompensation, HCC, and liver transplantation [3–6]. Recently, treatment options for HCV infection and its efficacy have improved with the development of direct antiviral agents (DAA).
The polymerase inhibitor sofosbuvir (SOF), associated with the second-generation protease inhibitor (PI) simeprevir (SMV), or the NS5A inhibitor daclatasvir (DCV), with or without ribavarin (RBV), allowed interferon(IFN)-free effective regimens, with SVR rates above 90% in clinical trials [7–9]. However, those studies excluded or included few patients with advanced liver disease, so real-life studies comprising this population are needed. Furthermore, clinical trials also demonstrated variances in SVR rates between different genotypes, with lower SVR rates amongst genotype 3 cirrhotic patients [9–11]. Our study aimed to assess SVR rates and to identify underlying related factors in a large real-world cohort, including patients with advanced liver disease treated with IFN-free regimens.
Materials and methods
Patient enrolment
We included adult (> 18 years) patients with HCV chronic infection that started IFN-free DAA therapy at Clinic Hospital, State University of Campinas (UNICAMP), Brazil, from December 2015 through July 2017. HCV genotypes 1, 2, and 3 were included. Chronic HCV infection was defined as the presence of HCV antibody (Abott AxSYM Anti-HCV 3.0; Abbott Laboratories, Wiesbaden, Germany) and detectable serum HCV RNA (Cobas Ampli Prep Taq Man; Roche Diagnostics Systems Inc., Almere, The Netherlands). Treatment-naive patients and those who previously failed to PEG-IFN and RBV or to PEG-IFN and RBV plus first generation PI were included. We excluded patients with HIV infection, post-liver transplant, and those who previously received SOF, DCV or SMV.
Stage of hepatic fibrosis evaluation
Stage of hepatic fibrosis was defined according to Metavir scoring system, transient hepatic elastography (Fibroscan®, Echosense, Paris, France) or upon the combination of clinical and laboratorial parameters [12]. For analysis purposes, the diagnosis of none or minimal fibrosis was made upon histological examination (F0 or F1 stage) or liver stiffness (LS) under 7.1 kPa; portal fibrosis was defined as Metavir F2 or LS between 7.1 and 9.5 kPa: bridging fibrosis comprised histological stage F3 or LS between 9.5 and 12.5 kPa. The diagnosis of cirrhosis was made upon histological examination (F4 stage) or LS 12.5 kPa and / or the presence of esophageal varices, ascites, and splenomegaly [12–14].
Treatment management and data collection
A questionnaire that included demographics, clinical characteristics and data about HCV infection was completed for each patient after medical appointment. The severity of medical conditions was estimated using Carlson’s comorbidity index (CCI) [15]. The estimation of glomerular filtration rate (eGFR) was performed using Modified Diet For Renal Disease [16]. Chronic kidney disease was classified according to the Kidney Disease Outcomes Quality Initiative criteria [17]. Clinical evaluation and laboratory tests were performed at baseline and every 4 weeks during treatment or more frequently, if needed. Serum biochemical and haematological analysis included haemoglobin (Hb), platelets, bilirubin, albumin, creatinine, aminotransferases, alaninotransferases, amylase, lipase, and prothrombin time. HCVRNA was performed at baseline, at treatment week 4, at the end of treatment (EOT) and post-treatment week 12 (PT12). Unquantifiable HCVRNA was defined as less than the lower limit of quantification. Among cirrhotic patients, Child-Pugh and Model for End-Stage Liver Disease (MELD) were calculated at baseline and at the EOT [18,19].
Safety was assessed by spontaneous adverse events (AE) reporting, by clinical evaluation and by laboratory data. Serious AE was defined as any AE that led to treatment discontinuation, decompensation of liver disease or grade 3 or 4 laboratory abnormalities. Mild anemia was defined as Hb 10.1–11.9 g/dL for women and Hb 10.1–12.9 g/dL for men; moderate and severe anemia was defined as Hb 8.6–10.0 g/dL and Hb ≤ 8.5 g/dL, respectively. Early therapy discontinuation was based on the decision of the physicians attending each patient. If treatment was interrupted by patients’ decision it was considered poor tolerability other than AE-related.
Treatment dose and duration
Treatment was proposed to patients following standard practices and national guidelines at the outpatient clinic, without influence from the study team [20,21]. Genotype 1 patients with Child-Pugh B or C cirrhosis or prior non-responders to first generation PI-based treatment received SOF (400mg daily) plus DCV (60mg daily) with or without RBV for 24 weeks; the rest of genotype 1 patients received SOF plus DCV or SMV (150mg daily) with or without RBV for 12 weeks. Genotype 2 patients were treated with SOF plus RBV for 12 weeks. Genotype 3 patients received SOF plus DCV with or without RBV for 12 weeks. Ribavirin was adjusted by weight (1000mg/day for patients <75 kg and 1250mg/day for patients ≥ 75kg) and by glomerular filtration rate (eGFR). Changes in RBV dosages were documented, and DAA dosage did not change during treatment.
Analysis population and endpoints
The treated population comprised all the patients that received at least 1 day of the purposed treatment. The primary endpoint was SVR, defined as unquantifiable HCVRNA at PT12. The primary analytic approach was an intention-to-treat (ITT) assessment. The secondary analytic approach was a modified intention-to-treat (mITT) assessment that excluded patients with missing virologic PT12 data due to loss to follow-up or death. Secondary endpoints comprised identification of factors associated with achievement of SVR and safety assessment.
Virologic failure was defined as absence of SVR due to no response (lack of achievement of unquantifiable HCVRNA during treatment), virologic breakthrough (quantifiable HCVRNA at EOT after an unquantifiable HCVRNA during treatment), or relapse (unquantifiable HCVRNA at EOT but quantifiable at PT12). In ITT assessment, non-virologic treatment failure included missing HCVRNA due to loss to follow-up or death on-or-after-treatment.
Statistical analysis
We performed statistical analysis using Epi Info™, version 7.1.2.0 (Center for disease Control and Prevention, Atlanta, Georgia, USA) and GraphPad®(GraphPad Software, La Jolla, California, USA). Baseline continuous data were reported as median, and categorical values as frequencies and percentages. Univariate analyses were performed using 2- tailed Fisher’s, and analysis of variation or Mann-Whitney, as appropriate. A p<0.05 was considered statistically significant. Variables with p<0.2 were selected for a backward logistic regression model.
Ethical considerations
Study design, protocols, patient enrolment, and data collection and storage were in accordance with ethical considerations supported by the Helsinki Declaration [22]. The study was reviewed and approved by the Ethics Committee for Research of the School of Medical Sciences, UNICAMP.
Results
Patients
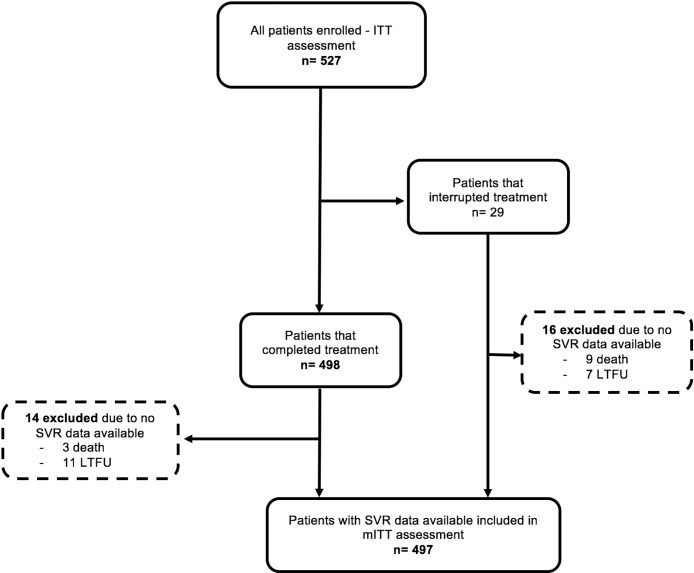
We included 527 patients treated with interferon-free DAA regimens, and 487 were included for mITT efficacy (Fig 1). Table 1 shows patients’ characteristics. Among all patients, median age was 56 years, most were male (59.8%), non-black (93.4%), and HCV-treatment-experienced (60.9%). Thirty-six patients (6.8%) had moderate chronic kidney impairment at baseline, and four patients were on haemodialysis. Cirrhosis was present in 51.6% of patients, most of them (81.6%) with compensated liver disease. Genotype 1 infection was the most prevalent (76.8%), followed by genotypes 3 (20.7%) and 2 (2.5%).
Fig 1. Derivation of the analysis population.
ITT = intention-to-treat; LTFU = loss to follow-up; SVR = sustained virological response; mITT = modified intention-to-treat.
Table 1. Baseline characteristics of patients treated with all-oral direct antiviral agents, Campinas, Brazil (n = 526).
|
Parameter, n (%) unless otherwise indicated |
All treated 527 (100%) |
Genotype 1 405 (76.9%) |
Genotype 3 109 (20.7) |
||||
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age, year | 56 (25–83) | 55 (25–81) | 63 (40–72) | 57 (36–83) | |||
| Male | 315 (59.8) | 252 (62.2) | 6 (46.2) | 57 (52.3) | |||
| Race | |||||||
| Non-black | 492 (93.4) | 376 (92.8) | 10 (77) | 106 (97.2) | |||
| Black | 35 (6.6) | 29 (7.2) | 3 (23) | 3 (2.8) | |||
| Medical History | |||||||
| Charlsson´s comorbidity index | 5 (1–12) | 5 (1–12) | 4 (1–8) | 5 (1–12) | |||
| Stage of liver fibrosis† | |||||||
| None or minimal fibrosis | 65 (12.3) | 51 (12.6) | 5 (38.5) | 9 (8,.2) | |||
| Portal fibrosis | 108 (20.5) | 86 (21.2) | 2 (15.4) | 20 (18.3) | |||
| Bridging fibrosis | 81 (15.4) | 69 (17.0) | 1 (7.6) | 12 (11.1) | |||
| Cirrhosis | 272 (51.6) | 199 (49.1) | 5 (38.5) | 68 (62.4) | |||
| Child-Pugh A | 222 (81.6) | 159 (79.9) | 5 (100) | 58 (85.3) | |||
| Child-Pugh B | 37 (13.6) | 31 (15.6) | - | 6 (8.6) | |||
| Child-Pugh C | 13 (4.8) | 9 (4.5) | - | 4 (5.9) | |||
| MELD | 9 (6–22) | 9 (6–22) | 8 (7–10) | 10 (6–18) | |||
| HCV treatment-experienced | 321 (60.9) | 248 (61.2) | 8 (61.5) | 65 (59.6) | |||
| Baseline laboratory values | |||||||
| Albumin, g/dL | 4.1 (2.1–5.1) | 4.1 (2.1–5.1) | 4.31(3.8–4.6) | 4.1 (2.3–4.7) | |||
| Bilirrubin, g/dL | 0.81 (0.14–5.15) | 0.82 (0.14–5.15) | 0.62 (0.40–1.22) | 0.85 (0.2–3.64) | |||
| INR | 1.11 (0.84–2.62) | 1.10 (0.84–2.62) | 1.11 (0.99–1.34) | 1.14 (0.93–2.12) | |||
| eGFR | 90 (4–191) | 91 (4–191) | 83 (59–131) | 90 (31–163) | |||
| Hemoglobin, g/dL | 14.6 (8.0–18.9) | 14.7 (8.0–18.9) | 14.0 (12.4–17.2) | 14.4 (9.9–17.6) | |||
| Platelets, 109/L | 156 (33–375) | 156 (33–375) | 211 (62–279) | 155 (35–298) | |||
| HCV viral load, log UI/mL | 5.84 (2.97–7.31) | 5.85 (2.97–6.44) | 6.08 (4.18–6.90) | 5.77 (3.07–7.31) | |||
| Treatment regimens | |||||||
| SOF + DCV + RBV | 12 wk | 202 (38.3) | 113 (27.9) | 89 (81.7) | - | ||
| 24 wk | 118 (22.4) | 118 (29.2) | - | - | |||
| SOF + DCV | 12 wk | 35 (6.6) | 15 (3.7) | 20 (18.3) | - | ||
| 24 wk | 13 (2.5) | 13 (3.2) | - | - | |||
| SOF + SMV + RBV | 12 wk | 11 (2.1) | 11 (2.7) | - | - | ||
| SOF + SMV | 12 wk | 135 (25.6) | 135 (33.3) | - | - | ||
| SOF + RBV | 12 wk | 13 (2.5) | - | - | 13 (100) | ||
Data presented as median and range, unless otherwise noted.
†One patient did not have evaluation of liver fibrosis and treatment was indicated because of extra hepatic manifestation.
MELD, Model for End-Stage Liver Disease; HCV, hepatitis C virus; INR, prothrombin international normalize ratio; eGFR, estimated glomerular renal function; SOF, sofosbuvir; DCV, daclatasvir; SMV, simeprevir; RBV, ribavirin; wk, weeks
Mean duration of treatment was 12 weeks (range 1–24). Table 1 illustrates treatment regimens and durations for each HCV genotype. Majority of patients received a combination of SOF + DCV + RBV (60.7%) followed by SOF + SMV (25.6%), and SOF + DCV (9.1%).
Sustained virological response
SVR outcomes for ITT and mITT are shown in Table 2 for all patients, and broken down by genotype, cirrhotic status, and treatment regimens. Among all patients, SVR was 90.5% for ITT and 96% for mITT. SVR was higher in non cirrhotic patients (94.2% in ITT and 96.8% in mITT) compared to cirrhotic patients (87.1% in ITT and 95.2% in mITT). In both ITT and mITT assessments, SVR was higher in patients with cirrhosis Child-Pugh A (88.7% and 95.7%, respectively) than in patients with cirrhosis Child-Pugh Child B or C (80% and 90%, respectively).
Table 2. Sustained virologic response derived by genotype, cirrhosis status, HCV prior treatment, and treatment regimen, in intention-to-treat and modified intention-to-treat assessment (n = 527).
| Overall | Genotype 1 | Genotype 2 | Genotype 3 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ITT (n = 527) | mITT (n = 497) | ITT (n = 405) | mITT (n = 381) | ITT (n = 13) | mITT (n = 12) | ITT (n = 109) | mITT (n = 104) | ||||||||||
| SVR, n/N (%) | 477/527 (90.5) | 477/497 (96.0) | 374/405 (92.1) | 374/381 (98.2) | 11/13 (84.6) |
11/12 (92.7) |
92/109) (84.4) |
92/104 (88.4) |
|||||||||
| Patients´ characteristics | |||||||||||||||||
| No cirrhosis | 240/255 (94.2) | 240/248 (96.8) | 195/206 (94.6) | 195/200 (97.5) | 7/8 (87.5) | 7/7 (100) | 38/41 (92.1) |
38/41 (92.1) |
|||||||||
| Cirrhosis | 237/272 (87.1) | 237/249 (95.2) | 179/199 (89.9) | 179/181 (98.9) | 4/5 (80.0) | 4/5 (80.0) | 54/68 (79.4) | 54/63 (85.7) | |||||||||
| Child-Pugh A | 197/222 (88.7) | 197/206 (95.6) | 145/159 (91.2) | 145/147 (98.6) | 4/5 (80.0) | 4/5 (80.0) | 48/58 (82.8) | 48/54 (88.9) | |||||||||
| Child-Pugh B or C | 40/50 (80.0) | 40/43 (93.0) | 34/40 (85.0) | 34/34 (100) | - | - | 6/10 (60.0) | 6/9 (66.7) | |||||||||
| HCV—Treatment naive | 182/206 (88.4) | 182/191 (95.3) | 143/157 (91.1) | 143/146 (97.9) | 4/5 (80.0) | 4/4 (100) | 35/44 (79.6) | 35/41 (85.4) | |||||||||
| HCV—Treatment experienced | 295/321 (91.9) | 295/306 (96.4) | 230/248 (92.7) | 231/235 (98.3) | 7/8 (87.5) | 7/8 (87.5) | 57/65 (87.7) | 57/63 (90.5) | |||||||||
| Treatment regimen | |||||||||||||||||
| SOF + DCV + RBV | 12 wk | 176/202 (87.1) | 176/185 (95.1) | 101/113 (89.4) | 101/101 (100) | NA | 75/89 (84.3) | 75/84 (89.3) | |||||||||
| 24 wk | 109/118 (92.4) | 109/111 (98.2) | 109/118 (92.4) | 109/111 (98.2) | NA | NA | |||||||||||
| SOF + DCV | 12 wk | 32/35 (91.4) | 32/35 (91.4) | 15/15 (100) | 15/15 (100) | NA | 17/20 (85.0) | 17/20 (85.0) | |||||||||
| 24 wk | 12/13 (92.3) | 12/12 (100) | 12/13 (92.3) | 12/12 (100) | NA | NA | |||||||||||
| SOF + SMV + RBV | 12 wk | 10/11 (90.1) | 10/10 (100) | 10/11 (90.1) | 10/10 (100) | NA | NA | ||||||||||
| SOF + SMV | 12 wk | 129/137 (94.2) | 129/134 (96.3) | 129/137 (94.2) | 129/134 (96.3) | NA | NA | ||||||||||
| SOF + RBV | 12 wk | 11/13 (84.6) | 11/12 (92.7) | NA | 11/13 (84.6) | 11/12 (92.7) | NA | ||||||||||
Data presented as median and range, unless otherwise noted.
HCV, hepatitis C virus; SVR, sustained virological response; ITT, intention-to-treat; mITT, modified intention-to-treat; SOF, sofosbuvir; DCV, daclatasvir; RBV, ribavarin; wk, weeks; SMV; simeprevir; NA, not applicable
In both ITT and mITT assessments, SVR was higher in patients infected with genotype 1 (n = 405, 92.1% and 98.2%), followed by a smaller group of genotype 2 (n = 13, 84.6% and 92.7%) and slightly lower in genotype 3 (n = 109, 84.4% and 88.4%).
Concerning the assorted treatment regimens for genotype 1- infected patients, SVR rates in ITT assessment for those treated with SOF + DCV + RBV for 12 and 24 weeks, and with SOF + SMV were 87.1% (176/202), 92.4% (109/118), and 94.2% (129/137), respectively. For patients with genotype 3, SVR rates were 84.3% (75/89) for patients treated with SOF + DCV + RBV, and 85% (17/20) for those who received SOF + DCV.
Regarding baseline characteristics among all patients in ITT assessment, lower CCI (p = 0.0014) and absence of cirrhosis (p = 0.0071) were associated with achievement of SVR. A sub-analysis in cirrhotic patients demonstrated that lower MELD (p = 0.0258), higher albumin (p = 0.0015), and higher eGFR (p = 0.0366) were related with SVR (Table 3.) There was no particular variable associated with SVR among non-cirrhotic patients. Multivariate analysis did not demonstrate any variable independently associated with SVR.
Table 3. Baseline characteristics associated with sustained virological response.
| All patients | |||
| n (%) | SVR, 477 (90.5) | No SVR, 50 (9.5) | p- Value |
| Age, years‡ | 56 (25–81) | 57.5 (30–83) | 0.1200 |
| Sex, male (vs female) | 282 (59.1) | 33 (66.0) | 0.3674 |
| Race, non- black (vs black) | 446 (93.5) | 46 (92.0) | 0.7628 |
| Charlson Comorbidity Index‡ | 5 (1–11) | 5.5 (1–12) | 0.0014 |
| Prior HCV tx, yes (vs no) | 295 (61.8) | 26 (52.0) | 0.2225 |
| Cirrhosis, yes (vs no) | 237 (49.7) | 35 (70.0) | 0.0071 |
| Ribavirin use, yes (vs no) | 305 (63.9) | 38 (76.0) | 0.1180 |
| eGFR, mL/min/m3‡ | 91 (4–191) | 85.5 (33–173) | 0.1884 |
| Haemoglobin, g/dL‡ | 14.6 (8.0–18.9) | 14.0 (9.4–18.2) | 0.5469 |
| HCVRNA, log‡ | 5.84 (3.07–7.44) | 5.88 (2.97–7.15) | 0.6717 |
| Cirrhotic patients | |||
| n (%) | SVR, 237 (87.1) | No SVR, 35 (12.9) | p- Value |
| Age, years‡ | 57 (29–81) | 60 (30–83) | 0.1610 |
| Sex, male (vs female) | 147 (62.0) | 23 (65.7) | 0.7128 |
| Race, non- black (vs black) | 221 (93.3) | 32 (91.4) | 0.7203 |
| Charlson Comorbidity Index‡ | 5 (2–11) | 6 (4–12) | 0.2191 |
| Prior HCV tx, yes (vs no) | 156 (65.8) | 21 (60.0) | 0.5695 |
| Ribavirin use, yes (vs no) | 207 (87.3) | 31 (88.6) | 1.000 |
| MELD‡ | 9 (6–22) | 10.5 (6–22) | 0.0258 |
| Child-Pugh A (vs B or C) | 197 (83.1) | 25 (71.4) | 0.1044 |
| Albumin, g/dL‡ | 3.9 (2.1–5.0) | 3.6 (2.5–4.7) | 0.0015 |
| Billirubin, g/dL‡ | 0.98 (0.14–4.40) | 1.17 (0.20–5.15) | 0.1054 |
| INR‡ | 1.17 (0.89–2.12) | 1.20 (0.98–2.23) | 0.1885 |
| eGFR, mL/min/m3‡ | 93 (4–191) | 81.5 (33–173) | 0.0366 |
| Haemoglobin, g/dL‡ | 14.4 (8.0–18.9) | 13.7 (9.4–17.1) | 0.3886 |
| Platelets, 109/L‡ | 111 (33–360) | 122 (38–375) | 0.4763 |
| HCVRNA, log‡ | 5.80 (3.07–7.34) | 5.78 (2.87–6.92) | 0.7907 |
| Non-cirrhotic patients | |||
| n (%) | SVR, 240 (94.1) | No SVR, 15 (5.9) | p- Value |
| Age, years‡ | 54 (25–81) | 55 (36–79) | 0.9019 |
| Sex, male (vs female) | 135 (56.3) | 10 (66.7) | 0.5927 |
| Race, non- black (vs black) | 225 (93.8) | 14 (93.3) | 1.0000 |
| Charlson Comorbidity Index‡ | 4 (1–9) | 4 (1–8) | 0.3447 |
| Prior HCV tx, yes (vs no) | 139 (57.9) | 5 (33.3) | 0.1044 |
| Ribavirin use, yes (vs no) | 98 (40.8) | 7 (46.7) | 0.7880 |
| eGFR, mL/min/m3‡ | 88 (4–180) | 98.5 (59–116) | 0.5078 |
| Haemoglobin, g/dL‡ | 14.8 (9.3–18.5) | 15.0 (12.8–18.2) | 0.2507 |
| HCVRNA, log‡ | 5.88 (3.18–7.44) | 5.99 (4.23–7.15) | 0.6717 |
‡Data shown in median and range. Bold values means statistically significant (p<0.05). SVR, sustained virological response; vs, versus; HCV, hepatitis C virus; eGFR, estimated glomerular filtration rate; MELD, model for end stage liver disease; INR, prothrombin international normalize ratio; tx = treatment.
Treatment failure
Fifty patients on ITT assessment did not achieve SVR due to virologic (n = 18) or non-virologic (n = 32) failure. Among virologic failures, there were 1 null-responder 2 breakthroughs, and 15 relapses. Among non-virological failures, there were 2 patients that interrupted treatment before achieving a non-quantifiable HCVRNA; 12 patients died (4 during treatment and 8 during follow-up period); and 18 patients lost follow-up (6 during treatment and 12 after the EOT). Individual characteristics of the 50 patients with treatment failure are shown in Table 4. Among virologic failures, most patients (61.1%) were infected with genotype 3, 55.5% were HCV-previously treated, and half (n = 9) had cirrhosis. Concerning non-virologic failures, most patients had genotype 1 infection (75%), half (n = 16) were HCV-treatment experienced, and most were cirrhotic (59.3%).
Table 4. Baseline characteristics of patients with treatment failure.
| # | Age | Sex | GT | Treatment Regimen | Actual Duration of Tx (weeks) |
Prior HCV Tx (Y/N) | Cirrhosis (Y/N) |
Child-Pugh Class | MELD Score | End of Treatment | Type of Failure |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Virologic Failure | |||||||||||
| 1 | 55 | M | 1a | SOF + DCV + RBV 24 wk | 24 | Y | Y | A | 9 | Complete | Relapse |
| 2 | 69 | M | 1 | SOF + DCV + RBV 24 wk | 24 | Y | N | - | - | Complete | Breakthrough |
| 3 | 56 | F | 1b | SOF + SMV 12 wk | 12 | Y | N | - | - | Complete | Null-responder |
| 4 | 41 | M | 1b | SOF + SMV 12 wk | 12 | N | N | - | - | Complete | Relapse |
| 5 | 79 | F | 1a | SOF + SMV 12 wk | 12 | N | N | - | - | Complete | Relapse |
| 6 | 55 | M | 1b | SOF + SMV 12 wk | 4 | Y | N | - | - | Inter. AE | Relapse |
| 7 | 63 | M | 2 | SOF + RBV 12 wk | 12 | Y | Y | A | 8 | Complete | Relapse |
| 8 | 58 | F | 3 | SOF + DCV + RBV 12 wk | 12 | N | Y | A | 15 | Complete | Relapse |
| 9 | 61 | F | 3 | SOF + DCV + RBV 12 wk | 12 | Y | Y | C | 18 | Complete | Relapse |
| 10 | 46 | M | 3 | SOF + DCV + RBV 12 wk | 12 | N | Y | B | 15 | Complete | Relapse |
| 11 | 52 | M | 3 | SOF + DCV + RBV 12 wk | 12 | Y | Y | A | 12 | Complete | Relapse |
| 12 | 56 | M | 3 | SOF + DCV + RBV 12 wk | 12 | Y | Y | A | NA | Complete | Relapse |
| 13 | 57 | M | 3 | SOF + DCV + RBV 12 wk | 12 | Y | Y | 5 | 9 | Complete | Relapse |
| 14 | 61 | F | 3 | SOF + DCV 12 wk | 12 | N | Y | A | 9 | Complete | Relapse |
| 15 | 36 | F | 3 | SOF + DCV + RBV 12 wk | 12 | N | N | - | - | Complete | Breakthrough |
| 16 | 52 | M | 3 | SOF + DCV + RBV 12 wk | 12 | Y | N | C | 18 | Complete | Relapse |
| 17 | 52 | M | 3 | SOF + DCV + RBV 12 wk | 12 | N | N | - | - | Complete | Relapse |
| 18 | 61 | M | 3 | SOF + DCV 12 wk | 12 | N | N | - | - | Complete | Relapse |
| Non-Virologic Failure | |||||||||||
| 19 | 54 | M | 1a | SOF + DCV + RBV 24 wk | 10 | Y | Y | C | 19 | Inter. AE | Death |
| 20 | 76 | F | 1b | SOF + DCV + RBV 12 wk | 8 | Y | Y | A | 9 | Inter. Death | Death |
| 21 | 44 | M | 1b | SOF + DCV + RBV 12 wk | 12 | N | Y | A | 8 | Complete | LTFU |
| 22 | 76 | M | 1a | SOF + DCV + RBV 24 wk | Unknown | Y | Y | A | 9 | LTFU | LTFU |
| 23 | 45 | M | 1a | SOF + DCV + RBV 12 wk | 12 | N | Y | A | 10 | Complete | LTFU |
| 24 | 55 | M | 1a | SOF + DCV + RBV 24 wk | Unknown | N | Y | C | 22 | LTFU | LTFU |
| 25 | 60 | M | 1b | SOF + DCV + RBV 12 wk | 12 | N | Y | A | 8 | Complete | LTFU |
| 26 | 64 | F | 1 | SOF + DCV + RBV 24 wk | 18 | Y | Y | C | 20 | Inter. EA | Death |
| 27 | 76 | F | 1b | SOF + DCV + RBV 12 wk | 4 | Y | Y | A | 14 | Inter. EA | Death |
| 28 | 55 | M | 1a | SOF + DCV + RBV 12 wk | 12 | Y | Y | A | 8 | Complete | LTFU |
| 29 | 30 | M | 1a | SOF + DCV + RBV 24 wk | 11 | Y | Y | A | 12 | Inter. EA | Death |
| 30 | 47 | F | 1b | SOF + DCV + RBV 24 wk | 4 | Y | Y | C | 21 | Inter. EA | Death |
| 31 | 41 | F | 1b | SOF + DCV + RBV 12 wk | Unknown | Y | Y | A | 8 | LTFU | LTFU |
| 32 | 67 | M | 1b | SOF + DCV + RBV 24 wk | 24 | Y | Y | C | 13 | Complete | Death |
| 33 | 78 | F | 1b | SOF + DCV + RBV 12 wk | 12 | N | Y | A | 11 | Complete | Death |
| 34 | 65 | M | 1a | SOF + DCV + RBV 12 wk | 8 | Y | Y | 5 | 8 | Inter. AE | Death |
| 35 | 65 | F | 1b | SOF + DCV + RBV 12 wk | 12 | N | Y | A | 11 | Complete | LTFU |
| 36 | 45 | M | 1b | SOF + DCV + RBV 24 wk | 24 | N | Y | B | 11 | Complete | Death |
| 37 | 76 | F | 1b | SOF + SMV 12 wk | 4 | N | Y | A | 11 | Inter. Intolerance | Non responder |
| 38 | 55 | M | 1a | SOF + DCV + RBV 12 wk | Unknown | Y | N | – | - | LTFU | LTFU |
| 39 | 45 | M | 1a | SOF + SMV + RBV 12 wk | 12 | N | N | - | - | Complete | LTFU |
| 40 | 60 | M | 1b | SOF + DCV + RBV 12 wk | 12 | N | N | - | - | Complete | LTFU |
| 41 | 66 | F | 1b | SOF + SMV 12 wk | 12 | Y | N | - | - | Complete | LTFU |
| 42 | 40 | M | 1b | SOF + SMV 12 wk | Unknown | N | N | - | - | LTFU | LTFU |
| 43 | 37 | M | 1 | SOF + SMV 12 wk | Unknown | N | N | - | - | LTFU | LTFU |
| 44 | 68 | M | 2 | SOF + RBV 12 wk | 12 | N | N | - | - | Complete | LTFU |
| 45 | 73 | F | 3 | SOF + DCV + RBV 12 wk | 4 | Y | Y | A | 6 | Inter. Other | Non responder |
| 46 | 57 | M | 3 | SOF + DCV 12 wk | 2 | Y | Y | A | 13 | Inter. AE | Death |
| 47 | 75 | F | 3 | SOF + DCV + RBV 12 wk | 8 | N | Y | B | 10 | Inter. Other | Death |
| 48 | 65 | F | 3 | SOF + DCV + RBV 12 wk | 1 | N | Y | A | 6 | Inter. Other | LTFU |
| 49 | 61 | M | 3 | SOF + DCV + RBV 12 wk | 12 | N | Y | A | 7 | Complete | LTFU |
| 50 | 83 | F | 3 | SOF + DCV + RBV 12 wk | 12 | Y | Y | A | 8 | Complete | LTFU |
GT, genotype; tx, treatment; HCV, hepatitis C virus; Y, yes; N, no; MELD, model for end stage liver disease; SOF, sofosbuvir; DCV, daclatasvir; RBV, ribavirin; wk, weeks; SMV, simeprevir; NA, not available; Inter, interrupted; AE, adverse events; LTFU, loss to follow-up.
Safety
Forty-five (8.5%) patients experienced 1 or more serious AE. Mild anemia was seen in 33.9% (n = 179), moderate anemia in 5.5% (n = 29), and severe anemia in 1.7% (n = 9) of patients. Twenty-two cirrhotic patients (8%) had clinical liver decompensation during treatment.
Fifteen (2.8%) patients interrupted treatment due to AE: 12 due to liver decompensation, 2 due to sepsis and 1 due to severe anemia. Seven patients interrupted treatment because of non-AE causes: 4 because of intolerance; 1 due to dysphagia caused by ischemic stroke; 1 due to hepatocellular carcinoma -related liver transplant, and 1 for misunderstanding of correct medication dosage. There were 12 on-and-off-treatment deaths: 2 due to ischemic stroke (considered possibly related to treatment), 3 of sepsis, and 7 caused by complication of advanced liver disease (all with decompensated cirrhosis and 1 also with variceal bleeding). All death were classified as non-virologic treatment failure.
Discussion
Our cohort comprised patients infected with diverse HCV genotypes and a high proportion of cirrhotic patients, including decompensated cirrhosis. We demonstrated high SVR rates in ITT assessment (90.5%), and even better in mITT (96%). SVR rates were higher among patients infected with genotype 1 and without cirrhosis. Among virologic failures, most patients had genotype 3 HCV-infection (63.6%) and half of them were cirrhotic.
Considering genotype 1-infected patients, SVR rates in our study (92.1% in ITT and 98.2% in mITT) were high and similar to those found in phase II Cosmos (92%), phase III OPTMIST-1 (97%), and phase III AI44040 (98%) clinical trials, even considering that those studies did not include or had few cirrhotic patients [7–9]. Our study had superior efficacy endpoint among patients that received SMV-based treatments (94.2% without RBV and 90.1% with RBV) compared to the TARGET cohort (84.2%) [23]. Moreover, we found that cirrhotic genotype 1 patients had lower SVR rate (89.9%) compared to non-cirrhotic patients (94.6%), which was also demonstrated by the HEPATHER study (87% and 98%, respectively) [24]. Our study included few patients with genotype 2 infection, consequently, we were not able to perform particular sub-analysis in this population. However, SVR rates (84.6% in ITT and 92.7% in mITT) among those patients were similar to another Brazilian cohort (88%) [25].
In our study, patients infected with genotype 3 had lower SVR rate compared to patients with genotypes 1 and 2. Efficacy outcome by ITT assessment for genotype 3 (84.4%) was slightly lower compared to the phase III studies ALLY-3 (89%) and ALLY-3+ (90%) [10,11]. This could be explained due to the low proportion of cirrhotic patients in the ALLY-3 (19.8%) compared to our study (62.4%). ALLY-3+ did not include decompensated cirrhosis and half of the patients received treatment for 16 weeks; while 15% of our cirrhotic patients had decompensated liver disease, and due to national guidelines, treatment duration was restricted to 12 weeks [10,11]. Among our findings, SVR in patients with genotype 3 and cirrhosis (79.4% in ITT and 85.7% in mITT) was somewhat lower than found among patients treated with SOF + DCV ± RBV the cirrhotic Spanish cohort (90.6 to100%), but comparable to the European compassionate study with 24 weeks duration treatment (88%), and to a Brazilian cohort (85%) [25–27]. We believe that genotype 3-infected patients, specially those with cirrhosis, are a difficult-to-treat populations that could benefit from treatment enlargement, as demonstrated in previous studies [26–27].
Prior studies revealed that HCV-treatment experienced patients achieved lower SVR rates [28,29]. Strikingly, in our study patients with prior HCV treatment had greater SVR rates (91.9% in ITT and 96.4% in mITT) compared to HCV-treatment naïve patients (88.4 in ITT and 95.3% in mITT). This was also demonstrated by the HEPATHER cohort, even for separate analysis between patients prior-null responders from prior relapsers and virologic breakthroughs. These results could be justified by different history of care and selection profiles, or even by compliance between treatment-experienced and treatment-naïve patients [24].
Besides cirrhosis status, we found that lower CCI index was associated with SVR (p = 0.0014). An Egyptian cohort showed that comorbidities were more frequent in patients with treatment failure (74.6%, p = 0.18), although CCI index was not performed [29]. Indeed, CCI index may be an important approach for individual patients before treatment. Higher CCI index is suitable with patients that need more attention while on-and- after treatment, due to the risk of drug-interactions and also treatment interruption [30–31].
Among cirrhotic patients, we demonstrated that higher albumin, lower MELD score and higher eGFR at baseline were associated with SVR achievement. Marcelin et al also showed that lower albumin was associated with treatment failure among patients with advanced fibrosis, and the TARGET cohort revealed that higher baseline albumin level was associated with SVR [23–28]. Although Child-Pugh A patients had superior SVR rate (88.7%) compared to Child-Pugh B and C (80%), Child-Pugh score was not an individual predictor of SVR achievement. Other previous studies also demonstrated that compensated cirrhotic patients had higher SVR rates compared to patients with decompensated liver disease, yet it was not statistically significant, except for one cohort that evaluated SVR among elderly patients [24,26,32]. Nevertheless, in our study lower MELD was independently associated with treatment response. Lastly, we found that higher eGFR was associated with SVR, which was not demonstrated by previous real-life studies[23,24,32]. Indeed, eGFR might be a confounded variable since it is included in MELD score. Although, higher eGFR could be associated with patients with a better health-status, explaining its association with SVR achievement. In despite of that, all the 4 patients with end-stage kidney disease included in our study achieved SVR12.
Our results showed that a small proportion of cirrhotic patients (8%) developed liver decompensation while on treatment. A British cohort including a large number decompensated cirrhotic patients (n = 409) demonstrated that 23% of those had worsening in MELD scores of 2 points or more [33]. Maan et al followed 433 cirrhotic patients treated with DAA and revealed that 11.5% of those experienced clinical liver decompensation, compared to 8% of cirrhotic patients in our study [34]. Decompensation of acute-on-chronic liver disease was also the main cause of treatment interruption due to AE (80%, n/N = 12/15) and death on-and-after treatment (58.3%, n/N = 7/12) in our casuistry. These data brings the attention to liver decompensation during treatment as an important cause of poor outcome.
Due to the observational nature of our study, no conclusion regarding superiority of one treatment regimen over another could be made. Also, genotype 1-infected patients with decompensated cirrhosis and those who previously failed from first-generation protease inhibitor received 24 weeks of DCV based-treatment, so groups that received 12 or 24 weeks of SOF + DCV ± RBV were not comparable. That said, no assessment between treatment duration could be done. Another important limitation of our study is that we do not have virologic analysis of failures. As most virologic failures were relapses rather than virologic breakthroughs and null-responders, we expect that treatment failures would be predominantly associated with resistance-associated variants [35]. Additional limitations of our study is missing data regarding Child-Pugh and MELD scores at EOT and the potential of under reporting of AE. However, it is unlikely that serious AE, which are clinically most relevant, were missed.
In conclusion, SVR rates amongst genotype 1 patients were high and similar to clinical trails and real-life cohorts, while SVR rates among genotype 3 patients were lower than those studies. Lower CCI index and absence of cirrhosis were associated with SVR achievement. Among cirrhotic patients, higher albumin, lower MELD and higher eGFR were related to treatment response. Nevertheless a small proportion of patients had liver decompensation, it was associated with poor outcome such as treatment interruption and death.
Acknowledgments
The authors would like to thank Dr Cecilia Amelia Fazzo Escanhoela for the histological analyses of the liver biopsies.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.World Health Organization. Global Hepatitis Report 2017. Available from: (http://www.who.int/hepatitis/publications/global-hepatitisreport2017/en/).
- 2.Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014. November;61(1 Suppl):S58–68. doi: 10.1016/j.jhep.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 3.Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distributions of hepatitis C virus infection. J Hepatol 2014; 61:45–57. [DOI] [PubMed] [Google Scholar]
- 4.Bruggmann P, Berg T, Øvrehus AL, Moreno C, Brandão Mello CE, Roudot-Thoraval F, et al. Historical epidemiology of hepatitis C virus (HCV) in selected countries. J Viral Hepatol 2014; 21 (Suppl 1): 5–33. [DOI] [PubMed] [Google Scholar]
- 5.Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol 2011;9:e501. [DOI] [PubMed] [Google Scholar]
- 6.Fontana RJ, Sanyal AJ, Ghany MG, Lee WM, Reid AE, Naishadham D, et al. Factors that determine the development and progression of gastroesopha- geal varices in patients with chronic hepatitis C. Gastroenterology 2010;138:2321–2331, 2331. doi: 10.1053/j.gastro.2010.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014. November 15;384(9956):1756–65. Erratum in: Lancet. 2014 Nov 15;384(9956):1748. doi: 10.1016/S0140-6736(14)61036-9 [DOI] [PubMed] [Google Scholar]
- 8.Kwo P, Gitlin N, Nahass R, Bernstein D, Etzkorn J, Rojter S, et al. Simeprevir Plus Sofosbuvir (12 and 8Weeks) in Hepatitis C Virus Genotype 1-Infected Patients Without Cirrhosis: OPTIMIST-1, a Phase 3, Randomized Study. Hepatology. 2016. August;64(2):370–80. doi: 10.1002/hep.28467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014. January 16;370(3):211–21. Erratum in: N Engl J Med. 2014 Apr 10;370(15):1469. doi: 10.1056/NEJMoa1306218 [DOI] [PubMed] [Google Scholar]
- 10.Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015. April;61(4):1127–35. doi: 10.1002/hep.27726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leroy V, Angus P, Bronowicki JP, Dore GJ, Hezode C, Pianko S, et al. Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: A randomized phase III study (ALLY-3+). Hepatology. 2016. May;63(5):1430–41. doi: 10.1002/hep.28473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedossa P. Presentation of a grid for computer analysis for compilation of histopathologic lesions in chronic viral hepatitis C. Cooperative study of the METAVIR group. Ann Pathol 1993; 13:260–265. [PubMed] [Google Scholar]
- 13.Rockey DC, Bissell DM. Noninvasive measures of liver fibrosis. Hepatology 2006; 43:S113–S120. doi: 10.1002/hep.21046 [DOI] [PubMed] [Google Scholar]
- 14.Mendes LC, Ferreira PA, Miotto N, Zanaga L, Goncales E, Lazarini MS, et al. Transient elastography and APRI score: looking at false positives and false negatives. Diagnostic performance and association to fibrosis staging in chronic hepatitis C. Braz J Med Biol. Res 2016; 49(9):e5432 doi: 10.1590/1414-431X20165432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson M, Szatrowski TP, Peterson J, Jeffrey GGLD. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47:1245–1251. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Greene T, Schluchter MD, Cleary PA, Teschan PE, Lorenz RA, et al. Glomerular filtration rate measurements in clinical trials. Modification of Diet in Renal Disease Study Group and the Diabetes Control and Complications Trial Research Group. J Am Soc Nephrol 1993. November; 4: 1159–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis 2002;39:Suppl 2:S1-S246. [PubMed]
- 18.Pugh RN, Murray-Lyon IM, Dawnson L, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1976. Aug; 60 (8): 646–49 [DOI] [PubMed] [Google Scholar]
- 19.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001; 33:464–470. doi: 10.1053/jhep.2001.22172 [DOI] [PubMed] [Google Scholar]
- 20.AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015; 62:932–954. doi: 10.1002/hep.27950 [DOI] [PubMed] [Google Scholar]
- 21.European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2016. J Hepatol 2017; 66:153–194. doi: 10.1016/j.jhep.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 22.Rickham PP. HUMAN EXPERIMENTATION. CODE OF ETHICS OF THE WORLD MEDICAL ASSOCIATION. DECLARATION OF HELSINKI. Br Med J. 1964. July 18;2(5402):177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sulkowski MS, Vargas HE, Di Bisceglie AM, Kuo A, Reddy KR, Lim JK, et al. Effectiveness of Simeprevir Plus Sofosbuvir, With or Without Ribavirin, in Real-World Patients With HCV Genotype 1 Infection. Gastroenterology. 2016. February;150(2):419–29. doi: 10.1053/j.gastro.2015.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pol S, Bourliere M, Lucier S, Hezode C, Dorival C, Larrey D, et al. Safety and efficacy of daclatasvir-sofosbuvir in HCV genotype 1-mono-infected patients. J Hepatol. 2017. January;66(1):39–47. doi: 10.1016/j.jhep.2016.08.021 Epub 2016 Sep 10. [DOI] [PubMed] [Google Scholar]
- 25.Cheinquer HH, Coelho HS, Aires RS, Quintela ED, Lobato C, Filho JEM, et al. New direct action antivirals containing regimes to treat patients with hepatitis C chronic infection: first results from a national real-world registry of the Brazilian Hepatology Society. J Hepatol. 2017. December; 66(1): S 508. [Google Scholar]
- 26.Alonso S, Riveiro-Barciela M, Fernandez I, Rincón D, Real Y, Llerena S, et al. Effectiveness and safety of sofosbuvir-based regimens plus an NS5A inhibitor for patients with HCV genotype 3 infection and cirrhosis. Results of a multicenter real-life cohort. J Viral Hepat. 2017. April;24(4):304–311. doi: 10.1111/jvh.12648 [DOI] [PubMed] [Google Scholar]
- 27.Welzel TM, Petersen J, Herzer K, Ferenci P, Gschwantler M, Wedemeyer H, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, achieved high sustained virological response rates in patients with HCV infection and advanced liver disease in a real-world cohort. Gut. 2016. November;65(11):1861–1870. Erratum in: Gut. 2016 Dec;65(12):2060. doi: 10.1136/gutjnl-2016-312444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kutala BK, Mouri F, Castelnau C, Bouton V, Giuily N, Boyer N, Asselah T, Marcellin P. Efficacy and safety of sofosbuvir-based therapies in patients with advanced liver disease in a real-life cohort. Hepat Med. 2017. December 18;9:67–73. doi: 10.2147/HMER.S149578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagaty A, Abd El-Wahab EW. Real-life results of sofosbuvir based therapy in chronic hepatitis C -naïve and -experienced patients in Egypt. PLoS One. 2017. October 5;12(10):e0184654 doi: 10.1371/journal.pone.0184654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez-Osorio I, Cid P, Morano L, Castro Á, Suárez M, Delgado M, et al. Real life experience with direct-acting antivirals agents against hepatitis C infection in elderly patients. J Clin Virol. 2017. March;88:58–61. doi: 10.1016/j.jcv.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 31.Miotto N, Mendes LC, Zanaga LP, Goncales ESL, Lazarini MSK, Pedro MN, et al. Predictors of early discontinuation of interferon-free direct antiviral agents in patients with hepatitis C virus and advanced liver fibrosis: results of a real-life cohort. Eur J Gastroenterol Hepatol. 2017. October;29(10):1149–1154. doi: 10.1097/MEG.0000000000000944 [DOI] [PubMed] [Google Scholar]
- 32.Conti F, Brillanti S, Buonfiglioli F, Vukotic R, Morelli MC, Lalanne C, et al. Safety and efficacy of direct-acting antivirals for the treatment of chronic hepatitis C in a real-world population aged 65 years and older. J Viral Hepat. 2017. June;24(6):454–463. doi: 10.1111/jvh.12663 [DOI] [PubMed] [Google Scholar]
- 33.Foster GR, Irving WL, Cheung MC, Walker AJ, Hudson BE, Verma S, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016. June;64(6):1224–31. doi: 10.1016/j.jhep.2016.01.029 [DOI] [PubMed] [Google Scholar]
- 34.Maan R, van Tilborg M, Deterding K, Ramji A, van der Meer AJ, Wong F, et al. Safety and Effectiveness of Direct-Acting Antiviral Agents for Treatment of Patients With Chronic Hepatitis C Virus Infection and Cirrhosis. Clin Gastroenterol Hepatol. 2016. December;14(12):1821–1830.e6. doi: 10.1016/j.cgh.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 35.Hézode C, Chevaliez S, Scoazec G, Soulier A, Varaut A, Bouvier-Alias M, et al. Retreatment with sofosbuvir and simeprevir of patients with hepatitis C virus genotype 1 or 4 who previously failed a daclatasvir-containing regimen. Hepatology. 2016. June;63(6):1809–16. doi: 10.1002/hep.28491 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.