Abstract
Background
Evidence for the preventative effects of percutaneous transluminal angioplasty and stenting (PTAS) on the recurrence of stroke in patients with severe intracranial vertebrobasilar stenoses (IVBS) varies, and the influence of study characteristics on the study outcomes have not been determined.
Methods
A study level based meta-analysis was performed to investigate the influence of baseline characteristics on the 30-day and follow-up stroke recurrence or death in symptomatic IVBS patients receiving PTAS. Relevant single center studies were retrieved by searching PubMed and Embase. A random effect model was applied to synthesize the outcomes. Meta-regression and subgroup analyses were performed to evaluate the potential influence of study characteristics on outcomes.
Results
Fifteen cohort studies comprising 554 symptomatic IVBS patients were included. PTAS was associated with an 8% incidence of stroke recurrence or death (95% CI: 5% to 12%) in IVBS patients within 30 days, and 8 per 100 person-years (95% CI: 5 to 11 per 100 person-years) of cumulative stroke recurrence or death during follow-up. Meta-regression indicated that the center volume, as defined by the numbers of cases per year, was negatively correlated with 30-day (regression coefficient = -0.09, p = 0.02) and follow-up (regression coefficient = -0.60, p = 0.01) stroke recurrence or death. Age, gender, or comorbidities have no significant effect on the outcomes.
Conclusions
Centers of higher procedural volume may be associated with better clinical outcomes for symptomatic IVBS patients receiving PTAS.
Introduction
Although ischemic events in the anterior cerebral circulation contribute to about 80% of all cerebral ischemic events, patients with impairment posterior cerebral circulation were considered to be at higher risk of recurrence of cerebral ischemic events [1]. Stroke was reported to recur in 8–10% of patients with a previous minor stroke or transient ischemic attach (TIA) [2]. However, an annual recurrence of 15% was reported in patients with impaired posterior cerebral circulation, and particularly those with symptomatic intracranial vertebrobasilar stenoses (IVBS), despite administration of the optimal medical therapy [3, 4]. Indeed, in a previous large study of Warfarin-Aspirin Symptomatic Intracranial Disease (WASID), stroke recurred in the posterior circulation in 23% and 10% of patients assigned to aspirin and warfarin therapy, while stroke in the anterior circulation recurred in 8% and 2% of these groups, respectively [5]. Novel strategies capable of preventing stroke are urgently sought, particularly for patients with symptomatic IVBS [6].
Early case reports and series indicated that endovascular treatment with percutaneous transluminal angioplasty and stenting (PTAS) may effectively prevent stroke in patients with severe intracranial arterial stenoses [7]. However, subsequent randomized controlled trials (RCTs) reported that PTAS was associated with poorer clinical outcomes in patients with severe intracranial arterial stenosis (ICAS) when compared with the aggressive medical therapy [8]. Indeed, the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMRIS) trial, in which 35% of enrolled patients had IVBS, was stopped prematurely because the 30-day rate of stroke or death was significantly higher in the PTAS group with the Wingspan stent system as compared with the medical group [9]. Moreover, a recent RCTs aiming to compare the efficacy of PTAS and medical treatment in patients with vertebral artery stenoses was also halted early because stenting of symptomatic vertebral artery stenosis was associated with a major periprocedural vascular complication [10]. However, the limited numbers of available RCTs makes it difficult to determine the key determinants of the poor clinical outcomes in IVBS patients undergoing PTAS. Moreover, the efficacies of PTAS in patients with IVBS varied considerably between previous cohort studies [11–27]. Moreover, the factors that contribute to clinical outcomes in patients with IVBS that receive PTAS have not been determined. In this study level based meta-analysis, we aimed to investigate the influence of center and baseline patient characteristics on 30-day and follow-up outcomes in symptomatic IVBS patients receiving PTAS, focusing on the volume of the individual center included.
Methods
The Meta-Analysis of Observational Studies in Epidemiology (MOOSE) [28] protocol and Cochrane Handbook [29] guidelines were followed throughout the design, implementation, analysis, and reporting of this systematic review and meta-analysis.
Literature search
We systematically searched the electronic databases PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) and Embase (Ovid from Wolters Kluwer) for relevant records using the search strategies outlined in S1 File. We used this broad strategy in order to incorporate as many relevant studies as possible. The search was limited to studies clinical studies published in English. The reference lists of original and review articles were also manually screened to identify additional records. The final literature search was performed on April 20th, 2018.
Inclusion and exclusion criteria
Studies were included in our systematic review and meta-analysis if they met the following criteria: 1) published as full-length article; 2) reported as a single center cohort study in humans (prospective or retrospective design); 3) including at least ten consecutive cases with symptomatic IVBS undergoing PTAS; 4) with a follow-up duration of ≥ 1 month; 5) reporting the composite outcome of recurrence of ischemic stroke or all-cause death within 30 days of the PTAS procedure or during subsequent follow-up. Studies published as editorial, letters, or conference abstracts were excluded. Studies including patients without IVBS, with specific requirements for stents used and subsequently included non-consecutive cases of IVBS were excluded, in addition to studies including IVBS patients that were confirmed to be caused by dissections, or including patient that underwent surgical treatment such as bypass.
Data extraction and quality evaluation
The literature search, data extraction, and quality assessment were independently performed by the two authors according to the predefined inclusion criteria. Discrepancies were resolved by consensus and discussion. To define the potential influence of center volume on the PTAS outcome, we extracted each consecutive IVBS patient’s data and the duration of the recruitment and procedures. The center volume was defined as the number of consecutive IVBS patients that received PTAS divided by the time of the recruitment and procedures (cases per year). Therefore, the extracted data included: 1) author, publishing year, design, and located country of each study; 2) baseline characteristics of the included patients: numbers, mean ages, proportions of male, hypertensive, diabetic, or hyperlipidemic, proportions of current smokers, proportions with lesions of basilar arteries; 3) other study characteristics, such as center volume as defined above, stent systems used and follow-up durations; 4) outcome data, including the patients with recurrent ischemic stroke or all-cause death within 30 days after the PTAS procedure or during subsequent follow-up. The quality of the included studies was evaluated using the modified Newcastle-Ottawa Scale, which addresses the following aspects [30], including the manner of data collection (prospective or retrospective), methods of detecting vascular stenoses, definitions of stroke recurrence and deaths, follow-up methods, and loss to follow-up. Each aspect was assigned 1 point, with 5 points as the highest score.
Statistical analysis
The purpose of the study was to evaluate the potential effects of center volume on the composite outcomes of stroke recurrence and death within 30 days after the PTAS procedure or during the follow-up. Therefore, the numbers of patients with the above outcomes were calculated. If the same patients suffered from both stroke recurrence and subsequent death, they were counted only once. The incidence of stroke recurrence and death within 30 days was calculated as follows: the number of patients with events within 30 days divided by the total numbers of patients included in each study (events / total patients). The incidence of stroke recurrence and death during follow-up was calculated as follows: number of patients with events at the end of the follow-up divided by total person-years (events / person-years). These outcomes were synthesized with a random-effect model to incorporate the potential heterogeneities among the included studies [29]. The weight (%) of each study was calculated based on the incidence of events and sample size in each study via the DerSimonian-Laird methods as previously described [31]. We used the Cochrane’s Q test to formally assess the inter-study heterogeneity, and a p value < 0.10 indicated the potential existence of significant heterogeneity [32, 33]. The I2 statistic was also calculated to assess the contribution of heterogeneity, as opposed to chance, to total variation [34]. A value of I2 > 50% indicated significant heterogeneity. Meta-regression and predefined subgroup analysis were performed to identify the source of heterogeneity, particularly focusing on the potential influence of center volume. Median and interquartile values of continuous variables were used as cut-off values for grouping studies. Potential publication bias was assessed by the visual inspection of the symmetry of the funnel plots, and the results of the Egger regression asymmetry test [35]. We used STATA software (Version 12.0; Stata Corporation, College Station, TX) for these statistical analyses.
Results
Database searching
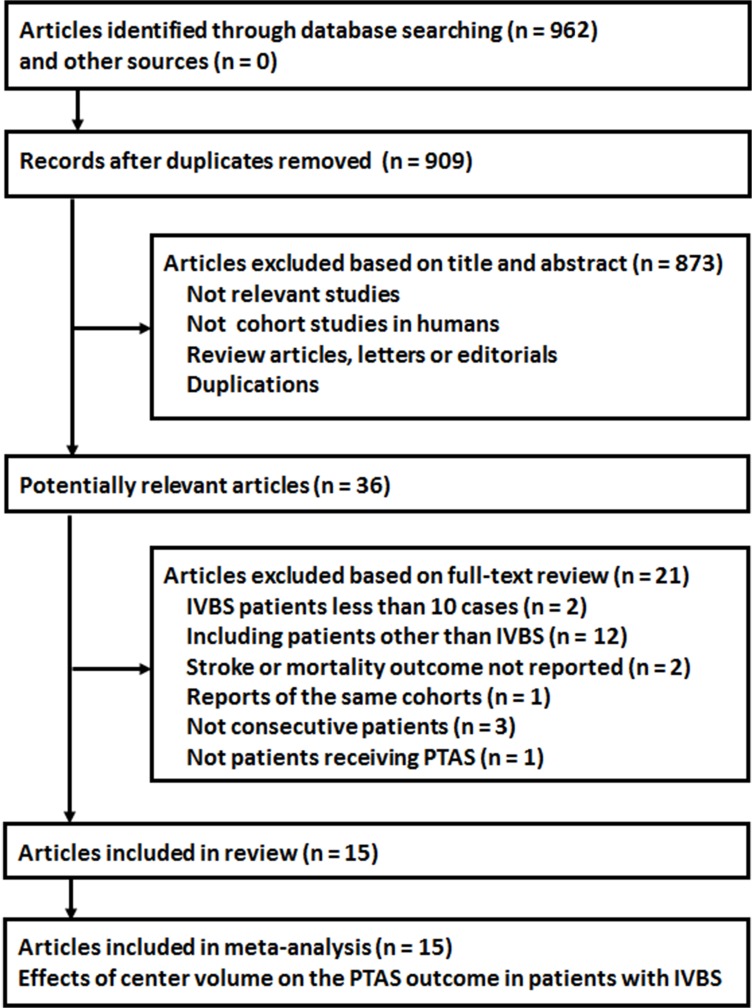
Relevant literature was identified as described in Fig 1. Briefly, 962 records were retrieved from the initial search of PubMed and Embase databases. After removing duplicate reports and primary screening of the included articles’ titles and abstracts 36 records were considered to be relevant. After full-text review, 15 studies [11–22, 24, 25, 27] were finally included in the current systematic review and meta-analysis after further exclusion of 21 records that patients without IVBS, enrolled non consecutively, included patients not receiving PTAS, did not report relevant outcomes, or enrolled duplicate cohorts.
Fig 1. The flowchart of database searching.
Study characteristics
The 15 included cohort studies [11–22, 24, 25, 27] enrolled a total of 554 symptomatic IVBS patients that underwent PTAS procedures. The characteristics of the included studies are listed in Table 1. These studies were published between the year 2000 and 2016. Most were retrospective cohort studies, except four [19, 21, 25, 27] which were prospective cohort studies. The study centers were distributed in North America [11, 12, 15–17], Europe [14, 19, 20], Asia [13, 21, 24, 25, 27], Argentina [22] and Australia [18]. The mean age of the included patients ranged from 59.0 to 68.3 years, and patient groups were 58.8% to 100% male. The mean stenoses of the targeted lesions ranged from 67.0% to 92.3%, and the proportions of patients with basilar stenoses ranged from 32% to 100%. The follow-up duration ranged from 1 to 43.5 months. Center volume differed significantly between studies, ranging from 2 to 97 patients per year. The early studies applied coronary stents [11–16, 18] for the PTAS procedures in patients with symptomatic IVBS, while the Wingspan and Apollo stent systems were applied in more recent studies [21, 24, 25, 27]. The quality of the included studies was generally optimal, with quality scores ranging from 4 to 5 points.
Table 1. Baseline characteristics of included studies.
| Study | Design | Location | Patient Number | Mean age | Male | HTN | DM | HD | Smoking | Mean stenosis | Center volume | Basilar artery lesions | Stents system | Follow-up | Quality scores |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| years | % | % | % | % | % | % | case/year | % | % | months | |||||
| Gomez 2000 | RC | USA | 12 | 62.6 | 83.3 | NR | NR | NR | NR | 71.4 | 12 | 100 | Coronary stents | 5.9 | 4 |
| Levy 2001 | RC | USA | 11 | 62.9 | 100 | 72.7 | 9 | 27.3 | 45.5 | > 70 | 5.5 | 72.7 | Coronary stents | 4 | 4 |
| Kim 2005 | RC | Korea | 17 | 64 | 58.8 | 100 | 35.3 | 29.4 | 35.3 | 76.1 | 5.7 | 46.2 | Coronary stents | 17 | 4 |
| Weber 2005 | RC | Germany | 21 | 67 | 71.4 | NR | NR | NR | NR | 92.3 | 11 | 40.9 | Coronary stents | 9 | 4 |
| Yu 2005 | RC | USA | 18 | 69 | 83.3 | 83 | 50 | 72 | 33 | 79.6 | 4.5 | 100 | Coronary stents | 12.1 | 4 |
| Abruzzo 2007 | RC | USA | 10 | 68.3 | 80 | 90 | 40 | 30 | 20 | 80.7 | 2.5 | 100 | Coronary stents | 31 | 4 |
| Steinfort 2007 | RC | Australia | 13 | 60 | 100 | NR | NR | NR | NR | 67 | 6.5 | 38.5 | Paclitaxel-coated stents | 10.3 | 4 |
| Fiorella 2007 | RC | USA | 44 | 64.8 | 79.5 | NR | NR | NR | NR | 82.5 | 7.3 | NR | Velocity, Duet, Tetra and Vision stents | 43.5 | 4 |
| Ralea 2008 | PC | France | 12 | 62.6 | 66.7 | 91.7 | 25 | 100 | 25 | 80 | 2 | 50 | Tsunami, INX, Cérébrence and Wingspan stents | 15 | 4 |
| Seifert 2009 | RC | Austria | 17 | 65.9 | 70.6 | NR | NR | NR | NR | NR | 3.4 | NR | Coronary stents or self-expanding nitinol stents | 12.7 | 4 |
| Povedano 2010 | RC | Argentina | 25 | 63 | 84 | 84 | 24 | 60 | 40 | NR | 2.5 | 32 | AVE INX and AVE GFX; Cypher, Sonic, and Velocity; Express and Neuroform; Taxus Express; Herculink and MultiLink Pixel; and Wingspan stents | 12 | 4 |
| Jiang 2010 | PC | China | 139 | 59 | 92.1 | 78.4 | 28.8 | 80.6 | 66.2 | 81 | 19.8 | 50 | Wingspan stents | 24.9 | 5 |
| Li 2012 | RC | China | 30 | 60.3 | 83.3 | 86.7 | 40 | 16.7 | 46.7 | 82.3 | 10 | 46.7 | Wingspan stents | 17.8 | 4 |
| Wang 2015 | PC | China | 88 | 62.6 | 75 | 77.3 | 52.3 | 50 | 34.1 | 82.2 | 14.6 | 40.9 | Wingspan stents | 29.3 | 5 |
| Liu 2016 | PC | China | 97 | 58.5 | 82.1 | 82.4 | 28.9 | 44.3 | 61.9 | 84.2 | 48.5 | 53.6 | Apollo and Wingspan stents | 1 | 5 |
RC, retrospective cohort; PC, prospective cohort; HTN, hypertension; DM, diabetes mellitus; HD, hyperlipidemia; NR, not reported
Stroke recurrence or death within 30 days or during follow-up after PTAS
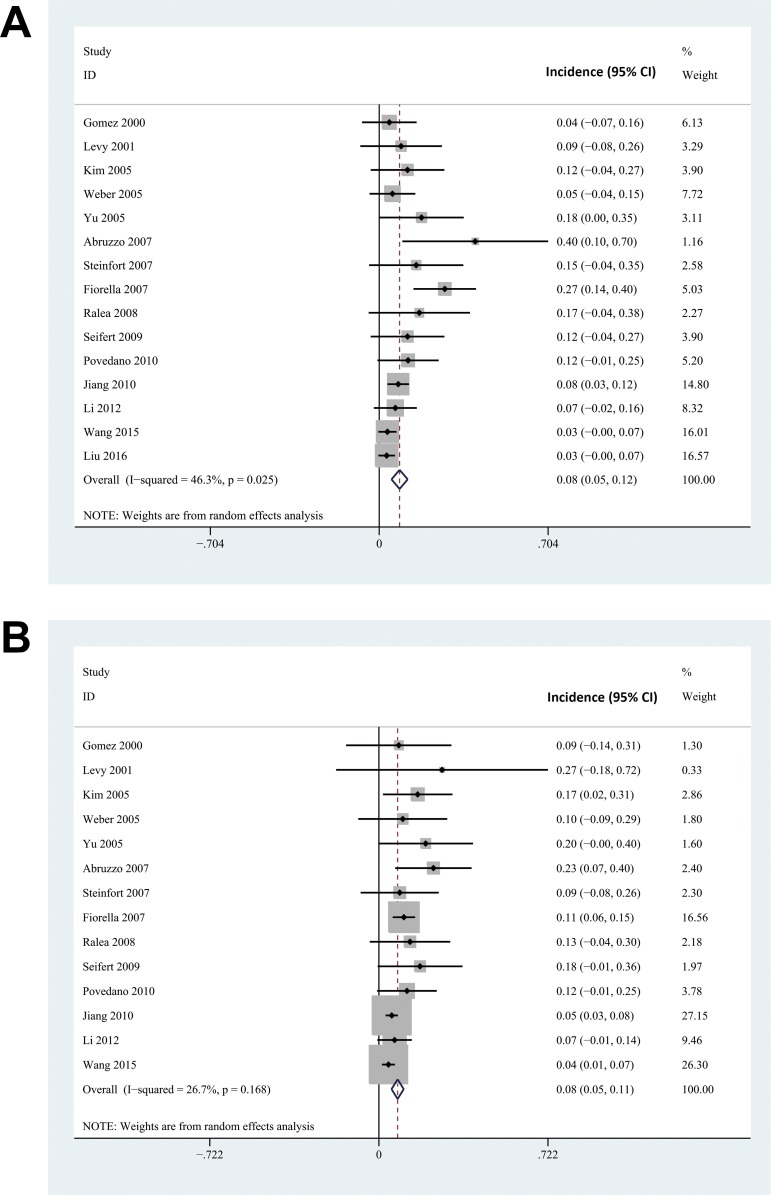
By combing the results of 15 included cohort studies with a random effect model, the meta-analysis indicated PTAS procedure was associated with an 8% incidence of stroke recurrence or death (95% CI: 5% to 12%) in IVBS patients within 30 days after the procedure. Considerable heterogeneities in incidence were noticed (Cochrane’s Q test: p = 0.03, I2 = 46.3%), varying from 3% to 40% among included studies (Fig 2A). Stroke recurrence or death during follow-up was reported in 14 studies [11–22, 24, 25]. The pooled results indicated that the risk of cumulative stroke recurrence or death during follow-up was 8 per 100 person-years for IVBS patients receiving PTAS (95% CI: 5 to 11 per 100 person-years). Although only moderate heterogeneity was noticed among the included studies (Cochrane’s Q test: p = 0.17, I2 = 26.7%), the risk of stroke recurrence or death during follow-up varied considerably from 4 to 23 per 100 person-years (Fig 2B).
Fig 2. The forest plots of the meta-analysis of incidences of combined outcome of stroke recurrence and death in symptomatic IVBS patients after PTAS.
A, forest plot for the incidence of combined outcome of stroke recurrence and death within 30 days after PTAS; forest plot for the incidence of combined outcome of stroke recurrence and death during follow-up (per person year).
Influence of study characteristics on stroke recurrence or death after PTAS
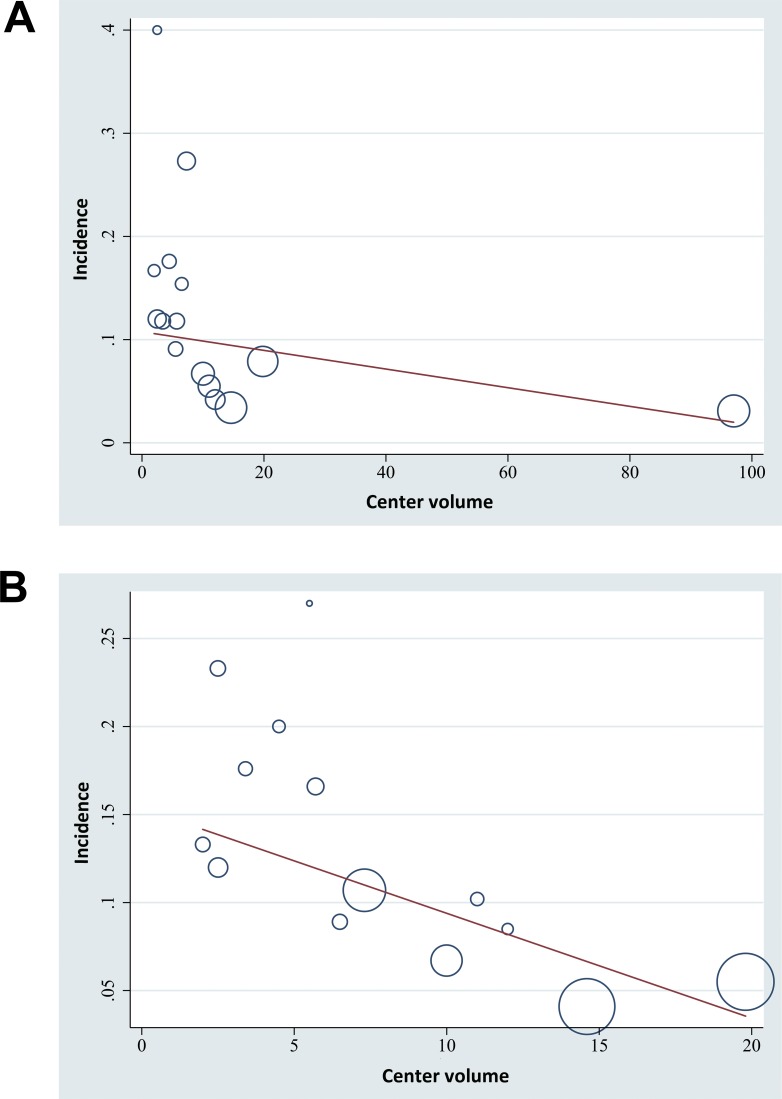
We subsequently evaluated the potential influence of study characteristics on the rate of stroke recurrence or death after PTAS via meta-regression analyses. Results showed that the center volume of the included studies as defined by the numbers of cases per year was negatively correlated with both the risk of stroke recurrence or death within 30 days after PTAS (regression coefficient = -0.09, 95% CI: -0.16 to -0.02, p = 0.02; Table 2 and Fig 3A) and that during follow-up (regression coefficient = -0.60, 95% CI: -1.06 to -0.13, p = 0.01; Table 2 and Fig 3B). Other study characteristics, such as the mean age of enrolled patients, the proportion of male hypertensive, diabetic, hyperlipidemic, or smoker patients, or the proportion of patients with the basilar artery lesions were not significantly associated with outcomes.
Table 2. Impact of study characteristics on the outcomes stroke recurrence or death within 30 days after PTAS or during clinical follow-up.
| 30 days after PTAS (%) | During clinical follow-up (per100 person-year) | |||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | p | Coefficient | 95% CI | p | |
| Mean age (years) | 1.28 | -0.32 to 2.89 | 0.10 | 1.59 | -0.42 to 3.60 | 0.11 |
| Males (%) | 0.02 | -0.49 to 0.52 | 0.93 | -0.16 | -0.73 to 0.42 | 0.55 |
| HTN (%) | 0.39 | -0.33 to 1.11 | 0.25 | 0.61 | -0.12 to 1.35 | 0.09 |
| DM (%) | -0.08 | -0.45 to 029 | 0.64 | -0.14 | -0.66 to 0.38 | 0.55 |
| HD (%) | 0.05 | -0.14 to 0.24 | 0.55 | -0.04 | -0.26 to 0.19 | 0.72 |
| Center volume (case per year) | -0.09 | -0.16 to -0.02 | 0.02 | -0.60 | -1.06 to -0.13 | 0.01 |
| Basilar artery lesions (%) | 0.21 | -0.08 to 0.50 | 0.14 | 0.10 | -0.06 to 0.26 | 0.13 |
HTN, hypertension; DM, diabetes mellitus; HD, hyperlipidemia
Fig 3. Influence of center volume on the risks of stroke recurrence or mortality in symptomatic IVBS patients after PTAS.
A, correlation of center volume with the incidence of stroke recurrence and death within 30 days after PTAS; B, correlation of center volume with the incidence of stroke recurrence and death during follow-up (per person year).
Impact of center volume on the risks of stroke recurrence or death after PTAS
The influence of center volume on the risks of stroke recurrence or death after PTAS in symptomatic IVBS patients was further confirmed by subgroup analyses. The first quartile, the median and the third quartile (4, 7, and 10 cases per year) were used as cut-offs for grouping of the patients. Studies with center volumes of ≥ 4 cases per year reported significantly lower risk of stroke recurrence or death during the follow-up (p for subgroup difference = 0.02), but not for that within 30 days after PTAS (p for subgroup difference = 0.10, Table 3). Studies with center volumes of ≥ 7 and 10 cases per year reported significantly lower risks of stroke recurrence or death within 30 days after PTAS (p for subgroup difference both < 0.05) or during follow-up (p for subgroup difference both < 0.01, Table 3).
Table 3. Impact of center volume on the clinical outcomes of PTAS.
| Case volume (case per year) | 30 days after PTAS | During clinical follow-up | ||||||
|---|---|---|---|---|---|---|---|---|
| Studies (patients), n | I2 | Incidence (%) | p for subgroup | Studies (patients), n | I2 | Incidence (per 100 peson-year) | p for subgroup | |
| First Quartile | ||||||||
| < 4 | 4 (64) | 0% | 15 (6–23) | 4 (64) | 0% | 16 (8–24) | ||
| ≥ 4 | 11 (490) | 46% | 7 (3–11) | 0.10 | 10 (393) | 39% | 7 (4–9) | 0.02 |
| Median | ||||||||
| < 7 | 8 (123) | 0% | 14 (8–20) | 8 (123) | 0% | 16 (10–22) | ||
| ≥ 7 | 7 (431) | 60% | 6 (3–10) | 0.04 | 6 (334) | 14% | 6 (4–8) | <0.01 |
| Third Quartile | ||||||||
| < 10 | 10 (188) | 16% | 14 (9–19) | 10 (188) | 0% | 13 (9–16) | ||
| ≥ 10 | 5 (366) | 0% | 4 (2–7) | 4 (269) | 0% | 5 (3–7) | <0.01 | |
Publication bias
Visual inspection indicated symmetrical funnel plots, implying a low chance of significant publication biases. These results were further confirmed by Egger’s regression tests (p = 0.52 for the outcome within 30 days, and p = 0.71 for the outcome during follow-up).
Discussion
In this meta-analysis of all available cohort studies, we evaluated the potential determinants of 30-day and cumulative stroke recurrence or death in patients with symptomatic IVBS that received PTAS procedures. We found that the volume of each center, as indicated by the numbers of cases enrolled per year, was negatively correlated to the stroke recurrence or death within 30 days of PTAS and during the subsequent follow-up. These results were further confirmed by subgroup analyses, grouping by stroke volume quartiles, which indicated that studies with larger volumes reported better clinical outcomes in IVBS patients receiving PTAS. Other characteristics, including the mean ages of the patients, the proportion of male, hypertensive, diabetic, hyperlipidemic, or currently smoking participants, or the proportion with basilar artery lesions were not significantly associated with outcomes. These results demonstrate that PTAS procedures performed in high volume centers may have better preventative efficacy for stroke recurrence or death in patients with IVBS. This observation suggests that the experience of the operator may be a key determinant of the clinical efficacy of PTAS procedure.
Since disappointing results were reported by RCTs, suggesting that PTAS may be associated with more frequent periprocedural and follow-up stroke recurrence and mortality [8–10], efforts have been made to characterize outcome determinants. In a previous retrospective cohort study of 583 patients with symptomatic ICAS undergoing PTAS, age, comorbidities of diabetes, and basilar arty lesions were potential independent contributors to increased risk of serious in-hospital adverse events [26]. However, our study level based meta-regression analyses did not find the above factors to be significant associated with clinical outcomes in patients receiving PTAS. Despite differences in the populations evaluated, we could not exclude potential determinative effects of these above factors, and the influence of these factors should be further investigated in cohort studies or meta-analyses of adequate data from individual patients. Future studies are warranted. Interestingly, previous studies also showed that the experience of the operator may also be a key determinant of the PTAS outcome. In a prospective cohort study of 95 patients with ICAS, a learning curve for mastering the safety precautions of Wingspan stenting for ICAS was identified, reflecting improved development of the techniques in each surgeon with each surgery performed [36]. Also, a retrospective study of 188 patients identified operator experience to be an independent predictor of periprocedural complications in patients with ICAS receiving PTAS [37]. Our analyses confirmed and extended these findings. To the best of our knowledge, our study is the first meta-analysis to systematically evaluate the potential influence of study characteristics on the clinical efficacy of PTAS in patients with IVBS. More importantly, we identified a potential negative association between center volume and the risk of 30-day and cumulative stroke recurrence and death during follow-up, highlighting that PTAS performed by an experienced operator in a qualified center may represent the most effective treatment for patients with symptomatic IVBS. As indicated by the results of a recently published subgroup analysis of SAMMPRIS [38], for patients with IVBS, the incidence of the combined outcome of recurrent stroke and death was reported to be 4.9 per 100 person-years in patients allocated to the aggressive medical therapy, while it was 13.5 per 100 person-years in PTAS group. Interestingly, in the two studies with the largest center volume [21, 25], the incidence of the combined outcome of recurrent stroke and death were reported to be 4 and 5 per 100 person-years after PTAS, respectively. These data, together with our finding that centers with higher procedural volume may be associated with better clinical outcomes for symptomatic IVBS patients receiving PTAS, may indicate that PTAS for IVBS may achieve equal or even better clinical efficacy than aggressive medical therapy where performed by experienced physicians at centers with large procedural volumes. Moreover, due to a lack of recourses and compliance, the goal of aggressive medical therapy and lifestyle intervention as applied in RCTs could not be achieved in real-world clinical practice. Therefore, if performed by experienced operators at centers with large procedural volumes, PTAS may confer equal or even better clinical efficacy than aggressive medical therapy in the real world. Further studies are needed to confirm these results.
Despite these strengths, our conclusions are limited by the scope of this study. Firstly, only few cohort studies were available for this current meta-analysis and meta-regression study, which provide inadequate statistical power to detect the significant influence of all relevant study characteristics to the efficacy of PTAS in IVBS patients. The influence of characteristics such as the age, gender, and comorbidities on the clinical efficacy of PTAS should be optimally evaluated in a large cohort study or meta-analysis of individual patients. Secondly, due to the limited number of cohort studies included, we were not able to confirm whether the influence of center volume on clinical outcomes of PTAS in symptomatic IVBS patients was independent from other potential factors. If adequate numbers of cohort studies can be identified in the future, multivariate analyses will be required. Thirdly, differences such as location of the lesions, severity of stenoses, and stent systems applied may contribute to the heterogeneity among the included studies, which may further confound the results. Indeed, the IVBS intervention location may affect the outcomes stroke recurrence and death. However, the influence of intervention location on the stroke recurrence and death could not be evaluated on the study-level because IVBS intervention location was rarely reported. Finally, our study is hypothesis generating. The determination of the standards for the qualification of the operators and centers of PTAS for symptomatic IVBS patients should be intensively discussed and investigated in real world practice to improve the comprehensive care of these patients.
Conclusions
In conclusion, our analyses indicated that PTAS procedures performed in high volume centers may have better preventative efficacy for stroke recurrence or death in patients with IVBS. These results highlight that the experience of the operator may be a key determinant of the clinical efficacy of PTAS procedure. Future studies are needed to determine the comparative efficacy of PTAS and aggressive medical therapy in certain subgroup of patients with ICAS with PTAS performed by experienced operators in centers with large procedural volumes. Moreover, in addition to RCTs, the comparative efficacy of PTAS performed by experienced operators and aggressive medical therapy should be investigated in real world studies.
Supporting information
(DOCX)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Gulli G, Khan S, Markus HS. Vertebrobasilar stenosis predicts high early recurrent stroke risk in posterior circulation stroke and TIA. Stroke. 2009;40(8):2732–7. Epub 2009/05/30. doi: 10.1161/STROKEAHA.109.553859 STROKEAHA.109.553859 [pii]. . [DOI] [PubMed] [Google Scholar]
- 2.Marquardt L, Kuker W, Chandratheva A, Geraghty O, Rothwell PM. Incidence and prognosis of > or = 50% symptomatic vertebral or basilar artery stenosis: prospective population-based study. Brain. 2009;132(Pt 4):982–8. Epub 2009/03/19. doi: 10.1093/brain/awp026 [pii]. . [DOI] [PubMed] [Google Scholar]
- 3.Qureshi AI, Ziai WC, Yahia AM, Mohammad Y, Sen S, Agarwal P, et al. Stroke-free survival and its determinants in patients with symptomatic vertebrobasilar stenosis: a multicenter study. Neurosurgery. 2003;52(5):1033–9; discussion 9–40. Epub 2003/04/18. . [PubMed] [Google Scholar]
- 4.Abuzinadah AR, Alanazy MH, Almekhlafi MA, Duan Y, Zhu H, Mazighi M, et al. Stroke recurrence rates among patients with symptomatic intracranial vertebrobasilar stenoses: systematic review and meta-analysis. Journal of neurointerventional surgery. 2016;8(2):112–6. doi: 10.1136/neurintsurg-2014-011458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chimowitz MI, Kokkinos J, Strong J, Brown MB, Levine SR, Silliman S, et al. The Warfarin-Aspirin Symptomatic Intracranial Disease Study. Neurology. 1995;45(8):1488–93. Epub 1995/08/01. . [DOI] [PubMed] [Google Scholar]
- 6.Turan TN, Smock A, Chimowitz MI. The challenge of stroke prevention with intracranial arterial stenosis. Curr Cardiol Rep. 2013;15(12):422 Epub 2013/10/10. doi: 10.1007/s11886-013-0422-y ; PubMed Central PMCID: PMC3870590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groschel K, Schnaudigel S, Pilgram SM, Wasser K, Kastrup A. A systematic review on outcome after stenting for intracranial atherosclerosis. Stroke. 2009;40(5):e340–7. Epub 2009/02/03. doi: 10.1161/STROKEAHA.108.532713 STROKEAHA.108.532713 [pii]. . [DOI] [PubMed] [Google Scholar]
- 8.Tsivgoulis G, Katsanos AH, Magoufis G, Kargiotis O, Papadimitropoulos G, Vadikolias K, et al. Percutaneous transluminal angioplasty and stenting for symptomatic intracranial arterial stenosis: a systematic review and meta-analysis. Ther Adv Neurol Disord. 2016;9(5):351–8. Epub 2016/09/02. doi: 10.1177/1756285616650357 [pii]. ; PubMed Central PMCID: PMC4994779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365(11):993–1003. Epub 2011/09/09. doi: 10.1056/NEJMoa1105335 ; PubMed Central PMCID: PMC3552515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Compter A, van der Worp HB, Schonewille WJ, Vos JA, Boiten J, Nederkoorn PJ, et al. Stenting versus medical treatment in patients with symptomatic vertebral artery stenosis: a randomised open-label phase 2 trial. Lancet Neurol. 2015;14(6):606–14. Epub 2015/04/25. doi: 10.1016/S1474-4422(15)00017-4 S1474-4422(15)00017-4 [pii]. . [DOI] [PubMed] [Google Scholar]
- 11.Gomez CR, Misra VK, Liu MW, Wadlington VR, Terry JB, Tulyapronchote R, et al. Elective stenting of symptomatic basilar artery stenosis. Stroke. 2000;31(1):95–9. [DOI] [PubMed] [Google Scholar]
- 12.Levy EI, Horowitz MB, Koebbe CJ, Jungreis CC, Pride GL, Dutton K, et al. Transluminal stent-assisted angiplasty of the intracranial vertebrobasilar system for medically refractory, posterior circulation ischemia: early results. Neurosurgery. 2001;48(6):1215–21; discussion 21–3. [DOI] [PubMed] [Google Scholar]
- 13.Kim DJ, Lee BH, Kim DI, Shim WH, Jeon P, Lee TH. Stent-assisted angioplasty of symptomatic intracranial vertebrobasilar artery stenosis: feasibility and follow-up results. AJNR American journal of neuroradiology. 2005;26(6):1381–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Weber W, Mayer TE, Henkes H, Kis B, Hamann GF, Schulte-Altedorneburg G, et al. Stent-angioplasty of intracranial vertebral and basilar artery stenoses in symptomatic patients. European journal of radiology. 2005;55(2):231–6. doi: 10.1016/j.ejrad.2004.11.010 [DOI] [PubMed] [Google Scholar]
- 15.Yu W, Smith WS, Singh V, Ko NU, Cullen SP, Dowd CF, et al. Long-term outcome of endovascular stenting for symptomatic basilar artery stenosis. Neurology. 2005;64(6):1055–7. doi: 10.1212/01.WNL.0000154600.13460.7B [DOI] [PubMed] [Google Scholar]
- 16.Abruzzo TA, Tong FC, Waldrop AS, Workman MJ, Cloft HJ, Dion JE. Basilar artery stent angioplasty for symptomatic intracranial athero-occlusive disease: complications and late midterm clinical outcomes. AJNR American journal of neuroradiology. 2007;28(5):808–15. [PMC free article] [PubMed] [Google Scholar]
- 17.Fiorella D, Chow MM, Anderson M, Woo H, Rasmussen PA, Masaryk TJ. A 7-year experience with balloon-mounted coronary stents for the treatment of symptomatic vertebrobasilar intracranial atheromatous disease. Neurosurgery. 2007;61(2):236–42; discussion 42–3. doi: 10.1227/01.NEU.0000255521.42579.31 [DOI] [PubMed] [Google Scholar]
- 18.Steinfort B, Ng PP, Faulder K, Harrington T, Grinnell V, Sorby W, et al. Midterm outcomes of paclitaxel-eluting stents for the treatment of intracranial posterior circulation stenoses. Journal of neurosurgery. 2007;106(2):222–5. doi: 10.3171/jns.2007.106.2.222 [DOI] [PubMed] [Google Scholar]
- 19.Ralea IC, Nighoghossian N, Tahon F, Derex L, Cakmak S, Trouillas P, et al. Stenting of symptomatic basilar and vertebral artery stenosis in patients resistant to optimal medical prevention: the lyon stroke unit experience. European neurology. 2008;60(3):127–31. doi: 10.1159/000144082 [DOI] [PubMed] [Google Scholar]
- 20.Seifert T, Augustin M, Klein GE, Horner S, Niederkorn K, Fazekas F. Symptomatic stenosis of the vertebrobasilar arteries: results of extra- and intracranial stent-PTA. European journal of neurology. 2009;16(1):31–6. doi: 10.1111/j.1468-1331.2008.02297.x [DOI] [PubMed] [Google Scholar]
- 21.Jiang WJ, Du B, Hon SF, Jin M, Xu XT, Ma N, et al. Do patients with basilar or vertebral artery stenosis have a higher stroke incidence poststenting? Journal of neurointerventional surgery. 2010;2(1):50–4. doi: 10.1136/jnis.2009.000356 [DOI] [PubMed] [Google Scholar]
- 22.Povedano G, Zuberbuhler P, Lylyk P, Ameriso SF. Management strategies in posterior circulation intracranial atherosclerotic disease. Journal of endovascular therapy: an official journal of the International Society of Endovascular Specialists. 2010;17(3):308–13. [DOI] [PubMed] [Google Scholar]
- 23.Short JL, Majid A, Hussain SI. Endovascular treatment of symptomatic intracranial atherosclerotic disease. Front Neurol. 2011;1:160 Epub 2011/03/02. doi: 10.3389/fneur.2010.00160 ; PubMed Central PMCID: PMC3040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Zhao ZW, Gao GD, Deng JP, Yu J, Gao L, et al. Wingspan stent for high-grade symptomatic vertebrobasilar artery atherosclerotic stenosis. Cardiovascular and interventional radiology. 2012;35(2):268–78. doi: 10.1007/s00270-011-0163-5 [DOI] [PubMed] [Google Scholar]
- 25.Wang ZL, Gao BL, Li TX, Cai DY, Zhu LF, Bai WX, et al. Symptomatic intracranial vertebral artery atherosclerotic stenosis (>/ = 70%) with concurrent contralateral vertebral atherosclerotic diseases in 88 patients treated with the intracranial stenting. European journal of radiology. 2015;84(9):1801–4. doi: 10.1016/j.ejrad.2015.05.033 [DOI] [PubMed] [Google Scholar]
- 26.Cheng L, Jiao L, Gao P, Song G, Chen S, Wang X, et al. Risk factors associated with in-hospital serious adverse events after stenting of severe symptomatic intracranial stenosis. Clin Neurol Neurosurg. 2016;147:59–63. Epub 2016/06/14. doi: 10.1016/j.clineuro.2016.05.019 S0303-8467(16)30193-7 [pii]. . [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Zhao X, Mo D, Ma N, Gao F, Miao Z. Stenting for symptomatic intracranial vertebrobasilar artery stenosis: 30-day results in a high-volume stroke center. Clinical neurology and neurosurgery. 2016;143:132–8. doi: 10.1016/j.clineuro.2016.02.029 [DOI] [PubMed] [Google Scholar]
- 28.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. Epub 2000/05/02. jst00003 [pii]. . [DOI] [PubMed] [Google Scholar]
- 29.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011; www.cochranehandbook.org. [Google Scholar]
- 30.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2010;http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 31.Malta M, Magnanini MM, Mello MB, Pascom AR, Linhares Y, Bastos FI. HIV prevalence among female sex workers, drug users and men who have sex with men in Brazil: a systematic review and meta-analysis. BMC Public Health. 2010;10:317 Epub 2010/06/10. doi: 10.1186/1471-2458-10-317 1471-2458-10-317 [pii]. ; PubMed Central PMCID: PMC2898825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. Epub 2002/07/12. doi: 10.1002/sim.1186 . [DOI] [PubMed] [Google Scholar]
- 33.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. Epub 2003/09/06. doi: 10.1136/bmj.327.7414.557 327/7414/557 [pii]. ; PubMed Central PMCID: PMC192859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37(5):1148–57. Epub 2008/04/22. doi: 10.1093/ije/dyn065 dyn065 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. Epub 1997/10/06. ; PubMed Central PMCID: PMC2127453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu SC, Leung TW, Lee KT, Wong LK. Learning curve of Wingspan stenting for intracranial atherosclerosis: single-center experience of 95 consecutive patients. J Neurointerv Surg. 2014;6(3):212–8. Epub 2013/03/22. doi: 10.1136/neurintsurg-2012-010593 neurintsurg-2012-010593 [pii]. ; PubMed Central PMCID: PMC3963535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai Q, Li Y, Xu G, Sun W, Xiong Y, Bao Y, et al. Learning curve for intracranial angioplasty and stenting in single center. Catheter Cardiovasc Interv. 2014;83(1):E94–100. Epub 2013/06/05. doi: 10.1002/ccd.25038 . [DOI] [PubMed] [Google Scholar]
- 38.Lutsep HL, Lynn MJ, Cotsonis GA, Derdeyn CP, Turan TN, Fiorella D, et al. Does the Stenting Versus Aggressive Medical Therapy Trial Support Stenting for Subgroups With Intracranial Stenosis? Stroke. 2015;46(11):3282–4. Epub 2015/09/19. doi: 10.1161/STROKEAHA.115.009846 STROKEAHA.115.009846 [pii]. ; PubMed Central PMCID: PMC4624506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.