Abstract
Introduction
Clinical characteristics of testicular germ cell tumours (GCTs) apparently change over time, and some vary geographically. The aim of this study is to document the clinical profile of contemporary GCT patients.
Patients and Methods
Four hundred twenty-two Caucasian GCT-patients treated in one German centre during 2000–2017, were analysed in terms of patient-age, laterality, histology, tumour-size, clinical stages (CS), pathological (pT)-stages and serum biomarker expression. The results were analysed descriptively and compared with the literature.
Results
Median age was 36 years and 60.2% had seminoma. Βeta-human chorionic gonadotropin was expressed in 37.9% and alpha Fetoprotein in 25.6%. CS1 presenting stage was 66.6% of all GCT patients, 79.1% in seminoma, and 47.6% in nonseminoma. Tumour size was significantly associated with pT-stages and CS. Patients >50 years had significantly more seminoma (77.6%) than younger ones (57.9%). Comparison with literature data revealed a shifting towards higher age, lower CS, higher proportion of seminoma and striking differences of characteristics among geographic regions.
Conclusions
A typical contemporary clinical profile of testicular GCTs is presented in this study. Median age, relative incidence of seminoma and proportion of CS1 appear to be increasing over time. Striking differences among ethnic groups regarding the characteristics of GCT require further investigation.
Keywords: Germ cell tumour, Seminoma, Nonseminoma, Clinical staging, Tumour markers, Tumour size
Introduction
Testicular germ-cell tumours (GCTs) represent a malignancy with many unusual features: The disease is rare with an incidence of only 8–10 per 100 thousand men per year in northern European countries [1, 2]. In contrast to most other malignancies, this neoplasm has its peak occurrence in young males aged 20–45 years, and most notably, more than 90% of cases can be cured [3, 4]. GCTs may involve a complex spectrum of characteristics with various histologies and several clinical and pathological (pT) stages with all of these features being relevant for therapeutic decision making [5]. Clinically, the most relevant characteristics comprise of histology, clinical and pT stages, primary tumour size, age, and serum biomarkers beta-human chorionic gonadotropin (bHCG), alpha-fetoprotein (AFP) and lactate dehydrogenase (LDH). Though each one of these characteristics is basically well recognized, possible interrelationships between the various parameters are far less well understood.
With regard to treatment, GCTs are divided into 2 histologic groups, pure seminomas and nonseminomas, the latter comprising the 4 pure histologic GCT patterns except pure seminoma, and the combinations of all 5 subtypes [5, 6, 7]. The relative frequency of seminoma and nonseminoma, respectively, is thought to be around 50% each [8, 9] though recent investigations indicated an increasing incidence of seminoma [10]. The presence of teratoma in the primary tumour may sometimes cause particular therapeutic considerations because this histologic subtype is resistant to radiotherapy and to chemotherapy [3]. The incidence of teratoma-elements in the primary tumour has been reported in pathologic evaluations but only rarely in clinical series.
It is well-established knowledge that nonseminoma presents more often with metastatic spread than seminoma [11]. However, in light of the repeatedly documented shifting of stages over time [12, 13], there is little data regarding the distribution of clinical stages (CS) in a modern unselected series of primary GCT patients.
Further, age of the patient, primary tumour size, pT stage, and expression of serum biomarkers bHCG, AFP, and LDH represent additional important parameters for clinical decision-making. Most of these parameters have been evaluated mainly in relation to treatment outcome either selectively or in various combinations of some of the factors and most of the reports relate to patient cohorts treated in the last century [8, 14, 15, 16]. Furthermore, due to the rarity of GCT, only <10% of all general hospitals comprise a volume of more than 10 cases of this disease annually [17]. Accordingly, the majority of clinical publications on GCT stem from institutions that usually represent secondary or even tertiary referral centres with highly selected patient samples featuring clinical profiles not generalizable to unselected cohorts of patients with GCT at the primary care level. Because of the paucity of recent data regarding the clinical profile of testicular tumours at the time of primary presentation, we aimed to perform a comprehensive descriptive analysis of the presenting features of a contemporary patient sample with statistical testing of associations among the various characteristics. We evaluated a series of consecutive patients treated in a single centre in Germany during the last 2 decades registering clinical and pathological characteristics at the time of diagnosis. We also looked to interrelationships between the various factors, particularly the association of histology and age with other factors. The basic aim of the study was to increase the understanding of the biological behavior of GCTs by means of a thorough analysis of their clinico-pathological features.
Patients and Methods
A consecutive series of 422 patients with GCT were retrospectively analysed with regard to clinico-pathological parameters observed at the time of first presentation. All of the patients were treated at Albertinen Krankenhaus Hamburg during 2000–2017, and all were of Northern European ancestry. The department is a designated institution for primary treatment of all common urologic diseases with a special focus on testicular diseases. Thus, more than 90% of the testis cancer patients presented for primary diagnosis and treatment of GCT, while <10% represented referral cases for secondary treatment. The following parameters were recorded: histology of the primary tumour (pure seminoma, nonseminoma, and nonseminoma with components of teratoma), age at presentation, laterality of primary tumour (left/right, bilateral, extragonadal), CS (Lugano classification), pathological (local) stage (pT) according to the UICC classification of 2002 [18], and size of primary tumour (in cm). The expression of serum tumour markers bHCG, AFP and LDH was registered (yes/no) too. A more detailed evaluation of the tumour marker expressions will be reported separately. pT-stages were categorized as pT1 and >pT1 (i.e., all pT stages higher than pT1). CS were categorized to CS1, CS2a, b, CS2c and CS3, and in a further analysis to CS1 vs. >CS1. We categorized pT-stages and CS for 2 reasons – first to discriminate between organ-confined and locally advanced disease (pT1 vs. >pT1) and local versus systemic disease (CS1 vs. >CS1), respectively, and second for statistical reasons because the lowest stages (i.e., pT1 and CS1) comprised of much more cases than the combined other stages.
Metastasized patients were additionally categorized according to the classification of the International Germ Cell Cancer Consensus Group (IGCCCG) [5, 19].
Individual data was initially registered in a database using MS Excel software. Final analysis was performed using SAS software package version 9.4 (SAS Institute, Cary, NC, USA) on windows platform.
First, tabulation of all data and a descriptive statistical analysis of all of the presenting characteristics were performed. We compared the sub-group of seminoma with nonseminoma regarding the frequencies of the parameters registered. We then examined possible associations of the size of the primary tumour with the various parameters. Finally, we evaluated the role of age by comparing patients older than 50 years with the younger ones regarding the frequencies of the various characteristics.
To document the frequencies of the various parameters, we calculated proportions (%) of enumerable factors. Medians and interquartile ranges (IQRs) were calculated regarding age and the size of primary tumour. When appropriate, results were displayed graphically. Pairwise comparisons were performed using the chi square test for categorical variables and the Mann-Whitney U test for continuous variables. No formal hypothesis testing was planned; all reported p values are descriptive. A p value <0.05 was considered statistical significant.
Results
Of the 422 patients with testicular GCT, 254 (60.2%) presented with pure seminoma, 168 (39.8%) had nonseminoma, histologically. Teratomatous elements were observed in 47.6% of the nonseminoma cases. The proportions of CS and pT- stages are listed in Table 1. Median age of the entire population is 36 years (IQR 31–45 years) with 31 years (IQR 26–37.5) in nonseminomas and 41 years (34–47 years) in seminomas. The difference is statistically significant (p < 0.0001). The primary tumour was located on the right side in 201 cases (47.6%), and in 200 on the left (47.4%); 15 were bilateral (3.5%) and 4 patients had primary extragonadal GCT – one mediastinal and 3 retroperitoneal. Median age was not significantly different among the CS. The median ages of patients with bilateral tumours and those with unilateral cancer were 34 and 37 years, respectively; the difference was not significant (p = 0.1617). The median primary tumour size was 3 cm (IQR 1.8–4.5 cm). The median tumour sizes in patients with teratoma and those without were 3.1 cm (IQR 2–4 cm) and 3.5 cm (IQR 1.9–5.5 cm) respectively (p > 0.05).
Table 1.
Clinical characteristics of patients with testicular germ cell tumours
| n | % | Age |
|||
|---|---|---|---|---|---|
| min | median (IQR) | max | |||
| All GCT | 422 | 100 | 14 | 36 (31–45) | 74 |
| Seminoma | 254 | 60.2 | 17 | 41 (34–47) | 66 |
| Nonseminoma | 168 | 39.8 | 14 | 31 (26–37.5) | 74 |
| pT1 | 222 | 52.6 | 17 | 37 (32–44) | 74 |
| >pT1 | 200 | 47.4 | 14 | 36 (29–45) | 66 |
| CS1 (all GCT) | 281 | 66.6 | 17 | 38 (32–45) | 74 |
| CS2a, b | 95 | 22.5 | 14 | 33 (27–44) | 66 |
| CS2c | 17 | 4.0 | 20 | 36 (31–42) | 59 |
| CS3 | 29 | 6.9 | 17 | 34 (25–44) | 55 |
| CS1 (seminoma) | 201 | 79.1 | 17 | 40 (34–46) | 64 |
| CS2a, b | 37 | 14.6 | 26 | 42 (32–47) | 66 |
| CS2c | 9 | 3.5 | 35 | 42 (36–46) | 59 |
| CS3 | 7 | 2.8 | 31 | 45 (31–48) | 55 |
| CS1 (nonseminoma) | 80 | 47.6 | 17 | 32 (27–38) | 74 |
| CS2a, b | 58 | 34.5 | 14 | 29 (26–37) | 53 |
| CS2c | 8 | 4.8 | 20 | 29.5 (22.5–34) | 44 |
| CS3 | 22 | 13.1 | 17 | 33 (22–42) | 51 |
| NS with teratoma | 80 | 47.6 | 17 | 31 (24–35.5) | 74 |
| No teratoma | 88 | 52.4 | 14 | 31 (27–39) | 64 |
| <25% teratoma | 18 | 10.7 | 20 | 31.5 (26–36) | 53 |
| >25–≤50% teratoma | 25 | 14.9 | 17 | 34 (27–38) | 74 |
| >50–≤75% teratoma | 13 | 7.7 | 18 | 27 (22–34) | 51 |
| >75% teratoma | 24 | 14.3 | 17 | 28 (22–33.5) | 58 |
| Tumour on right side | 201 | 47.6 | 14 | 37 (32–45) | 66 |
| Left side | 200 | 47.4 | 17 | 36 (29–44.5) | 74 |
| Bilateral synchronously | 9 | 2.1 | 17 | 33 (30–36) | 47 |
| Bilateral sequential | 6 | 1.4 | 23 | 34 (28–39) | 44 |
| Extragonadal | 4 | 0.9 | 47 | 52 (47–57) | 57 |
GCT, germ cell tumour; CS, clinical stages; IQR, interquartile range; pT, pathological; NS, nonseminoma.
In the entire GCT cohort, the expression rates of serum tumour markers were as follows: bHCG 37.9%, AFP 25.6 and LDH 32.9%.
Table 2 shows the grouping of the 141 patients with metastases according to the IGCCCG classification.
Table 2.
Classification of patients with metastases according to IGCCCG
| n | Percentage of metastasized cases (n = 141) | Percentage of all GCT patients (n = 422) | |
|---|---|---|---|
| Good prognosis | 113 | 80.1 | 26.8 |
| Intermediate prognosis | 17 | 12.1 | 4.0 |
| Poor prognosis | 11 | 7.8 | 2.6 |
IGCCCG, International Germ Cell Cancer Consensus Group; GCT, germ cell tumour.
Table 3 provides the results of the comparisons of seminoma with nonseminoma. The 2 histological subtypes are significantly different from each other regarding age, proportions of CS, pT-stages and expression of tumour markers. Seminoma presented more frequently on the right side (53.5%) than nonseminoma (45.0%); however, this difference was not significant. The median tumour sizes were not different among the groups.
Table 3.
Comparison of seminoma with nonseminoma
| Parameter | Seminoma (n = 254) | Nonseminoma (n = 168) | p value | Test |
|---|---|---|---|---|
| Age, years, median (IQR) | 41 (34–47) | 31 (26–37.5) | <0.0001 | MWU |
| Proportion CS1, n (%) | 79.1 | 47.6 | <0.0001 | Chi-square |
| Proportion pT1, n (%) | 59.1 | 42.9 | 0.0011 | Chi-square |
| Tumour size, cm, median (IQR) | 2.8 (1.6–4.5) | 3.2 (2–5) | 0.1352 | MWU |
| Tumour on right side, n (%) | 53.5 | 45.0 | 0.6504 | Chi-square |
| Expression rate of bHCG, n (%) | 28.0 | 53.0 | <0.0001 | Chi-square |
| Expression rate of AFP, n (%) | 2.8 | 60.1 | <0.0001 | Chi-square |
| Expression rate of LDH, n (%) | 29.1 | 38.7 | 0.0367 | Chi-square |
MWU, Mann-Witney U test; IQR, interquartile range; CS, clinical stages; pT, pathological; bHCG, beta-human chorionic gonadotropin; AFP, alpha-fetoprotein; LDH, lactate dehydrogenase.
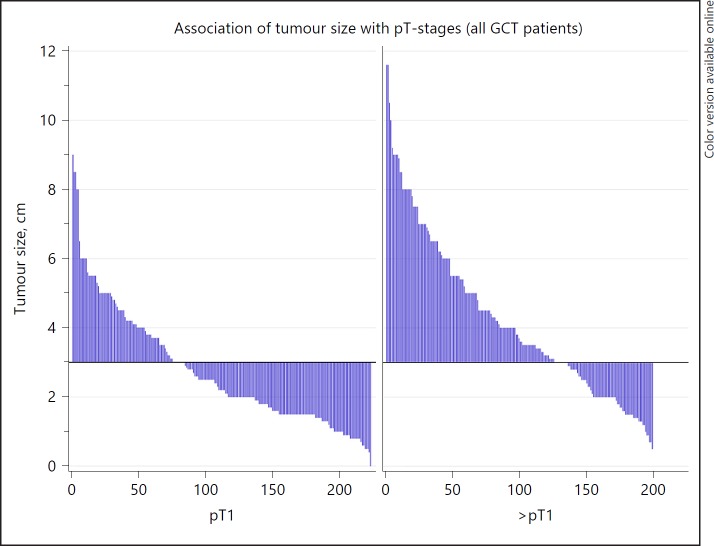
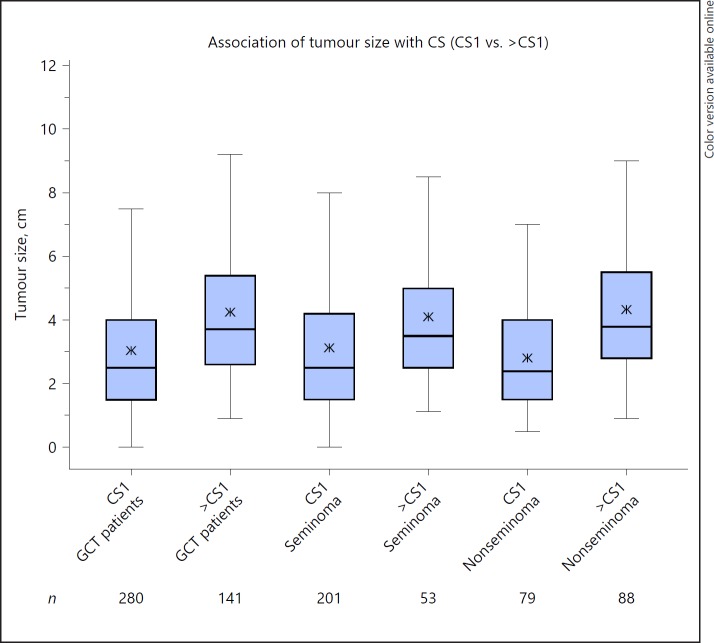
Table 4 shows the significant association of larger primary tumour sizes with advanced pT-staging (Fig. 1) and also with clinical staging in both seminoma and nonseminoma (Fig. 2). Notably, the median size of left-sided tumours is significantly larger than that of right-sided tumours (3.3 vs. 2.8 cm; p = 0.0008). Tumour size is not associated with age and histology.
Table 4.
Associations of primary tumour size with various parameters
| Parameter (first factor vs. second) | Patients, n, median tumour size (IQR), cm |
p value* | |
|---|---|---|---|
| patients with first factor | patients with second factor | ||
| Seminoma vs. nonseminoma | 254, 2.8 (1.6–4.5) | 168, 3.2 (2.0–5.0) | 0.1352 |
| Age ≤50 vs. >50 years | 373, 3 (1.8–4.5) | 49, 2.9 (1.5–4.2) | 0.5056 |
| Right vs. left side | 201, 2.8 (1.6–4.3) | 200, 3.3 (2.0–5.0) | 0.008 |
| CS1 vs. >CS1 (all GCT) | 281, 2.5 (1.5–4.0) | 141, 3.7 (2.6–5.4) | <0.0001 |
| CS1 vs. >CS1 (seminoma only) | 201, 2.5 (1.5–4.2) | 53, 3.5 (2.5–5.0) | 0.0031 |
| CS1 vs. >CS1 (nonseminoma only) | 80, 2.4 (1.5–4.0) | 88, 3.8 (2.8–5.5) | <0.0001 |
| pT1 stage vs. >pT1 (all GCT) | 222, 2.2 (1.5–3.8) | 200, 3.7 (2.4–5.5) | <0.0001 |
| pT1 stage vs. >pT1 (seminoma) | 150, 2.0 (1.5–3.7) | 104, 4.0 (2.75–6.0) | <0.0001 |
| pT1 stage vs. >pT1 (nonseminoma) | 72, 2.6 (1.5–4.5) | 96, 3.4 (2.0–5.2) | 0.0448 |
Mann-Whitney U test.
GCT, germ cell tumour; CS, clinical stages; IQR, interquartile range; pT, pathological.
Fig. 1.
Association of tumour-size with pathological (pT)-stage. Waterfall plot showing the association of tumour-size with pT-stage. X-axis denotes patient numbers; y-axis denotes tumour-size in cm. The horizontal line represents the median tumour size of all cases. Each case is represented by one vertical bar. Cases are ranked according to tumour size. The plot illustrates that among the patients with >pT1, markedly more cases are located above the median line than in the pT1 group. Also, in the >pT1 group, more cases have very large tumours (>8 cm size).
Fig. 2.
Association of tumour size with clinical stages (CS). Box plot showing the associations of tumour size with CS. Each box represents one particular subgroup of the patient population. Horizontal line within box denotes median tumour size; upper and lower limits of boxes denote interquartile ranges (IQRs). Whiskers show lowest and highest values within a range of 1.5 IQR. Stars within boxes denote mean values of tumour size in the corresponding subgroups. The plot illustrates the markedly higher tumour sizes in patients with CS > CS1. This finding is almost identical in all groups examined: entire group of germ cell tumours (GCT), seminomas and nonseminomas.
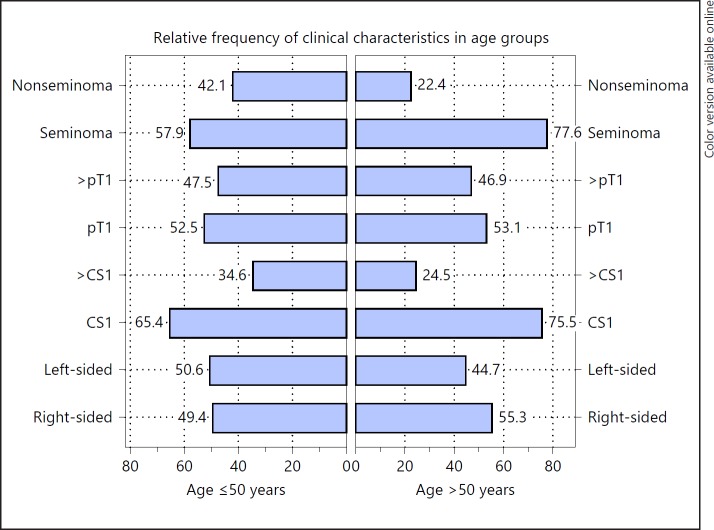
Of the entire GCT cohort, 49 patients (11.6%) were aged >50 years. Comparisons of the characteristics of patients >50 years with the younger ones are summarized in Table 5 and Figure 3. There were significantly more seminomas in the older age group (77.6 vs. 57.9%, p = 0.0083). Elderly patients had more frequently localized disease (CS1; 75.5 vs. 65.4%), but this difference was not significant (p = 0.1590). All of the other characteristics were distributed almost equally among the 2 age categories.
Table 5.
Clinical characteristics in age categories ≤50 and >50 years
| ≤50 years, n (%) | >50 years, n (%) | p value | |
|---|---|---|---|
| All GCT | 373 (88.4) | 49 (11.6) | |
| Seminoma | 216 (57.9) | 38 (77.6) | |
| Nonseminoma | 157 (42.1) | 11 (22.5) | 0.0083 |
| Local pathological stage | |||
| pT1 | 196 (52.5) | 26 (53.1) | |
| >pT1 | 177 (47.5) | 23 (46.9) | 0.9460 |
| Local stage seminoma | |||
| pT1 | 129 (59.7) | 21 (55.3) | |
| >pT1 | 87 (40.3) | 17 (44.7) | 0.6062 |
| Local stage nonseminoma | |||
| pT1 | 67 (42.7) | 5 (45.5) | |
| >pT1 | 90 (57.3) | 6 (54.5) | 0.8571 |
| CS distribution (all GCT) | |||
| CS1 | 244 (65.4) | 37 (75.5) | |
| CS2a, b | 87 (23.3) | 8 (16.3) | |
| CS2c | 15 (4.0) | 2 (4.1) | |
| CS3 | 27 (7.2) | 2 (4.1) | 0.5254 |
| CS distribution (seminoma) | |||
| CS1 | 171 (79.2) | 30 (78.9) | |
| CS2a, b | 32 (14.8) | 5 (13.2) | |
| CS2c | 7 (3.2) | 2 (5.3) | |
| CS3 | 6 (2.8) | 1 (2.6) | 0.9326 |
| CS distribution (nonseminoma) | |||
| CS1 | 73 (46.5) | 7 (63.6) | |
| CS2a, b | 55 (35.0) | 3 (27.3) | |
| CS2c | 8 (5.1) | 0 (0) | |
| CS3 | 21 (13.4) | 1 (9.1) | 0.6781 |
| CS1 vs. >CS1 (all GCT) | |||
| CS1 | 244 (65.4) | 37 (75.5) | |
| >CS1 | 129 (34.6) | 12 (24.5) | 0.1590 |
| CS1 vs. >CS1 seminoma | |||
| CS1 | 171 (79.2) | 30 (78.9) | |
| >CS1 | 45 (20.8) | 8 (21.1) | 0.9755 |
| CS1 vs. >CS1 nonseminoma | |||
| CS1 | 73 (46.5) | 7 (63.6) | |
| >CS1 | 84 (53.5) | 4 (36.4) | 0.2712 |
| Nonseminoma with teratoma components | |||
| No teratoma | 82 (52.2) | 6 (54.6) | |
| With teratoma | 75 (47.8) | 5 (45.5) | 0.8818 |
| Tumour right-sided (all GCT) | 175 (49.4) | 26 (55.3) | 0.2943 |
| Tumour right-sided(seminoma) | 108 (52.7) | 21 (58.3) | 0.0821 |
| Tumour right-sided (nonseminoma) | 67 (45.0) | 5 (45.5) | 0.9885 |
GCT, germ cell tumour; CS, clinical stages; pT, pathological.
Fig. 3.
Comparison of age groups ≤50 and >50 years. Horizontal boxes denote relative proportions (%) of clinical characteristics in age groups ≤50 and >50 years.
Discussion
There is a paucity of studies looking specifically to the presenting characteristics of testis cancer patients. Most of the recent reports on GCT focus on specific clinical issues in selected patient populations. Therefore, our dataset is somehow unique because it reveals the current and typical spectrum of clinical features of testicular GCTs arising in Central Europe. These data may thus serve as a contemporary standard GCT sample.
The following are the 4 core results: (1) the clinical profile of GCTs is multifaceted and as shown in the discussion, there appears to be geographical variation and temporal shifting of some characteristics. (2) Seminoma and nonseminoma have significantly different presenting features. (3) Primary tumour size is associated with clinical and pT-stages and curiously, with laterality. (4) Patients older than 50 years present with a somehow different clinical profile than the younger ones.
The median age of the entire patient group is 36 years which is almost identical with the median age of 35 years reported from a large multicentric study on contralateral biopsies conducted in Germany in the first decade of this century [20] and with a report from Regional Cancer Registries of Eastern Germany [21]. It is also in line with the observation of increasing age of testis cancer patients reported recently [10]. Notably, reports from southern European countries documented lower mean ages of 30.8 years [22, 23] and 31.8 years [24] and this finding is consistent with that of a recent report from the United States, where mean ages of 29.7 and 35.7 years, respectively, were found in GCT patients of Hispanic descent and in non-Hispanic white patients (p < 0.05) [25]. Although a formal comparison of the data reported here with ours is not reasonable because mean values would be compared with medians, the differences outlined here appear quite substantial and thus significant from a clinical point of view. The New Zealand Cancer Registry reported a median age of 36 years of the patients of European descent, while Maori patients with GCT presented at a significantly younger median age of 32 years [26]. Notably, a recent study from Japan reported a somewhat higher median age of 37 years [27].
The relative frequency of seminoma among all GCTs is 60.2%. This figure is slightly higher than the rate of 56% observed in Germany in the first decade of this century [10] and our finding probably confirms the ongoing shift to the increasing incidence of seminoma relative to nonseminoma [21, 28, 29]. The relative frequencies of seminoma of 60, 56 and 58% reported from the New Zealand white population [26], the United States [29], and the United Kingdom [12], respectively, are quite close to the finding of the present study. Corresponding to the divergent median patients' ages observed in various geographic regions, the relative incidence of seminoma appears to be lower in southern European countries [16, 23, 30]. Likewise, among American GCT patients of Hispanic descent, nonseminoma was predominant with 56.3% of cases compared to 43.7% in non-Hispanic white patients [25]. Interestingly, the relative incidence of seminoma is probably highest in Japan where rates of 62.7–63.4% are reported [27, 31]. By contrast, GCT patients from the Pacific area usually have nonseminoma-rates of 50% or more [26]. The geographic variations of age and of the distributions of histologic subtypes are noteworthy, epidemiologically, and certainly deserve further investigation to explore the impact of demographic, ethnic and socioeconomic factors on the pathogenesis and clinical course of testicular GCT.
Of the nonseminoma-patients, 47.6% were shown to have teratomatous components in the primary tumour, which is close to the proportion of 55% reported from another recent German series [32] and it is only a little different from the rates of 58.2% observed in a large population-based series in Germany in the 1970s [33], and likewise found in the Indiana University series of 644 patients [34]. The different prevalence rates of teratoma observed must be weighted cautiously because the detection of teratoma is highly dependent on histopathological technique and on the particular experience of the pathologist. Accordingly, histopathological classification of GCTs was found to be significantly different among pathologists with limited and with specific experience, respectively, regarding testicular pathology [35, 36]. Since teratoma is insensitive to chemotherapy and radiotherapy, knowledge about its presence may influence clinical decision-making [34]. As a result of the present study, it should be noted that teratoma may be encountered in roughly half of the nonseminomatous tumours.
Two thirds (66.6%) of all GCT patients presented with CS1. This finding is consistent with the rates of 69.3% [37], 71% [12], 71.5% [38] and 74% [20] reported from recent European trials, and with a rate of 68.0% reported from the United States in non-Hispanic white men [25], and it is also close to the rate of 71.3% observed in Japan [31]. An even higher proportion of 78% CS1 cases was documented in New Zealand white patients [26]. By contrast, Hispanic patients in the United States presented with a clearly lower frequency of CS1 of 57.4% [25], and accordingly, 2 series from Spain reported proportions of CS1 of 56.6 and 47.1% respectively [16, 30]. One US military hospital series comprising a high proportion of Hispanic men also reported a rate of <50% of localized stages [39]. In all, there appear to be ethnic differences with respect to primary staging mirroring the variations found with age at presentation and the relative incidence of seminomas. Another aspect is that the frequency of localized disease found in contemporary cohorts is clearly higher than the proportion of 50% usually reported from a series of patients treated some decades ago [9, 33, 40]. Thus, our data corroborate the observation of a shifting towards lower stages over time as reported previously [13, 38, 41, 42, 43].
Among the metastasized patients, 80% were considered to have good prognosis according to the IGCCCG classification, while 12 and 8% had intermediate and poor prognosis respectively. These results are in accordance with the US National Cancer Data Base, where frequencies of 82, 7, and 11%, respectively, are recorded for the good, intermediate, and poor prognosis groups [25]. However, these results are clearly at variance with the proportions of 60, 26 and 14% reported in the classical IGCCCG series [19] and with the rates of 63, 22 and 16% observed in Mannheim, Germany [32]. The differences most probably relate to markedly lower proportions of seminoma cases of only 11.2% in the classical IGCCCG series and even zero in the Mannheim series, while 37.6% of the present cohort had seminoma. Accordingly, a proportion of 69.2% of patients with good prognosis was reported in a large multi-institutional series in Spain where the proportion of seminoma were <20% in metastasized cases [16]. Probably, the original IGCCCG records overestimated the true incidence of the poor prognosis category because that original series was composed of patients from selected renowned tertiary referral centres [19]. In a primary care setting, the relative frequency of the good prognosis category is probably much higher than originally estimated. Moreover, the original IGCCCG data stems from patients treated in the 1980s and 1990s of the last century. In view of the stage-shifting towards lower stages during the last decades [12, 42], a higher proportion of the good prognosis category must be expected in contemporary series. In all, the proportions of prognostic categories found in our cohort appear to reflect the contemporary practice patterns on the primary care level.
The 2 subtypes of GCT, seminoma and nonseminoma are distinct entities, biologically and clinically, despite their common origin from germ cell neoplasia in situ. On average, seminoma patients of the present series were 10 years older than nonseminoma patients. While all other reports also found higher ages in seminoma patients [25, 29], a difference of more than 8 years between the 2 subtypes has only been reported from 2 Nordic series [37, 44], and 2 recent German investigations [10, 21]. The age difference between seminoma and nonseminoma seems to be markedly lower in southern European countries where differences of 5.9 years [22, 23] and even 3 years [45] have been documented. The geographic variation of the age differences among GCT subtypes points again to the apparent ethnic dissimilarities of GCTs. Generally, the reason for the large difference of age between seminoma and nonseminoma as observed in this study and in the Nordic countries remains elusive. One might hypothesize that the known shift towards higher ages of GCTs could be more active in the seminoma subtype.
Seminoma is known to follow a less aggressive course than nonseminoma. Accordingly, we found significantly more CS1 cases and localized primary tumours (pT1 stage) in seminoma than in nonseminoma. This finding is in line with that of all previous reports [16, 29]. The median size of the primary tumour was somewhat smaller in seminoma than in nonseminoma, but that difference was not significant, statistically. There was a trend towards a higher proportion of right-sided tumours among the seminoma patients (53.5 vs. 45.0% in nonseminoma); however, this difference was not significant either. All of the 3 classical serum tumour markers were significantly more expressed in nonseminomas than in seminomas. We noted a rather high expression of beta HCG in 28% of the seminoma patients. Previous investigations had reported expression rates of 18–21% [16, 24, 37, 46]. However, rates of 30–31% had been reported likewise [47, 48]. AFP was expressed in 60% of nonseminomas, while only isolated patients with seminoma had unspecific elevations of this marker [49]. The expression rate of LDH was also significantly higher in nonseminomas, which represents an unexpected finding [50, 51]. In aggregate, seminomas and nonseminomas are different from each other with regard to most of the clinical characteristics.
Primary tumour size is significantly larger in advanced clinical (>CS1) and in higher local pT stages (>pT1), both in seminoma and in nonseminoma. This finding corresponds to the experience made in seminoma, where increasing tumour size is significantly associated with the risk of progression [52]. Bhardwa et al. [53] noted larger tumour sizes in metastasized patients, but that association was not significant. No association between tumour size and metastatic spread was observed in a small German study conducted in the 1980s [54]. In all, the present data may point to some interrelationship of tumour size with the potential of metastatic spread also in nonseminoma. It is therefore of note that the trend towards decreasing tumour size observed during the last decades [13, 55] does obviously synchronize with the down-shifting of CS during the same time-span [12, 38]. A curious finding is the documentation of significantly larger median tumour size in left-sided disease in comparison to right-sided GCT (3.3 vs. 2.8 cm). Though the p-value is as low as 0.0008, the numerical difference between the 2 median values is only 0.5 cm and the inter IQRs are widely overlapping (right: 1.6–4.3; left: 2.0–5.0). Thus, a chance finding must be considered. But, histology might also contribute to the finding because seminomas tend to be smaller than nonseminoma and more seminomas were on the right side, though these 2 latter findings were not significant statistically. Nonetheless, the significant association of laterality with tumour size is noteworthy and deserves further attention. To date, no further data are available regarding this issue.
It is a well-settled experience that elderly patients with GCT are faced with lower over-all survival rates than younger individuals [38, 56, 57, 58, 59]. Also well acknowledged is the increasing age of presentation of GCT patients during the last decades [10, 21]. Therefore, patients' age is gaining increasing attention among care-givers and likewise, the parameter “age” has been considered an independent prognostic factor [60]. In a landmark study conducted in Germany in the 1970s, a proportion of only 7.2% of GCT patients were found to be aged >50 years [33]. Consistent with the age drift among GCT patients during the last decades, we noted as many as 11.6% of patients to be aged >50 years. Our figure is identical with a recent report from Japan [31] and close to data of the California Tumour Registry series, where 10% of GCT patients were older than 55 years [61]. We observed a significantly higher proportion of seminomas among the elderly patients (77.6 vs. 57.9% in the younger ones) confirming previous experience [58, 59, 62]. In accordance with other reports, we also noted a markedly higher proportion of organ-confined disease in patients aged >50 years than in the younger ones (75.5 vs. 65.4%). The predominance of CS1 is even more distinct in nonseminomas (63.6 vs. 46.5%). However, the differences are not significant statistically probably because of low numbers of nonseminomas. Primary tumour size and pT stages are not different among the age categories. We did not look to outcome in the present study. The higher proportions of seminoma and the higher frequency of localized disease stages among the elderly would point to a more favourable prognosis of this subgroup. But conversely, the survival rates of the elderly are evidently worse and as demonstrated herein, this shortfall cannot be explained with the presenting characteristics of the GCT patients. Obviously, treatment-related problems may translate into inferior survival rates of the elderly GCT-patients.
Limitations of the present study relate to the retrospective design with the imminent risk of selection bias. Although the overall sample size is certainly appropriate, some subgroups involve only small patient numbers, thus precluding significant statistical results. The lack of treatment results may be another shortfall, however, this study specifically aimed to analyse the presenting clinical features of GCT patients. One possible strength of the study is the homogeneous composition of the patient sample with consecutively accrued patients with testicular GCT at first presentation. Another one could be the large number of clinical data relating to each individual patient as well as the completeness of data allowing for statistical testing of associations among the various factors.
In conclusion, the present study documents the presenting clinical characteristics of testicular GCTs. We confirmed the predominance of low stages mainly in seminoma. The 2 histologic subtypes of GCT are different in terms of age, clinical staging, pT-staging and serum marker expressions. The size of the primary tumour appears to be associated with CS, pT-stages, and laterality. The comparison of the present results with data reported from other geographic regions reveals striking differences among ethnic groups regarding the clinical characteristics of GCT. These differences are still not understood and clearly deserve further clinical and epidemiological investigations.
Statement of Ethics
The work reported here does not involve research involving Human Participants and/or Animals. All patients were managed in accordance with the World Medical Association Declaration of Helsinki (2013).
Disclosure Statement
This study did not receive any funding. None of the authors has any potential conflicts of interest (financial or non-financial) with publishing the present report.
Acknowledgements
Prof. Thomas Loening and Prof. Guido Sauter and their coworkers provided valuable help in ascertaining and interpreting the histopathologic reports. Dr. Inken Dralle-Filiz and Dr. Benjamin Soyka-Hundt provided valuable help in ascertaining clinical data of the patients. Prof. Andreas Stang, Essen, gave valuable advice for preparing the manuscript.
References
- 1.Znaor A, Lortet-Tieulent J, Jemal A, Bray F. International variations and trends in testicular cancer incidence and mortality. Eur Urol. 2014;65:1095–1106. doi: 10.1016/j.eururo.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Stang A, Bray F, Dieckmann KP, Lortet-Tieulent J, Rusner C. Mortality of testicular cancer in east and west Germany 20 years after reunification: a gap not closed yet. Urol Int. 2015;95:160–166. doi: 10.1159/000381883. [DOI] [PubMed] [Google Scholar]
- 3.Rajpert-De Meyts E, McGlynn KA, Okamoto K, Jewett MA, Bokemeyer C. Testicular germ cell tumours. Lancet. 2016;387:1762–1774. doi: 10.1016/S0140-6736(15)00991-5. [DOI] [PubMed] [Google Scholar]
- 4.Adra N, Einhorn LH. Testicular cancer update. Clin Adv Hematol Oncol. 2017;15:386–396. [PubMed] [Google Scholar]
- 5.Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, Horwich A, Laguna MP, Nicolai N, Oldenburg J. Guidelines on testicular cancer: 2015 update. Eur Urol. 2015;68:1054–1068. doi: 10.1016/j.eururo.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 6.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 who classification of tumours of the urinary system and male genital organs-part a: renal, penile, and testicular tumours. Eur Urol. 2016;70:93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Beyer J, Albers P, Altena R, Aparicio J, Bokemeyer C, Busch J, Cathomas R, Cavallin-Stahl E, Clarke NW, Claßen J, Cohn-Cedermark G, Dahl AA, Daugaard G, de Giorgi U, de Santis M, de Wit M, de Wit R, Dieckmann KP, Fenner M, Fizazi K, Flechon A, Fossa SD, Germá Lluch JR, Gietema JA, Gillessen S, Giwercman A, Hartmann JT, Heidenreich A, Hentrich M, Honecker F, Horwich A, Huddart RA, Kliesch S, Kollmannsberger C, Krege S, Laguna MP, Looijenga LH, Lorch A, Lotz JP, Mayer F, Necchi A, Nicolai N, Nuver J, Oechsle K, Oldenburg J, Oosterhuis JW, Powles T, Rajpert-De Meyts E, Rick O, Rosti G, Salvioni R, Schrader M, Schweyer S, Sedlmayer F, Sohaib A, Souchon R, Tandstad T, Winter C, Wittekind C. Maintaining success, reducing treatment burden, focusing on survivorship: Highlights from the third European consensus conference on diagnosis and treatment of germ-cell cancer. Ann Oncol. 2013;24:878–888. doi: 10.1093/annonc/mds579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy BJ, Schmidt JD, Winchester DP, Peace BL, Natarajan N, Mettlin C. National survey of patterns of care for testis cancer. Cancer. 1987;60:1921–1930. doi: 10.1002/1097-0142(19871015)60:8<1921::aid-cncr2820600842>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 9.Mead GM. Testicular cancer and related neoplasms. BMJ. 1992;304:1426–1429. doi: 10.1136/bmj.304.6839.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruf CG, Isbarn H, Wagner W, Fisch M, Matthies C, Dieckmann KP. Changes in epidemiologic features of testicular germ cell cancer: age at diagnosis and relative frequency of seminoma are constantly and significantly increasing. Urol Oncol. 2014;32(33):e1–e6. doi: 10.1016/j.urolonc.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Osswald M, Harlan LC, Penson D, Stevens JL, Clegg LX. Treatment of a population based sample of men diagnosed with testicular cancer in the united states. Urol Oncol. 2009;27:604–610. doi: 10.1016/j.urolonc.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powles TB, Bhardwa J, Shamash J, Mandalia S, Oliver T. The changing presentation of germ cell tumours of the testis between 1983 and 2002. BJU Int. 2005;95:1197–1200. doi: 10.1111/j.1464-410X.2005.05504.x. [DOI] [PubMed] [Google Scholar]
- 13.Heinzelbecker J, Katzmarzik M, Weiss C, Trojan L, Michel MS, Haecker A. Changes of stage, predictive factors and adjuvant treatment modalities in seminomatous testicular cancer from 1987 to 2007 and their impact on the status of metastasis, recurrence-free and overall survival: A single-center analysis. Urol Int. 2011;87:282–287. doi: 10.1159/000329768. [DOI] [PubMed] [Google Scholar]
- 14.Fosså SD, Aass N, Kaalhus O. Testicular cancer in young Norwegians. J Surg Oncol. 1988;39:43–63. doi: 10.1002/jso.2930390110. [DOI] [PubMed] [Google Scholar]
- 15.Bosl GJ, Geller N, Cirrincione C, Hajdu SI, Whitmore W, Jr, Nisselbaum J, Vugrin D, Golbey RB. Interrelationships of histopathology and other clinical variables in patients with germ cell tumors of the testis. Cancer. 1983;51:2121–2125. doi: 10.1002/1097-0142(19830601)51:11<2121::aid-cncr2820511128>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Germa-Lluch JR, Garcia del Muro X, Maroto P, Paz-Ares L, Arranz JA, Guma J, Alba E, Sastre J, Aparicio J, Fernandez A, Barnadas A, Terrassa J, Saenz A, Almenar D, Lopez-Brea M, Climent MA, Sanchez MA, Lasso de la Vega R, Berenguer G, Perez X. Clinical pattern and therapeutic results achieved in 1490 patients with germ-cell tumours of the testis: The experience of the Spanish germ-cell cancer group (GG) Eur Urol. 2002;42:553–563. doi: 10.1016/s0302-2838(02)00439-6. [DOI] [PubMed] [Google Scholar]
- 17.Woldu SL, Matulay JT, Clinton TN, Singla N, Krabbe LM, Hutchinson RC, Sagalowsky A, Lotan Y, Margulis V, Bagrodia A. Impact of hospital case volume on testicular cancer outcomes and practice patterns. Urol Oncol. 2018;36:14.e7–14.e15. doi: 10.1016/j.urolonc.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Wittekind C. TNM-Klassifikation der Hodentumoren Definitionen und Voraussetzungen einer richtigen Anwendung. Pathologe. 2014;35:252–255. doi: 10.1007/s00292-014-1904-4. [DOI] [PubMed] [Google Scholar]
- 19.International Germ Cell Collaborative Group International germ cell consensus classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- 20.Dieckmann KP, Kulejewski M, Pichlmeier U, Loy V. Diagnosis of contralateral testicular intraepithelial neoplasia (TIN) in patients with testicular germ cell cancer: systematic two-site biopsies are more sensitive than a single random biopsy. Eur Urol. 2007;51:175–185. doi: 10.1016/j.eururo.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 21.Stang A, Rusner C, Stabenow R. Changing epidemiologic features of testicular germ cell cancer in Germany: corroboration at population level. Urol Oncol. 2013;31:1839–1840. doi: 10.1016/j.urolonc.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Bonet AS, Muñoz-Delgado EG, Vico FJ, Ruiz JC, Chapado MS. Analysis of clinical-pathologic variables, staging and prognostic groups, and therapeutic results of 106 germ-cell testicular tumors. Arch Esp Urol. 2011;64:972–980. [PubMed] [Google Scholar]
- 23.Dusaud M, Durand X, Desfemmes FR, Molimard B, Bayoud Y, Audouin M, Houlgatte A. A 20-year epidemiological review of testis cancer at a French military hospital. Mil Med. 2015;180:1184–1188. doi: 10.7205/MILMED-D-14-00604. [DOI] [PubMed] [Google Scholar]
- 24.Lorenzo L, Marzullo L, Luján S, Rogel R, Broseta E, Boronat F. Clinical and survival patterns among patients with primary testicular cancer. Rev Int Androl. 2017;15:39–44. [Google Scholar]
- 25.Woldu SL, Aydin AM, Rao AV, Hutchinson RC, Singla N, Clinton TN, Krabbe LM, Passoni NM, Raj GV, Miller DS, Amatruda JF, Sagalowsky AI, Lotan Y, Arriaga Y, Margulis V, Bagrodia A. Differences at presentation and treatment of testicular cancer in hispanic men: Institutional and national hospital-based analyses. Urology. 2018;112:103–111. doi: 10.1016/j.urology.2017.08.059. [DOI] [PubMed] [Google Scholar]
- 26.Gurney JK, Sarfati D, Stanley J. Obscure etiology, unusual disparity: the epidemiology of testicular cancer in New Zealand. Cancer Causes Control. 2015;26:561–569. doi: 10.1007/s10552-015-0533-4. [DOI] [PubMed] [Google Scholar]
- 27.Miki T, Kamoi K, Fujimoto H, Kanayama HO, Ohyama C, Suzuki K, Nishiyama H, Eto M, Naito S, Fukumori T, Kubota Y, Takahashi S, Mikami K, Homma Y. Clinical characteristics and oncological outcomes of testicular cancer patients registered in 2005 and 2008: the first large-scale study from the Cancer Registration Committee of the Japanese Urological Association. Int J Urol. 2014;21:S1–S6. doi: 10.1111/iju.12441. [DOI] [PubMed] [Google Scholar]
- 28.Trabert B, Chen J, Devesa SS, Bray F, McGlynn KA. International patterns and trends in testicular cancer incidence, overall and by histologic subtype, 1973–2007. Andrology. 2015;3:4–12. doi: 10.1111/andr.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sokoloff MH, Joyce GF, Wise M, Urologic diseases in America project Testis cancer. J Urol. 2007;177:2030–2041. doi: 10.1016/j.juro.2007.01.127. [DOI] [PubMed] [Google Scholar]
- 30.Molina Saera J, Aparicio Urtasun J, Díaz Beveridge R, Palomar Abad L, Giménez Ortiz A, Ponce Lorenzo J, Montalar Salcedo J. Epidemiological pattern and time trends in testicular germ-cell tumors: a single institution 20-year experience. Clin Transl Oncol. 2006;8:588–593. doi: 10.1007/s12094-006-0064-2. [DOI] [PubMed] [Google Scholar]
- 31.Kawai T, Tanaka Y, Cancer Registration Committee of the Japanese Urological Association Clinical characteristics of testicular germ cell tumors in patients aged 50 years and older: a large-scale study from the Cancer Registration Committee of the Japanese Urological Association. Int J Urol. 2017;24:124–128. doi: 10.1111/iju.13268. [DOI] [PubMed] [Google Scholar]
- 32.Heinzelbecker J, Katzmarzik M, Weiss C, Trojan L, Haecker A. During twenty years of cisplatin-based therapy the face of nonseminomatous testicular germ cell tumors is still changing: an evaluation of presentation, management, predictive factors and survival. Int Braz J Urol. 2013;39:10–21. doi: 10.1590/S1677-5538.IBJU.2013.01.03. [DOI] [PubMed] [Google Scholar]
- 33.Weißbach L, Hildenbrand G. München: W. Zuckschwerdt Verlag; 1982. Register und Verbundstudie für Hodentumoren - Bonn. [Google Scholar]
- 34.Beck SD, Foster RS, Bihrle R, Ulbright T, Koch MO, Wahle GR, Einhorn LH, Donohue JP. Teratoma in the orchiectomy specimen and volume of metastasis are predictors of retroperitoneal teratoma in post-chemotherapy nonseminomatous testis cancer. J Urol. 2002;168:1402–1404. doi: 10.1016/S0022-5347(05)64458-8. [DOI] [PubMed] [Google Scholar]
- 35.Sesterhenn IA, Weiss RB, Mostofi FK, Stablein DM, Rowland RG, Falkson G, Rivkind SE, Vogelzang NJ. Prognosis and other clinical correlates of pathologic review in stage I and II testicular carcinoma: a report from the testicular cancer intergroup study. J Clin Oncol. 1992;10:69–78. doi: 10.1200/JCO.1992.10.1.69. [DOI] [PubMed] [Google Scholar]
- 36.Sharma P, Dhillon J, Agarwal G, Zargar-Shoshtari K, Sexton WJ. Disparities in interpretation of primary testicular germ cell tumor pathology. Am J Clin Pathol. 2015;144:289–294. doi: 10.1309/AJCPJTX8R6CVWSRW. [DOI] [PubMed] [Google Scholar]
- 37.Sundström J, Salminen E, Nurmi M, Toppari J, Pöllänen P, Pelliniemi LJ, Huhtala S, Rajala P, Laato M. Management of testicular cancer - 16 years' experience from southwest Finland. Scand J Urol Nephrol. 2001;35:21–25. doi: 10.1080/00365590151030723. [DOI] [PubMed] [Google Scholar]
- 38.Verhoeven RH, Gondos A, Janssen-Heijnen ML, Saum KU, Brewster DH, Holleczek B, Crocetti E, Rosso S, Hakulinen T, Aareleid T, Brenner H, EUNICE Survival Working Group Testicular cancer in Europe and the USA: survival still rising among older patients. Ann Oncol. 2013;24:508–513. doi: 10.1093/annonc/mds460. [DOI] [PubMed] [Google Scholar]
- 39.Cooper DE, L'esperance JO, Christman MS, Auge BK. Testis cancer: a 20-year epidemiological review of the experience at a regional military medical facility. J Urol. 2008;180:577–581. doi: 10.1016/j.juro.2008.04.032. discussion 581–582. [DOI] [PubMed] [Google Scholar]
- 40.MacKay EN, Sellers AH. A statistical review of malignant testicular tumours based on the experience of the Ontario Cancer Foundation Clinics, 1938–1961. Can Med Assoc J. 1966;94:889–899. [PMC free article] [PubMed] [Google Scholar]
- 41.Schultz HP, Arends J, Barlebo H, Brincker H, Stroyer-Christoffersen I, Engelholm SA, Gammelgaard PA, Genster H, Hansen HH, Krag Jacobsen G, et al. Testicular carcinoma in Denmark 1976–1980. Stage and selected clinical parameters at presentation. Acta Radiol Oncol. 1984;23:249–253. doi: 10.3109/02841868409136020. [DOI] [PubMed] [Google Scholar]
- 42.Hernes EH, Harstad K, Fossa SD. Changing incidence and delay of testicular cancer in southern norway (1981–1992) Eur Urol. 1996;30:349–357. doi: 10.1159/000474195. [DOI] [PubMed] [Google Scholar]
- 43.Bosl GJ, Geller NL, Chan EY. Stage migration and the increasing proportion of complete responders in patients with advanced germ cell tumors. Cancer Res. 1988;48:3524–3527. [PubMed] [Google Scholar]
- 44.Agnarsson BA, Gudbjartsson T, Einarsson GV, Magnusson K, Thoroddsen A, Bergthorsson JT, Amundadottir L, Barkardottir RB, Björnsson J. Testicular germ cell tumours in Iceland: a nationwide clinicopathological study. APMIS. 2006;114:779–783. doi: 10.1111/j.1600-0463.2006.apm_468.x. [DOI] [PubMed] [Google Scholar]
- 45.Lallave Martín F, Lomas Garrido M, Laguna Alvarez E, Asuar Aydillo S, Murillo Mirat J, Ramírez Zambrana A, Molina Suarez JL. [Testicular germ cell tumours: descriptive study of 13 years of experience in the health care area of Badajoz] Arch Esp Urol. 2007;60:531–537. doi: 10.4321/s0004-06142007000500005. [DOI] [PubMed] [Google Scholar]
- 46.Neumann A, Keller T, Jocham D, Doehn C. [Human placental alkaline phosphatase (HPlAP) is the most frequently elevated serum marker in testicular cancer] Aktuelle Urol. 2011;42:311–315. doi: 10.1055/s-0031-1271545. [DOI] [PubMed] [Google Scholar]
- 47.Rüther U, Rothe B, Grunert K, Bader H, Sessler R, Nunnensiek C, Rassweiler J, Lüthgens M, Eisenberger F, Jipp P. Role of human chorionic gonadotropin in patients with pure seminoma. Eur Urol. 1994;26:129–133. doi: 10.1159/000475361. [DOI] [PubMed] [Google Scholar]
- 48.Hartmann M, Pottek T, Bussar-Maatz R, Weissbach L. Elevated human chorionic gonadotropin concentrations in the testicular vein and in peripheral venous blood in seminoma patients. An analysis of various parameters. Eur Urol. 1997;31:408–413. doi: 10.1159/000474498. [DOI] [PubMed] [Google Scholar]
- 49.Dieckmann KP, Anheuser P, Simonsen H, Höflmayer D. Pure testicular seminoma with non-pathologic elevation of alpha fetoprotein: a case series. Urol Int. 2017;99:353–357. doi: 10.1159/000478706. [DOI] [PubMed] [Google Scholar]
- 50.von Eyben FE. Laboratory markers and germ cell tumors. Crit Rev Clin Lab Sci. 2003;40:377–427. doi: 10.1080/10408360390247814. [DOI] [PubMed] [Google Scholar]
- 51.Gilligan TD, Seidenfeld J, Basch EM, Einhorn LH, Fancher T, Smith DC, Stephenson AJ, Vaughn DJ, Cosby R, Hayes DF. American society of clinical oncology clinical practice guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol. 2010;28:3388–3404. doi: 10.1200/JCO.2009.26.4481. [DOI] [PubMed] [Google Scholar]
- 52.Boormans JL, Mayor de Castro J, Marconi L, Yuan Y, Laguna Pes MP, Bokemeyer C, Nicolai N, Algaba F, Oldenburg J, Albers P. Testicular tumour size and rete testis invasion as prognostic factors for the risk of relapse of clinical stage I seminoma testis patients under surveillance: a systematic review by the testicular cancer guidelines panel. Eur Urol. 2017 doi: 10.1016/j.eururo.2017.09.025. pii S0302-2838(17)30826-6. [DOI] [PubMed] [Google Scholar]
- 53.Bhardwa JM, Powles T, Berney D, Baithun S, Nargund VH, Oliver RT. Assessing the size and stage of testicular germ cell tumours: 1984–2003. BJU Int. 2005;96:819–821. doi: 10.1111/j.1464-410X.2005.05748.x. [DOI] [PubMed] [Google Scholar]
- 54.Bussar-Maatz R, Weissbach L. Beziehungen zwischen Primärtumor und Metastasierung. Beitr Onkol (Karger Verlag, Basel) 1988;28:170–1770. [Google Scholar]
- 55.McGuinness LA, Obeidat S, Hickerton B, Long R. Has increasing public health awareness influenced the size of testicular tumours among adult populations over the last 40 years? J Public Health (Oxf) 2017;39:90–94. doi: 10.1093/pubmed/fdw014. [DOI] [PubMed] [Google Scholar]
- 56.Bach D, Weissbach L, Tschubel K, Muller R, Vahlensieck W, Gedigk P. [Particular aspects of testicular tumor in the elderly (author's transl)] MMW Munch Med Wochenschr. 1977;119:291–296. [PubMed] [Google Scholar]
- 57.Hatton MQ, Paul J, Harding M, MacFarlane G, Robertson AG, Kaye SB. Changes in the incidence and mortality of testicular cancer in Scotland with particular reference to the outcome of older patients treated for non-seminomatous germ cell tumours. Eur J Cancer. 1995;31A:1487–1491. doi: 10.1016/0959-8049(95)00298-w. [DOI] [PubMed] [Google Scholar]
- 58.Inci K, Dogan HS, Akdogan B, Ergen A, Tasar C, Ozen H. Does age affect the prognosis of patients with testicular germ cell tumor? Urol Int. 2007;79:117–123. doi: 10.1159/000106323. [DOI] [PubMed] [Google Scholar]
- 59.Spermon JR, Witjes JA, Kiemeney LA. Difference in stage and morphology-adjusted survival between young and elderly patients with a testicular germ cell tumor. Urology. 2002;60:889–893. doi: 10.1016/s0090-4295(02)01886-1. [DOI] [PubMed] [Google Scholar]
- 60.Abdel-Rahman O. Incorporating age into international germ cell consensus classification (IGCCC): a time to move forward? Expert Rev Anticancer Ther. 2018;18:101–105. doi: 10.1080/14737140.2018.1403321. [DOI] [PubMed] [Google Scholar]
- 61.Krain LS. Testicular cancer in California from 1942 to 1969: the California Tumor Registry experience. Oncology. 1973;27:45–51. doi: 10.1159/000224718. [DOI] [PubMed] [Google Scholar]
- 62.Wheater MJ, Manners J, Nolan L, Simmonds PD, Hayes MC, Mead GM. The clinical features and management of testicular germ cell tumours in patients aged 60 years and older. BJU Int. 2011;108:1794–1799. doi: 10.1111/j.1464-410X.2011.10252.x. [DOI] [PubMed] [Google Scholar]