Abstract
Purpose
To characterize prednisone use in pregnant women with rheumatoid arthritis using individual-level heat-maps and clustering individual trajectories of prednisone dose, and to evaluate the association between prednisone dose trajectory groups and gestational length.
Methods
This study included pregnant women with rheumatoid arthritis who enrolled in the MotherToBaby Autoimmune Diseases in Pregnancy Study (2003-2014)before gestational week 20 and reported prednisone use without another oral glucocorticoid during pregnancy (n=254). Information on medication use and pregnancy outcomes was collected by telephone interview plus by medical record review. Prednisone daily dose and cumulative dose were plotted by gestational day using a heat-map for each individual. K-means clustering was used to cluster individual trajectories of prednisone dose into groups. The associations between trajectory group and demographics, disease severity measured by the Health Assessment Questionnaire at enrollment, and gestational length were evaluated.
Results
Women used prednisone 3 to 292 days during pregnancy, with daily doses ranging from <1-60 mg. Total cumulative dose ranged from 8-6,225 mg. Disease severity, non-biologic disease modifying anti-rheumatic drug (nb-DMARD) use, and gestational length varied significantly by trajectory group. After adjusting for disease severity, nb-DMARD use, and other covariates, the highest vs lowest daily dose trajectory group was associated with reduced gestational age at delivery (β: -2.3weeks (95%: -3.4, -1.3)), as was the highest vs lowest cumulative dose trajectory group (β: -2.6weeks (95%: -3.6, -1.5)).
Conclusions
In pregnant women with rheumatoid arthritis, patterns of higher prednisone dose were associated with shorter gestational length compared with lower dose.
Keywords: cluster analysis, glucocorticoids, prednisone, pregnancy, rheumatoid arthritis, gestational age
Introduction
Oral glucocorticoids are used to treat symptoms caused by several chronic diseases, including asthma, rheumatoid arthritis, and other autoimmune diseases. For pregnant women with these conditions, oral glucocorticoids may be used to manage flares or when other treatments regarded as riskier for chronic management are discontinued.1-4 Approximately 1-2% of pregnant women,5-7 and 20-40% of pregnant women with rheumatoid arthritis8,9 use oral glucocorticoids.
Despite frequent use of oral glucocorticoids during pregnancy among women with autoimmune diseases, the medications have been associated with a range of adverse perinatal outcomes in some studies including cleft lip with or without cleft palate, maternal infection, gestational diabetes, preeclampisa, preterm birth, and gestational age at delivery.10-21 Several studies have reported that oral glucocorticoid use during pregnancy is associated with an approximately two-fold or higher increased risk of preterm birth compared with women not using oral glucocorticoids.16-21 Notably, higher rheumatoid arthritis severity/activity, which impacts prednisone use and dose, is also associated with an increased risk of preterm birth.22 The literature contains limited information regarding the relation between oral glucocorticoid dose and gestational length.23,24
Oral glucocorticoid duration and dosage of use depends on a number of factors including disease severity, patient and physician preference, and whether the medication is being used to treat or prevent a flare.1,2 Defining medication exposure according to trimester of use removes exposure information, including dose changes, intensity of use, and coverage gaps, that is important for understanding how the medications are used during pregnancy and how these factors impact pregnancy outcomes.
To better understand oral glucocorticoid utilization patterns and exposure levels in women with rheumatoid arthritis during pregnancy, we aimed to characterize prednisone use according to daily and cumulative dose using individual plots and clustering individual trajectories. We also aimed to evaluate whether patterns of prednisone use were associated with gestational age at delivery. We were interested in the oral route of glucocorticoids because of their systemic effects. Moreover, we focused on prednisone use in particular because it is the most commonly used oral glucocorticoid during pregnancy,5,7 and it accounted for 98% of all oral glucocorticoid use in the study population.
Methods
Data source
This study used data collected by the MotherToBaby Autoimmune Diseases in Pregnancy Study, an ongoing prospective cohort study that aims to evaluate the effects of select medications and autoimmune diseases during pregnancy.22,25 Participants were from the United States and Canada and were self-referred, referred by their health care providers, or referred by MotherToBaby, a free counseling service of the Organization of Teratology Information Specialists (OTIS) that provides evidence-based information regarding medications and other exposures during pregnancy and lactation.
Trained study staff conducted semi-structured interviews with participants via telephone up to four times: at the time of study enrollment, at approximately 24 and 32 weeks' gestation, and after delivery. Interviewers collected data from participants regarding demographics, socio-economic status (Hollingshead categories based on maternal and paternal education and occupation; possible range from highest=1 to lowest=5)26 maternal health and behavior, names, dates, and dose of each medication used during pregnancy, and pregnancy outcomes. Additionally, interviewers administered the Health Assessment Questionnaire Disability Index (HAQ; possible range from 0=no disability to 3=completely disabled), a validated self-assessment questionnaire to measure rheumatoid arthritisseverity27,28 Medical records were requested from all participants to help verify maternal report.
Study population
Pregnant women with a last menstrual period between 2003 and 2014 were eligible for this study if they reported having rheumatoid arthritis and enrolled in the MotherToBaby Autoimmune Diseases in Pregnancy Study before gestational week 20 (Supplemental Figure 1). Women were excluded who had a spontaneous or therapeutic abortion, were lost to follow up or withdrew, or had incomplete pregnancy outcome information. Of the 526 women meeting the basic inclusion criteria, 55% reported using prednisone on at least one day during pregnancy. We excluded 239 (45%) women because they did not report any oral glucocorticoid use during pregnancy, 6(1%) women because they used oral glucocorticoids other than prednisone, 6 (1%) women because they were missing all prednisone dose information, and 21 (4%) women who had another Autoimmune Diseases in Pregnancy Study-qualifying diagnosis, i.e., ankylosing spondylitis, Crohn's disease, psoriasis, or psoriatic arthritis, because prednisone may have been used for these indications rather than rheumatoid arthritis. In total, there were 254 women included in the current study.
Exposure information
The total number of days of prednisone use during pregnancy and prednisone daily dose and cumulative dose since the beginning of pregnancy were calculated for each woman on each gestational day using women's reported start and stop dates, frequency of use during start and stop dates, and doses. There were 6 women who were missing prednisone dose for some, but not all, of the days on which they reported using prednisone (see paragraph below). Median, minimum, and maximum total number of days of prednisone use, mean daily dose for days on which prednisone use was reported, and total cumulative dose during pregnancy were calculated.
Individual plots
Prednisone daily dose and cumulative dose on each gestational day were plotted using a heat-map graphic for each individual, with gestational day as the x-axis. The plot legends were capped at ≥50 mg of prednisone per day and ≥2,000 mg of cumulative prednisone dose. The 6 women with incomplete prednisone dose information were identified with asterisks on the individual plots.
Trajectory groups
We grouped women in K clusters of similar individual trajectories of prednisone dose using k-means clustering in R statistical software29 (with default settings) on our longitudinal dataset that contained dose per gestational day. This approach classifies each woman into the cluster in which the Euclidean distance between her dose on each day and the cluster mean dose on each day is minimized. This approach requires that women have the same number of follow-up days. To avoid including postpartum days for women with shorter gestational lengths, a uniform window of observation that ended before delivery was needed. Therefore, the k-means clustering analysis was restricted to women who did not deliver before 32 gestational weeks and was limited to prednisone use during the first 32 gestational weeks. The algorithm was considered for K=2 to K=10. To select the number K of clusters for the daily dose analysis, we required the cluster to have no fewer than 5% of the total number of women and required the trajectories to be clinically meaningful. Setting K=4 satisfied these criteria. We also set K=4 for the cumulative dose analysis for consistency between the daily dose and cumulative dose clustering analyses. We plotted the observed daily mean and 95% CI for each cluster, i.e., trajectory group, on each gestational day. Then we plotted the dose on each gestational day for the first 32 gestational weeks using heat maps for each individual stratified by trajectory group. For each trajectory group, we calculated the mean of members' mean daily dose of prednisone on days of use and the mean total cumulative dose of prednisone during the first 32 gestational weeks.
We evaluated the associations between the trajectory groups and gestational age at enrollment (continuous), maternal age (continuous), race and ethnicity (non-Hispanic White versus other), socio-economic status (Hollingshead categories 1 and 2 vs 3, 4 and 5), pre-pregnancy overweight/obesity (body mass index ≥25 vs <25), rheumatoid arthritis severity measured by the HAQ at the time of enrollment (continuous), any use of the following medications during the first 32 gestational weeks, separately: biologic disease modifying antirheumatic drug (b-DMARD), non-biologic disease modifying antirheumatic drug (nb-DMARD), nonsteroidal anti-inflammatory drug (NSAID), and gestational age at delivery (continuous) were evaluated with chi-square and ANOVA tests. Using linear regression, we estimated beta coefficients and (CI) for the association between the trajectory groups and gestational age at delivery adjusting for the variables above as well multiple gestation, as it is a strong predictor of preterm birth. For these models, the reference groups were those with lowest daily and cumulative doses, and three women with missing covariate values were excluded.
The MotherToBaby Pregnancy Studies were approved by the University of California, San Diego Institutional Review Board and the current study was declared exempt.
Results
Cohort characteristics
The mean gestational age at enrollment was 11.1 weeks (standard deviation: 4.5 weeks) and the mean maternal age was 32.4 years (standard deviation: 4.6 years) (Table 1). Most women were non-Hispanic White (76.8%) and had the highest 2 levels of socio-economic status (78.0%). At the time of enrollment, the mean HAQ score was 0.7. Moreover, use of nb-DMARDs, b-DMARDs, and NSAIDs was common.
Table 1.
Summary of study population characteristics and prednisone use.
| Characteristics and Prednisone Use | N=254 |
|---|---|
| Gestational Week at Study Enrollment, mean (SD) | 11.1 (4.5) |
| Maternal age, mean (SD) | 32.4 (4.6) |
| Race/Ethnicity, n (%) | |
| Non-Hispanic White | 195 (76.8) |
| Other | 32 (12.6) |
| Missing or Not Available* | 27 (10.6) |
| Highest Socioeconomic Status, Hollingshead Categories 1 or 2, n (%)† | 198 (78.0) |
| Primiparous, n (%) | 126 (49.6) |
| Pre-pregnancy Overweight or Obese, n (%) | 84 (33.1) |
| Multiple Gestation, n (%) | 16 (6.3) |
| Health Assessment Questionnaire at Enrollment, mean (SD)‡ | 0.7 (0.7) |
| Biologic Disease Modifying Antirheumatic Drug, n (%)§ | 196 (77.2) |
| Non-Biologic Disease Modifying Antirheumatic Drug, n (%)§ | 122 (48.0) |
| Non-Steroidal Anti-Inflammatory Drug, n (%)§ | 134 (52.8) |
| Total Cumulative Days of Prednisone During Pregnancy, median (min, max) | 199.5 (3, 292) |
| Average Daily Dose (mg) of Prednisone During Pregnancy, median (min, max)‖ | 7 (<1, 60) |
| Total Cumulative Dose (mg) of Prednisone During Pregnancy, median (min, max) | 1,234 (8, 6,225) |
Abbreviations: min, minimum; max, maximum; SD, standard deviation.
Not available due to change in data collection of race and ethnicity.
Calculated using Hollingshead categories based on maternal and paternal education and occupation; Possible Range: 1, highest to 5, lowest. Missing for 2 women.
Missing for 1 woman.
Use during pregnancy before gestational week 32.
Average daily dose was calculated for days on which prednisone was used.
Total cumulative days of use, daily dose, and total cumulative dose of prednisone
Women used prednisone on 3 to 292 days during pregnancy (Table 1), with a median total of 199.5 days. The range of prednisone daily doses varied widely with some women taking less than 1 mg and others taking up to 60 mg, and the median daily dose was 7 mg. Total cumulative dose during pregnancy ranged from 8-6,225 mg, and the median total cumulative dose was 1,234 mg.
Individual plots
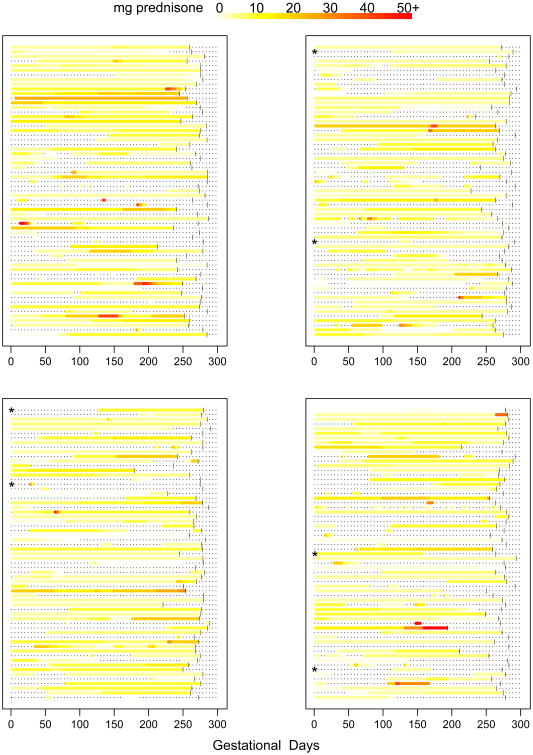
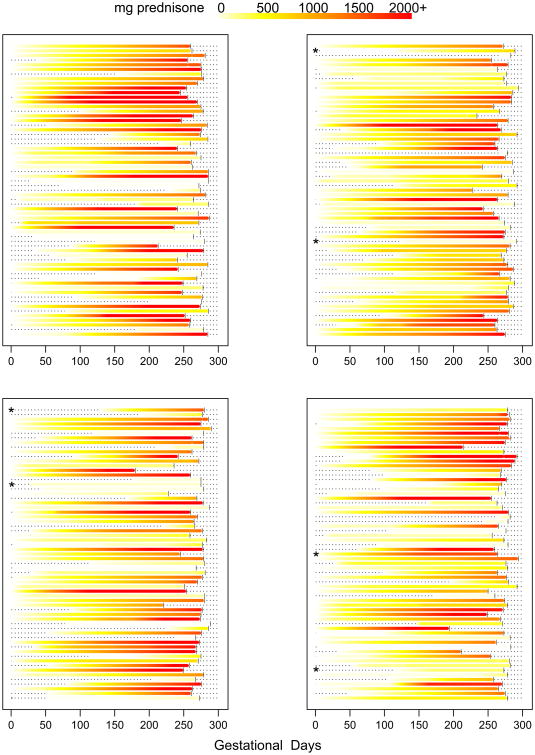
Prednisone timing of use, daily dose, and cumulative dose during pregnancy was variable across individuals (Figures 1-2). After initiating prednisone use during pregnancy, total cumulative dose tended to accumulate gradually for most women.
Figure 1.
Daily dose of prednisone during pregnancy for each individual by gestational day. Within the graphs, each horizontal line represents a woman's pregnancy. The colors encode the reported dose of prednisone used on each gestational day. Vertical bars represent the delivery day. The dashes represent days during pregnancy with no prednisone use. Asterisks indicate women with incomplete prednisone dose information. The color legend for daily dose is depicted at the top of the figure.
Figure 2.
Cumulative dose of prednisone during pregnancy for each individual by gestational day. Within the graphs, each horizontal line represents a woman's pregnancy. The colors encode the reported cumulative dose of prednisone used since the start of pregnancy on each gestational day. Vertical bars represent the delivery day. The dashes to the left of the colors represent days before prednisone was initiated during pregnancy. Asterisks indicate women with incomplete prednisone dose information. The color legend for cumulative dose is depicted at the top of the figure.
Trajectory groups
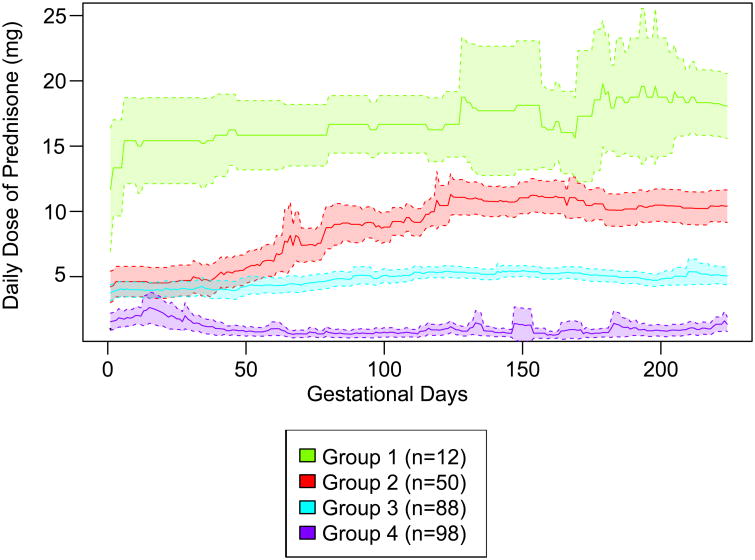
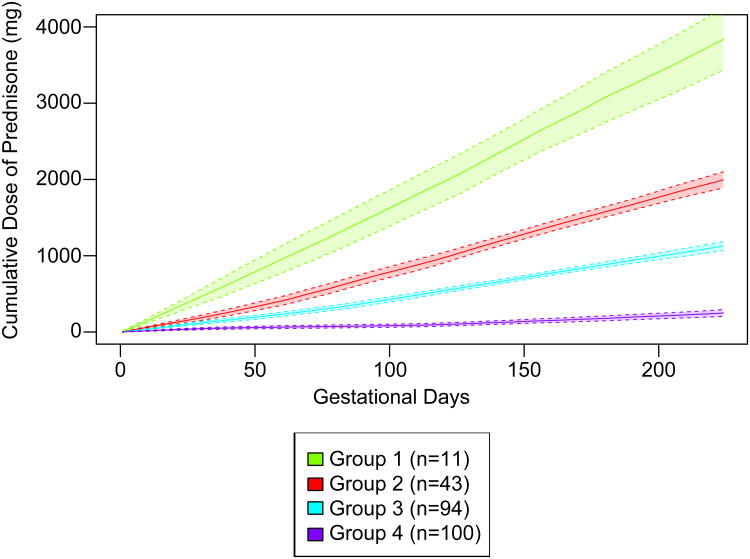
There were 248 women who delivered at or after 32 gestational weeks and were eligible for k-means clustering. The trajectory group means for members' mean daily dose of prednisone and mean total cumulative dose of prednisone during the first 32 gestational weeks are reported in Table 2. With four daily dose trajectory groups (Figure 3 and Supplemental Figures 2-5), the k-means model identified women who had on average 1) highest and slightly increasing prednisone doses (group 1, 5% of women), 2) increased prednisone doses in the later part of pregnancy (group 2, 20% of women), 3) relatively low and stable prednisone doses (group 3, 35% of women), and 4) relatively low prednisone doses with some fluctuations (group 4, 40% of women) during the first 32 gestational weeks. There was variability in daily dose patterns within each group (Supplemental Figures 2-5). With four cumulative dose trajectory groups (Figure 4 and Supplemental Figures 6-9), the k-means model identified women who tended to have 1) high cumulative dose early in pregnancy (group 1, 4% of women), 2) intermediate cumulative dose (group 2, 17% of women), 3) low cumulative dose (group 3, 38% of women), and 4) very low cumulative dose (group 4, 40% of women) during the first 32 gestational weeks.
Table 2.
Prednisone use during the first 32 gestational weeks and characteristics associated with trajectory groups, by trajectory group, n=248.
| Daily Dose Trajectory | Cumulative Dose Trajectory | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics and Prednisone Use | Group 1 | Group 2 | Group 3 | Group 4 | Group 1 | Group2 | Group 3 | Group 4 |
| n=12 | m=50 | n=88 | n=98 | n=11 | n=43 | n=94 | n=100 | |
| Total Cumulative Days of Prednisone During Pregnancy, mean (SD) | 223.4 (1.4) | 193.9 (40.6) | 193.2 (43.8) | 57.3 (61.5) | 223.5 (1.5) | 202.3 (38.6) | 190.1 (45.0) | 59.6 (62.5) |
| Average Daily Dose of Prednisone During the First 32 Gestational Weeks (mg), mean (SD)* | 16.9 (3.2) | 10.5 (3.3) | 5.8 (1.7) | 6.9 (7.8) | 17.2 (3.2) | 10.4 (3.5) | 6.3 (2.8) | 6.8 (7.5) |
| Total Cumulative Dose of Prednisone During the First 32 Gestational Weeks (mg), mean (SD) | 4,262.2 (863.5) | 2,355.0 (489.8) | 1,336.1 (311.5) | 331.9 (278.7) | 4,345.9 (853.0) | 2,394.6 (553.3) | 1,395.3 (413.5) | 370.7 (348.5) |
| HAQ at Enrollment, mean (SD)† | 0.9 (0.7) | 1.0 (0.6) | 0.5 (0.6) | 0.5 (0.6) | 0.8 (0.8) | 0.9 (0.6) | 0.6 (0.7) | 0.5 (0.6) |
| Non-Biologic Disease Modifying Antirheumatic Drug, n (%)§ | 1 (8.3) | 24 (48.0) | 51 (58.0) | 43 (43.9) | 1 (9.1) | 32 (48.8) | 54 (57.4) | 43 (43.0) |
| Gestational Age at Delivery (weeks), mean (SD) | 35.7 (1.4) | 37.6 (1.9) | 38.5 (1.7) | 38.8 (1.6) | 35.7 (1.5) | 37.8 (1.7) | 38.4 (1.8) | 38.7 (1.7) |
Abbreviation: HAQ,Health Assessment Questionnaire, SD, standard deviation. Daily dose: ANOVA p<0.01 for gestational age at delivery, HAQ at enrollment, and non-biologic disease modifying antirheumatic drugs. Cumulative dose: ANOVA p<0.01 for gestational age at delivery and HAQ at enrollment, ANOVA p=0.01 for non-biologic disease modifying antirheumatic drugs.
Average daily dose was calculated for days on which prednisone was used.
Missing for 1 woman.
Use during pregnancy before gestational week 32.
Figure 3.
Prednisone daily dose trajectory groups. The solid lines represent the observed mean dose on each gestational day for each group and the shaded areas represent 95% confidence intervals.
Figure 4.
Prednisone cumulative dose trajectory groups. The solid lines represent the observed mean cumulative dose on each gestational day for each group and the shaded areas represent 95% confidence intervals.
Most individuals fell in the same group based on both daily and cumulative dose; such that 92% of daily dose group 1 was in cumulative dose group 1, 72% of daily dose group 2 was in cumulative dose group 2, 83% of daily dose group 3 was in cumulative dose group 3, and 93% of daily dose group 4 was in cumulative dose group 4.
Daily dose and cumulative dose trajectory groups were not associated with gestational age at enrollment, maternal age, race/ethnicity, socio-economic status, pre-pregnancy overweight/obesity, biologic DMARDs, or NSAIDs. HAQ scores varied across trajectory groups (p-values <0.01) as did the frequency of nb-DMARD use before gestational week 32 (p<0.01 for daily dose trajectories and p=0.01 for cumulative dose trajectories), which was lowest in Group 1 for daily and cumulative dose trajectories. Furthermore, gestational age at delivery varied across trajectory groups (p-values <0.01); for daily dose trajectory groups the mean gestational age at delivery was 35.7 weeks for group 1, 37.6 weeks for group 2, 38.5 weeks for group 3, and 38.8 weeks for group 4 (Table 2). After adjusting for HAQ score at enrollment, nb-DMARD use, and other covariates, the highest two daily dose trajectory groups were associated with shorter gestational age at delivery compared with the lowest daily dose trajectory group (Table 3; Group 1: β: -2.3weeks (95% CI: -3.4, -1.3); Group 2: -0.6 weeks (95% CI: -1.3, 0.0)), as was the highest cumulative dose trajectory group compared with the lowest cumulative dose trajectory group (Group 1 β: -2.6 weeks (95% CI: -3.6, -1.5 weeks).
Table 3.
Multivariable adjusted* association between trajectory groups and gestational age at delivery, n=245.†
| Trajectory Group | n | Gestational Age at Delivery (weeks) β(95% Confidence Interval) |
|---|---|---|
| Daily dose | ||
| Group 1 | 12 | -2.3 (-3.4, -1.3) |
| Group 2 | 50 | -0.6 (-1.3, 0.0) |
| Group 3 | 88 | Reference |
| Group 4 | 95 | 0.3 (-0.2, 0.8) |
| Cumulative dose | ||
| Group 1 | 11 | -2.6 (-3.6, -1.5) |
| Group 2 | 43 | -0.5 (-1.2, 0.1) |
| Group 3 | 94 | -0.4 (-0.9, 0.1) |
| Group 4 | 97 | Reference |
Adjusted for gestational age at enrollment, maternal age, race and ethnicity, socio-economic status, pre-pregnancy overweight/obesity, Health Assesment Questionnaire score at enrollment, any use of each of the medications during the first 32 gestational weeks: biologic disease modifying antirheumatic drug, non-biologic disease modifying antirheumatic drug, nonsteroidal anti-inflammatory drug, and multiple gestation.
Excluding 3 women with missing covariate values.
Discussion
In this study of prospectively followed pregnancies in women with rheumatoid arthritis, 55% reported using prednisone. The individual-level plots in this study illustrate variability in prednisone dose, length of courses, and timing of courses during pregnancy. They also call attention to the high cumulative doses of prednisone that some women are exposed to during pregnancy. While prednisone doses varied greatly across women, clusters of individual trajectories of prednisone dose were associated with disease activity, nb-DMARD use, and gestational length. Women in the lowest daily dose trajectory group (Group 3), averaging 6 mg per day of prednisone use before gestational week 32, had relatively low levels of disease activity and had an average gestational age at delivery of nearly 39 weeks. Compared with this group, after adjusting for disease severity at enrollment, nb-DMARD use, and other characteristics, the 12 women in the highest daily dose trajectory group (Group 1), averaging 17 mg per day of prednisone use before gestational week 32, had shorter gestational lengths by more than two weeks on average. Furthermore, women in the second highest daily dose trajectory group (Group 2), averaging more than 10 mg per day of prednisone use before gestational week 32, had shorter gestational lengths by approximately four days.
Our study comparing prednisone dose profiles head-to-head and gestational length builds upon previous smaller studies unadjusted for potential confounders.23,24 Among pregnant women with systemic lupus erythematosus, Clark et al reported that significantly more women with preterm deliveries had prednisone dose >10 mg/day than women with term deliveries.23 However, De Mann et al found no association between prednisone dose > 7.5mg/day versus ≤ 7.5 mg/day and gestational age at delivery among women with rheumatoid arthritis.24
Previous studies have used clustered trajectories to summarize complex individual-level medication utilization trajectories, but only two such studies summarized medication use during pregnancy.30-33 Hurault-Delarue et al used k-means methodology to identify trajectories of psychotropic medications dispensed during pregnancy.32 Moreover, the authors linked trajectories of high exposure to anxiolytics or hypnotics with an adverse neonatal outcome compared with no exposure.33
Although trajectory groups provide more information regarding medication utilization than binary trimester categorization of medication use, each trajectory group contains heterogeneity. Within-group heterogeneity was especially apparent for the prednisone daily dose trajectory groups (compared with cumulative dose trajectory groups) in this study, e.g., daily dose group means were always non-zero although individuals had gaps in prednisone use.
This study has some limitations to consider. First, because changes in dose are tied to disease severity, residual confounding by disease severity is a concern. Although we adjusted for disease severity at enrollment, as well as the use of other rheumatoid arthritis-related medications, the association between the highest dose trajectory groups and shorter gestational length could reflect residual confounding by changes in disease severity later in pregnancy. Second, some of the trajectory groups contained few women, which could result in chance findings. This is especially a concern for the highest cumulative and daily dose trajectories, which included only 11 or 12 women. Third, errors in a woman's reported dates of prednisone use and dose could result in misclassification into a trajectory group other than the one that would best fit her actual trajectory of use. Furthermore, reporting errors across women in the cohort could change the shape of the trajectories from what they would have been without errors. Any measurement error would be expected to attenuate associations between the trajectory groups and gestational age at delivery, assuming that the errors were independent of the outcome. To minimize measurement error in this study, maternal report of prednisone use was obtained primarily during pregnancy as opposed to months after delivery. Finally, generalizability is a concern for this study because women were primarily non-Hispanic White, had relatively high socio-economic status, and older maternal age. The trajectory patterns may not generalize to other populations if prednisone use during pregnancy varies by such demographic factors.
In head-to-head comparison of prednisone dose profiles in women with rheumatoid arthritis, the highest prednisone daily dose and cumulative dose trajectories compared with the lowest dose trajectories were associated with shorter gestational age at delivery after adjusting for disease severity at enrollment, DMARDs, NSAIDs, and other factors. As increasing dose is expected to be associated with increasing disease severity, results may reflect residual confounding by disease severity late in pregnancy. Given that the median cumulative dose of prednisone exposure during pregnancy exceeded 1,200 mg for women with rheumatoid arthritis in this study, it is crucial to consider the impact of such doses on maternal and fetal outcomes in future studies, while accounting for disease severity.
Supplementary Material
Supplemental Figure 1. Study cohort diagram.
Supplemental Figure 2. Observed daily dose of prednisone for each individual by gestational day in daily dose trajectory group 1.
Supplemental Figure 3. Observed daily dose of prednisone for each individual by gestational day in daily dose trajectory group 2.
Supplemental Figure 4. Observed daily dose of prednisone for each individual by gestational day in daily dose trajectory group 3.
Supplemental Figure 5. Observed daily dose of prednisone for each individual by gestational day in daily dose trajectory group 4.
Supplemental Figure 6. Observed cumulative dose of prednisone for each individual by gestational day in cumulative dose trajectory group 1.
Supplemental Figure 7. Observed cumulative dose of prednisone for each individual by gestational day in cumulative dose trajectory group 2.
Supplemental Figure 8. Observed cumulative dose of prednisone for each individual by gestational day in cumulative dose trajectory group 3.
Supplemental Figure 9. Observed cumulative dose of prednisone for each individual by gestational day in cumulative dose trajectory group 4.
Key Points.
Prednisone timing of use, daily dose, and cumulative dose during pregnancy were variable across women with rheumatoid arthritis.
Prednisone dose trajectory groups were associated with rheumatoid arthritis severity, non-biologic disease modifying anti-rheumatic drug use, and gestational age at delivery.
Trajectory groups with the highest daily and cumulative doses of prednisone, compared with the lowest doses, were associated with shorter gestational age at delivery after adjusting for rheumatoid arthritis severity, disease modifying anti-rheumatic drug use, nonsteroidal anti-inflammatory drug use, and other factors.
Given that the median cumulative dose of prednisone exposure during pregnancy was >1,200 mg in this study, it is crucial to consider the impact of such doses on maternal and fetal outcomes.
Acknowledgments
This project was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health K99HD082412 (PI: Palmsten). The MotherToBaby Pregnancy Studies are supported by a research contracts with American Academy of Allergy, Asthma and Immunology, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, GlaxoSmithKline; Janssen Pharmaceuticals, Pfizer, Inc., Hoffman La Roche-Genentech, Genzyme Sanofi-Aventis, Seqirus, Takeda Pharmaceutical Company Limited, and UCB, USA. The funders of the MotherToBaby Pregnancy Studies had no role in the current study's design, in the data collection, in the analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Footnotes
Prior Presentation: Results from this study were presented in poster format at the Health Care Systems Research Network Conference, San Diego, California, March 2017.
References
- 1.Ostensen M, Forger F. Management of RA medications in pregnant patients. Nat Rev Rheumatol. 2009;5(7):382–390. doi: 10.1038/nrrheum.2009.103. [DOI] [PubMed] [Google Scholar]
- 2.Ateka-Barrutia O, Khamashta MA. The challenge of pregnancy for patients with SLE. Lupus. 2013;22(12):1295–1308. doi: 10.1177/0961203313504637. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson CB, Mahsud-Dornan S, Patterson RN. Inflammatory bowel disease in pregnancy. BMJ. 2008;337:a427. doi: 10.1136/bmj.39566.681458.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Namazy JA, Schatz M. The safety of asthma medications during pregnancy: an update for clinicians. Ther Adv Respir Dis. 2014;8(4):103–110. doi: 10.1177/1753465814540029. [DOI] [PubMed] [Google Scholar]
- 5.Andrade SE, Gurwitz JH, Davis RL, et al. Prescription drug use in pregnancy. Am J Obstet Gynecol. 2004;191(2):398–407. doi: 10.1016/j.ajog.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 6.Palmsten K, Hernandez-Diaz S, Kuriya B, Solomon DH, Setoguchi S. Use of disease-modifying antirheumatic drugs during pregnancy and risk of preeclampsia. Arthritis Care Res (Hoboken) 2012;64(11):1730–1738. doi: 10.1002/acr.21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmsten K, Hernandez-Diaz S, Chambers CD, et al. The Most Commonly Dispensed Prescription Medications Among Pregnant Women Enrolled in the U.S. Medicaid Program. Obstet Gynecol. 2015;126(3):465–473. doi: 10.1097/AOG.0000000000000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Man YA, Dolhain RJ, van de Geijn FE, Willemsen SP, Hazes JM. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum. 2008;59(9):1241–1248. doi: 10.1002/art.24003. [DOI] [PubMed] [Google Scholar]
- 9.Kuriya B, Hernandez-Diaz S, Liu J, Bermas BL, Daniel G, Solomon DH. Patterns of medication use during pregnancy in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011;63(5):721–728. doi: 10.1002/acr.20422. [DOI] [PubMed] [Google Scholar]
- 10.Bandoli G, Palmsten K, Forbess Smith CJ, Chambers CD. A Review of Systemic Corticosteroid Use in Pregnancy and the Risk of Select Pregnancy and Birth Outcomes. Rheum Dis Clin North Am. 2017;43(3):489–502. doi: 10.1016/j.rdc.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62(6):385–392. doi: 10.1002/1096-9926(200012)62:6<385::AID-TERA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 12.Skuladottir H, Wilcox AJ, Ma C, et al. Corticosteroid use and risk of orofacial clefts. Birth Defects Res A Clin Mol Teratol. 2014;100(6):499–506. doi: 10.1002/bdra.23248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung YP, Kaplan GG, Coward S, et al. Intrapartum corticosteroid use significantly increases the risk of gestational diabetes in women with inflammatory bowel disease. J Crohns Colitis. 2015;9(3):223–230. doi: 10.1093/ecco-jcc/jjv006. [DOI] [PubMed] [Google Scholar]
- 14.Desai RJ, Bateman BT, Huybrechts KF, et al. Risk of serious infections associated with use of immunosuppressive agents in pregnant women with autoimmune inflammatory conditions: cohort study. BMJ. 2017;356:j895. doi: 10.1136/bmj.j895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schatz M, Zeiger RS, Harden K, Hoffman CC, Chilingar L, Petitti D. The safety of asthma and allergy medications during pregnancy. J Allergy Clin Immunol. 1997;100(3):301–306. doi: 10.1016/s0091-6749(97)70241-0. [DOI] [PubMed] [Google Scholar]
- 16.Schatz M, Dombrowski MP, Wise R, et al. The relationship of asthma medication use to perinatal outcomes. J Allergy Clin Immunol. 2004;113(6):1040–1045. doi: 10.1016/j.jaci.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Gur C, Diav-Citrin O, Shechtman S, Arnon J, Ornoy A. Pregnancy outcome after first trimester exposure to corticosteroids: a prospective controlled study. Reprod Toxicol. 2004;18(1):93–101. doi: 10.1016/j.reprotox.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Bakhireva LN, Jones KL, Schatz M, Johnson D, Chambers CD Organization Of Teratology Information Services Research G. Asthma medication use in pregnancy and fetal growth. J Allergy Clin Immunol. 2005;116(3):503–509. doi: 10.1016/j.jaci.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 19.Chakravarty EF, Colon I, Langen ES, et al. Factors that predict prematurity and preeclampsia in pregnancies that are complicated by systemic lupus erythematosus. Am J Obstet Gynecol. 2005;192(6):1897–1904. doi: 10.1016/j.ajog.2005.02.063. [DOI] [PubMed] [Google Scholar]
- 20.Al Arfaj AS, Khalil N. Pregnancy outcome in 396 pregnancies in patients with SLE in Saudi Arabia. Lupus. 2010;19(14):1665–1673. doi: 10.1177/0961203310378669. [DOI] [PubMed] [Google Scholar]
- 21.Broms G, Granath F, Stephansson O, Kieler H. Preterm birth in women with inflammatory bowel disease - the association with disease activity and drug treatment. Scand J Gastroenterol. 2016;51(12):1462–1469. doi: 10.1080/00365521.2016.1208269. [DOI] [PubMed] [Google Scholar]
- 22.Bharti B, Lee SJ, Lindsay SP, et al. Disease Severity and Pregnancy Outcomes in Women with Rheumatoid Arthritis: Results from the Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project. J Rheumatol. 2015;42(8):1376–1382. doi: 10.3899/jrheum.140583. [DOI] [PubMed] [Google Scholar]
- 23.Clark CA, Spitzer KA, Nadler JN, Laskin CA. Preterm deliveries in women with systemic lupus erythematosus. J Rheumatol. 2003;30(10):2127–2132. [PubMed] [Google Scholar]
- 24.de Man YA, Hazes JM, van der Heide H, et al. Association of higher rheumatoid arthritis disease activity during pregnancy with lower birth weight: results of a national prospective study. Arthritis Rheum. 2009;60(11):3196–3206. doi: 10.1002/art.24914. [DOI] [PubMed] [Google Scholar]
- 25.Palmsten K, Hulugalle A, Bandoli G, et al. Agreement Between Maternal Report and Medical Records During Pregnancy: Medications for Rheumatoid Arthritis and Asthma. Paediatr Perinat Epidemiol. 2018 Jan;32(1):68–77. doi: 10.1111/ppe.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollingshead AB. Four factor index of social status, unpublished working paper. Yale University; New Haven, CT: 1975. https://artlesstanzim.files.wordpress.com/2014/05/hollinghead-four-factors-2.pdf. [Google Scholar]
- 27.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23(2):137–145. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 28.Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: dimensions and practical applications. Health Qual Life Outcomes. 2003;1:20. doi: 10.1186/1477-7525-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.K-Means Clustering. [Accessed May 2, 2017]; https://stat.ethz.ch/R-manual/R-devel/library/stats/html/kmeans.html.
- 30.Riegel B, Lee CS, Ratcliffe SJ, et al. Predictors of objectively measured medication nonadherence in adults with heart failure. Circ Heart Fail. 2012;5(4):430–436. doi: 10.1161/CIRCHEARTFAILURE.111.965152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franklin JM, Shrank WH, Pakes J, et al. Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence. Med Care. 2013;51(9):789–796. doi: 10.1097/MLR.0b013e3182984c1f. [DOI] [PubMed] [Google Scholar]
- 32.Hurault-Delarue C, Chouquet C, Savy N, et al. How to take into account exposure to drugs over time in pharmacoepidemiology studies of pregnant women? Pharmacoepidemiol Drug Saf. 2016;25(7):770–777. doi: 10.1002/pds.4000. [DOI] [PubMed] [Google Scholar]
- 33.Hurault-Delarue C, Chouquet C, Savy N, et al. Interest of the trajectory method for the evaluation of outcomes after in utero drug exposure: example of anxiolytics and hypnotics. Pharmacoepidemiol Drug Saf. 2017;26(5):561–569. doi: 10.1002/pds.4199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Study cohort diagram.
Supplemental Figure 2. Observed daily dose of prednisone for each individual by gestational day in daily dose trajectory group 1.
Supplemental Figure 3. Observed daily dose of prednisone for each individual by gestational day in daily dose trajectory group 2.
Supplemental Figure 4. Observed daily dose of prednisone for each individual by gestational day in daily dose trajectory group 3.
Supplemental Figure 5. Observed daily dose of prednisone for each individual by gestational day in daily dose trajectory group 4.
Supplemental Figure 6. Observed cumulative dose of prednisone for each individual by gestational day in cumulative dose trajectory group 1.
Supplemental Figure 7. Observed cumulative dose of prednisone for each individual by gestational day in cumulative dose trajectory group 2.
Supplemental Figure 8. Observed cumulative dose of prednisone for each individual by gestational day in cumulative dose trajectory group 3.
Supplemental Figure 9. Observed cumulative dose of prednisone for each individual by gestational day in cumulative dose trajectory group 4.