Abstract
High blood pressure (BP) is a major cardiovascular risk factor that is treatable, yet hypertension awareness and control rates are low. Ubiquitous BP monitoring technology could improve hypertension management, but existing devices require an inflatable cuff and are not compatible with such anytime, anywhere measurement of BP. We extended the oscillometric principle, which is used by most automatic cuff devices, to develop a cuff-less BP monitoring device using a smartphone. As the user presses her/his finger against the smartphone, the external pressure of the underlying artery is steadily increased while the phone measures the applied pressure and resulting variable-amplitude blood volume oscillations. A smartphone application provides visual feedback to guide the amount of pressure applied over time via the finger pressing and computes systolic and diastolic BP from the measurements. We prospectively tested the smartphone-based device for real-time BP monitoring in human subjects to evaluate usability (n = 30) and accuracy against a standard automatic cuff-based device (n = 32). We likewise tested a finger cuff device, which uses the volume-clamp method of BP detection. About 90% of the users learned the finger actuation required by the smartphone-based device after one or two practice trials. The device yielded bias and precision errors of 3.3 and 8.8 mmHg for systolic BP and −5.6 and 7.7 mmHg for diastolic BP over a 40 to 50 mmHg range of BP. These errors were comparable to the finger cuff device. Cuff-less and calibration-free monitoring of systolic and diastolic BP may be feasible via a smartphone.
INTRODUCTION
High blood pressure (BP) is a major risk factor for strokes and heart disease (1) that is treatable with lifestyle changes and medication (2). However, hypertension awareness and control rates are low (3). Only ~55% of hypertensives in developed nations and ~45% of hypertensives in developing nations are aware of their condition, and ~15% of hypertensives have their BP under control. Ubiquitous BP monitoring technology could improve hypertension awareness by providing serial measurements from the mass population during daily life (4) and enhance hypertension control by providing continual feedback to the individual patient (5). However, existing noninvasive devices require an inflatable cuff and therefore are not feasible for such anytime, anywhere monitoring of BP.
We proposed to extend the oscillometric principle, which is the basis of most automatic cuff-based BP measurement devices (6, 7), for cuff-less BP measurement using a smartphone. In this scenario, the user serves as the actuator (instead of the cuff) by pressing her/his finger against the phone to vary the external pressure of the underlying artery, whereas the phone serves as the sensor (rather than the cuff) to measure the resulting variable-amplitude blood volume variations or oscillations and applied pressure. The phone also provides visual feedback to guide the amount of finger pressure applied over time and computes BP from the measurements.
To investigate the oscillometric finger-pressing method, we developed a smartphone-based device to implement the method in real time. We then prospectively tested the device in human subjects for usability and accuracy against a standard cuff device. We likewise tested a finger cuff device, which uses the volume-clamp method, to determine BP (8). Our results indicate that smartphone-based BP monitoring is easily performed via finger actuation and can measure BP with accuracy similar to the finger cuff device.
RESULTS
Smartphone-based BP measurement device: Concept, prototype, and usage
Concept
The smartphone-based device represents an extension of the oscillometric principle for cuff-less BP monitoring. As shown in Fig. 1A, in conventional oscillometry, the cuff serves as an actuator to vary the external pressure of an artery and as a sensor to measure this pressure and the resulting variable-amplitude blood volume oscillations within the artery. BP is then computed from the oscillation amplitudes as a function of the applied pressure (henceforth called the “oscillogram”). As shown in Fig. 1B, for the smartphone-based device, the user serves as the actuator by pressing her/his finger against the phone to steadily increase the external pressure of the underlying artery (transverse palmar arch artery), whereas the phone, embedded with photoplethysmography (PPG) and force transducers, serves as the sensor to measure the blood volume oscillations and applied pressure. PPG is a well-known optical technique in which a tissue sample is illuminated and the changes mainly in light absorption due to the pulsatile blood volume within the tissue are measured (9). The phone also provides visual feedback to guide the amount of finger pressure applied over time, as shown in Fig. 1C, and then likewise computes BP from the oscillogram, as shown in Fig. 1D.
Fig. 1. From conventional cuff-based blood pressure measurement to cuff-less BP monitoring using a smartphone.
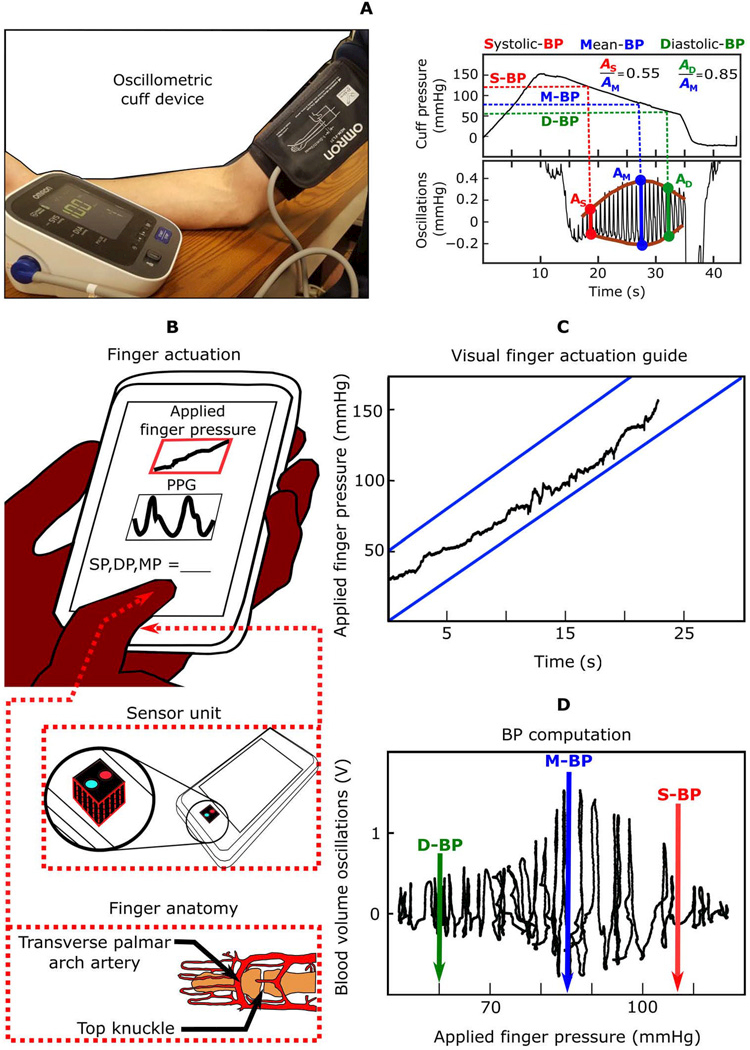
(A) Image of a conventional cuff-based oscillometric device and diagram of representative blood pressure (BP) measurement. (B) Schematic diagrams of the proposed oscillometric finger-pressing method for cuff-less BP monitoring using a smartphone, in which the user serves as the actuator instead of the cuff, to vary the external pressure of the transverse palmar arch artery by finger pressing, whereas the phone serves as the sensor to measure blood volume oscillations and applied pressure similar to a cuff, provides a visual display of the applied finger pressure over time to guide the actuation (C), and computes BP similar to a cuff (D). Image of finger anatomy adapted from (35).
Prototype
Figure 2 (A and B) shows the smartphone-based device. The prototype device is a three-dimensional (3D)–printed case affixed to the back of a smartphone. The case houses a PPG sensor on top of a force transducer to measure the blood volume oscillations and applied finger pressure, as well as circuitry to acquire and transmit the measurements to the smartphone (Fig. 2A). The smartphone runs an application to visually guide the finger actuation and compute systolic, diastolic, and mean BP at the brachial artery from the finger blood volume oscillation and finger pressure measurements (Fig. 2B).
Fig. 2. Smartphone-based device for real-time monitoring of BP via the oscillometric finger-pressing method.
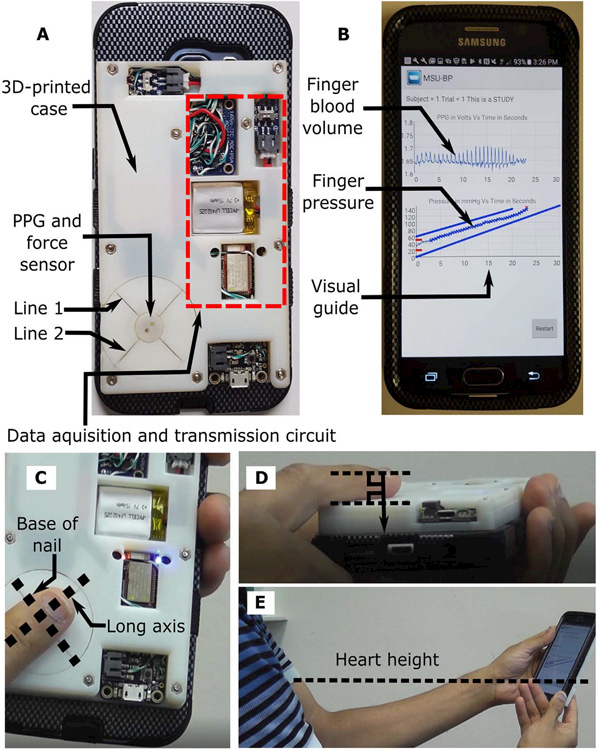
(A) Photograph of the smartphone-based device. A three-dimensional (3D)–printed case was affixed to the back of a smartphone. The case includes visual line indicators to guide finger placement and houses photoplethysmography (PPG) and force sensors along with other circuitry to acquire and transmit the finger blood volume oscillation and applied finger pressure measurements to the phone. (B) Photograph of an application running on the phone to provide visual guidance for the finger actuation and display the finger measurements. Photographs illustrating that a user places her/his finger on the sensor according to the line indicators (C), rests the same finger on the surface of the case to apply force in the normal direction with respect to the case (D), and holds the device at the same height as the heart (E).
Usage
As shown in Fig. 2 (C to E), a user interacts with the device to measure BP in three steps. First, the user places her/his index finger on the sensor so that the base of the finger nail is aligned with “line 1” on the back of the phone and that the long axis of the finger is centered on “line 2” (Fig. 2C). In this way, measurement from the transverse palmar arch artery may be targeted (Fig. 1B). The user also rests a portion of the same finger below the top knuckle on the case surface to ensure force application in the normal direction relative to this surface (Fig. 2D). Second, the user holds the device at the same height as the heart to eliminate hydrostatic effects while viewing the smartphone screen (Fig. 2E). Third, the user presses her/his finger against the sensor to steadily increase the external pressure of the artery, such that the external pressure application acts similar to a cuff to press the artery against the supporting bone (Fig. 1C). The user maintains the applied pressure within the target blue lines: Pressure is displayed as it evolves in real time via the smartphone application (Fig. 2B). After sufficient finger pressure is achieved, the measurement automatically terminates, and the BP measurements are displayed. If the applied pressure falls outside the target lines or the oscillogram quality is deemed inadequate due to a measurement or computation failure, then the device asks the user to try again. See movie S1 for a video demonstration of the device.
Device testing: Usability and accuracy
Usability
To test device usability, 30 new users (age, 39 ± 10 years; height, 168 ± 8 cm; weight, 79 ± 18 kg; 67% females) participated. Each user was allowed practice trials to learn the finger actuation procedure. Figure 3A shows a histogram of the number of practice trials required for each user to correctly execute the finger actuation, maintaining the applied finger pressure within the target blue lines on the smartphone application. About 90% of the users learned the finger actuation after one or two trials. After learning the finger actuation, each user then performed the finger actuation two to four times with the aim of obtaining a pair of close or three BP measurements. Figure 3B shows a histogram of the output of the device (BP measurement or “try again” message) overall measurements. About 60% of the measurements were successful. Figure 3C shows a histogram of the number of try again messages outputted by the device for each user. The device did not output a try again for about 50% of the users and yielded multiple BP measurements for about 80% of the users. However, the device did not output any BP measurements for 2 of the 30 users. Figure 3D shows a histogram of the reasons for the try again messages. Almost 60% of the try again messages were due to a computation failure. The remaining try again messages were almost exclusively due to a measurement failure. Actuation failure was rare. Computation failure is relatively easy to correct and was the reason that the device did not produce any BP measurement in one of the users.
Fig. 3. Device usability results (n = 30 new users).
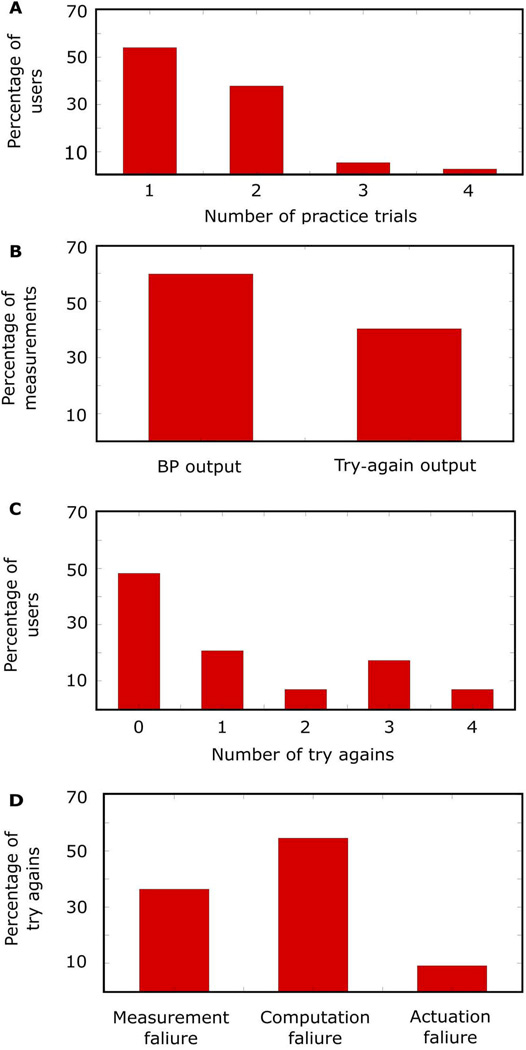
Histograms of the (A) number of practice trials needed to learn the requisite finger actuation for all users; (B) percentage of BP measurements versus try again messages outputted by the device over all users; (C) number of try again messages per user; and (D) reasons for the try again messages.
Accuracy
To test device accuracy, the same 30 new users and 5 additional experienced users (age, 33 ± 8 years; height, 173 ± 4 cm; weight, 72 ± 5 kg; 0% females) participated. The latter five users also obtained multiple BP measurements but held the device well below the heart to raise their BP. Device measurements during this hydrostatic challenge may be thought of as BP from a brachial artery situated beneath the heart. The BP measurements from the device were averaged when more than one measurement was available and were assessed against the average of two measurements from a standard oscillometric arm cuff device. Figure 4 (A to D) shows the correlation and Bland-Altman plots for the systolic and diastolic BP measurements from the 32 users for which the smartphone-based device yielded BP measurements, and the reference device produced valid BP values. The smartphone-based device yielded bias errors (µ) and precision errors (σ) of 3.3 and 8.8 mmHg for systolic BP and −5.6 and 7.7 mmHg for diastolic BP over a 40- to 50-mmHg range of BP. Figure 4 (E to H) shows corresponding plots for a finger cuff device, which uses the volume-clamp method and likewise computes brachial BP from finger measurements. The smartphone-based device showed BP measurement accuracy similar to the finger cuff device with respect to the standard arm cuff device. However, unlike the smartphone-based device, the finger cuff device always yielded BP measurements. Table S1 shows the individual subject-level results of the device testing, including anthropomorphic information, number of practice trials, and all of the BP measurements.
Fig. 4. Device accuracy results (n = 32 users).
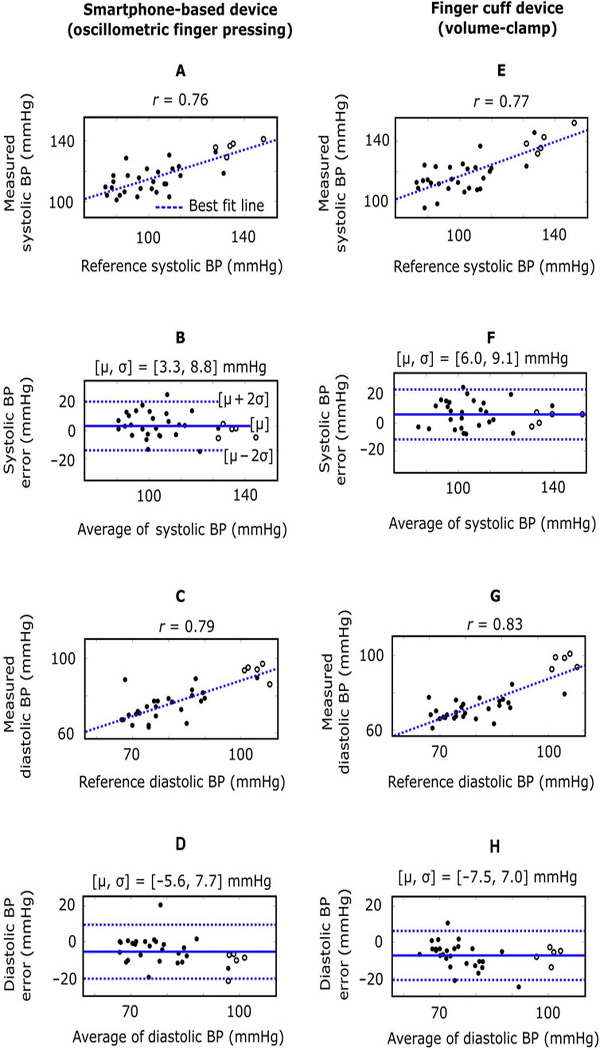
Correlation and Bland-Altman plots comparing the brachial BP measurements from the smartphone-based device [oscillometric finger-pressing method (A to D)] and the brachial BP measurements from a finger cuff device [volume-clamp method (E to H)], with each relative to a standard arm cuff device. The filled circles are data points from new users holding both finger devices at the same height as the heart, whereas the unfilled circles are data points from experienced users holding both finger devices below the heart to raise the BP. r, correlation coefficient; m, bias error (mean of the errors); s, precision error (SD of the errors); solid line in Bland-Altman plots, bias error; dashed lines in Bland-Altman plots, limits of agreement.
DISCUSSION
We proposed the oscillometric finger-pressing method for cuff-less BP monitoring using a smartphone. This method may be implemented with a PPG sensor, which measures pulsatile blood volume (9), and a force sensor. These sensors are already integrated in many smartphones (10, 11), although some customization of the sensor architecture is necessary to enable BP measurements. Because the user serves as the actuator to apply external pressure to the transverse palmer arch artery in her/his index finger, the requisite hardware that performs BP measurements is miniaturized and greatly simplified compared to possible alternative methods that would automatically vary the external pressure. Therefore, it may be relatively easy to incorporate the components required by the oscillometric finger-pressing method in smartphone encasings, which are commonly used to protect the phone against damage due to drops and otherwise or within the phones themselves. For example, a thin-filmed force sensor could be placed on top of an existing PPG sensor on the back of the phone.
Other cuff-less BP measurement modalities are being widely pursued at present. Pulse transit time (PTT) is the most popular method (9). PTT often varies inversely with BP in a person and can be measured simply as the relative timing between proximal and distal waveforms indicative of the arterial pulse. Hence, PTT could potentially permit convenient BP monitoring. However, PTT in units of milliseconds must be calibrated to BP in units of millimeters of mercury, and PTT, as a single value, cannot independently track systolic and diastolic BP. As a result, accuracy is the concern for the PTT-based approach. Ultrasound may allow for other methods. The most popular ultrasound method measures the arterial diameter waveform along with the local PTT (in the form of pulse wave velocity) and then applies the Bramwell-Hill equation to compute the absolute pulse pressure (systolic BP-diastolic BP) (12–14). Diastolic BP may also be measured via calibration of the PTT measurement. However, convenience is generally the concern for ultrasound systems. Arterial tonometry is a long-standing method (15) also worth mentioning. In theory, this method can measure a BP waveform without using a cuff by pressing a force sensor on an artery. The sensor must flatten or applanate the artery so that its wall tension is perpendicular to the probe. However, manual applanation and automatic applanation have proven to be difficult, so the measured waveform is routinely calibrated with cuff BP values in practice (16).
The oscillometric finger-pressing method may overcome the short-comings of other cuff-less BP measurement modalities. First, it can independently measure systolic and diastolic BP without any calibration and may therefore be sufficiently accurate. Second, it offers a convenience advantage over automatic cuff devices: People in low-resource settings may not have any access to cuff devices; others must go to pharmacies or other specified locations to use these devices; and people who own a device are unlikely to carry it with them wherever they go. By contrast, smartphones are readily available to many. About 3 billion people around the world are predicted to have smartphones by 2020 (17). It is also anticipated that smartphones will become widely used in low-income nations in the near future due to reduced costs resulting from more competition in the marketplace (18). Furthermore, smartphones are constantly in use. For example, adults in the U.S. use these devices almost 3 hours a day on average (19).
An oscillometric method for cuff-less BP monitoring was previously proposed and was demonstrated in a pilot subject (20). In that study, a person raised her/his hand to lower the transmural pressure of the hand arteries via the hydrostatic effect while wearing a ring embedded with PPG and force sensors on a finger to measure the resulting variable-amplitude blood volume oscillations and the pressure applied by the ring on the finger. The main concern with this interesting method is that the extent of the pressure reduction is limited by the arm length. Hence, for most people, the ring must be applied on the finger at a pressure that does not deviate considerably from the mean BP of the person so that the oscillogram may be interrogated over the crucial zero–transmural pressure regime. Furthermore, extra sensors for measuring the height of the hand relative to the heart are required, or assumptions about the relative height must be made; motion artifact may be problematic when the hand raising is performed relatively quickly, or smooth muscle contraction may be a factor when the hand raising is performed very slowly (15); and hand-raising may be awkward for users in public settings. The proposed oscillometric finger-pressing method overcomes these limitations, although it is vulnerable to BP measurement error when the device is not held at the same height as the heart.
We developed a smartphone-based device to implement the oscillometric finger-pressing method in real time. The prototype device includes a PPG and a force sensor unit to acquire the requisite measurements from the finger, a visual display on a smartphone application to guide the finger actuation, and an empirical algorithm to compute systolic and diastolic BP at the brachial artery from the finger measurements. It is BP at the brachial artery rather than the finger that is the proven cardiovascular risk factor (1). The device outputs a try again message if the actuation is unsuccessful or the oscillogram quality is deemed inadequate. We tested the usability of the device and its accuracy against a standard automatic arm cuff device in 35 human subjects while likewise assessing a finger cuff device often used in research that has achieved approval from the U.S. Food and Drug Administration for measuring brachial BP (21). This finger cuff device applied the volume-clamp method as follows. First, the finger cuff device slowly increases the cuff pressure while also measuring the blood volume via a PPG sensor within the cuff to compute mean BP according to the oscillometric principle. Then, the device continually varies the cuff pressure to maintain the “unloaded” blood volume (the blood volume at which the cuff pressure equals the mean BP) throughout the cardiac cycle via a fast servo-control system. The cuff pressure may therefore yield the finger BP waveform. This BP waveform is then converted to a brachial BP waveform via an empirical algorithm (22).
We found that all new users could execute the finger actuation required by the smartphone-based device and that most of these users could do so after one or two practice trials. We suspect that the finger actuation becomes second nature with increasing device usage. After the new users learned the finger actuation, the device yielded BP measurements much more often than not. When the device produced the try again messages, the cause was usually due to computation and measurement failures rather than actuation failure. Computation failures may be easily corrected in the future as more data are collected and with software updates. It may also be possible to reduce the frequency of try again errors due to measurement failure without compromising accuracy by lowering the standard for measurement quality. Although the device did not yield BP measurements in two users, the reason was computation failure for one of the users (and measurement failure for the other user).
The smartphone-based device could measure systolic and diastolic BP with promising accuracy. The device yielded bias and precision errors relative to the automatic arm cuff device that were close to the AAMI (Association for the Advancement of Medical Instrumentation) limits of 5 and 8 mmHg, but an AAMI data collection protocol was not used. Furthermore, the device measured BP as accurately as the finger cuff device.
Here, all of the subjects used the device correctly. In practice, users may not always be so compliant. However, a key advantage of a smartphone-based BP monitoring device is that many measurements can be made over time with the ubiquitous system (23). These measurements could be averaged to eliminate error caused by random variations in finger placement on the sensor and in the height at which the device is held, as well as to mitigate error caused by imperfect BP computation. Averaging many measurements also abolishes the substantial BP variations that occur within a person due to stress, physical activity, recent ingestion of a meal, and other factors (24). In this way, the device may be able to indicate a sufficiently reliable BP measurement for hypertension detection despite large errors in any single measurement. Screening for hypertension may be the main clinical application of the device, especially in the 20- to 50-year-old segment of the population who are often technology savvy and health conscious but may be at risk for early development of hypertension (25, 26).
Our study has limitations, and future efforts are needed to bring the oscillometric finger-pressing method to practice. One limitation is that the oscillometric finger-pressing method can neither make nighttime BP measurements, which are clinically important (27), nor be performed by all people, such as those lacking fine motor control. However, even cuff-based methods may not be suitable for everyone (for example, morbidly obese people). Another limitation is that the smartphone-based device was not tested according to an AAMI data collection protocol, which involves a subject population that covers a prescribed range of BP values (28). However, by also studying experienced users during a hydrostatic challenge, we were able to extend the tested range of each BP measurement to 40 to 50 mmHg. The device may be improved by leveraging additional sensing to confirm correct device usage, by mitigating the adverse effect of finger vascular tone changes via inclusion of a temperature sensor to assess cold-induced finger vasoconstriction, or by applying a physics-based algorithm to compute both BP and the arterial compliance curve (rather than an empirical algorithm, which may implicitly assume invariant arterial compliance curves despite finger vascular tone changes) (29). The smartphone could also warn users of high BP, securely transmit the measured BP to caregivers, and send text reminders to patients with uncontrolled BP to take their medications.
In summary, we studied the oscillometric finger-pressing method for cuff-less BP monitoring using a smartphone. Although various form factors for implementing this method may be envisaged, the smartphone form may allow the method to reach the most people while being conveniently housed within a single, portable device. In this way, a complete hypertension management system would be available in the pockets of many.
MATERIALS AND METHODS
Study design
We investigated the oscillometric finger-pressing method for cuff-less BP monitoring using a smartphone. We performed informal and formal human studies under protocols approved by the Michigan State University Institutional Review Board and with written, informed consent from each subject. The informal study facilitated the development of a single prototype device, whereas the formal study allowed for objective testing of this device. The formal study followed a prospective design, in which the real-time output of the device was assessed (as opposed to a retrospective design in which an offline output, as determined by first recording the finger blood volume oscillation and pressure measurements of the device and then analyzing the measurements, is assessed). The study was therefore necessarily blinded to all cuff BP measurements. This study included the following predefined components: number of subjects (n = 35) that is about half of the AAMI study population (28) and comparable to similar studies in the field for demonstrating proof of concept (9); number of measurements per subject; and subject and data inclusion/exclusion criteria. No outliers were excluded.
Device development: Informal human study
To develop the hardware component of the device and a basic visual display for finger actuation guidance, we qualitatively explored various options in about 10 human subjects. We then collected a training data set to define the software component of this device, including finger measurements, via the device held at the same height as the heart and reference BP measurements via a standard automatic arm cuff (BP7650N, Omron) from 31 human subjects (age, 31 ± 7 years; height, 170 ± 8 cm; weight, 68 ± 10 kg; 39% females). Reference systolic and diastolic BP in this data set ranged from 90 to 124 mmHg and 60 to 89 mmHg, respectively. We computed reference mean BP, which was not outputted by the automatic arm cuff device, from systolic and diastolic BP according to the 0.4/0.6 rule (30).
Device development: Prototype
Hardware
We built a physical device consisting of a 3D-printed case attached to a smartphone. The case (Vero White material; 112 mm × 68 mm × 11.6 mm dimensions; printed by Objet350 Connex, Stratasys) was attached using screws to the back of a standard smartphone encasing (SAMS6HPCTUFF2DIM1, MyBat), which housed the smartphone (Galaxy S6, Samsung). The components within the case include a sensor unit, data acquisition and transmission circuitry, and a power supply, as shown in Fig. 5. The sensor unit consists of PPG and force transducers. The PPG sensor was custom-built, comprising a light-emitting diode and photodetector pair operating in reflectance-mode and at an infrared wavelength (940 nm) to penetrate beneath the skin (9) and to provide superior signal quality in lower–skin perfusion conditions (dark skin pigmentations and cold temperatures) (31). The sensor surface, which constitutes the finger pressing area, is a 10-mm-diameter circle. The force sensor (CS15–4.5N, SingleTact) is a thin-filmed, capacitive transducer that measures normal direction force, with specifications congruent with BP measurement (0.9-mmHg resolution and 430-mmHg range). The force-sensitive area is a 15-mm-diameter circle. The PPG sensor is positioned on top of the force sensor with a rigid-structure-rubber sheet (TangoBlack material; 15 mm diameter) between the two, which allows the force applied on the PPG sensor, but not elsewhere on the case surface, to reach the force-sensitive area and be uniformly distributed on it. A one-time calibration of the force sensor was performed while residing in the completed case via placement of high-density weights (WHST13, United Scientific Supplies) on the PPG sensor. The relationship from the voltage (V) measured by the force sensor to the known pressure (P, the force exerted by each weight divided by the area of a 10-mm-diameter circle) was represented with a piece-wise linear function (P = 560.1V − 281.9 if V < 0.74 or P = 225.2V − 32.1 otherwise).
Fig. 5. Smartphone-based device hardware.
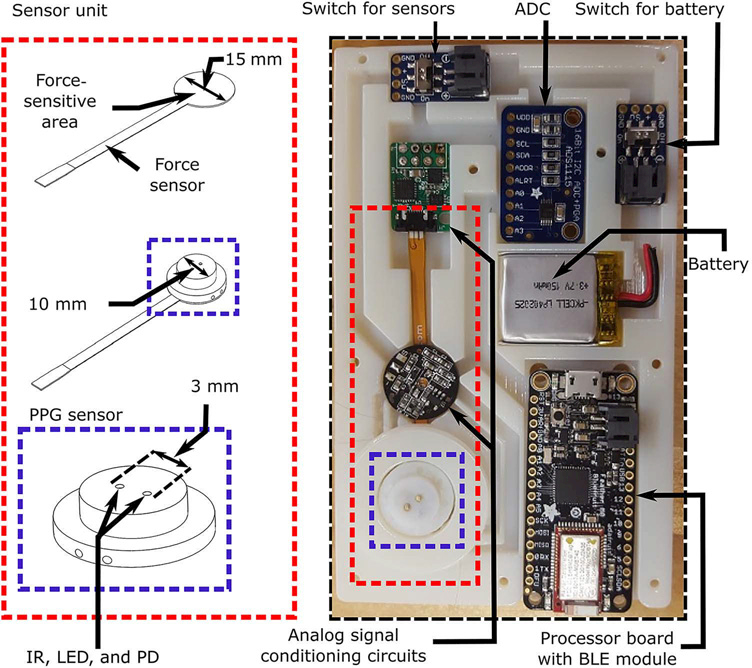
Schematic diagram of the PPG and force sensor unit and photograph of this unit, data acquisition and transmission circuitry, and power supply housed within the 3D-printed case affixed to the back of the phone. IR, infrared; LED, light-emitting diode; PD, photodetector; ADC, analog-to-digital converter; BLE, Bluetooth low energy.
The blood volume waveform outputted by the PPG sensor is amplified and filtered via a band-pass filter with cutoff frequencies of 1.8 and 4.3 Hz (analog signal conditioning) to differentiate the blood volume waveform with respect to time while also attenuating high-frequency noise. The applied pressure outputted by the force sensor is conditioned using circuitry provided with the sensor. The two measurements are then passed through an analog-to-digital converter (ADS1115, Adafruit) with 16-bit resolution and at a 40-Hz sampling rate. The digital signals are finally transmitted to the smartphone via a development board with a processor (ATSAMD21G18, Arm) interfaced to a Bluetooth low-energy module (nRF51822, Nordic Semiconductor). All components are powered with a rechargeable lithiumion polymer battery (3.7 V, 150 mA·hour), and switches are included to shut down the battery and sensors.
Software
We created an Android application to run on the smartphone. Figure 6 shows a flow chart of the application along with the important equations for computing BP. The application includes a visual display to guide the finger actuation and an algorithm to compute and output BP or ask the user to try again. The application uses various thresholds and BP computation formulas that were defined on the basis of the training data set. Formulas that optimized the agreement between the smartphone-derived BP measurements and the reference arm cuff device measurements were selected. Thresholds were selected qualitatively by choosing what we considered to be reasonable values and then by confirming that these values allowed for what we considered to be a good balance between BP measurement accuracy and percentage of try again messages.
Fig. 6. Smartphone-based device software.
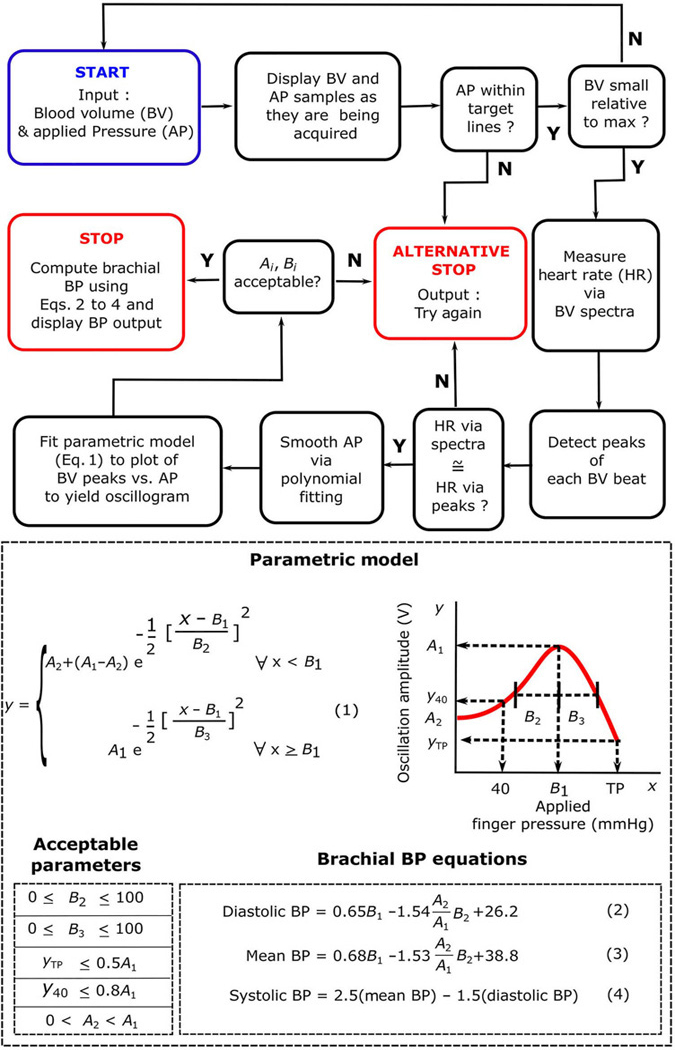
Flowchart of the smartphone application and important equations for computing BP running on the phone wherein the input is the measured blood volume waveform and applied pressure and the output is brachial BP values or a try again message. The blue box indicates the beginning of the flowchart, whereas the red boxes indicate the two possible ends of the flowchart. The plot illustrates a parametric model of the oscillogram [blood volume oscillation amplitude (y) as a function of the applied finger pressure (x)] from which BP is computed. BP computation details are provided in the “Software” subsection of Materials and Methods.
The visual display depicts separate graphs of the blood volume wave-form and applied pressure both plotted against time. Each sample of these measurements is displayed on its respective plot as it is being acquired. The applied pressure displayed specifically represents a 1-s moving average. The applied pressure versus time plot also includes a pair of blue lines, indicating a target rate range of 4.7 to 6.0 mmHg/s for the pressure increase. The magnitude of the blood volume oscillations is monitored in real time via a 1.33-s moving average of the SD of the blood volume waveform. If this SD falls below 20% of its maximum attained value, then the measurement automatically terminates because enough data have been obtained. Moreover, if three successive samples of the applied pressure fall outside of the two target blue lines, then the application will ask the user to try again.
The algorithm first constructs the oscillogram from the zero-mean blood volume waveform and the applied pressure obtained during a successful finger actuation. The measurements are analyzed over the time interval for which the applied pressure ranges from 40 mmHg to the termination pressure (TP). The average heart rate is determined from the blood volume waveform based on its spectral peaks within the frequency range of 0.5 to 3 Hz. The peaks of each beat of the blood volume waveform are then detected by leveraging the average heart rate and the local maxima of the waveform. Because the PPG sensor measures a differentiated blood volume waveform, the waveform peaks are reflective of the peak-to-peak amplitudes of the blood volume oscillations. If the average of the reciprocal of the peak-to-peak intervals is not within 10% of the average heart rate determined via spectral analysis (in hertz), then the application will ask the user to try again because the blood volume waveform may be contaminated by artifact or the wave-form beats may not have been well detected. The applied pressure measurement is thereafter smoothed via a third-order polynomial fit. A discrete oscillogram is then formed by plotting the blood volume peaks versus the corresponding pressure and smoothing the plot via a three-point moving average. A final, continuous oscillogram is constructed by fitting the parametric function in Eq. 1 in Fig. 6 to the discrete oscillogram. In this equation, x and y are the abscissa and ordinate, respectively, of the oscillogram, and Ai and Bi are the parameters that define the oscillogram. This equation models the oscillogram as an asymmetric function, as justified elsewhere (29), via two half Gaussian functions. As illustrated to the right of Eq. 1 in Fig. 6, the parameter A2 represents the starting value of the oscillogram; A1 and B1 represent the maximal amplitude of the oscillogram and the applied pressure at which it is maximal, respectively; and B2 and B3 represent the width of the oscillogram over the pressure range to the left and right of its maximum, respectively. Note that the parameter A2 is needed, because the device asks the user to maintain a relatively constant pressure before beginning the actuation (movie S1) such that the oscillogram is often flat initially. These five parameters are estimated via nonlinear least-squares fitting.
The algorithm then computes BP from the final oscillogram or asks the user to try again. Empirical methods are used, similar to cuff-based devices that use fixed-ratio or similar methods to compute brachial BP from an arm oscillogram (6, 7). In the fixed-ratio method, mean BP is first obtained as the cuff pressure at which the oscillogram is maximal, and systolic and diastolic BP are then determined as the cuff pressure at which the oscillogram is some fixed ratio of its maximal value. Similarly, finger cuff devices based on the volume-clamp method convert the measured finger BP to brachial BP via a population average transfer function and a regression equation (22). The brachial BP values are specifically computed from the finger oscillogram model parameters via the empirical linear regression formulas in Eqs. 2 to 4 in Fig. 6. We arrived at these formulas using stepwise regression (32), which deter-mined both the model parameters that are statistically significant regressors (P < 0.05) of the reference cuff BP values and the associated coefficients in the regression model for computing BP. The diastolic BP formula is conceptually similar to the fixed-ratio method, and the constant term therein also accounts for brachial diastolic BP being systematically higher than finger diastolic BP (22, 33). The mean BP formula includes both B1 and (which is a measure of oscillogram width) and is therefore similar to an existing method designed to handle relatively flat or wide oscillograms, in which the mean BP is determined as the lowest external pressure at which the oscillogram is still close to maximal (34). The constant term in the formula likewise accounts for brachial mean BP being systematically higher than finger mean BP (22, 33). The systolic BP formula is based on the 0.4/0.6 rule for computing brachial mean BP from brachial systolic and diastolic BP (30). Although stepwise regression yielded a different formula for systolic BP, the degree of significance of the regressors was borderline (P ≅ 0.05). Furthermore, the difference between brachial systolic BP and finger systolic BP is not only due to the resistive pressure drop but also due to arterial wave reflection and is therefore more complicated. Hence, the simple formula here may generalize better. Finally, as shown to the left of Eqs. 2 to 4 in Fig. 6, if B2 or B3 is greater than 100 mmHg (the oscillogram is excessively wide), A2 is less than 0 or greater than A1 (the oscillogram is negative or monotonically decreasing), the oscillogram amplitude at an applied pressure of 40 mmHg (y40) is greater than 0.8A1, or the oscillation amplitude at the TP (yTP) is greater than 0.5A1 (the oscillogram has not been interrogated over a sufficiently wide pressure range), then the application will not output BP and instead will ask the user to try again. Note that these empirical thresholds and formulas are expected to change because more data are added to the training data set.
Device testing: Formal human study
We prospectively tested the smartphone-based device for usability and accuracy against a standard automatic arm cuff device.
Experimental subjects
We recruited 30 users who had not used the device before and 5 experienced users. These five users were part of the training data set subject cohort but performed an intervention to change their BP. The inclusion criteria were: (i) from ages 21 to 60 years; (ii) right-handed (because the device was designed for such users but could be easily extended for both right- and left-handed users); (iii) no cardiovascular disorders other than hypertension; and (iv) no problems with fine motor control. The exclusion criterion for the accuracy testing was invalid automatic cuff BP measurements defined as: (i) a poorly fit cuff on the user’s arm or (ii) cuff BP measurements (mean via 0.4/0.6 rule) deviating by >10 mmHg (because BP was assumed to be stable throughout the protocol). Only one user was excluded because of invalid cuff BP measurements.
Experimental measurements and protocol
Figure 7 shows the BP measurement devices and protocol. The BP measurement instruments were the smartphone-based device (Fig. 7A), a standard oscillometric arm cuff device (BP7650N, Omron; Fig. 7B), and a finger cuff device based on the volume-clamp method that transforms a measured finger BP waveform into a brachial BP wave-form (Finometer Model 2, Finapres Medical Systems; Fig. 7C). The protocol included an initial learning phase (for new users only) and a data collection phase (for all users; Fig. 6D). During the learning phase, use of the smartphone-based device was demonstrated, and users were allowed to practice with the device until they were able to perform the index finger actuation correctly in terms of keeping the applied finger pressure between the target blue lines. During the data collection phase, a series of BP measurements were made as follows: BP with the standard cuff device placed properly on the right arm; multiple cuff-less BP measurements using the smartphone-based device with at least 1 min between each measurement; brachial BP waveform with the finger cuff device for 1 min, with the cuff positioned on the index finger of the right hand; and BP with the standard arm cuff device. Smartphone data collection was terminated once (i) two measurements yielded mean BP values within 10 mmHg; (ii) three measurements produced BP values; or (iii) four measurements were made. New users held the smartphone-based device and finger cuff device at the same height as the heart, whereas the experienced users (n = 5) held both devices at the same height but well below the heart to raise their BP via the hydrostatic effect. The finger cuff device also included a sensor to measure the BP offset caused by the hydrostatic effect (ρgh, where ρ is blood density, g is gravity, and h is the height between the heart and finger).
Fig. 7. Human study design for device testing.
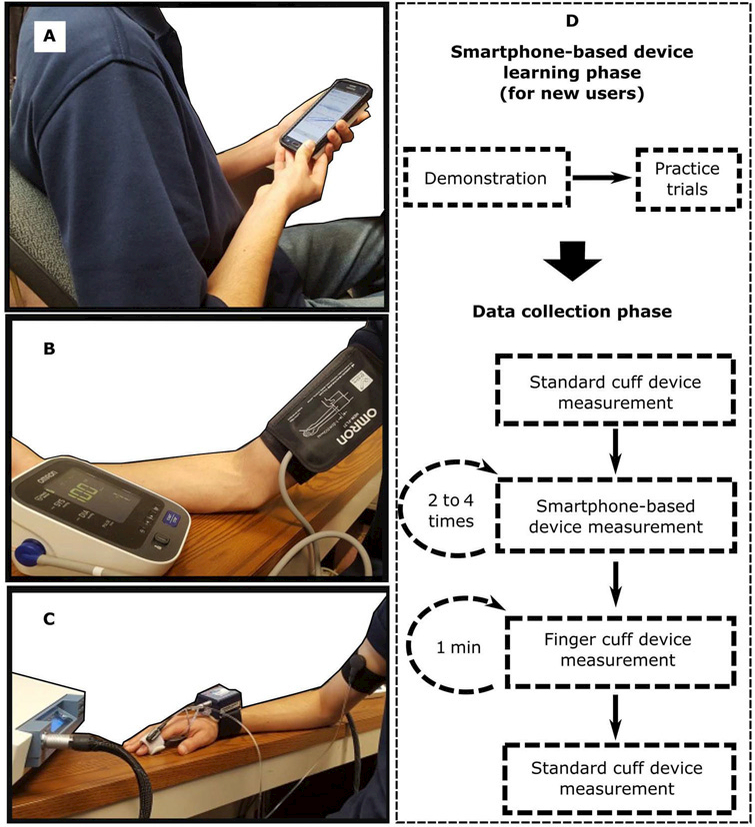
Photographs of the three BP measurement devices for study: (A) the smartphone-based device, (B) a standard automatic arm cuff device (the reference device), and (C) a finger cuff device (a competing device). (D) Diagram of the experimental protocol for BP measurement using the devices shown in (A) to (C). The protocol included a learning phase for new users to become familiar with the smartphone-based device and a data collection phase involving a measurement with the reference device, two to four measurements with the smartphone-based device, 1 min of measurement with the finger cuff device, and a final measurement with the reference device. Study details are provided in the “Device testing: Formal human study” subsection of Materials and Methods.
Data analysis
To test usability, we recorded the number of practice trials required for each new user to successfully execute the finger actuation, documented the number of BP values obtained and try again messages outputted by the device for each of the users, and determined the reason for each of the try agains via post hoc visual inspection of the data. To test accuracy, we averaged the cuff-less BP measurements of the smartphone-based device when multiple measurements were available for the user and averaged the pair of measurements from the arm cuff device. For the experienced users, we then added the ρgh measurement to the systolic and diastolic BP measurements from the arm cuff device. We did not assess the mean BP measurements from the smartphone-based device because the reference device did not output this BP value, and use of the 0.4/0.6 rule for computing reference mean BP may bias the results.
Statistical analysis
We used standard analyses to assess the systolic and diastolic BP measurements of the smartphone-based device against the reference BP measurements from the arm cuff device. In particular, we assessed the accuracy visually using correlation and Bland-Altman plots and quantitatively using the correlation coefficient (r), bias error (µ, mean of the errors), and precision error (σ, SD of the errors). For comparison, we assessed the average brachial BP values obtained using the finger cuff device against the reference BP measurements from the standard cuff device. The results of the devices were similar enough that statistical comparisons were not necessary (Fig. 4).
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/10/431/eaap8674/DC1
Table S1. Anthropomorphic information, number of practice trials, and all BP measurements per subject.
Movie S1. Video demonstration of the smartphone-based BP monitoring device.
Supplementary Material
Acknowledgments
Funding: This work was supported by the NIH under grant EB-018818 and the Michigan State University Office of the Vice President for Research and Graduate Studies under a Targeted Support Grant for Technology Development.
Footnotes
Author contributions: A.C. developed the smartphone-based device, performed the human studies, analyzed the collected data to assess the device, and coprepared the manuscript. C.-S.K. helped in the early development of both the hardware and the software components of the device. M.N. helped in the final development of the software component and in the human studies. K.N. helped in the human studies. J.-O.H. helped in advising the study and editing the manuscript. R.M. guided the study and coprepared the manuscript.
Competing interests: A.C., J.-O.H., and R.M. are inventors on patent application PCT/US2017/020739 submitted by Michigan State University and University of Maryland that covers the oscillometric finger-pressing method. The patent has been exclusively licensed to Digitouch Health LLC. They also provided initial, unpaid consulting to transition the method to the company. All other authors declare that they have no competing interests.
Data and materials availability: All data for interpreting the manuscript have been included. Additional information may be requested from R.M. (rama@egr.msu.edu).
REFERENCES
- 1.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration, Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360, 1903–1913 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Psaty BM, Smith NL, Siscovick DS, Koepsell TD, Weiss NS, Heckbert SR, Lemaitre N, Wagner EH, Furberg CD, Health outcomes associated with antihypertensive therapies used as first-line agents: A systematic review and meta-analysis. JAMA 277, 739–745 (1997). [PubMed] [Google Scholar]
- 3.Ibrahim MM, Damasceno A, Hypertension in developing countries. Lancet 380, 611–619 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Pickering TG, Shimbo D, Haas D, Ambulatory blood-pressure monitoring. N. Engl. J. Med 354, 2368–2374 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Agarwal R, Bills JE, Hecht TJW, Light RP, Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: A systematic review and meta-analysis. Hypertension 57, 29–38 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Alpert BS, Quinn D, Gallick D, Oscillometric blood pressure: A review for clinicians. Am. Soc. Hypertens 8, 930–938 (2014). [DOI] [PubMed] [Google Scholar]
- 7.van Montfrans GA, Oscillometric blood pressure measurement: Progress and problems. Blood Press. Monit 6, 287–290 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Imholz BPM, Wieling W, van Montfrans GA, Wesseling KH, Fifteen years experience with finger arterial pressure monitoring: Assessment of the technology. Cardiovasc. Res 38, 605–616 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Mukkamala R, Hahn J-O, Inan OT, Mestha LK, Kim C-S, Töreyin H, Kyal S, Toward ubiquitous blood pressure monitoring via pulse transit time: Theory and practice. IEEE Trans. Biomed. Eng 62, 1879–1901 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.How to use the heart rate monitor on the Galaxy S5 (2017); www.androidcentral.com/ how-use-heart-rate-monitor-galaxy-s5.
- 11.eeNews Europe: Samsung leads the adoption of pressure sensors in Smartphones, for floor-accurate indoor geolocation (2017); www.electronics-eetimes.com/en/samsung-leads-the-adoption-of-pressure-sensors-in-smartphones-for-floor-accurate-indoor-geolocation.html?cmp_id=7&news_id=222916211.
- 12.Seo J, Pietrangelo SJ, Lee H-S, Sodini CG, Noninvasive arterial blood pressure waveform monitoring using two-element ultrasound system. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 62, 776–784 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Beulen BWAMM, Bijnens N, Koutsouridis GG, Brands PJ, Rutten MCM, van de Vosse N, Toward noninvasive blood pressure assessment in arteries by using ultrasound. Ultrasound Med. Biol 37, 788–797 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Vappou J, Luo J, Okajima K, Di Tullio M, Konofagou EE, Non-invasive measurement of local pulse pressure by pulse wave based ultrasound manometry (PWUM). Physiol. Meas 32, 1653–1662 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pressman GL, Newgard PM, A transducer for the continuous external measurement of arterial blood pressure. IEEE Trans. Biomed. Eng 10, 73–81 (1963). [DOI] [PubMed] [Google Scholar]
- 16.Hansen S, Staber M, Oscillometric blood pressure measurement used for calibration of the arterial tonometry method contributes significantly to error. Eur. J. Anaesthesiol 23, 781–787 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Statista: Number of smartphone users worldwide from 2014 to 2020 (billions) (2017); www.statista.com/statistics/330695/number-of-smartphone-users-worldwide.
- 18.Bastawrous A, Armstrong MJ, Mobile health use in low- and high-income countries: An overview of the peer-reviewed literature. J. R. Soc. Med 106, 130–142 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.How much time do people spend on their mobile phones in 2017 (2017); https://hackernoon.com/how-much-time-do-people-spend-on-their-mobile-phones-in-2017-e5f90a0b10a6.
- 20.Shaltis PA, Reisner AT, Asada HH, Cuffless blood pressure monitoring using hydrostatic pressure changes. IEEE Trans. Biomed. Eng 55, 1775–1777 (2008). [DOI] [PubMed] [Google Scholar]
- 21.FMS Finapres Medical Systems. The Finapres NOVA has received 510(k) clearance from the US FDA! (2017); www.finapres.com.
- 22.Gizdulich P, Prentza A, Wesseling KH, Models of brachial to finger pulse wave distortion and pressure decrement. Cardiovasc. Res 33, 698–705 (1997). [DOI] [PubMed] [Google Scholar]
- 23.Mukkamala R, Hahn J-O, Toward ubiquitous blood pressure monitoring via pulse transit time: Predictions on maximum calibration period and acceptable error limits. IEEE Trans. Biomed. Eng 10.1109/TBME.2017.2756018 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosner B, Polk BF, Predictive values of routine blood pressure measurements in screening for hypertension. Am. J. Epidemiol 117, 429–442 (1983). [DOI] [PubMed] [Google Scholar]
- 25.Yano Y, Stamler J, Garside DB, Daviglus ML, Franklin SS, Carnethon MR, Liu K,Greenland P, Lloyd-Jones DM, Isolated systolic hypertension in young and middle-aged adults and 31-year risk for cardiovascular mortality: The Chicago Heart Association Detection Project in Industry Study. J. Am. Coll. Cardiol 65, 327–335 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber MA, Interpreting blood pressure in young adults. J. Am. Coll. Cardiol 65, 336–338 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Kuznetsova T, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Wang J, Sandoya E, O’Brien E, Staessen JA; International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes (IDACO) investigators, Prognostic accuracy of day versus night ambulatory blood pressure: A cohort study. Lancet 370, 1219–1229 (2007). [DOI] [PubMed] [Google Scholar]
- 28.ISO 81060–2:2013 Non-invasive sphygmomanometers—Part 2: Clinical investigation of automated measurement type; www.iso.org/standard/57977.html.
- 29.Liu J, Cheng H-M, Chen C-H, Sung S-H, Hahn J-O, Mukkamala R, Patient-specific oscillometric blood pressure measurement: Validation for accuracy and repeatability. IEEE J. Transl. Eng. Health Med 5, 1900110 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bos WJ, Verrij E, Vincent HH, Westerhof BE, Parati G, van Montfrans GA, How to assess mean blood pressure properly at the brachial artery level. J. Hypertens 25, 751–755 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Lemay M, Bertschi M, Sola J, Renevey P, Parak J, Korhonen I, in Wearable Sensors: Fundamentals, Implementation and Applications, Sazonov E, Neuman MR, Eds. (Academic Press, 2014), chap. 2.3. [Google Scholar]
- 32.Draper NR, Smith H, in Applied Regression Analysis (Wiley-Interscience, 1998), pp. 307–312. [Google Scholar]
- 33.Wesseling KH, Settels JJ, van der Hoeven GMA, Nijboer JA, Butijn MWT, Dorlas JC, Effects of peripheral vasoconstriction on the measurement of blood pressure in a finger. Cardiovasc. Res 19, 139–145 (1985). [DOI] [PubMed] [Google Scholar]
- 34.Ursino M, Cristalli C, A mathematical study of some biomechanical factors affecting the oscillometric blood pressure measurement. IEEE Trans. Biomed. Eng 43, 761–778 (1996). [DOI] [PubMed] [Google Scholar]
- 35.Strauch B, de Moura W, Arterial system of the fingers. J. Hand Surg. Am 15, 148–154 (1990). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


