Abstract
Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used for their anti-inflammatory, analgesic, and antipyretic effects. NSAIDs generally work by blocking the production of prostaglandins (PGs) through the inhibition of two cyclooxygenase enzymes. PGs are key factors in many cellular processes, such as gastrointestinal cytoprotection, hemostasis and thrombosis, inflammation, renal hemodynamics, turnover of cartilage, and angiogenesis. Interest has grown in the various effects of NSAIDs during the last decade. Epidemiological studies have revealed the reduced risk of several cancer types and neurodegenerative diseases by prolonged use of NSAIDs. Recent advances in the understanding of the cellular and molecular mechanisms of NSAIDs will accelerate the processes of discovery and clinical implementation. This review summarizes the molecular mechanisms of NSAIDs on the body systems.
Keywords: Nonsteroidal anti-inflammatory drugs, mechanism, cyclooxygenase, molecular
Introduction
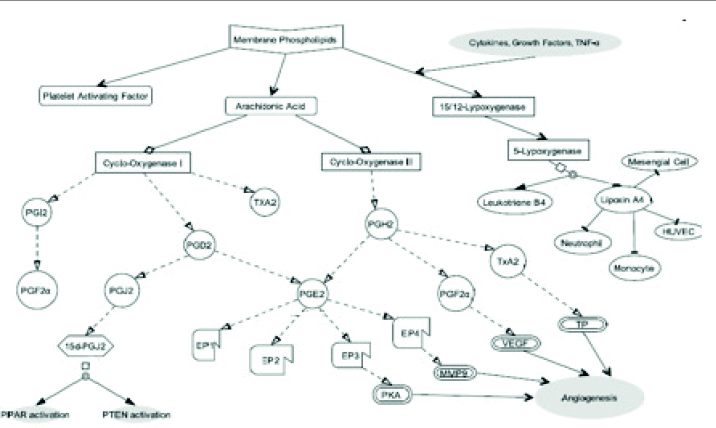
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most prescribed drugs worldwide. It is estimated that 30 million people/day around the world take NSAIDs [1]. These drugs are used due to their potent analgesic, anti-inflammatory, and antipyretic effects. Inhibition of cyclooxygenase (COX) enzyme, which takes part in the biosynthesis of prostaglandins (PGs) and thromboxane (TX), is the mechanism of action of NSAIDs [2]. PGs and TXs are important mediators of fever, pain, and inflammation. Inflammation has a major role in the pathophysiology of various diseases. NSAIDs affect the synthesis and action of inflammatory mediators including PGs, coagulation cascade-derived peptides, interleukin (IL)-2, IL-6, and tumor necrosis factor (TNF). The synthesis of prostanoids that are produced from arachidonic acid causes inflammatory pain [3]. Arachidonic acid mainly exists as esterified phosphatidylcholine and phosphatidylethanolamine phospholipid forms in the membranes. It is released from the cell membrane by phospholipase A2 (PLA2), which is the overall rate-limiting step for eicosanoids. There are two isoforms of PLA2 classified as secretory and cytoplasmic. This multiple isoform existence allows for various biological responses for different tissues. PLA2 isoforms can be stimulated by TNF-α, granulocyte-macrophage colony-stimulating factor, interferon (IFN), and various growth factors, such as mitogen-activated protein kinase (MAPK) and phosphokinase C. COX enzymes convert arachidonic acid to PGs, prostacyclins, and TXs. The first member of this cascade starts with the formation of PGG2 then PGH2. PGH2 is converted into various PG isoforms, such as PGE2, PGD2, PGF2alpha, PGI2, or TXA2 via tissue-specific isomerases. In another pathway, arachidonic acid is converted into leukotrienes by three forms of lipoxygenases (5-LOX, 12-LOX, and 15-LOX) (Figure 1). Various types of cells produce leukotrienes including mast cells, white blood cells (leukocytes, which give the compounds their names), brain, lung, spleen, and heart. 12-LOX and 15-LOX play roles in the production of lipoxins.
Figure 1.
Schematic presentation of cyclooxygenase and lipoxygenase pathway
PGH2: prostaglandin H2; PGF2: prostaglandin F2; PGD2: prostaglandin D2; PGE2: prostaglandin E2; PGI: prostacyclin; TXA2: thromboxane A2; PHJ2: prostaglandin J2; PGF2α: prostaglandin F2α; MMP9: matrix metalloproteinase 9; VEGF: vascular endothelial growth factor; TP: thromboxane receptor; EP1, 2, 3, and 4: prostaglandin E receptors; HUVEC: human umbilical vein endothelial cell; PPAR: peroxisome proliferator-activated receptor; PTEN: phosphatase and tensin homolog
There are three isoforms of COX enzyme, namely COX-1, COX-2, and COX-3. Human chromosome 9 (loci 9q32–33.3 and 1q25.2–25.3) contains COX-1 coding gene, and chromosome 1 has COX-2 coding gene [4]. COX-3 is the product of the same gene, such as COX-1, which is expressed in the spinal cord and cerebral cortex and is found in the endothelial cells, monocytes, and heart in smaller quantities. It may play a role in the perception of pain, but its function is not fully understood yet. COX-1 and COX-2 are also called PGG/H synthase 1 and PGG/H synthase 2. COX-1 is found in the platelets, blood vessels, mesothelial cells, stomach, kidneys, and other tissues [5]. It is a constitutive enzyme and participates in the production of prostanoids that adjust physiologic processes (e.g., hemostasis). Generally, it has non-inducible capacity, but human chorionic gonadotropin can upregulate COX-1 expression in amniotic fluid. COX-2, an inducible form of COX, is present in inflamed tissues through stimulation by cytokines, lipopolysaccharide (LPS), and TNF-α, and protein expression increases up to 80% upon induction. It can also be found in healthy organs in smaller quantities, including the kidneys [6].
NSAIDs act in many different events in the cells. In addition, some NSAIDs participate in calcium-mediated intracellular response, suppression of free radicals and superoxide, downregulation of the production of IL-1, and inhibition of chemotaxis [7]. Arachidonic acid metabolism that is inhibited by NSAIDs also regulates Rho/Rho kinase pathway directly [8]. Consequently, Rho/Rho kinase pathway regulatory pathway of survival, cytoskeleton, and actin–myosin interaction could be intervened with NSAIDs that can explain more mechanism of intercellular responses. Certain NSAIDs can bind to members of the peroxisome proliferator-activated receptor (PPAR) family and other intracellular receptors and activate them. Activation of PPAR is believed to moderate anti-inflammatory activities. More accordingly, a study about nimesulide, which is also a COX-2 inhibitor, showed that PPAR-δ activity that mediated through COX-2 and further 15d-PGJ2 suppresses hepatic inflammation [9]. NSAIDs have also been shown to increase heat shock protein (HSP) response [10]. Although NSAIDs exert their effects on COX pathways, there are many studies about their effects on more complicated mechanisms. The first evidence about these mechanisms emanating from acetylsalicylic acid reported nuclear factor-kappa beta (NF-κB) inhibition [11, 12]. Studies also showed that indomethacin, aspirin, and phenylbutazone enhance TNF-α release induced by LPS [13]. Moreover, in their study, they also showed that phenylbutazone, sulindac, and acetylsalicylic acid significantly increase nitric oxide (NO) release.
Effects of NSAIDs on Systems
Central nervous system
PGs have been found in many regions of the brain. NSAIDs have anti-pyretic and analgesic effects in humans and animals. These activities are thought to be the result of the inhibition of PG formation in the central nervous system (CNS). Most of the COX-1 is in the microglia, though it has been found in the hippocampal and cerebral cortical neurons as well. Although it is clear that COX-3 is expressed abundantly in the cerebral cortex, its enzymatic activity has not yet been elucidated. In contrast to most tissues, inducible COX-2 is also constitutively expressed in the cervical lumbar sections of the spinal cord and several brain regions, such as the cerebral cortex, hippocampus, and amygdala in discrete populations of neurons and but not in glia. COX-1 expression at the spinal level is not wide-ranging and is localized in the cytoplasm of glial cells [14, 15].
NSAIDs are thought to delay the onset of Alzheimer’s disease. COX-2 inhibition interferes with β-amyloid cascade that mediates the suppression of memory and synaptic plasticity. According to data, selective COX-2 inhibitors may have protective effects against Alzheimer’s disease by reducing PGE2 response at synapses due to COX-2 inhibition [15]. PPAR-γ activation that mediated through 15-deoxy PGJ2 also inhibits LPS-stimulated inducible nitric oxide synthase expression and TNF-α production as well as IFN-γ-induced expression of major histocompatibility complex class II antigens, by preventing the activation of the transcription factors signal transducer and activator of transcription 1 and NF-κB, which is also a regulator of microglial inflammation and functions. In animal models, treatment with ibuprofen or indomethacin reduced microglial activation, with a concomitant reduction in amyloid β (Aβ) plaques and inflammatory markers. Activation of PPAR-γ inhibits the Aβ-stimulated activation of the microglia and monocytes and their neurotoxic products. PPAR-γ agonists act to inhibit the Aβ-stimulated expression of IL-6 and TNF-α [16].
On the other hand, NSAIDs inhibit gamma secretase activity, reducing the formation and accumulation of Aβ in animal models and neurodegeneration [17]. In addition, they modulate microglial phagocytosis, contribute to plaque and debris removal and to lower plaque burden and gliosis, and had seen long-term NSAID treatment [18, 19]. In vitro study evaluating the effects on microglial activation of opsonization of Aβ deposits with anti-Aβ IgG (immunoglobulin G), as a strategy to enhance microglial clearance of Aβ deposits, indomethacin had negligible effects on microglial migration and phagocytosis of Aβ, but it did significantly inhibit microglial secretion of IL-6 following opsonization [20]. Another possible mechanism about PGs in Alzheimer’s disease, PGE2 production elevated in the early stages of pathology. This also indicates PGE production of several inflammation molecules, such as IL-6 and chemokines [16].
Two meta-analyses have been published about the useful effects of COX-2 inhibitors in schizophrenia. NSAIDs are thought to have beneficial effects in the prevention of Parkinson’s disease since neuroinflammation can have important an role in the neurodegeneration associated with Parkinson’s disease [21].
Fever is a complex response triggered by inflammatory and infectious diseases. It generally occurs when peripheral endogenous pyrogens come into contact with the hypothalamus. Having a cytokine structure, these pyrogens are generally synthesized by leukocytes and other cells, the most well-known of which are TNF-α, IL-1 β, and IL-6. They can be synthesized in the CNS as well. The preoptic area is near the rostral hypothalamus and is important in controlling thermoregulation. Specialized cells, called temperature-sensitive neurons, regulate thermoneutral set point temperature [22]. Pyrogens cause an increase in set point temperatures, and fever occurs [23]. In febrile response, these pyrogens are not the final mediators. These cytokines induce COX-2 production that increases the synthesis of PGs. These prostanoids adjust hypothalamic temperature control by increasing heat production. The mechanism of action of antipyretics is based on reducing PG production. Alleviation of discomfort, prevention of febrile seizures, reduction of cognitive impairment, and reduction of morbidity and mortality are among the reasons why antipyretics are used [24].
Gastrointestinal system
PGI2 and PGE2 inhibit gastric acid secretion and have vasodilator effects on the vessels of the gastric mucosa. PGs also stimulate mucus secretion. On the other hand, NSAIDs could inhibit PG-mediated effects on the gastrointestinal tract. This effect includes the inhibition of mucin production, HCO3 secretion, and mucosal proliferation. NSAIDs can cause gastrointestinal damage due to the deterioration of this mechanism [25]. This injurious effects can be caused by PG or non-PG-mediated mechanisms. Several studies suggested that increased endothelin-converting enzyme, NO hydrogen sulfide, IL-1β, and calcitonin gene-related peptide (CGRP) and decreased constitutive nitric oxide synthase and polyamines support NSAID-mediated non-PG-dependent injurious mechanism [26]. However, this effect can be seen in supratheurapeutic concentrations of NSAIDs.
COX-1 inhibition by the use of NSAIDs causes gastric hypermotility. This mechanism is unclear but could assume restricted blood flow with high amplitude, resulting in tissue hypoxia and microvascular damage. Gastric lesion that formed eventually because of increased mucosal permeability and myeloperoxidase activity comes up with this enhanced gastric hypermotility [26]. NSAIDs have been shown to upregulate various stress proteins in in vitro and in vivo studies including heat shock protein 72 (HSP-72), glucose-regulated protein 78 (GRP-78), and HO-1 (Heme oxygenase-1, also known as HSP-32) [27]. Stress proteins protect the gastric mucosa from NSAID-induced apoptosis and may therefore play an important role in NSAID-induced cytoprotection and adaptive gastroprotection when PGs are suppressed [26].
Urinary system
NSAIDs can inhibit both constitutive COX-1 in the kidney and intravascular volume-dependent inducible COX-2. Apart from glomerular filtration rate and renal hemodynamics dependence on COX-1, salt and water excretion is mainly under control of COX-2. PGE2, which is the product of COX-induced reaction, controls NaCl transport in the loop of Henle and modulates water transport. PGI2, which is also the product of the same reaction, modulates glomerular filtration rate and renin release. It is already known that COX-2 activates the renin system, but increased activity of the renin system inhibits COX-2. Additionally, PGI2 and PGE2 increase potassium secretion, sensing NaCl concentration at the distal end of the loop of Henle serves tubuloglomerular feedback, thus both of these effects are modulated by PG, which is derived from the macula densa. Angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists that interfere with the angiotensin cascade increase the expression of COX-2 in the kidney [28]. In addition, MAPK and p38 regulate the COX-2 expression in the renal cortex [29]. Consequently, partial mechanism of NSAID on sodium and water retention is mediated through PGE2 and PGI2.
Several studies have investigated the role of COX enzymes and the relationship with glomerular diseases. Monocyte chemoattractant protein-1 (MCP-1) is expressed in animals and humans with glomerulonephritis. Endogenous PGs can reduce MCP-1 expression, which is primarily involved in monocyte/macrophage infiltration, suggesting a role for COX-1 and COX-2 enzymes in renal inflammation. In addition, it has been shown that NSAIDs, especially indomethacin, have a potential in different types of glomerulonephritis and nephrotic syndrome [30]. A study about indomethacin in podocytes also showed that inhibition of inflammatory response, which is dependent on TNF-α, triggers the activation of NF-κB [31].
COXs are locally produced in numerous sites in the kidney. It is already known that COX-1, which assumes PGE2, is primarily synthesized in the tubules, whereas the glomeruli synthesize both PGE2 and PGI2. PGE2 plays a role in modulating vasopressin effects on the reabsorption of water in the collecting duct system of the kidneys. PGs play essential roles in the regulation of renal hemodynamic, renin release, as well as water and salt balance [32]. Renal PGs predominantly have vasodilator effects on the kidneys. On the other hand, PGs are increased to maintain renal perfusion and reduce ischemia in case of hypotension and reduced renal perfusion due to vasoconstriction stimulated by norepinephrine, angiotensin II, vasopressin, or endothelin [33]. The most common renal effect of NSAIDs is increased sodium reabsorption that causes peripheral edema through the inhibition of PGE2. NSAID-caused electrolyte imbalance is not fed to sodium though; inhibition of PG biosynthesis in the kidneys causes hyperkalemia as well. The decrease in renal blood flow due to NSAID may result in kidney failure [34]. Long-term NSAID consumption can induce analgesic nephropathy, which is identified by chronic nephritis and renal papillary necrosis.
Cardiovascular system
PGs and TXs are essential in vascular function. In normal conditions, COX-dependent vasodilators, such as PGI2, regulate vascular tone. Nonetheless, COX-dependent vasoconstriction (triggered by TX and/or its precursor, PGH2) takes part in some vascular pathologies including cerebral ischemia, diabetes, systemic hypertension, and aging [35].
The fact that NSAIDs inhibit PGs may affect cardiovascular regulation. PGE2 and PGI2 are shown to have glomerular vasodilatory effects in the kidney. PGE2 has direct natriuretic effects. Inhibition of the synthesis of PGs by NSAIDs may cause sodium and water retention and worsen hypertension [36]. Selective COX inhibitors can induce thrombosis risk due to decreased synthesis of PGI2, which plays a critical role in vasodilation and platelet inhibition in endothelial cells. Consequently, imbalance can occur between PGI2 and TXA2 that is a vasoconstrictive substance and contribute to platelet aggregation and thrombus formation. TXA2 is released from platelets and plays a role in aggregation, whereas PGI2 is released from the endothelium and plays a role in the inhibition of aggregation.
Aspirin inhibits platelet aggregation by blocking not only COX enzyme but also synthesis of TXA2 and PGI2. Although aspirin inhibits TXA2 synthesis in low doses (100 mg), it inhibits both TXA2 and PGI2 in high doses. Moreover, the inhibition of platelet aggregation by aspirin may inhibit the release of certain platelet-associated substances in the venous circulation, including fibrinogen, platelet factors III and IV, von Willebrand factor, factors V and XIII, thrombospondin, serotonin, and calcium ions among other substances that aid with the improvement of venous thrombosis, and COX-2 selective NSAIDs have little or no role on platelet function.
Bone metabolism
PGs take part in the control of osteoblast and osteoclast functions, and the inhibition of PG synthesis restrains bone formation [37]. They directly affect osteoclasts that lead to increased bone resorption by a mitogenic effect and increase their functional activity. They exert this range of action through a variety of receptors expressed. These receptors belong to the G protein-coupled receptor family and have four subtypes (EP1, EP2, EP3, and EP4) [38]. Although the role of each receptor is not fully explored, studies suggested that the PGE2 binding to EP4 can stimulate osteoclastogenesis and osteoblastic differentiation, and animal models lacking the EP2 and EP4 receptors had defects in bone metabolism [39] Although all NSAIDs have similar effects, certain NSAIDs can modulate the behavior of osteoblasts, such as proliferation, differentiation, adhesion, and migration.
However, PGs may increase the multiplication and differentiation of osteoblasts and reveal anabolic effects on the bone (Ippokratis Pountos et al. [40]). In bone tissue, local regulation of PGs is conducted through COX-2 activation [41]. On the contrary, PG’s exact mechanism on bone cells is considered intricate but, it was found that PGE2 regulates bone morphogenetic protein-2 (BMP-2), BMP-7, and receptor activator of nuclear factor-kappa B ligand expression [42], [38]. It can also increase cell numbers through suppression of apoptosis without direct effect on proliferation. There is also a theory about the inhibition of COX-2 deviating from the arachidonic acid cascade from lipoxygenase pathway that negatively affects bone healing [43]. This pathway negatively influences osteoblast proliferation and stimulation of osteoclast activity [43] COX-2 selective inhibitors also showed an increase in aggrecan and type II collagen mRNA levels that indicate chondrocyte differentiation and, consequently, failed to hypertrophy, which impeded the healing process of another mechanism [43]
Studies about NSAIDs, well known as immunoregulators, on lymphocytes, potent immune elements, also showed a different regulation of cytokine production in human IFN-δ and IL-2. A randomized open label trial about cytokine concentration and signal transduction pathways in the synovial fluid also showed increased vascular endothelial growth factor, IL-6, and TNF-α concentrations. They also evaluated the effects of NSAIDs on MAPK, extracellular signal-regulated kinase (ERK), Jun kinase (JNK), p38, and RAS phosphorylation signal transduction pathways on the synovial membrane. However, they only tested NSAIDs on the highest doses and showed that NSAID treatment significantly inhibits ERK, JNK, p38, and RAS phosphorylation along with caspase-3 activation [44]. Studies with chemical-induced animal models of inflammation and open label trial on patients showed different cytokine modulation with NSAIDs. It should be noted that NSAIDs exert different effects, thus these cellular signal pathways need more research and clinical-based evidences.
Respiratory system
Inhibition of COX-1 causes a decrease in some PGs, which exert important regulatory effects on respiratory epithelial cells. Decreased PG production can induce leukotriene pathway, causing bronchoconstriction. When used in high doses, salicylates damage the process of oxidative phosphorylation, causing an increase in plasma carbon dioxide levels (COX-independent effects) followed by hyperventilation. Higher doses can result in depression of respiration.
A special case about COX inhibition on the respiratory system, which was named aspirin-exacerbated respiratory disease consisting of asthma, aspirin sensitivity, and nasal polyps, came to be known as Samter’s triad. It is also known as NSAID-exacerbated disease, and the mechanism of this pathology is not clearly understood. Reduction of PGE2, a potent inhibitor of leukotriene pathway, by COX is considered as the mechanism responsible for the progression of the disease.
Cancer
In the research of various mechanisms about cancer progression and metastasis, anti-inflammatory drugs are also investigated. Epidemiological studies have showed that the use of NSAID is inversely related to the incidence of some cancer types [45]. Long-term NSAID users who have colorectal cancer have showed lower mortality rates than non-NSAID users [46]. In addition, sulindac, ibuprofen, piroxicam, and aspirin have been shown to decrease the recurrence, incidence, mortality rate, and number of cancer cells in a significant manner. [47], [28]. Some clinical trials for several malignancies have showed that selective COX-2 inhibitors, particularly celecoxib have potential cancer chemopreventive effect [48].
The effects of NSAIDs on tumor inhibitions and prevention of metastasis have not been fully understood yet [49]. It is widely accepted that an extreme amount of the production of PGs and cytokines by tumors is associated with their metabolism, proliferation, angiogenesis, invasion ability, resistance to apoptosis, and suppression of antitumor immunity.
In tumorigenesis, transcriptional upregulation of the COX-2 gene in colorectal adenomas and carcinomas has been observed, although COX-1 is not affected [50] Upregulated COX-2 in colorectal carcinoma and, consequently, enhanced PGE2 signaling via PGE-EP receptors is 3–4-fold higher than that in healthy tissue. It has been shown in other studies that PGE2 inhibits apoptosis and stimulates tumor growth and angiogenesis via the activation of β-catenin/T-cell factor dependent transcription [51]. On the other hand, PPAR-δ, which inhibits tumorigenesis, is inhibited with sulindac and indomethacin.
The known inhibitory effects of cyclooxygenase pathways prompted the investigation on NSAIDs through COX-2 specific and non-COX pathway for cancer research. From these studies, the NF-κB pathway initially emerges. NF-κB activation is inhibited with acetylsalicylic acid without interfering gene transcription [52]. In addition, sulindac inhibits NF-κB pathway [53]. Another family of intercellular pathways, WNT pathway, which is associated with carcinogenesis, most notably that of colorectal cancer, can also be inhibited by sulindac [54].
Generally, NSAIDs are used prevalently for their analgesic effects. Although cyclooxygenase inhibition is the main NSAIDs in use today, they are also associated with safety and tolerability concerns. However, further investigation of NSAID’s mechanism of action disclosed complicated molecular pathways. It is important to understand which pathological pathways they are able to modulate and how these pathways interact with multiple targets. Molecular approaches may further enhance our understanding of the pathological pathways, improve the identification of risks, and aid in the design of novel treatment strategies. Several mechanisms are determined, but the remainder needs to be clarified. How can NSAIDs counter inflammation in other cellular pathways? Are NSAIDs the new research area for diseases with high mortality such as Alzheimer’s disease and cancer? Although this answers far away from our knowledge, the exact cellular pathways will aid us to learn more about their mechanisms. Results of recent studies suggested that both Cox-dependent and Cox-independent mechanisms are involved in various pharmacological activities of NSAIDs.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - C.G., S.B.; Design - C.G., S.B.; Supervision - C.G., S.B.; Resources - C.G., S.B.; Materials - C.G., S.B.; Data Collection and/or Processing - C.G., S.B.; Analysis and/or Interpretation - C.G., S.B.; Literature Search - C.G., S.B.; Writing Manuscript - C.G., S.B.; Critical Review - C.G., S.B.; Other - C.G., S.B.
Conflict of Interest: Authors have no conflict of interest to declare.
Financial Disclosure: The authors declare that this study has received no financial support.
References
- 1.Bhala N, Emberson J, Merhi A, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769–79. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacchi S, Palumbo P, Sponta A, Coppolino MF. Clinical pharmacology of non-steroidal anti-inflammatory drugs: a review. Antiinflamm Antiallergy Agents Med Chem. 2012;11:52–64. doi: 10.2174/187152312803476255. [DOI] [PubMed] [Google Scholar]
- 3.Samad TA, Sapirstein A, Woolf CJ. Prostanoids and pain: unraveling mechanisms and revealing therapeutic targets. Trends Mol Med. 2002;8:390–6. doi: 10.1016/S1471-4914(02)02383-3. [DOI] [PubMed] [Google Scholar]
- 4.Birmingham B, Buvanendran A. Nonsteroidal anti-inflammatory drugs, Acetaminophen, and COX-2 inhibitors. In: Turk DC, Argoff CE, Hurley RW, editors. Practical Management of Pain. Philadelphia: Elsevier; 2014. pp. 553–68. e5. [Google Scholar]
- 5.Zidar N, Odar K, Glavac D, et al. Cyclooxygenase in normal human tissues-is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J Cell Mol Med. 2009;13:3753–63. doi: 10.1111/j.1582-4934.2008.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Consalvi S, Biava M, Poce G. COX inhibitors: a patent review (2011–2014) Expert Opin Ther Pat. 2015;25:1357–71. doi: 10.1517/13543776.2015.1090973. 2015. [DOI] [PubMed] [Google Scholar]
- 7.Borazan NH, F D. Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, nonopioid analgesics, & drugs used in gout. In: Katzung BG, Trevor AJ, editors. Basic and Clinical Pharmacology. 13 ed. New York: McGraw-Hill Education; 2015. p. 618. [Google Scholar]
- 8.Liu J, Gao HY, Wang XF. The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system. Neural Regen Res. 2015;10:1892–6. doi: 10.4103/1673-5374.170325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsujimoto S, Kishina M, Koda M, et al. Nimesulide, a cyclooxygenase-2 selective inhibitor, suppresses obesity-related non-alcoholic fatty liver disease and hepatic insulin resistance through the regulation of peroxisome proliferator-activated receptor γ. Int J Mol Med. 2016;38:721–8. doi: 10.3892/ijmm.2016.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batulan Z, Nalbantoglu J, Durham HD. Nonsteroidal anti-inflammatory drugs differentially affect the heat shock response in cultured spinal cord cells. Cell Stress Chaperones. 2005;10:185–96. doi: 10.1379/CSC-30R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hovens MM, Snoep JD, Tamsma JT, Huisman MV. Aspirin in the prevention and treatment of venous thromboembolism. J Thromb Haemost. 2006;4:1470–5. doi: 10.1111/j.1538-7836.2006.01928.x. [DOI] [PubMed] [Google Scholar]
- 12.Wu KK. Aspirin and other cyclooxygenase inhibitors: new therapeutic insights. Semin Vasc Med. 2003;3:107–12. doi: 10.1055/s-2003-40668. [DOI] [PubMed] [Google Scholar]
- 13.Cho JY. Immunomodulatory effect of nonsteroidal anti-inflammatory drugs (NSAIDs) at the clinically available doses. Arch Pharm Res. 2007;30:64–74. doi: 10.1007/BF02977780. [DOI] [PubMed] [Google Scholar]
- 14.Lee CH, Yoo KY, Choi JH, et al. Cyclooxygenase-2 immunoreactivity and protein level in the gerbil hippocampus during normal aging. Neurochem Res. 2010;35:99–106. doi: 10.1007/s11064-009-0037-2. [DOI] [PubMed] [Google Scholar]
- 15.Yagami T, Koma H, Yamamoto Y. Pathophysiological roles of cyclooxygenases and prostaglandins in the central nervous system. Mol Neurobiol. 2016;53:4754–71. doi: 10.1007/s12035-015-9355-3. [DOI] [PubMed] [Google Scholar]
- 16.Krause DL, Müller N. Neuroinflammation, microglia and implications for anti-inflammatory treatment in Alzheimer’s disease. Int J Alzheimers Dis. 2010;14:2010. doi: 10.4061/2010/732806. pii: 732806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cudaback E, Jorstad NL, Yang Y, Montine TJ, Keene CD. Therapeutic implications of the prostaglandin pathway in Alzheimer’s disease. Biochem Pharmacol. 2014;88:565–72. doi: 10.1016/j.bcp.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koenigsknecht-Talboo J, Landreth GE. Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by pro-inflammatory cytokines. J Neurosci. 2005;25:8240–9. doi: 10.1523/JNEUROSCI.1808-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim GP, Yang F, Chu T, et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J Neurosci. 2000;20:5709–14. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strohmeyer R, Kovelowski CJ, Mastroeni D, et al. Microglial responses to amyloid beta peptide opsonization and indomethacin treatment. Journal of Neuroinflammation. 2005;2:18. doi: 10.1186/1742-2094-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rees K, Stowe R, Patel S, et al. Non-steroidal anti-inflammatory drugs as disease-modifying agents for Parkinson’s disease: evidence from observational studies. Cochrane Database Syst Rev. 2011:CD008454. doi: 10.1002/14651858.CD008454.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Aronoff DM, Neilson EG. Antipyretics: mechanisms of action and clinical use in fever suppression. Am J Med. 2001;111:304–15. doi: 10.1016/S0002-9343(01)00834-8. [DOI] [PubMed] [Google Scholar]
- 23.Boulant JA. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis. 2000;31(Suppl 5):S157–61. doi: 10.1086/317521. [DOI] [PubMed] [Google Scholar]
- 24.Greisman LA, Mackowiak PA. Fever: beneficial and detrimental effects of antipyretics. Curr Opin Infect Dis. 2002;15:241–5. doi: 10.1097/00001432-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Matsui H, Shimokawa O, Kaneko T, et al. The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J Clin Biochem Nutr. 2011;48:107–11. doi: 10.3164/jcbn.10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musumba C, Pritchard DM, Pirmohamed M. Review article: cellular and molecular mechanisms of NSAID-induced peptic ulcers. Aliment Pharmacol Ther. 2009;30:517–31. doi: 10.1111/j.1365-2036.2009.04086.x. [DOI] [PubMed] [Google Scholar]
- 27.Mizushima T. Various stress proteins protect gastric mucosal cells against non-steroidal anti-inflammatory drugs. Inflammopharmacology. 2007;15:67–73. doi: 10.1007/s10787-006-1560-2. [DOI] [PubMed] [Google Scholar]
- 28.Harris RE, Chlebowski RT, Jackson RD, et al. Breast cancer and nonsteroidal anti-inflammatory drugs: prospective results from the Women’s Health Initiative. Cancer Res. 2003;63:6096–101. [PubMed] [Google Scholar]
- 29.Kömhoff M, Wang JL, Cheng HF, et al. Cyclooxygenase-2-selective inhibitors impair glomerulogenesis and renal cortical development. Kidney Int. 2000;57:414–22. doi: 10.1016/S0085-2538(15)46757-2. [DOI] [PubMed] [Google Scholar]
- 30.Hörl WH. Nonsteroidal Anti-Inflammatory Drugs and the Kidney. Pharmaceuticals (Basel) 2010;3:2291–321. doi: 10.3390/ph3072291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamura M, Takano Y, Hiramatsu N, et al. Suppression of cytokine responses by indomethacin in podocytes: a mechanism through induction of unfolded protein response. Am J Physiol Renal Physiol. 2008;295:F1495–503. doi: 10.1152/ajprenal.00602.2007. [DOI] [PubMed] [Google Scholar]
- 32.Norregaard R, Kwon TH, Frokiaer J. Physiology and pathophysiology of cyclooxygenase-2 and prostaglandin E2 in the kidney. Kidney Res Clin Pract. 2015;34:194–200. doi: 10.1016/j.krcp.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadowski J, Badzynska B. Intrarenal vasodilator systems: NO, prostaglandins and bradykinin. An integrative approach. J. Physiol Pharmacol. 2008;59(Suppl 9):105–34. [PubMed] [Google Scholar]
- 34.Davidge ST. Prostaglandin H synthase and vascular function. Circ Res. 2001;89:650–60. doi: 10.1161/hh2001.098351. [DOI] [PubMed] [Google Scholar]
- 35.Weir MR. Renal effects of nonselective NSAIDs and coxibs. Cleve Clin J Med. 2002;69(Suppl 1):SI53–8. doi: 10.3949/ccjm.69.Suppl_1.SI53. [DOI] [PubMed] [Google Scholar]
- 36.Vuolteenaho K, Moilanen T, Moilanen E. Nonsteroidal anti-inflammatory drugs, cyclooxygenase-2 and the bone healing process. Basic Clin Pharmacol Toxicol. 2008;102:10–4. doi: 10.1111/j.1742-7843.2007.00149.x. [DOI] [PubMed] [Google Scholar]
- 37.Paralkar VM, Grasser WA, Mansolf AL, et al. Regulation of BMP-7 expression by retinoic acid and prostaglandin E(2) J Cell Physiol. 2002;190:207–17. doi: 10.1002/jcp.10048. [DOI] [PubMed] [Google Scholar]
- 38.Machwate M, Harada S, Leu CT, et al. Prostaglandin receptor EP(4) mediates the bone anabolic effects of PGE(2) Mol Pharmacol. 2001;60:36–41. doi: 10.1124/mol.60.1.36. [DOI] [PubMed] [Google Scholar]
- 39.Pountos I, Georgouli T, Calori GM, Giannoudis PV. Do Nonsteroidal anti-ınflammatory drugs affect bone healing? A critical analysis. Scientific World Journal. 2012;2012:606404. doi: 10.1100/2012/606404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerstenfeld LC, Thiede M, Seibert K, et al. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2003;21:670–5. doi: 10.1016/S0736-0266(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 41.Arikawa T, Omura K, Morita I. Regulation of bone morphogenetic protein-2 expression by endogenous prostaglandin E2 in human mesenchymal stem cells. J Cell Physiol. 2004;200:400–6. doi: 10.1002/jcp.20031. [DOI] [PubMed] [Google Scholar]
- 42.Cottrell J, O’Connor JP. effect of non-steroidal anti-ınflammatory drugs on bone healing. Pharmaceuticals (Basel) 2010;3:1668–93. doi: 10.3390/ph3051668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maxis K, Delalandre A, Pelletier JM, et al. The shunt from the cyclooxygenase to lipoxygenase pathway in human osteoarthritic subchondral osteoblasts is linked with a variable expression of the 5-lipoxygenase-activating protein. Arthritis Res Ther. 2006;8:R181. doi: 10.1186/ar2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gallelli L, Galasso O, Falcone D, et al. The effects of nonsteroidal anti-inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis. A randomized open label trial. Osteoarthritis Cartilage. 2013;21:1400–8. doi: 10.1016/j.joca.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 45.Arun B, Goss P. The role of COX-2 inhibition in breast cancer treatment and prevention. Semin Oncol. 2004;31(2 Suppl 7):22–9. doi: 10.1053/j.seminoncol.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 46.Rayburn ER, Ezell SJ, Zhang R. Anti-Inflammatory Agents for Cancer Therapy. Mol Cell Pharmacol. 2009;1:29–43. doi: 10.4255/mcpharmacol.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cruz-Correa M, Hylind LM, Romans KE, Booker SV, Giardiello FM. Long-term treatment with sulindac in familial adenomatous polyposis: a prospective cohort study. Gastroenterology. 2002;122:641–5. doi: 10.1053/gast.2002.31890. [DOI] [PubMed] [Google Scholar]
- 48.Gong L, Thorn CF, Bertagnolli MM, et al. Celecoxib pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012;22:310–8. doi: 10.1097/FPC.0b013e32834f94cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Umar A, Steele VE, Menter DG, Hawk ET. Mechanisms of nonsteroidal anti-inflammatory drugs in cancer prevention. Semin Oncol. 2016;43:65–77. doi: 10.1053/j.seminoncol.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 50.Kargman SL, O’neill GP, Vickers PJ, et al. Expression of prostaglandin G/H synthase-1 and -2 protein in human colon cancer. Cancer Res. 1995;55:2556–9. [PubMed] [Google Scholar]
- 51.Shao J, Jung C, Liu C, Sheng H. Prostaglandin E2 Stimulates the beta-catenin/T cell factor-dependent transcription in colon cancer. J Biol Chem. 2005;280:26565–72. doi: 10.1074/jbc.M413056200. [DOI] [PubMed] [Google Scholar]
- 52.Stark LA, Din FV, Zwacka RM, Dunlop MG. Aspirin-induced activation of the NF-kappaB signaling pathway: a novel mechanism for aspirin-mediated apoptosis in colon cancer cells. FASEB J. 2001;15:1273–5. doi: 10.1096/fj.00-0529fje. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–42. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boon EM, Keller JJ, Wormhoudt TA, et al. Sulindac targets nuclear beta-catenin accumulation and wnt signalling in adenomas of patients with familial adenomatous polyposis and in human colorectal cancer cell lines. Br J Cancer. 2004;90:224–9. doi: 10.1038/sj.bjc.6601505. [DOI] [PMC free article] [PubMed] [Google Scholar]