Abstract
Objective
To assess the efficacy of a home care program designed to improve access to medical care for older adults with multiple chronic conditions who are at risk for hospitalization.
Study Design
Randomized controlled trial in which participants were assigned to the home care intervention (Choices for Healthy Aging [CHA]) program or usual care.
Methods
The intervention group consisted of 298 older adults at risk of hospitalization as determined by a risk stratification tool. Measures included satisfaction with medical care, medical service use, and costs of medical care.
Results
The intervention group reported significantly greater satisfaction with care than usual care recipients (t test = 2.476; P = .014). CHA patients were less likely than usual care patients to be admitted to the hospital (25.6% and 37.1%, respectively; P = .02). There were no differences in terms of costs of care between the home care and usual care groups.
Conclusions
Provision of home care to older adults at high risk of hospitalization may improve satisfaction with care while reducing hospitalizations. Lack of difference in medical costs suggests that managed care organizations need to consider targeting rather than using risk stratification measures when designing programs for high-risk groups.
Advanced age is often associated with greater likelihood of acquiring chronic disease. Previous studies have reported that by age 65 years, the majority of individuals have multiple chronic conditions1 that could require services from an array of medical providers and higher healthcare expenditures. In addition, many chronically ill older people perceive managing their illness as beyond their ability.2 Many aspects of the current healthcare system have been ineffectively designed to monitor and treat chronic conditions that may involve multiple medical specialists. The relative lack of attention to care coordination has led to poorer health outcomes in the United States relative to costs of care.3
The Patient Protection and Affordable Care Act holds significant implications for healthcare providers regarding improvement in quality of care.4 Through a series of incentives and mandated reporting on quality indicators, healthcare providers will have greater accountability for the quality and costs of care provided. For example, beginning in 2012, managed care providers, health plans, and hospitals will be fiscally accountable for hospital readmissions. As this deadline approaches, effective methods to improve and manage care for high-risk patients with multiple chronic conditions are urgently needed. Additionally, the Patient Protection and Affordable Care Act seeks to improve both quality and continuity of care through the development and testing of new models, including service delivery models, that use home-based primary care physician and nurse practitioner teams to achieve higher quality and lower costs of care. These recommendations arise from a growing body of literature attesting to the need for home-based healthcare delivery systems to better respond to individuals with complex healthcare issues.5,6 Providing in-home primary care to high-risk, chronically ill patients has been found to improve quality of care and patient satisfaction, although impact on healthcare costs is mixed.7,8
This study assessed the effects of an interdisciplinary team that provided care in the home for chronically ill patients at high risk for hospitalization. We hypothesized that patients who received care via the Choices for Healthy Aging (CHA) program would report greater satisfaction with services and have fewer hospitalizations and emergency department (ED) visits, resulting in reduced costs of care compared with the costs for usual care recipients.
METHODS
A randomized controlled trial was conducted to test the effectiveness of the CHA program for reducing medical service use and improving satisfaction with care among a high-risk group of patients enrolled in a managed care organization located in Southern California. This study was approved by the institutional review board of the research organization working with the managed care health plan.
Research Site
This research was conducted within a management services organization that manages and operates medical groups and independent physician networks nationally. The study was conducted among 3 Los Angeles County regions of the management services organization.
Eligibility and Enrollment
Potential participants were identified from a pool of patients by using an electronic risk assessment screening process developed and tested by SCAN Health Plan (for more information see Predicting the Financial Risks of Seriously Ill Patients).9 The assessment tool identified frail older adults at high risk for use of medical services by using an algorithm that considered variables such as age, sex, number of medications, number and types of chronic conditions, and use of EDs and inpatient hospital services. Patients were initially contacted by telephone and were provided with information about the study. Interested participants provided written informed consent for study participation. Participants were randomized into either an intervention or control group using a computer-generated randomization chart.
Our primary hypotheses included improving satisfaction with medical care and reducing hospitalizations and costs of medical care. Based on the cost savings reported by Brumley et al,7 with a 2-sided, 2-sample t test at an alpha level of .05, a power level of 0.80, and an effect size of r = .16, we required a total of 216 patients to complete the study, 290 after adjusting for attrition rates.7
Study Groups
Intervention Group
The CHA program was adapted from an evidence-based home-based palliative care program found to be effective in improving patient satisfaction, decreasing deaths in the hospital, and reducing cost of care among patients in the last year or two of life.7,10 The home-based palliative care program also demonstrated that provision of interdisciplinary care was effective in shifting the locale of care from acute care settings to home and community environments, a place of care much more aligned with the needs and wishes of many severely ill patients.
Goals of the CHA program were built on the home-based palliative care program model and included the following activities: (1) early identification and treatment of exacerbation of the illness, (2) patient-specific health education, (3) self-management or caregiver management of the disease, and (4) advance care planning and other psychosocial issues. Care was delivered via an interdisciplinary team, with core team members consisting of a physician, nurse practitioner, nurse care manager, and a social worker.
Within 5 days of a patient consenting to the program, an initial home visit was made by a home care physician, nurse care manager, and social worker. The physician conducted an initial medical assessment and provided acute treatment needed for stabilization and palliation. The nurse followed with patient and family education, advance care planning, assessment of medications management need, and treatment adherence of patients. The social worker conducted a biopsychosocial evaluation, including an assessment of the patient’s living condition, level of caregiver support, and mental status. Treatment plans were developed in consultation with the patient and family. The plan addressed care for acute and chronic conditions experienced by each patient, with special attention to palliative care as needed. In addition, all medications were reviewed and monitored to ensure that participants were taking correct medications and dosages and were aware of potential side effects.
A physician/nurse practitioner team, in coordination with a nurse care manager, was responsible for conducting follow-up home visits at least once a month. During these visits, the nurse provided medication reconciliation, disease education, and advance care planning, and assessed the patient for warning signs of hospitalization. A social worker also visited the home once a month, on average, and provided patients and their families with information and referrals to supportive services in the community. Other team members made less frequent visits and telephone calls. The clinical team conducted weekly meetings to ensure continuity of care and coordinated treatment plans among the various healthcare providers treating the patient, including the patient’s primary care physician, specialists, and other providers/services such as durable medical equipment, home healthcare, pharmacy, and case management. Finally, a physician with a nurse care manager, medical assistant, and social worker acted as personal care advocates of the patients, facilitating coordination of appointments with specialists and other service providers.
The home care physician was available to visit 24 hours a day, 7 days a week, and also made regularly scheduled home visits as medically appropriate. Patients and caregivers were provided with the physician’s cell phone number, enabling easy access, and patients and families were encouraged to contact their home care physician any time to ask questions about their healthcare or address exacerbations. The initial intake was conducted within 1 to 2 home visits, and each intervention participant received at least 1 visit once per month, more depending on need and acuity.
Usual Care Group
Patients assigned to the usual care group received the standard care for which they were eligible, provided by their medical group. That included the usual primary care, home healthcare, hospice, ED, and hospital care.
Measures and Data Collection
Data were collected from patient interviews and from the electronic databases maintained by the medical group. Interviews were conducted via telephone immediately following receipt of the informed consent form (at enrollment) to collect demographic and satisfaction information at baseline; these interviews were repeated at 6 months. Undergraduate- and graduate-level research assistants, blinded to group assignments, were recruited and trained to conduct telephone interviews with patients. Interviews were approximately 15 minutes long.
Sociodemographics
Sociodemographic characteristics of patients were collected during the baseline survey, including participants’ age, sex, marital status, race/ethnicity, education level, income level, living arrangement, and social support. Living arrangement referred to the type of housing and household composition. Social support was determined by asking patients who their primary caregiver was.
Satisfaction With Medical Care
Satisfaction with medical care was measured using the Home Care Satisfaction Measure.11,12 The scale measures patient satisfaction with the overall primary care team and was administered to participants in both the treatment and control groups. Dimensions of care assessed by the Home Care Satisfaction Measure included choice, information/education, emotional support, coordination/continuity of care, problem solving, and overall quality.
Medical Service Utilization and Costs
Service utilization data were collected retrospectively from the management services organization electronic databases at 12 months following program enrollment. Service data included number of ED visits, physician office visits, and hospital days. These variables were coded and analyzed as continuous variables and dichotomized for logistic regressions. Due to varying payment mechanisms for hospital claims as well as clinician costs, actual costs were not available. Estimates for total costs of medical services were computed using proxy costs that represented average expenditures per utilization of inpatient and ED care. CHA intervention visits were recorded in an electronic database (for physicians and nurse practitioners) and through the use of a program electronic log for social workers, aides, and case managers. Proxy costs for intervention visits by discipline were calculated based on actual time recorded per visit for social workers and case managers, and for average visit and commute/travel times for nurse practitioners and physicians. Average hourly salary for each discipline (including benefits) was multiplied by either the actual hours recorded (social workers and care managers) or the average visit time (physicians and nurse practitioners). Clinician time included telephone, travel, and in-person time incurred in provision of patient care.
Analyses
Analyses were conducted using SPSS version 19.0. Descriptive statistics, including means, standard deviations, and percentages, were calculated for sociodemographic and key outcome variables. Bivariate analysis (Pearson’s χ2 and t tests) were computed to evaluate differences in sociodemographic characteristics and baseline measures between study groups. Differences in satisfaction with services were normally distributed and compared using independent samples t tests. As medical group administrators often look at per-member per-month ratios, we calculated this variable by multiplying the number of admissions (or hospital days) by 12,000 (1000 members × 12 months) and dividing by the actual sample size multiplied by the actual months of data collected.12 One outlier cost figure was nearly double the value of the high end of the remaining distribution and was therefore removed from analysis. As medical cost data are typically skewed, the nonparametric Mann-Whitney U test was conducted to compare mean rank of total costs of care between study groups.
Logistic regression analyses were conducted to determine the effect of the intervention group on ED use and hospitalization, adjusting for demographic variables and deaths occurring during the study period. Costs of medical care were aggregated into an overall cost variable that included intervention costs. Differences in total costs were compared in bivariate analysis and in regression analysis, adjusting for demographics and medical conditions. Negative binomial regression, well suited for nonnormally distributed data with a variance that exceeds the mean,13 was conducted to determine factors associated with medical care costs.
RESULTS
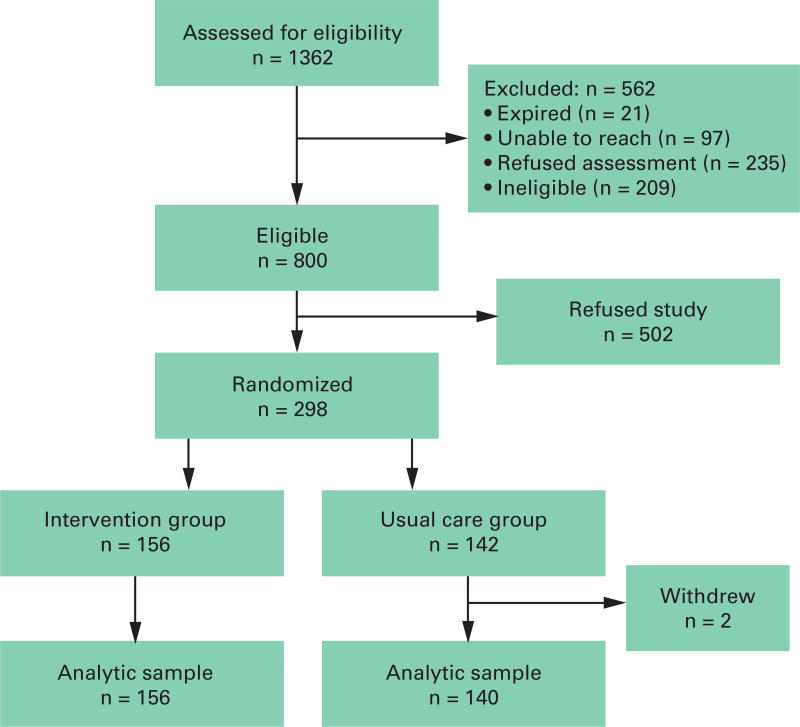
From January to July 2008, 298 patients were enrolled in the study; 156 were randomized to the CHA group and 142 to usual care (Figure). The mean age of study participants was 80.8 years (standard deviation [SD] 8.3 years), and 66.9% were female. More than 61% of the sample was white, 11.9% black, 18.0% non-white Hispanic, and 8.5% reported some other race/ethnicity. About 37% of the sample reported being married, with 43.5% reporting widowhood as their marital status (not shown). Educational attainment levels among study participants varied, with many reporting that they had engaged in some college work (27.3%), graduated from high school or received a General Educational Development equivalent (31.8%), or attained less than a high school degree (40.8%). There were no significant differences between the intervention and usual care groups, suggesting that randomization was successful in evenly distributing patients between groups (Table 1). Most participants reported living in their own homes or apartments (80.7%). About one-third resided with their spouse (35.1%) and about one-fourth lived alone (26.0%) or with a child (23.3%). Most participants reported that their primary caregiver was either their child (29.7%) or spouse (28.0%). Finally, 22 (7.4%) patients died during the study period, 8 (5.7%) in the study group and 14 (9%) in the usual care group (χ2= 1.14, P = .39).
Figure.
Study Enrollment Consort Diagram
Table 1.
Descriptive Statistics and Test Statistics for Difference Between Intervention and Control Groups on Sociodemographic Variables (N = 296)
| Characteristic | CHA | Control | Total | Statistic | P |
|---|---|---|---|---|---|
| Sex | n = 156 | n = 140 | N = 296 | χ2 = 1.32 | .25 |
| Male | 47 (30.1%) | 51 (36.4%) | 98 (33.1%) | ||
| Female | 109 (69.9%) | 89 (63.6%) | 198 (66.9%) | ||
| Age | n = 155 | n = 140 | N = 295 | t = −0.578 | .56 |
| Mean (SD), y | 81.1 (7.9) | 80.6 (8.7) | 80.8 (8.3) | ||
| Race/ethnicity | n = 155 | n = 139 | N = 294 | χ2 = 1.62 | .65 |
| White | 93 (60.0%) | 88 (63.3%) | 181 (61.6%) | ||
| Black | 18 (11.6%) | 17 (12.2%) | 35 (11.9%) | ||
| Non-white Hispanic | 32 (20.6%) | 21 (15.1%) | 53 (18.0%) | ||
| Other | 12 (7.7%) | 13 (9.4%) | 25 (8.5%) | ||
| Marital status | n = 154 | n = 140 | N = 294 | χ2 = 1.15 | .28 |
| Married | 61 (39.6%) | 47 (33.6%) | 108 (36.7%) | ||
| Unmarried | 93 (60.4%) | 93 (66.4%) | 186 (63.3%) | ||
| Education | n = 149 | n = 140 | N = 289 | χ2 = 0.21 | .90 |
| Less than high school | 62 (41.6%) | 56 (40.0%) | 118 (40.8%) | ||
| High school or GED | 48 (32.2%) | 44 (31.4%) | 92 (31.8%) | ||
| More than high school | 39 (26.2%) | 40 (28.6%) | 79 (27.3%) | ||
| Diagnosis | n = 156 | n = 140 | N = 296 | ||
| Diabetes | 81 (51.9%) | 74 (52.9%) | 155 (52.4%) | χ2 = 0.03 | .87 |
| Coronary artery disease | 51 (32.7%) | 43 (30.7%) | 94 (31.8%) | χ2 = 0.13 | .72 |
| Congestive heart failure | 81 (51.9%) | 58 (41.4%) | 139 (47.0%) | χ2 = 3.26 | .07 |
| Chronic obstructive pulmonary disease | 63 (40.4%) | 49 (35.0%) | 112 (37.8%) | χ2 = 0.91 | .34 |
| Cardiovascular disease | 29 (18.6%) | 24 (17.1%) | 53 (17.9%) | χ2 = 0.11 | .75 |
| Renal failure | 86 (55.1%) | 86 (61.4%) | 172 (58.1%) | χ2 = 1.20 | .27 |
| Chronic conditions | n = 156 | n = 140 | N = 296 | ||
| Mean (SD) | 2.51 (1.34) | 2.39 (1.47) | 2.45 (1.40) | t = −0.74 | .46 |
CHA indicates Choices for Healthy Aging; GED, General Educational Development; SD, standard deviation..
Satisfaction With Care
Table 2 shows mean levels of satisfaction with care at baseline and follow-up, as well as a change in score indicating the difference between these time points. Baseline satisfaction was collected among 293 study participants with no significant differences in mean satisfaction with care between groups (t = −1.50; P = .136); however, 6 months following enrollment (n = 253), the intervention group reported significantly higher mean satisfaction with care than the usual care group (t = 2.24; P = .026). The CHA group showed significantly greater mean change in overall satisfaction with care compared with the usual care group (10.92 vs 1.93 respectively; t = 3.21; P = .002). This represents an 18% increase in mean satisfaction score for the intervention group compared with a 3.7% increase for usual care group.
Table 2.
Descriptive Statistics and Test Statistics for Differences Between Intervention and Control Groups on Satisfaction With Medical Care, Utilization of Care, and Cost of Medical Care
| CHA | Control | Total | Statistic | P | |
|---|---|---|---|---|---|
| Satisfaction | |||||
| Baseline (n = 293), mean (SD) | 68.08 (20.3) | 71.68 (20.7) | 69.79 (20.6) | t = −1.50 | .14 |
| Six-month follow-up (n = 253), mean (SD) | 80.05(19.0) | 74.37 (21.4) | 77.38 (20.3) | t = 2.24 | .03 |
| Change in score (n = 250), mean (SD) | 10.91 (21.9) | 1.93 (22.4) | 6.67 (22.5) | t = 3.21 | <.01 |
| Inpatient utilization, n (%) | χ2 = 4.56 | .02 | |||
| Never | 116 (74.4) | 88 (62.9) | 204 (68.9) | ||
| Once or more | 40 (25.6) | 52 (37.1) | 92 (31.1) | ||
| ED utilization, n (%) | χ2 = 1.09 | .19 | |||
| Never | 130 (83.3) | 110 (78.6) | 240 (81.1) | ||
| Once or more | 26 (16.7) | 30 (21.4) | 56 (18.9) | ||
| Physician office visits, mean (SD) | 6.87 (4.38) | 7.09 (4.21) | 6.97 (4.30) | t = .45 | .65 |
| Total costs, mean (SD), $ | 7328.86 (12,735.88) | 9374.78 (16,683.98) | 8299.81 (14,751.74) | U = 2.51 | .01 |
| Range, $ | 133–88,509 | 0–85,962 | 0–88,509 |
CHA indicates Choices for Healthy Aging; ED, emergency department; SD, standard deviation.
Inpatient/ED Utilization
Table 2 shows results of analyses comparing study groups on dichotomous measures of inpatient and ED utilization. Percentages of participants in the CHA and usual care groups who utilized 1 or more hospital inpatient days in the 12 months following study enrollment were 25.6% and 37.1%, respectively, a significant difference (χ2 = 4.56, P = .02). Similarly, a smaller proportion of participants in the CHA group (16.7%) than in the usual care group (21.4%) utilized ED services at least once in the 12 months following enrollment, although this difference was not statistically significant (χ2 = 1.09, P = .19).
Rate per Thousand
Admission rate per thousand and hospital days per thousand were also compared by study group. The CHA group had a total of 78 hospital admissions for a 12-month admission rate per thousand of 500; the usual care group had 93 hospital admissions and an admission rate per thousand of 664.29. Similarly, the CHA group had an aggregate total of 254 inpatient days for a 12-month hospital day rate per thousand of 1628.21; the usual care group had 316 inpatient days and a 12-month hospital day rate per thousand of 2257.14.
Predictors of Medical Service Use
Logistic regression analyses were conducted to determine factors associated with ED use and hospitalizations. Analysis revealed that for ED use, only 1 variable was significant. Odds of ED use were 2.85 for females compared with males (Table 3). Factors associated with hospitalizations included sex, race, and study group. Specifically, higher odds of having a hospitalization were positively associated with female sex (odds ratio [OR] = 1.86, P = .04), white race (OR = 2.77, P <.01), and receiving usual care (OR = 1.72, P = .04).
Table 3.
Logistic Regression of Predictors of Emergency Department Use and Hospitalization
| Characteristic | Emergency Department Use | Hospitalizations | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR | 95% CI | P | OR | 95% CI | P | |
| Age | .975 | .940–1.012 | .183 | .984 | .952–1.018 | .354 |
|
| ||||||
| Female (vs male) | 2.850 | 1.33–6.122 | .007 | 1.864 | 1.039–3.344 | .037 |
|
| ||||||
| White (vs other) | 1.584 | .749–3.353 | .229 | 2.742 | 1.395–5.392 | .003 |
|
| ||||||
| Black (vs other) | .721 | .226–2.297 | .580 | 1.472 | .565–3.830 | .429 |
|
| ||||||
| Diagnosis (vs CHF) | ||||||
|
| ||||||
| Renal failure | .653 | .234–1.820 | .415 | 1.418 | .588–3.420 | .437 |
|
| ||||||
| Diabetes | .920 | .363–2.334 | .861 | 1.285 | .584–2.823 | .533 |
|
| ||||||
| CAD | .544 | .179–1.655 | .283 | .896 | .359–2.238 | .814 |
|
| ||||||
| COPD | .518 | .184–1.458 | .213 | 1.020 | .435–2.395 | .963 |
|
| ||||||
| CVD | .709 | .244–2.061 | .527 | 1.459 | .599–3.555 | .405 |
|
| ||||||
| No. of chronic conditions | 1.350 | .691–2.638 | .380 | 1.029 | .583–1.816 | .922 |
|
| ||||||
| Died | .470 | .101–2.191 | .337 | 1.318 | .496–3.497 | .580 |
|
| ||||||
| Usual care group (vs CHA group) | 1.493 | .806–2.63 | .202 | 1.731 | 1.731–2.918 | .039 |
CAD indicates coronary artery disease; CHA, Choices for Healthy Aging; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; OR, odds ratio.
Boldfaced items indicate P <.05.
Costs of Medical Care
Bivariate analysis of medical care costs (including intervention costs) revealed significantly lower costs of care among those enrolled in CHA, with costs on average more than $2000 lower than costs of care for those enrolled in usual care (see Table 2). An adjusted regression model examining intervention effect on costs of medical care revealed that, when adjusted for demographic variables, assignment to the CHA group was not significantly associated with medical costs of care. Being of white race was positively associated with higher costs of care, while age, male sex, and a diagnosis of coronary artery disease were associated with lower medical care costs (Table 4).
Table 4.
Adjusted Negative Binomial Regression of Factors Associated With Medical Care Costs
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Age | .975 | .955–.996 | .019 |
| Male (vs female) | .703 | .505–.979 | .037 |
| White (vs other) | 2.140 | 1.470–3.115 | .000 |
| Black (vs other) | .756 | .443–1.288 | .304 |
| Diagnosis (vs CHF) | |||
| Renal failure | .819 | .497–1.349 | .434 |
| Diabetes | .600 | .351–1.028 | .063 |
| CAD | .569 | .333–.973 | .039 |
| COPD | .996 | .591–1.678 | .988 |
| CVD | .880 | .514–1.505 | .640 |
| No. of chronic conditions | 1.375 | .976–1.937 | .068 |
| Died | 1.575 | .860–2.886 | .141 |
| CHA group (vs usual care) | .904 | .662–1.235 | .527 |
CAD indicates coronary artery disease; CHA, Choices for Healthy Aging; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; OR, odds ratio.
Boldfaced items indicate P <.05.
DISCUSSION
The CHA study demonstrated that a program using a home-based interdisciplinary team of medical and social service providers can improve patient satisfaction with healthcare provided by a managed care organization. In addition, findings of significant reductions in hospital days and the decreased probability of hospitalization for CHA intervention patients suggest that improved home care and support may reduce use of costly acute medical service. These finding are consistent with results from Brumley and colleagues7,14 in that more home care was positively associated with decreased hospital use.
Despite significantly lower hospital use among the intervention group, this finding did not translate to a corresponding reduction in overall healthcare costs when adjusted for demographics and health conditions. That may be because the risk stratification statistical program utilized for patient selection identified patients with fewer medical needs than expected. Brumley and colleagues found that among their seriously ill patients, hospitalization rates were 36% for the intervention group and 59% for the usual care group7; by contrast, we found much lower rates of medical service use, with 27% of intervention and 37% of usual care patients hospitalized. Thus, our population may have had a wide spectrum of medical needs with a range of complexity. Further investigation is needed to determine whether risk stratification is an effective method of determining need for interdisciplinary home care services. Another reason for the lack of reduction in total cost may be that the intervention costs with the interdisciplinary team were too high for the care required for the patient.
The data presented here show that the sample for this study may not have been as seriously ill (7.4% died) as participants in Brumley and colleagues’ home-based palliative care study, which found that average life expectancy following study enrollment was about 220 days, with 75% dying during the study period.7 The intensity of the intervention may have outweighed the medical need for some of the patients. In fact, following the data collected for this trial, both the clinical guidelines and the patient selection process were modified to provide a broader range of care in response to the needs of patients who are selected based on higher severity of illness. Additionally, staffing has evolved to be a nurse practitioner driven–model under a medical director with support from social work and nurse care managers; telephone monitoring has replaced in-person care for patients demonstrating less need and more stability.
Limitations
The relatively small number of participants in this study and the managed care sample may limit the generalization of findings. Use of proxy costs also comes with limitations, particularly for ED use and hospitalizations. Although our proxy cost value was an average cost generated from a range of charges for managed care participants, the average could potentially be a very conservative estimate and not reflect actual costs for our population. For example, several studies have shown that hospitalization costs are very high for seriously ill patients in their last year of life and terminal hospitalizations are even higher.15 These high costs oftentimes are derived from intensive care unit days, data that were unavailable for our sample. Thus, the lack of significance in our adjusted differences in costs of medical care may be due to the lack of actual cost figures as well as the large distribution of costs for this sample.
This study provided a unique approach to identification and management of high-risk patients in their homes. Although the results demonstrated significantly lower odds of hospitalization and greater satisfaction with medical care for the CHA group, there was no difference in costs of care by study group after adjusting for sample characteristics. Additional research is needed to determine better methods to identify high-risk patients efficiently to improve clinical and service outcomes and reduce the cost of care.
Take-Away Points.
Potential benefits of providing medical care in the home include reduction of hospitalizations and improved patient satisfaction with healthcare.
-
■
Compared with usual care patients, managed care patients receiving a home care intervention were less likely to be admitted to the hospital and had greater satisfaction with care.
-
■
However, there were no differences in terms of costs of care between the home care and usual care groups, suggesting that managed care organizations need to consider targeting when designing programs for high-risk groups.
Acknowledgments
Dr Levine, Dr Jung, and Ms Attaway all report employment with Healthcare Partners, the funder of the study. Dr Enguidanos reports receiving consultancies from Healthcare Partners.
Funding Source: Healthcare Partners.
Footnotes
Author Disclosures: Dr Steinman reports no relationship or financial interest with any entity that would pose a conflict of interest with the subject matter of this article.
Authorship Information: Concept and design (SL, BAS, KA, TJ, SE); acquisition of data (SL, KA, SE); analysis and interpretation of data (SL, BAS, KA, TJ, SE); drafting of the manuscript (SL, BAS, KA, SE); critical revision of the manuscript for important intellectual content (SL, BAS, KA); statistical analysis (BAS, KA, SE); provision of study materials or patients (SL, KA); obtaining funding (SL, KA); administrative, technical, or logistic support (SL, KA); and supervision (SL, KA, TJ).
References
- 1.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162(20):2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 2.Germain CB, Gitterman A. Helping individuals, families, and groups with stressful life transitions and traumatic events. In: Germain CB, Gitterman A, editors. The Life Model of Social Work Practice: Advances in Theory and Practice. 2. New York: Columbia University Press; 1996. pp. 108–145. [Google Scholar]
- 3.Schoen C, Davis K, How SKH, Schoenbaum SC. U.S. health system performance: a national scorecard. Health Aff (Millwood) 2006;25(6):w257–w475. doi: 10.1377/hlthaff.25.w457. [DOI] [PubMed] [Google Scholar]
- 4.111th US Congress. Patient Protection and Affordable Care Act. 2010:318–319. Pub L No. 111-148, §2702, 124 Stat 119. [Google Scholar]
- 5.Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001. [Google Scholar]
- 6.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511–544. [PubMed] [Google Scholar]
- 7.Brumley R, Enguidanos S, Jamison P, et al. Increased satisfaction with care and lower costs: results of a randomized trial of in-home palliative care. J Am Geriatr Soc. 2007;55(7):993–1000. doi: 10.1111/j.1532-5415.2007.01234.x. [DOI] [PubMed] [Google Scholar]
- 8.Hughes SL, Weaver FM, Giobbie-Hurder A, et al. Department of Veterans Affairs Cooperative Study Group on Home-Based Primary Care. Effectiveness of team-managed home-based primary care: a randomized multicenter trial. JAMA. 2000;284(22):2877–2885. doi: 10.1001/jama.284.22.2877. [DOI] [PubMed] [Google Scholar]
- 9.Levine SH, Adams J, Attaway K, et al. Predicting the Financial Risks of Seriously Ill Patients. Oakland, CA: California HealthCare Foundation; 2011. [Google Scholar]
- 10.Enguidanos SM, Cherin D, Brumley R. Home-based palliative care study: site of death, and costs of medical care for patients with congestive heart failure, chronic obstructive pulmonary disease, and cancer. J Soc Work End Life Palliat Care. 2005;1(3):37–56. doi: 10.1300/J457v01n03_04. [DOI] [PubMed] [Google Scholar]
- 11.Bertram L, Tanzi RE. The genetic epidemiology of neurodegenerative disease. J Clin Invest. 2005;115(6):1449–1457. doi: 10.1172/JCI24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geron SM, Smith K, Tennstedt S, Jette A, Chassler D, Kasten L. The home care satisfaction measure: a client-centered approach to assessing the satisfaction of frail older adults with home care services. J Gerontol B Psychol Sci Soc Sci. 2000;55(5):S259–S270. doi: 10.1093/geronb/55.5.s259. [DOI] [PubMed] [Google Scholar]
- 13.Long JS. Regression Models for Categorical and Limited Dependent Variables. Thousand Oaks, CA: Sage Publications; 1997. [Google Scholar]
- 14.Brumley R, Enguidanos S, Cherin D. Effectiveness of a home-based palliative care program for end-of-life. J Palliat Med. 2003;6(5):715–724. doi: 10.1089/109662103322515220. [DOI] [PubMed] [Google Scholar]
- 15.Morrison RS, Penrod JD, Cassel JB, et al. Palliative Care Leadership Centers’ Outcomes Group. Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med. 2008;168(16):1783–1790. doi: 10.1001/archinte.168.16.1783. [DOI] [PubMed] [Google Scholar]