Abstract
Background:
The prognostic impact of pathologic response to preoperative therapy in patients with duodenal adenocarcinoma (DA) and ampullary adenocarcinoma (AMPA) has not been established.
Methods:
A retrospective review of 266 patients who underwent curative resection for DA (n=97) or AMPA (n=169) during 1993–2015 was performed. For patients who underwent preoperative therapy, pathologic response was systematically evaluated and classified as major (0%−49% of viable residual tumor cells) or minor (≥50% of viable residual tumor cells). Univariable and multivariable analyses were performed to identify predictors of pathologic response and disease-specific survival (DSS).
Results:
In the 79 patients treated with preoperative therapy (DA n=34; AMPA n=45), concomitant use of radiation (67/79, 80%) was the sole independent predictor of major pathologic response (odds ratio: 8.17, 95% CI: 1.85–58.2, P=0.005). Patients with major pathologic response had a better 5-year DSS rate than patients with minor pathologic response (DA, 65% vs. 25%, P=0.028; AMPA, 85% vs. 43%, P=0.016). On multivariable analysis of DSS in the 79 patients who underwent preoperative therapy, major pathologic response was the sole predictor of improved DSS (hazard ratio: 2.88, 95% CI: 1.41–5.98, P=0.004). On multivariable analysis of DSS in the entire cohort, pathologic stage ≤II was the sole predictor of better DSS.
Conclusion:
Major pathologic response to preoperative therapy predicted improved DSS after resection of DA and AMPA and might represent a new prognosticator after resection of DA and AMPA.
Keywords: pathologic response, preoperative therapy, ampullary adenocarcinoma, duodenal adenocarcinoma
INTRODUCTION
Periampullary cancers are a heterogeneous group of malignancies that include all neoplasms derived from the pancreaticobilio-digestive junction. Cancers usually classified as periampullary include ductal cancers from the pancreatic head, cholangiocarcinomas from the common bile duct, duodenal cancers, and ampullary cancers.1 Of those, duodenal adenocarcinoma (DA) and ampullary adenocarcinoma (AMPA) are rare, accounting for 0.4% and 2.0% of gastrointestinal cancers, respectively.2, 3 AMPAs are histopathologically further subclassified as intestinal or pancreaticobiliary type.4 While several reports have suggested that pancreaticobiliary-type AMPA has a worse prognosis than intestinal-type AMPA,5–7 other reports did not find an impact of subtype on outcome.3, 8
While today, resection remains the only potentially curative treatment for DA and AMPA, the precise role of perioperative therapy in the treatment of these diseases has not been established.9 Since prospective randomized studies of postoperative adjuvant chemotherapy for periampullary adenocarcinoma failed to show a survival advantage of postoperative chemotherapy in patients with DA and AMPA,8, 10 postoperative chemotherapy options for these diseases are extrapolated from the literature on pancreatic- and colon cancer. Similarly, because of the rarity of DA and AMPA, little has been reported in the literature concerning the impact of preoperative therapy for these diseases.2, 3 Previous retrospective study regarding localized AMPA demonstrated safety and feasibility of preoperative chemotherapy and/or chemoradiation, but suggested a limited role for the routine administration of preoperative therapies prior to intended resection.11 In clinical practice at our institution, patients with DA or AMPA who frequently have bulky disease and/or regional adenopathy are likely to undergo preoperative therapy as recommended by the multidisciplinary care team.12
When preoperative therapy is used, pathologic response (as shown in other gastrointestinal cancers) to preoperative therapy correlates significantly with prognosis after surgery and can be used as a prognosticator; however, whether pathologic response to preoperative therapy correlates with prognosis in patients with DA and AMPA remains unclear.13–16 The aim of the current study was to determine the prognostic value of pathologic response to preoperative therapy in patients with DA and AMPA.
MATERIAL AND METHODS
Study Population
The Institutional Review Board of The University of Texas MD Anderson Cancer Center approved this study protocol (PA16–0530). A prospectively maintained hepatopancreatobiliary database of the Department of Surgical Oncology was reviewed to identify patients who underwent curative resection for DA and AMPA between January 1993 and December 2015. Patients with periampullary cancer considered to represent primary biliary or pancreatic adenocarcinoma and patients with Lynch syndrome diagnosed by Amsterdam II criteria were excluded. A total of 266 patients (97 with DA; 169 with AMPA) were identified (Figure 1). Among those patients, we identified patients treated with preoperative therapy followed by curative resection. The following data were extracted from electronic patient medical records: sex, age, body mass index, presence of diabetes mellitus, preoperative therapy characteristics (chemotherapy regimen and duration, and dose of radiation), operative characteristics (surgical procedure and margin status [R0, all margins clear of tumor on microscopic examination; R1, cancer on inked common bile duct, pancreatic neck, or superior mesenteric artery margin on microscopic examination, but margins clear of tumor on macroscopic examination]),17–19 primary tumor characteristics (differentiation, subtypes of AMPA [intestinal or pancreaticobiliary],4 perineural and lymphovascular invasion), lymph node metastases, pathologic stage based on the American Joint Committee on Cancer staging system, 7th edition,20 and postoperative therapy characteristics. AMPA with mixed intestinal and pancreaticobiliary components was categorized on the basis of the predominant component.5 Pathologic response to preoperative therapy was systematically evaluated according to the method by Ribero et al21 and reported as major or minor according to the mean of the percentage of viable residual cancer cells within each tumor (major response, 0% to 49% of viable residual cancer cells; minor response, ≥50% of viable residual cancer cells).13 Hematoxylin-eosin stained sections were reviewed by two gastrointestinal pathologists (H.W. and J.Z.). The pathologists were blinded with respect to clinical information, treatment regimen, outcome, and the goal of the study.
Figure 1.
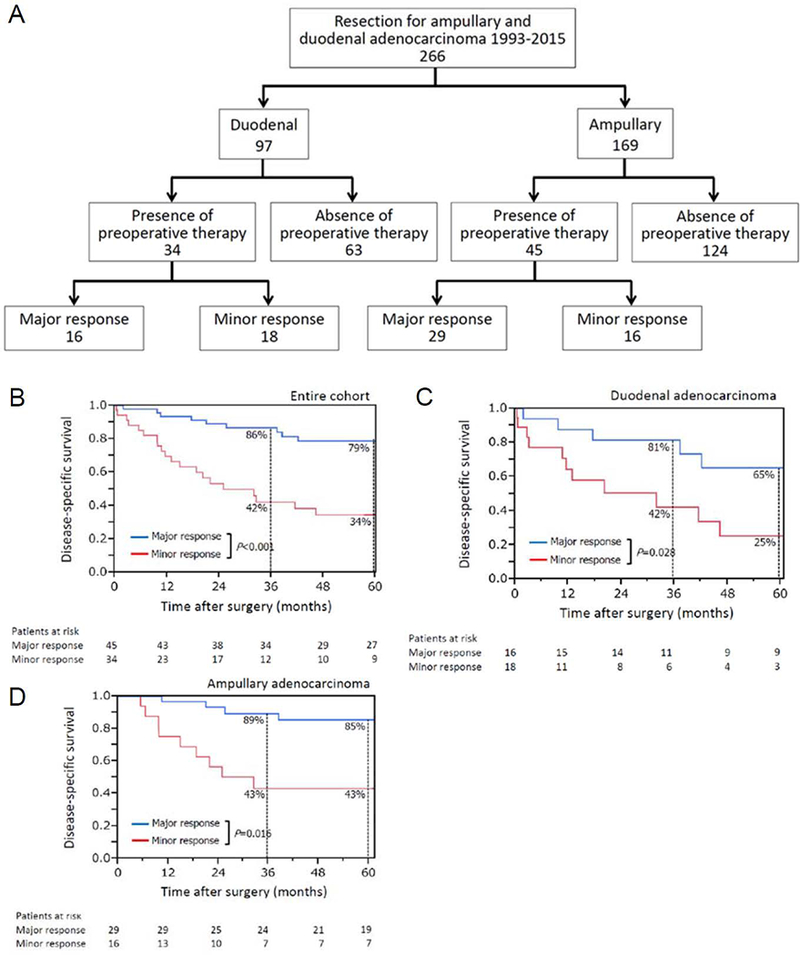
(A) Study population. Disease-specific survival by pathologic response to preoperative therapy in (B) all patients with duodenal adenocarcinoma (DA) and ampullary adenocarcinoma (AMPA) (n=279), (C) patients with DA (n=34), and (D) patients with AMPA (n=45).
Treatment
Decisions about the treatment strategy for each patient, including whether to use preoperative therapy, were made at a weekly specialty multidisciplinary conference. In general, patients were considered candidates for up-front resection when they were medically fit and able to undergo macroscopically curative resection. For patients with 1) pre-existing comorbidity, 2) disease perceived to be biologically advanced due to one or more well-defined clinical parameters (e.g., regional lymphadenopathy, advanced T stage, indeterminate liver/lung lesions), or 3) treatment of disease believed to be pancreatic ductal adenocarcinoma on the basis of existing clinical workup,11, 22, 23 preoperative therapy (chemotherapy alone or chemoradiation) was administered.11, 12, 24 Preoperative chemotherapy primarily consisted of regimens containing fluorouracil, capecitabine, or gemcitabine administered for 4 to 6 months.25, 26 When administered, external beam radiation was delivered using either a hypofractionated (10 fractions) or standard fractionated (28 fractions) regimen with concurrent fluorouracil, capecitabine, or gemcitabine. External beam radiation was delivered 5 days per week (Monday-Friday). CT-based three-dimensional conformal treatment planning was routinely used. Following preoperative therapy, the patient was restaged, and surgery was recommended in the absence of clinical or radiographic evidence of disease progression, the patient maintained an adequate performance status, and if a complete resection could be performed. Postoperative chemotherapy alone or chemoradiation was administered selectively based on the decision by multidisciplinary team of medical, surgical and radiation oncologists. In general, these therapeutic modalities were recommended for patients with high risk features associated with their cancer, such as positive lymph nodes, advanced T stage, R1 margin status, poor tumor differentiation or lymphovascular/perineural invasion.11 Patients were followed routinely after surgery with history, physical examination, laboratory evaluations, and axial imaging every 3 to 4 months for the first 2 years and every 4 to 6 months for the subsequent 3 years.
Statistical Analyses
Continuous variables were compared using the Wilcoxon rank-sum test, and categorical variables were compared using the χ2 test. For the evaluation of predictors of pathologic response, univariable and multivariable analyses were performed by logistic regression analysis. Disease-specific survival (DSS) was measured from the date of resection to the date of death due to disease (DA or AMPA) or last follow-up. Patients who died of an unrelated cause were censored at the time of death. Survival curves were generated using the Kaplan-Meier method, and differences in survival were evaluated with the log-rank test. Univariable and multivariable analyses to identify predictors of DSS were performed by Cox proportional hazards regression models. Variables with P<0.1 in univariable analysis were entered into each multivariable analysis. P<0.05 was considered statistically significant in all analyses. Statistical analyses were performed with IBM SPSS software (version 23.0; SPSS Inc., Chicago, IL, USA).
RESULTS
Patient Characteristics
Table 1 lists the clinicopathologic and operative data for the 97 patients with DA and 169 patients with AMPA. Patients with AMPA were more likely than patients with DA to be male. In the present study period, there were 97 patients (41 with DA; 56 with AMPA) who were planned to receive preoperative therapy followed by curative resection. However, of those, 18 (7 with DA; 11 with AMPA) did not undergo planned resection due to disease progression (4 with DA; 6 with AMPA) and poor general status (3 with DA; 5 with AMPA). These 18 patients were therefore excluded from the analysis. The final study cohort exits of 79 patients treated with preoperative therapy and curative resection (34 with DA; 45 with AMPA). Among them, patients with AMPA vs. DA were more likely to receive chemoradiation and less likely to receive chemotherapy alone (Table 1). In patients with AMPA undergoing preoperative therapy followed by resection, 13% of cases (6/45) were considered preoperatively pancreatic ductal adenocarcinoma, which was significantly more frequent compared to patients with DA (0/34, P=0.027).
Table 1.
Clinicopathologic characteristics of patients undergoing resection for duodenal adenocarcinoma and ampullary adenocarcinoma, 1993–2015*
| Characteristic | Total | Duodenal adenocarcinoma | Ampullary adenocarcinoma | P value† |
|---|---|---|---|---|
| All patients | 266 | 97 (36) | 169 (64) | |
| Sex, M: F | 153: 113 | 48: 49 | 105: 64 | 0.045 |
| Age, y, median (range) | 64 (28–88) | 63 (28–88) | 64 (28–88) | 0.764‡ |
| Body mass index, kg/m2, median (range) | 27 (15–41) | 27 (18–41) | 26 (15–41) | 0.631‡ |
| Diabetes mellitus | 43 (16) | 15 (15) | 28 (17) | 0.814 |
| Preoperative therapy | 79 (30) | 34 (35) | 45 (27) | 0.148 |
| Chemotherapy alone | 12 (15) | 10 (29) | 2 (4.4) | 0.002 |
| Chemoradiation | 67 (85) | 24 (71) | 43 (96) | 0.002 |
| Biologic equivalent dose, Gy, median (range) | 45 (30–63) | 35 (30–56) | 45 (30–63) | 0.908‡ |
| Chemotherapy regimen | ||||
| Fluorouracil-based | 39 (49) | 21 (62) | 18 (40) | 0.079 |
| Capecitabine-based | 37 (47) | 13 (38) | 24 (53) | |
| Gemcitabine-based | 3 (3.8) | 0 | 3 (6.7) | |
| Duration of preoperative chemotherapy, months, median (range) | 2.7 (1.0–9.7) | 2.8 (1.0–9.7) | 2.7 (1.0–7.4) | 0.344 |
| Pathologic response | ||||
| Major (viable tumor 0–49%) | 45 (57) | 16 (47) | 29 (64) | 0.122 |
| Complete (viable tumor 0%) | 2 (2.5) | 1 (2.9) | 1 (2.2) | 0.840 |
| Minor (viable tumor 50–100%) | 34 (43) | 18 (53) | 16 (36) | 0.122 |
| Surgical procedure | ||||
| Pancreaticoduodenectomy | 250 (94) | 82 (85) | 168 (99) | <0.001 |
| Other | 16 (6.0) | 15 (15) | 1 (0.6) | |
| Differentiation, well/moderate/poor | 17/182/67 | 5/67/25 | 12/115/42 | 0.816 |
| Subclassification of ampullary carcinoma | ||||
| Intestinal type dominant | - | - | 59 (35) | - |
| Pancreaticobiliary type dominant | - | - | 92 (54) | |
| Undetermined | - | - | 18 (11) | |
| Perineural invasion | ||||
| Positive/negative | 131/135 | 49/48 | 82/87 | 0.754 |
| Lymphovascular invasion | ||||
| Positive/negative | 131/135 | 51/46 | 80/89 | 0.411 |
| Surgical margin status | ||||
| R0/R1 | 259/7 | 92/5 | 167/2 | 0.057 |
| Pathologic stage (AJCC staging system, 7th edition) | ||||
| 0 | 8 | 0 | 8 | <0.001 |
| I | 69 | 18 | 51 | |
| II | 100 | 33 | 67 | |
| III | 81 | 39 | 42 | |
| IV | 8 | 7 | 1 | |
| Lymph node metastases | ||||
| Positive/negative | 139/127 | 53/44 | 86/83 | 0.555 |
| Postoperative therapy | 91 (34) | 30 (31) | 61 (36) | 0.393 |
| Chemotherapy alone | 30 (33) | 10 (33) | 20 (33) | 0.958 |
| Chemoradiation | 61 (67) | 20 (67) | 41 (67) | 0.958 |
Values in table are number of patients (percentage) unless indicated otherwise.
χ2 test unless indicated otherwise.
Wilcoxon rank-sum test.
AJCC, American Joint Committee on Cancer.
The rate of major pathologic response after preoperative therapy did not differ significantly between patients with AMPA (64%) and DA (47%) (P=0.122). There was no significant difference between the 2 groups with respect to preoperative chemotherapy regimen and duration. Among the 169 patients with AMPA, histologic subtyping (intestinal vs. pancreaticobiliary) was determined in 151 patients (89%), 35 of whom underwent preoperative therapy. There was no significant difference among patients with DA, intestinal AMPA, and pancreaticobiliary AMPA with respect to preoperative chemotherapy regimen (Supplementary Table 1). Patients with AMPA were more likely than those with DA to undergo pancreaticoduodenectomy. Among patients with DA, pathologic stage III was predominant; among patients with AMPA, pathologic stage II was predominant.
Supplementary Table 2 lists the clinicopathologic characteristics of the patients who did (n=79) and did not undergo preoperative therapy (n=187). The patients who underwent preoperative therapy had significantly more poorly differentiated tumors. There was no other significant difference identified between the 2 groups.
Predictors of Pathologic Response
Among the 79 patients who underwent preoperative therapy, major pathologic response was observed in 45 patients (57%). Table 2 lists the results of univariable and multivariable analyses of potential predictors of major pathologic response in patients who underwent preoperative therapy. The singular independent predictor of major pathologic response was concomitant use of radiation. Among the patients with AMPA who underwent preoperative therapy, there was no significant difference in terms of rate of major pathologic response by histopathologic subtype (Table 2).
Table 2.
Univariable and multivariable analyses of major pathologic response in patients undergoing preoperative therapy (n=79)
| Variable | n | Major pathologic response, n (%) | Univariable P value | Odds ratio (95% confidence interval) | Multivariable P value |
|---|---|---|---|---|---|
| All patients | 79 | 45 (57) | |||
| Sex* | |||||
| M | 50 | 32 (64) | 0.097 | 2.41 (0.86–7.06) | 0.094 |
| F | 29 | 13 (45) | |||
| Age, years* | |||||
| >60 | 49 | 32 (65) | 0.056 | 2.74 (0.99–7.97) | 0.052 |
| ≤60 | 30 | 13 (43) | |||
| Body mass index, kg/m2 | |||||
| ≤25 | 24 | 13 (54) | 0.740 | – | – |
| >25 | 55 | 32 (58) | |||
| Diabetes mellitus | |||||
| Yes | 15 | 9 (60) | 0.792 | – | – |
| No | 64 | 36 (56) | |||
| Entity | |||||
| Duodenal adenocarcinoma | 34 | 16 (47) | 0.122 | – | – |
| Ampullary adenocarcinoma | 45 | 29 (64) | |||
| Subclassification of ampullary carcinoma | |||||
| Intestinal | 8 | 5 (63) | 0.724 | – | – |
| Pancreaticobiliary | 27 | 15 (56) | |||
| Undetermined | 10 | 9 (90) | |||
| Type of preoperative therapy* | |||||
| Chemoradiation | 67 | 43 (64) | 0.002 | 8.17 (1.84–58.2) | 0.005 |
| Chemotherapy alone | 12 | 2 (17) | |||
| Preoperative chemotherapy regimen | |||||
| Fluorouracil-based | |||||
| Yes | 39 | 21 (54) | 0.581 | – | – |
| No | 40 | 24 (60) | |||
| Capecitabine-based | |||||
| Yes | 37 | 23 (62) | 0.381 | – | – |
| No | 42 | 22 (52) | |||
| Duration of preoperative chemotherapy | |||||
| <3 | 49 | 29 (59) | 0.610 | – | – |
| ≥3 | 30 | 16 (53) |
Variables entered into the multinomial logistic regression analyses.
Predictors of Survival
The median follow-up duration was 57 months (range 1.0–267 months). On multivariable analysis of potential predictors of DSS after curative resection in the entire cohort (n=266), the sole independent predictor of worse DSS was pathologic stage ≥III (Supplementary Table 3). Preoperative therapy independent of pathologic response was not a significant predictor of DSS in the entire cohort or in the subgroups of patients with DA and AMPA (Supplementary Figure 1). Similarly, presence of postoperative therapy was not a significant predictor of DSS in the entire cohort or in the subgroups of patients undergoing preoperative therapy (Supplementary Table 3 and Table 3).
Table 3.
Univariable and multivariable analysis of disease-specific survival in patients treated with preoperative therapy (n=79)
| Disease-specific survival (%)* | ||||||
|---|---|---|---|---|---|---|
| Variable | N | 3 years | 5 years | Univariable P value† | Hazard ratio (95% confidence interval) |
Multivariable P value‡ |
| All patients | 79 | 68 | 60 | - | - | - |
| Sex | ||||||
| M | 50 | 70 | 62 | 0.305 | - | - |
| F | 29 | 65 | 57 | |||
| Age, years | ||||||
| ≤60 | 30 | 62 | 62 | 0.660 | - | - |
| >60 | 49 | 72 | 60 | |||
| Body mass index, kg/m2 | ||||||
| ≤25 | 24 | 58 | 58 | 0.269 | - | - |
| >25 | 55 | 73 | 61 | |||
| Diabetes mellitus | ||||||
| Yes | 15 | 65 | 65 | 0.958 | - | - |
| No | 64 | 69 | 60 | |||
| Entity | ||||||
| Duodenal adenocarcinoma | 34 | 62 | 45 | 0.246 | - | - |
| Ampullary adenocarcinoma | 45 | 73 | 70 | |||
| Type of preoperative therapy | ||||||
| Chemotherapy alone | 12 | 74 | 74 | 0.936 | - | - |
| Chemoradiation | 67 | 68 | 59 | |||
| Preoperative chemotherapy regimen | ||||||
| Fluorouracil-based | ||||||
| Yes | 39 | 71 | 59 | 0.715 | - | - |
| No | 40 | 65 | 62 | |||
| Capecitabine-based | ||||||
| Yes | 37 | 65 | 61 | 0.604 | - | - |
| No | 42 | 71 | 59 | |||
| Duration of preoperative chemotherapy, months | ||||||
| <3 | 49 | 74 | 67 | 0.122 | - | - |
| ≥3 | 30 | 59 | 48 | |||
| Pathologic response to preoperative therapy§ | ||||||
| Minor (viable tumor 50-100%) | 34 | 42 | 34 | <0.001 | 2.88 (1.41-5.98) | 0.004 |
| Major (viable tumor 0-49%) | 45 | 86 | 79 | |||
| Differentiation | ||||||
| Well/moderate | 50 | 77 | 67 | 0.246 | - | - |
| Poor | 29 | 52 | 48 | |||
| Perineural invasion | ||||||
| Yes | 33 | 69 | 69 | 0.780 | - | - |
| No | 46 | 68 | 55 | |||
| Lymphovascular invasion | ||||||
| Yes | 35 | 64 | 60 | 0.962 | - | - |
| No | 44 | 72 | 61 | |||
| Lymph node metastases | ||||||
| Yes | 35 | 64 | 56 | 0.857 | - | - |
| No | 44 | 72 | 64 | |||
| Surgical margin status | ||||||
| R1 | 2 | 100 | 0 | 0.832 | - | - |
| R0 | 77 | 67 | 61 | |||
| Pathologic stage (AJCC staging system, 7th edition)§ | ||||||
| ≥III | 28 | 52 | 47 | 0.052 | 1.30 (0.63-2.65) | 0.467 |
| ≤II | 51 | 77 | 68 | |||
| Postoperative chemotherapy alone | ||||||
| Yes | 5 | 60 | 60 | 0.910 | - | - |
| No | 74 | 69 | 60 | |||
| Postoperative chemoradiation | ||||||
| Yes | 16 | 66 | 66 | 0.800 | - | - |
| No | 63 | 69 | 60 | |||
| Postoperative therapy | ||||||
| Yes | 21 | 65 | 65 | 0.886 | - | - |
| No | 58 | 70 | 60 | |||
Kaplan-Meier analysis.
Log-rank test.
Cox regression model.
Variables entered into the Cox regression model.
AJCC, American Joint Committee on Cancer.
However, among the 79 patients treated with preoperative therapy, DSS was significantly worse in patients with minor vs. major pathologic response (Figure 1B). Similar results were found in subgroup analyses of patients with DA (Figure 1C) and AMPA (Figure 1D). On multivariable analysis of factors associated with DSS after curative resection in patients undergoing preoperative therapy, major pathologic response was identified as the sole independent predictor of better DSS (Table 3).
Among the 187 patients without preoperative therapy, on multivariable analysis of factors associated with DSS after curative resection, absence of diabetes mellitus, R0 resection, and pathologic stage ≤II were identified as the independent predictors of better DSS (Supplementary Table 4).
DISCUSSION
In this study, we found that among patients with DA and AMPA who underwent preoperative therapy, patients with major vs. minor pathologic response had a better 5-year DSS rate overall and in the DA and AMPA subgroups. Previous reports have shown the prognostic effects of pathologic response to preoperative therapy in patients with several other gastrointestinal cancers,13–16 but to the best of our knowledge, this is the first report to show the impact of pathologic response to preoperative therapy on survival after curative resection of DA and AMPA.
We also found that in our cohort of resected DA and AMPA, concurrent radiotherapy was an independent predictor of major pathologic response among patients who underwent preoperative therapy. Previous reports have hinted towards an optimal pathological response of chemoradiation for DA and AMPA. Yeung et al reported a phase II study demonstrating the efficacy of preoperative chemoradiation for DA, which led to a resectability rate of 80% and revealed extensive necrosis and hyalinization in all cases undergoing resection.27 Others have shown in patients with AMPA that downstaging secondary to preoperative chemoradiation was achieved in 67% (28%, pathologically complete response).28 Further, patients with AMPA significantly more often received preoperative chemoradiation compared to patients with DA. This is in keeping with previous report suggesting a favorable chemoradiation sensitivity of AMPA.28 Previous studies of unresectable biliary tract cancer suggested that chemoradiation produced better long-term outcomes than chemotherapy alone.29 In recent years, a number of reports have shown the feasibility and tolerability of chemoradiation for locally advanced biliary tract cancer and DA.19, 30, 31 In the present study, histopathologic subtype of AMPA (intestinal or pancreaticobiliary) was not a predictor of major pathologic response to preoperative therapy. Previous studies that have addressed this topic have produced inconsistent results; some have shown that histomorphologic subtypes predict response to specific chemotherapy,9, 32 while others have shown no impact of subtype on chemotherapy response.3, 8, 33
In the current study, on multivariable analysis of potential predictors of DSS in the entire cohort, use of preoperative therapy did not have a positive impact on prognosis, which is in line with the published literature.11 However, this finding must be viewed in light of a possible selection bias: resectable patients with advanced disease were thought to possibly benefit from preoperative therapy due their comparatively higher disease burden. In fact, pathologic review of specimens following preoperative therapy and resection confirmed that poorly differentiated tumors were more common in patients with preoperative therapy compared to patients without preoperative therapy. A prospective randomized trial would provide robust evidence regarding the prognostic impact of preoperative therapy for DA and AMPA, but conducting such a trial would be challenging owing to the rarity of these diseases.2, 3
Our results also suggested that the impact of traditional predictors of DSS after resection in patients with DA and AMPA might be mitigated by the effects of modern preoperative therapy. The multivariable analysis of DSS in the 187 patients without preoperative therapy identified R0 resection and pathologic stage ≤II as the independent predictors of improved DSS. Conversely, on multivariable analysis of DSS in the 79 patients who underwent preoperative therapy, major pathologic response was the sole predictor of improved DSS instead of such traditional factors. Further research would be required to optimally chose postoperative therapy based on pathologic response to preoperative therapy rather than traditional prognosticators, which may aid clinicians in stratifying the need for postoperative therapy.
This study has several limitations. First, it is limited by its retrospective nature and associated biases, including selection bias since the analyzed cohort was limited to patients only who completed preoperative therapy followed by planned curative resection (79 of 97 [81%]). Second, histopathologic subtyping was not determined in a proportion of patients with AMPA (18/169, 11%), and the population of patients with histopathologic subtyping who were also treated with preoperative therapy was limited (n=35). Thus, our finding that histopathologic subtype of AMPA was not associated with pathologic response to preoperative therapy (Table 2) might potentially be underpowered to detect a difference. Third, our analyses grouped DA and AMPA together due to the rarity of these clinicopathologic disease entities, similar to previously published series1–3. Fourth, among patients treated with preoperative therapy, patients with AMPA were less likely than patients with DA to receive chemotherapy alone and more likely than patients with DA to receive chemoradiation. The higher rate of chemoradiation in the AMPA vs. DA group may have in part been due to some AMPA having been considered to be pancreatic ductal adenocarcinoma preoperatively34 and concerns regarding duodenal toxicity in concomitant radiation when treating DA35. Finally, the present study did not include information concerning the genotype of tumors, including information about KRAS status, and also lacked information regarding microsatellite instability status. In the field of surgery for colorectal liver metastases, previous literature has demonstrated an association between mutant KRAS and suboptimal pathologic response to preoperative chemotherapy.36 In addition, a study on AMPA37 identified KRAS mutation as an independent predictor of poor prognosis following curative resection and others have suggested microsatellite instability was associated with improved survival in patients with AMPA and DA.38, 39 Further research is needed to identify biomarkers which can predict pathologic response to preoperative therapy in patients with AMPA and DA. This would not only allow patients who would benefit from preoperative therapy to be stratified towards receiving it, it would also help to avoid unnecesary preoperative therapy in patients who do not derive a survival advantage from it.
In conclusion, in patients with DA and AMPA treated with preoperative therapy followed by curative resection, pathologic response predicted postoperative DSS. Delivery of radiotherapy concurrently with preoperative chemotherapy was associated with major pathologic response. Pathologic response to preoperative therapy may be a new predictor of prognosis after resection of DA and AMPA.
Supplementary Material
Disease-specific survival (DSS) by presence or absence of preoperative therapy in (A) the entire cohort of patients with duodenal adenocarcinoma (DA) and ampullary adenocarcinoma (AMPA) (n=266), (B) patients with DA (n=97), and (C) patients with AMPA (n=169).
Acknowledgments
The authors thank Stephanie Deming, an employee of the Department of Scientific Publications at MD Anderson Cancer Center, for copyediting the manuscript. This research was supported in part by the National Institutes of Health through The University of Texas MD Anderson Cancer Center’s Cancer Center Support Grant, CA016672.
Research support for this study: The University of Texas MD Anderson Cancer Center is supported in part by the NIH/NCI under award number P30CA016672.
Footnotes
Conflicts of interest: The authors report no conflicts of interest relevant to this article.
REFERENCES
- 1.Bourgouin S, Ewald J, Mancini J, et al. Predictors of survival in ampullary, bile duct and duodenal cancers following pancreaticoduodenectomy: a 10-year multicentre analysis. J Gastrointest Surg. 2015;19:1247–1255. [DOI] [PubMed] [Google Scholar]
- 2.Berberat PO, Künzli BM, Gulbinas A, et al. An audit of outcomes of a series of periampullary carcinomas. Eur J Surg Oncol. 2009;35:187–191. [DOI] [PubMed] [Google Scholar]
- 3.Shaib WL, Sharma R, Brutcher E, et al. Treatment utilization and surgical outcome of ampullary and duodenal adenocarcinoma. J Surg Oncol. 2014;109:556–560. [DOI] [PubMed] [Google Scholar]
- 4.Kimura W, Futakawa N, Yamagata S, et al. Different clinicopathologic findings in two histologic types of carcinoma of papilla of Vater. Jpn J Cancer Res. 1994;85:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schueneman A, Goggins M, Ensor J, et al. Validation of histomolecular classification utilizing histological subtype, MUC1, and CDX2 for prognostication of resected ampullary adenocarcinoma. Br J Cancer. 2015;113:64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang DK, Jamieson NB, Johns AL, et al. Histomolecular phenotypes and outcome in adenocarcinoma of the ampulla of vater. J Clin Oncol. 2013;31:1348–1356. [DOI] [PubMed] [Google Scholar]
- 7.Adsay V, Ohike N, Tajiri T, et al. Ampullary region carcinomas: definition and site specific classification with delineation of four clinicopathologically and prognostically distinct subsets in an analysis of 249 cases. Am J Surg Pathol. 2012;36:1592–1608. [DOI] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147–156. [DOI] [PubMed] [Google Scholar]
- 9.Schiergens TS, Reu S, Neumann J, et al. Histomorphologic and molecular phenotypes predict gemcitabine response and overall survival in adenocarcinoma of the ampulla of Vater. Surgery. 2015;158:151–161. [DOI] [PubMed] [Google Scholar]
- 10.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cloyd JM, Wang H, Overman M, et al. Influence of preoperative therapy on short- and long-term outcomes of patients with adenocarcinoma of the ampulla of Vater. Ann Surg Oncol. 2017, in press. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman JP, Cooper HS, Young NA, et al. Preoperative chemotherapy of chemoradiotherapy for the treatment of adenocarcinoma of the pancreas and ampulla of Vater. J Hepatobiliary Pancreat Surg. 1998;5:251–254. [DOI] [PubMed] [Google Scholar]
- 13.Blazer DG 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. [DOI] [PubMed] [Google Scholar]
- 14.Swisher SG, Hofstetter W, Wu TT, et al. Proposed revision of the esophageal cancer staging system to accommodate pathologic response (pP) following preoperative chemoradiation (CRT). Ann Surg. 2005;241:810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ajani JA, Mansfield PF, Crane CH, et al. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol. 2005;23:1237–1244. [DOI] [PubMed] [Google Scholar]
- 16.Chatterjee D, Katz MH, Rashid A, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer. 2012;118:3182–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cloyd JM, Katz MH, Prakash L, et al. Preoperative therapy and pancreatoduodenectomy for pancreatic ductal adenocarcinoma: a 25-year single-institution experience. J Gastrointest Surg. 2017;21:164–174. [DOI] [PubMed] [Google Scholar]
- 18.Cloyd JM, Crane CH, Koay EJ, et al. Impact of hypofractionated and standard fractionated chemoradiation before pancreatoduodenectomy for pancreatic ductal adenocarcinoma. Cancer. 2016;122:2671–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L, Katz MH, Lee SM, et al. Superior mesenteric artery margin of posttherapy pancreaticoduodenectomy and prognosis in patients with pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2015;39:1395–1403. [DOI] [PubMed] [Google Scholar]
- 20.Edge S, Byrd D, Compton C, et al. AJCC cancer staging manual New York: Springer; 2010. [Google Scholar]
- 21.Ribero D, Wang H, Donadon M, et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer. 2007;110:2761–2767. [DOI] [PubMed] [Google Scholar]
- 22.Katz MH, Lee JE, Pisters PW, et al. Retroperitoneal dissection in patients with borderline resectable pancreatic cancer: operative principles and techniques. J Am Coll Surg. 2012;215:e11–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onkendi EO, Boostrom SY, Sarr MG, et al. Neoadjuvant treatment of duodenal adenocarcinoma: a rescue strategy. J Gastrointest Surg. 2012;16:320–324. [DOI] [PubMed] [Google Scholar]
- 25.Overman MJ, Varadhachary GR, Kopetz S, et al. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol. 2009;27:2598–2603. [DOI] [PubMed] [Google Scholar]
- 26.Cereda S, Passoni P, Reni M, et al. The cisplatin, epirubicin, 5-fluorouracil, gemcitabine (PEFG) regimen in advanced biliary tract adenocarcinoma. Cancer. 2010;116:2208–2214. [DOI] [PubMed] [Google Scholar]
- 27.Yeung RS, Weese JL, Hoffman JP, et al. Neoadjuvant chemoradiation in pancreatic and duodenal carcinoma. A Phase II Study. Cancer. 1993;72:2124–2133. [DOI] [PubMed] [Google Scholar]
- 28.Palta M, Patel P, Broadwater G, et al. Carcinoma of the ampulla of Vater: patterns of failure following resection and benefit of chemoradiotherapy. Ann Surg Oncol. 2012;19:1535–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YI, Park JW, Kim BH, et al. Outcomes of concurrent chemoradiotherapy versus chemotherapy alone for advanced-stage unresectable intrahepatic cholangiocarcinoma. Radiat Oncol. 2013;8:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Autorino R, Mattiucci GC, Ardito F, et al. Radiochemotherapy with gemcitabine in unresectable extrahepatic cholangiocarcinoma: long-term results of a phase II study. Anticancer Res. 2016;36:737–740. [PubMed] [Google Scholar]
- 31.Lee KJ, Yi SW, Cha J, et al. A pilot study of concurrent chemoradiotherapy with gemcitabine and cisplatin in patients with locally advanced biliary tract cancer. Cancer Chemother Pharmacol. 2016;78:841–846. [DOI] [PubMed] [Google Scholar]
- 32.Kim HS, Shin SJ, Kim JH, et al. Better outcome of XELOX chemotherapy in patients with advanced intestinal-type adenocarcinoma of the ampulla of Vater. Tohoku J Exp Med. 2013;231:21–28. [DOI] [PubMed] [Google Scholar]
- 33.Shoji H, Morizane C, Hiraoka N, et al. Twenty-six cases of advanced ampullary adenocarcinoma treated with systemic chemotherapy. Jpn J Clin Oncol. 2014;44:324–330. [DOI] [PubMed] [Google Scholar]
- 34.Cloyd JM, Wang H, Egger ME, et al. Association of clinical factors with a major pathologic response following preoperative therapy for pancreatic ductal adenocarcinoma. JAMA Surg. 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly P, Das P, Pinnix CC, et al. Duodenal toxicity after fractionated chemoradiation for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2013;85:e143–e149. [DOI] [PubMed] [Google Scholar]
- 36.Mise Y, Zimmitti G, Shindoh J, et al. RAS mutations predict radiologic and pathologic response in patients treated with chemotherapy before resection of colorectal liver metastases. Ann Surg Oncol. 2015;22:834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valsangkar NP, Ingkakul T, Correa-Gallego C, et al. Survival in ampullary cancer: potential role of different KRAS mutations. Surgery. 2015;157:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruemmele P, Dietmaier W, Terracciano L, et al. Histopathologic features and microsatellite instability of cancers of the papilla of vater and their precursor lesions. Am J Surg Pathol. 2009;33:691–704. [DOI] [PubMed] [Google Scholar]
- 39. Fu T, Pappou EP, Guzzetta AA, et al. CpG island methylator phenotype-positive tumors in the absence of MLH1 methylation constitute a distinct subset of duodenal adenocarcinomas and are associated with poor prognosis Clin Cancer Res. 2012;18:4743–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disease-specific survival (DSS) by presence or absence of preoperative therapy in (A) the entire cohort of patients with duodenal adenocarcinoma (DA) and ampullary adenocarcinoma (AMPA) (n=266), (B) patients with DA (n=97), and (C) patients with AMPA (n=169).


