Abstract
Personality is a complex, yet partially heritable, trait. Although some Mendelian diseases like Williams-Beuren syndrome are associated with a particular personality profile, studies have failed to assign the personality features to a single gene or pathway. As a family of monogenic disorders caused by mutations in the Ras/MAPK pathway known to influence social behavior, RASopathies are likely to provide insight into the genetic basis of personality.
80 subjects diagnosed with cardiofaciocutaneous syndrome, Costello syndrome, neurofibromatosis type I and Noonan syndrome were assessed using a parent-report BFQ-C (Big-Five Questionnaire for Children) evaluating agreeableness, extraversion, conscientiousness, intellect/openness, and neuroticism, along with 55 unaffected sibling controls. A short questionnaire was added to assess sense of humor. RASopathy subjects and sibling controls were compared for individual components of personality, multidimensional personality profiles, and individual questions using Student tests, analysis of variance, and principal component analysis.
RASopathy subjects were given lower scores on average compared to sibling controls in agreeableness, extraversion, conscientiousness, openness and sense of humor, and similar scores in neuroticism. When comparing the multidimensional personality profile between groups, RASopathies showed a distinct profile from unaffected siblings, but no difference in this global profile was found within RASopathies, revealing a common profile for the Ras/MAPK-related disorders. In addition, several syndrome-specific strengths or weaknesses were observed in individual domains. We describe for the first time an association between a single pathway and a specific personality profile, providing a better understanding of the genetics underlying personality, and new tools for tailoring educational and behavioral approaches for individuals with RASopathies.
Keywords: Personality, RASopathies, Costello syndrome, Noonan syndrome, neurofibromatosis type I
INTRODUCTION
Personality plays a key role in the interactions between individuals, in their daily life, behavior, and achievements (Segel-Karpas et Lachman 2016). Although personality is influenced by environmental factors, it has been proven to be a partially heritable trait (Power et Pluess 2015) (Jang, Livesley, et Vemon 1996) (Vukasović et Bratko 2015), with highly probable genetic basis. Identifying specific genes or loci with major influence on aspects of personality could lead to better understanding of the determinants of personality. We hypothesize that monogenic Mendelian disorders with distinct personality profiles could help to identify biological pathways or processes critical to personality.
Williams-Beuren syndrome (WBS) is probably the most outstanding example of a genetic condition associated with a distinct personality profile. Individuals with WBS are often described as gregarious and people-oriented (Ng, Järvinen, et Bellugi 2014) (Lough et al. 2016) (Klein-Tasman et Mervis 2003) (Gosch et Pankau 1997). The social dimension of the personality profile in WBS has sometimes been contrasted with Prader-Willi syndrome (Di Nuovo et Buono 2011), known for its propensity to affect emotional regulation (Avrahamy et al. 2015). Another notable genetic condition giving rise to a singular personality profile is microdeletion 17q21.31, also known as Koolen-De Vries syndrome, generating hypersociability and a high level of frustration tolerance (D. A. Koolen et al. 2008). The highly penetrant personality features of WBS, and Prader-Willi syndromes each result from deletion of a locus containing many genes. Although the gene KANSL1 alone is considered to cause the clinical manifestations of Koolen-De Vries syndrome (David A. Koolen et al. 2012), due to impact on chromatin, this gene has implications in the expression of many additional genes. This explains why very few studies have yet succeeded in narrowing down to a critical gene specific for personality features. However, if a monogenic disorder were associated with a specific personality profile, the responsible gene would be open to further study.
In the general population, genome-wide association studies (GWAS) have had difficulties in pinpointing or replicating loci associated with personality (Terracciano et al. 2010) (Calboli et al. 2010) (de Moor et al. 2012). However, a recent GWAS meta-analysis identified 6 loci associated with conscientiousness, extraversion, and neuroticism (Lo et al. 2017). Interestingly, some of the GWAS loci and pathway analyses have implicated Ras/MAPK genes or signaling as being associated with personality (de Moor et al. 2012) (Kim et al. 2015).
The RASopathies are a family of monogenic disorders involving germline mutation in a gene encoding a component or regulator of the Ras/MAPK pathway; although each individual has a single autosomal dominant mutation, many genes across the signaling pathway can cause RASopathies (Rauen 2013). RASopathies have already been associated with autism-related behavioral traits like social responsiveness (Adviento et al. 2014) (Plasschaert et al. 2015) (Garg, Green, et al. 2013) (Alfieri et al. 2014). Beyond the context of behavioral disorders, we wanted to generally assess personality in a series of monogenic syndromes that could be analyzed together to draw conclusions about the Ras/MAPK pathway. We hypothesized that gain-of-function in the Ras/MAPK pathway could lead to specific personality profiles in RASopathies, with shared or distinct features among RASopathies.
RASopathies
The Ras/MAPK pathway is an intracellular signaling pathway that plays a key role in the regulation of cell differentiation, migration and apoptosis, best known for the role of somatic mutations in cancer. However, when mildly dysregulated by germline mutations, the Ras/MAPK pathway is responsible for syndromic RASopathies (Tidyman and Rauen 2016). The RASopathies include cardiofaciocutaneous syndrome (CFC, OMIM 115150), Costello syndrome (CS, OMIM 218040), neurofibromatosis type 1 (NF1, OMIM 162200, including Legius syndrome, OMIM 611431), and Noonan syndrome (NS, OMIM 163950, including NS with multiple lentigines, OMIM 151100). A common underlying mechanism leads to some overlapping clinical features among RASopathies, but distinct mutations also lead to specific features. All of the RASopathies are rare disorders (<1/1 000), mostly segregating in an autosomal dominant mode, although frequently de novo. They often present with intellectual disability, cardiac abnormalities (except for NF1), skin conditions, and a predisposition for developing tumors. CFC, CS and NS are distinguishable by specific facial features.
CFC is usually caused by a mutation in BRAF, KRAS (Niihori et al. 2006), MEK1, or MEK2 (Rodriguez-Viciana et al. 2006) genes and affects around 1:810,000 individuals (Japan (Abe et al. 2012)). CS is the result of a mutation in the HRAS gene (Aoki et al. 2005) (90%) affecting 1:380,000 live births. NF1 affects from 1/2 600 to 1/3 000 live births (Lammert et al. 2005), and is caused by a loss-of-function mutation in the NF1 gene (>95%), encoding an inhibitor of the pathway. NS affects between 1:1 000 and 1:2 500 live births (Tartaglia, Zampino, et Gelb 2010). About 50% of affected individuals show a mutation in the PTPN11 gene (Tartaglia, Zampino, et Gelb 2010), but a number of other genes can hold causal mutations like SOS1 (Lepri et al. 2011), NRAS, KRAS, BRAF, SHOC2, RAF1 (Tartaglia, Zampino, et Gelb 2010), RIT1 (Aoki et al. 2013) and CBL (Martinelli et al. 2010), and other less common causal genes are continuing to emerge.
Cognitive and social skills in RASopathies
Intellectual disability (ID) is highly variable among the RASopathies, and between affected individuals within a disorder. Some cognitive delay or disability in CFC is usually seen in affected individuals (Niihori et al. 2006), even though it can range from mild to severe, and 70% of CS subjects show an intellectual disability (Axelrad et al. 2009). The intellectual impairment in NF1 is less penetrant, ranging from normal IQ to severe learning disabilities (Hyman, Shores, et North 2005). NS is also associated with a variable IQ, ranging from a mild intellectual impairment to normal cognitive functioning (Acosta, Gioia, et Silva 2006) (Wingbermühle et al. 2012).
A 2014 study (Alfieri et al. 2014) showed that although some differences could be identified between RASopathies in externalizing scale disorders and social or attention problems, these disorders globally presented an increased risk of psychopathological issues and underdiagnosed autistic traits. The Ras/MAPK signaling pathway has been associated with autism spectrum disorder (ASD) risk (Wen, Alshikho, et Herbert 2016) (Pinto et al. 2010) (Hérault et al. 1993). Moreover, RASopathies are associated with increased qualitative and quantitative ASDs (Morris et al. 2016)(Adviento et al. 2014) (Garg, Green, et al. 2013) (Constantino et al. 2015) (Garg et al. 2015) (Garg, Lehtonen, et al. 2013) (Walsh et al. 2013).
Temperament and personality traits
When it comes to temperament and personality, individuals with CS are considered to be highly sociable and happy, despite an excessive shyness and a hypersensitivity, which may disappear after 2–4 years of age (Kawame et al. 2003). They also show low adaptive scores in socialization and daily living skills based on the parent-rated Child Behavior Checklist, the Peabody Picture Vocabulary Test III, and the Vineland adaptive behavior questionnaire (Axelrad et al. 2004). Children with NF1 have been described as more dependent and irritable, scoring low on conscientiousness and openness on the California Child Q-Set (which includes the five-factor model used in this study) (Prinzie et al. 2003). They also are subject to anxiety and dysthymia according to the Comprehensive Psychopathological Rating Scale and Karolina scales of Personality Inventory and self-evaluation scales (Samuelsson et Riccardi 1989) (Zöller et Rembeck 1999). Although the childhood delay in language and motor development often appear to be no longer present in adulthood in NS, the affected individuals still present a specific profile, with a psychosocial immaturity, alexithymia, and amenable traits in a retrospective study (Wingbermuehle et al. 2009).
Most of these descriptive studies of genetic syndromes have not utilized the most standard formal assessment of personality, the Big Five Questionnaire (BFQ). From a very exhaustive and complex taxonomy of personality (Allport et Odbert 1936) (Norman 1967) compiled into dimensional traits (Eysenck 1953) (Barrett et Eysenck 1984), previous studies have eventually come to an optimized model of five orthogonal dimensions in the description of personality traits (T. A. Widiger et Costa 1994), whose robustness to replicated analysis (Digman 1989) (Digman 1990) eventually made them known as the “Big Five” (Goldberg 1990). The five-factor structure of the BFQ explores. I. Extraversion vs Introversion; II. Agreeableness or Friendliness vs Hostility; III. Conscientiousness or Will; IV. Neuroticism vs Emotional Stability; and V. Intellect or Openness to experience (Caprara et al. 1993). Those traits can be evaluated from the subject’s reactions, behavior and choices, or expressed feelings. The five-factor model was used in the Diagnostic and Statistical Manual of Mental Disorders (T. A. Widiger et Costa 1994), to eventually embrace a trait-based taxonomy of personality pathology, as it proved to be suited for the delineation of normal and abnormal personality features (Costa et McCrae 2010) (Thomas A. Widiger et Costa 2012). The children’s version of the Big Five questionnaire (BFQ-C) proved to have a clear-cut factor structure, good internal consistency, and sufficient validity (Muris, Meesters, et Diederen 2005). Analyzing personality in a large cohort of individuals with multiple RASopathies has never been done before, and could ascertain the differences and common features between and among CFC, CS, NF1 and NS.
Sense of humor
As explained in The Sense of Humor: Explorations of a personality characteristic (Ruch 2007), sense of humor can be described as a five-dimensional trait, including cognitive, motivational, emotional, social and behavioral components, addressing some of the Big-Five factors (e.g. cognitive humor related to openness, motivational humor related to agreeableness). Heritability in humor styles has been assessed, and those studies concluded that the positive component of humor was essentially heritable, and correlated to some of the Big Five factors (Vernon et al. 2008) (Mendiburo-Seguel, Páez, et Martínez-Sánchez 2015) (R. A. Martin et al. 2003). As humor can be a prominent factor in self-fulfillment and social development, we wanted to assess humor as part of describing a genetic personality profile in the RASopathies.
Purpose of the study
The objective of our present study was to assess the five personality factors and sense of humor in individuals with RASopathies and a control group of unaffected siblings. On the basis of the previous observations of behavior and personality traits in individual RASopathies, our primary hypothesis was that personality profiles would show differences between RASopathies, considered together, and sibling controls. If so, we sought to further determine whether a common personality profile can be defined among CFC, CS, NF1, and NS in response to their common underlying biological background and/or whether disorder-specific profiles exist. The existence of association between personality traits and monogenic disorders may lead to better understanding of the biological mechanisms underlying personality in the general population.
SUBJECTS AND METHODS
Subjects
Inclusion criteria were diagnosis of NF1, Noonan, Costello, or CFC by a medical geneticist, or diagnosis of NF1 by a neurologist. Subjects with RASopathies were recruited at the University of California, San Francisco (UCSF) NF/Ras Pathway Genetics Clinic and three national RASopathy meetings (Berkeley, California, USA, July 2009; Chicago, Illinois, USA, July 2011; Orlando, Florida, USA, August 2013). Additional families were recruited at two UCSF NF Symposia (November 2011 and February 2014), and through RASopathy groups: NF, Inc., Children’s Tumor Foundation, Noonan Foundation, CFC International, Costello Syndrome Family Support Network, and Costello Kids.
In total, 135 subjects (60 females, 75 males) were evaluated by one of their parents, including 80 RASopathy subjects and 55 sibling controls: 21 CFC subjects, 14 of which had one unaffected sibling evaluated, 23 CS subjects (17 sibling controls), 19 NF1 subjects (10 sibling controls), and 17 NS subjects (14 sibling controls). In families with several available siblings, the closest in age was chosen to be included in the study. The mean age of the recruited probands was 12.8 years old, with a deviation of 9.4 years in the total sample (Supp. Table I). The ages ranged from 2 to 39 for cases and from 3 to 43 for sibling controls. No significant difference was observed in ages between the groups. In case the inclusion of adults (4 subjects over 15 years old in the CFC group, 7 for CS, 6 for NF1, 4 in NS and 14 in the control group) biased our analyses, we compared mean personality trait scores with adult subjects included or excluded, and found no substantive differences..
All subjects had parents or guardians with fluency in English to complete the questionnaire. Written informed consent was obtained for all participants. This study was approved by the UCSF Human Research Protection Program (CHR #10-02794).
Methods
The Big-Five Questionnaire Children (BFQ-C) and sense of humor scale for children have been filled out by one parent for each affected child and one of his/her unaffected siblings, when available. The BFQ-C includes a total of 65 questions, with 13 questions per factor, specifically developed to evaluate children using parent-report (Barbaranelli et al. 2003). The BFQ lies on the Likert scale model, built from gradual answers ranking from 1 to 5 (corresponding to “Hardly ever”, “Rarely”, “Sometimes”, “Often”, and “Almost always”).
The BFQ-C questions were addressed to parents, taking the form of simple assertions about the child’s behavioral habits and expected typical reactions and feelings. When the subjects were adults, the parents were asked to fill in the questionnaire based on the behavior shown by their offspring as children. Five questions were used to assess sense of humor, including one already present in the BFQ-C. The added questions were obtained from a simplified version of the humor styles questionnaire (Ruch 2007) adapted for younger subjects (James et Fox 2016). Average scores were calculated for each question, then for each BFQ factor and sense of humor, and these factors were analyzed independently.
Missing data
An additional “too young” column let the parent skip the question if it was tackling a situation they have not been able to evaluate yet. The Cronbach’s α reliability coefficient was calculated for each factor-specific subset of questions (Supp. Table II), and determined the maximal amount of missing data to be considered as an exclusion criterion (α<0.70).
Correlation amongst siblings
Linear regression was performed with sibling-paired samples according to the least squares method by Microsoft Excel, and corresponding p-values were calculated for each personality factor and sense of humor, and for each disorder.
Score comparison between groups
The scores for each personality factor were compared across groups using a bilateral Student test. The significant results from samples counting less than 20 individuals, with uncertain heteroscedasticity according to the Fisher-Snedecor test, or challenging the normal hypothesis by their skewness and kurtosis parameters, were confirmed by a Wilcoxon-Mann-Whitney test, using the UCLA Statistics Online Computational Resource (SOCR).
Analysis of variance amongst disorders
One-way and two-way ANOVA (analysis of variance) were performed using the “car” package of R 3.3.0 software, using the type II sum of squares to take into account the unbalanced sizes of each group. The two-way ANOVA was done using Pillai’s criterion with respect to the sibling’s potential similarities. Two sets of analyses were performed, the first on the variances between all the groups, including the control group, and a second one between the four RASopathies only. The same design was used to perform both one-way and two-way MANOVA, combining the results of the six factors to detect a significant personality profile. The RASopathy group results were compared to the control group results, and each RASopathy was then compared to the three remaining syndromes using a two-way MANOVA.
Gender
To assess the potential effect of gender on the results, gender-specific mean scores were retrieved for each personality factor, and groups were compared using simple and multivariate analysis of variance (ANOVA and MANOVA). No significant difference was found between males and females from each group on any personality factor, nor on a combined personality profile, so this variable was not considered further.
Principal component analysis by personality factors, and by questions
A principal component analysis was performed using R statistical built-in tools, in combination with the “factoextra” library. A first analysis used the scores of unaffected siblings, retrieving the coordinates of each proband on the first and second principal components, built from each personality parameter. The coordinates for the probands in each of the RASopathy groups were then introduced one by one to that first analysis and their coordinates on the first principal component were compared to those of the control distribution by both Fisher and Student tests. The results showing an inequality of variance on the Fisher test were then confirmed by a Welch test. The same approach was used to compare each syndrome to the 3 other RASopathies. A second PCA was performed on the 69-question vector, and the same comparisons were performed, between the RASopathy group and the control group first, then within RASopathies.
RESULTS
The RASopathies group, taken together, scored significantly lower than controls on agreeableness (T132=2.26, p=0.025), extraversion (T131=4.58, p<10−4), conscientiousness (T128=4.29, p<10−4), intellect/openness (T127=6.10, p<10−7), and sense of humor (T128=2.91, p=0.004). Yet, no significant difference was noted between the two groups for neuroticism (Fig. 1.a and 1.b). Thus, we observed a difference in personality profile for individuals with RASopathies compared with control siblings. The mean scores of each group by traits are presented in Table I.
Figure 1.
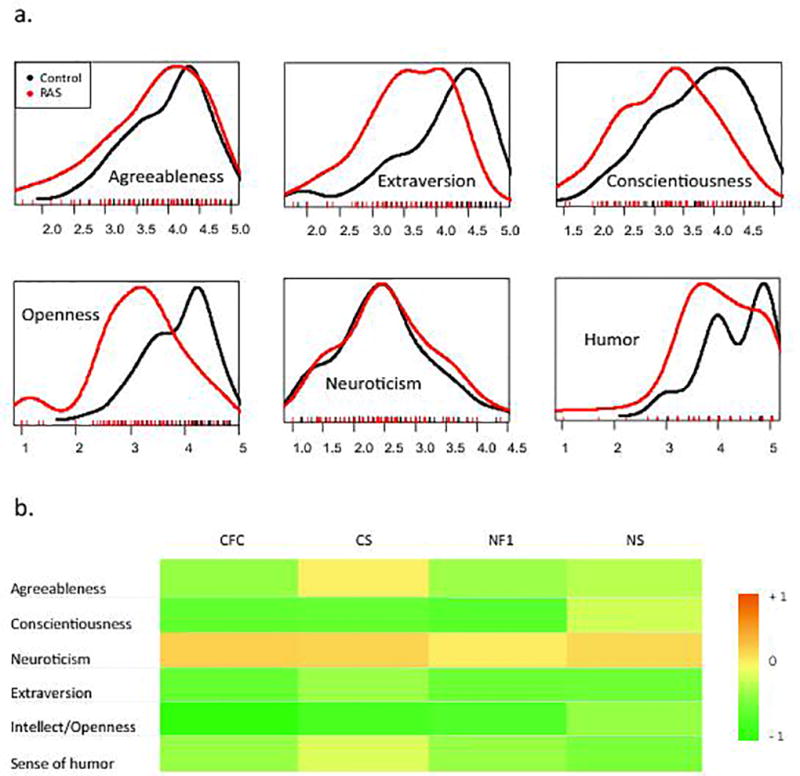
Table 1. BFQ-C and Sense of Humor Mean Scores.
Scores for each personality factor and sense of humor. Scores were rated out of 5 and represent the average of the parent rating on the Likert-scale for each subset of questions. The standard deviations (SD) of the scores are provided for each trait.
| BFQ-C | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Agreeableness | Extraversion | Conscientiousness | Openness | Neuroticism | Humor | |||||||
|
|
||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| CFC | 3.59 | 0.76 | 3.46 | 0.61 | 3.04 | 0.66 | 2.85 | 0.69 | 2.64 | 0.82 | 3.90 | 0.85 |
| CS | 4.02 | 0.65 | 3.70 | 0.59 | 3.06 | 0.66 | 3.08 | 0.72 | 2.61 | 0.57 | 4.15 | 0.60 |
| NF1 | 3.63 | 0.96 | 3.50 | 0.79 | 3.00 | 0.98 | 3.15 | 1.07 | 2.47 | 0.88 | 3.90 | 0.91 |
| NS | 3.73 | 0.70 | 3.51 | 0.70 | 3.50 | 0.60 | 3.47 | 0.73 | 2.58 | 0.72 | 3.77 | 0.94 |
| Controls | 4.02 | 0.59 | 4.11 | 0.70 | 3.71 | 0.74 | 3.90 | 0.59 | 2.45 | 0.72 | 4.31 | 0.62 |
|
| ||||||||||||
| RASopathies | 3.76 | 0.72 | 3.55 | 0.73 | 3.14 | 0.83 | 3.14 | 0.83 | 2.58 | 0.73 | 3.95 | 0.87 |
| Total Mean Score | 3.87 | 0.72 | 3.79 | 0.73 | 3.38 | 0.80 | 3.46 | 0.82 | 2.53 | 0.73 | 4.10 | 0.76 |
To assess whether RASopathies exhibited common personality traits, we first determined whether any of the five groups, including the control group, was distinct compared to the others in an analysis of variance (ANOVA). Comparing the scores of the five groups confirmed a difference in extraversion (F4=5.756, p<10−3), conscientiousness (F4=5.991, p<10−3), openness (F4=10.169, p<10−6), and sense of humor (F4=2.680, p=0.035). However, when considering the four RASopathy groups only, no differences were observed, confirming that the main driver of the increased variance among the five groups was the control group. These results were validated by pairing matched siblings in two-way ANOVA analyses, with significant results in each of the above areas (Supp. Table III).
When analyzing each RASopathy individually by Student tests to determine if each contributed equally to group differences from controls, all of them appeared to score lower than the control group on extraversion and openness. They also showed low scores on conscientiousness, except for the NS group, which showed no difference with the control group (T70=120, p=0.233), while scoring significantly higher than the three other RASopathies taken together (T128=2.51, p=0.013). Although no difference from control siblings was observed for CFC, CS, and NF1 on sense of humor, the NS subjects also scored significantly lower than the control group (T70=2.13, p = 0.037) (Fig. 1.a and 1.b). (Supp. fig. 1).
When making comparisons amongst RASopathies to determine whether distinct syndrome-specific strengths and weaknesses might exist, NS showed more conscientiousness than CFC (T31=2.09, p=0.045) and CS (T38=2.16, p=0.037), and higher openness than CFC (T31=2.49, p=0.018). We noted that CS subjects had higher scores than the other RASopathies on agreeableness (T132=2.13, p=0.035) (Table II).
Table II. Compared Mean Scores Between Groups for the Big Five Factors and Sense of Humor.
Results of the pairwise comparisons of the scores between groups are summarized by showing p-values resulting from a bilateral and heteroscedastic Student test. Bolded significance (p-value < 0.05) has been confirmed by a Wilcoxon-Mann-Whitney test. The rightmost column shows a pairwise test between one RASopathy and the combination of the other three RASopathies or controls compared with the combination of all four RASopathies.
| Big Five Factors | CS | NF1 | NS | vs Controls | vs Other RAS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| Tdf | p | Tdf | p | Tdf | p | Tdf | p | Tdf | p | ||
| Agreeableness | CFC | T41=1.98 | 0.055 | T37=0.15 | 0.885 | T35=0.57 | 0.570 | T73=2.23 | 0.029 | T132=2.00 | 0.274 |
| CS | T40=1.49 | 0.144 | T38=1.34 | 0.188 | T76=0.02 | 0.982 | T132=2.13 | 0.035 | |||
| NF1 | T34=0.35 | 0.730 | T72=1.62 | 0.109 | T132=0.67 | 0.503 | |||||
| NS | T70=1.53 | 0.130 | T132=0.17 | 0.865 | |||||||
|
|
|||||||||||
| Controls | T132=2.26 | 0.025 | |||||||||
|
|
|||||||||||
| Extraversion | CFC | T40=1.30 | 0.199 | T36=0.17 | 0.870 | T34=0.23 | 0.819 | T72=3.68 | <10−3 | T131=0.73 | 0.469 |
| CS | T40=0.93 | 0.359 | T38=0.91 | 0.366 | T76=2.61 | 0.011 | T131=1.37 | 0.172 | |||
| NF1 | T34=0.05 | 0.960 | T72=2.85 | 0.006 | T131=0.35 | 0.725 | |||||
| NS | T70=2.93 | 0.005 | T131=0.28 | 0.783 | |||||||
|
|
|||||||||||
| Controls | T131=4.58 | <10−4 | |||||||||
|
|
|||||||||||
| Conscientiousness | CFC | T37=0.12 | 0.907 | T33=0.14 | 0.890 | T31=2.09 | 0.045 | T69=3.27 | 0.001 | T128=0.66 | 0.510 |
| CS | T40=0.24 | 0.809 | T38=2.16 | 0.037 | T76=3.67 | <10−3 | T128=0.62 | 0.535 | |||
| NF1 | T34=1.85 | 0.072 | T72=2.74 | 0.007 | T128=0.77 | 0.442 | |||||
| NS | T70=1.20 | 0.233 | T128=2.51 | 0.013 | |||||||
|
|
|||||||||||
| Controls | T128=4.29 | <10−4 | |||||||||
|
|
|||||||||||
| Intellectual openness | CFC | T37=1.00 | 0.325 | T32=0.99 | 0.331 | T31=2.49 | 0.018 | T69=4.66 | <10−4 | T127=1.73 | 0.085 |
| CS | T39=0.25 | 0.802 | T38=1.67 | 0.104 | T76=4.47 | <10−4 | T127=0.43 | 0.665 | |||
| NF1 | T33=1.01 | 0.319 | T71=2.62 | 0.011 | T127=0.08 | 0.937 | |||||
| NS | T70=2.12 | 0.037 | T127=1.98 | 0.049 | |||||||
|
|
|||||||||||
| Controls | T127=6.10 | <10−7 | |||||||||
|
|
|||||||||||
| Neuroticism | CFC | T41=0.12 | 0.908 | T37=0.61 | 0.546 | T35=0.22 | 0.825 | T73=0.89 | 0.377 | T132=0.39 | 0.700 |
| CS | T40=0.60 | 0.553 | T38=0.15 | 0.884 | T76=1.04 | 0.302 | T132=0.30 | 0.764 | |||
| NF1 | T34=0.41 | 0.524 | T72=0.08 | 0.934 | T132=0.63 | 0.531 | |||||
| NS | T70=0.64 | 0.683 | T132=0.02 | 0.984 | |||||||
|
|
|||||||||||
| Controls | T132=0.98 | 0.326 | |||||||||
|
|
|||||||||||
| Sense of Humor | CFC | T39=1.08 | 0.288 | T33=0.01 | 0.994 | T33=0.42 | 0.677 | T71=1.85 | 0.068 | T128=0.28 | 0.779 |
| CS | T38=1.01 | 0.320 | T38=1.46 | 0.152 | T76=1.06 | 0.293 | T128=1.69 | 0.093 | |||
| NF1 | T32=0.39 | 0.696 | T70=1.71 | 0.093 | T128=0.27 | 0.789 | |||||
| NS | T70=2.13 | 0.037 | T128=0.90 | 0.370 | |||||||
|
|
|||||||||||
| Controls | T128=2.91 | 0.004 | |||||||||
Genotype-phenotype correlation
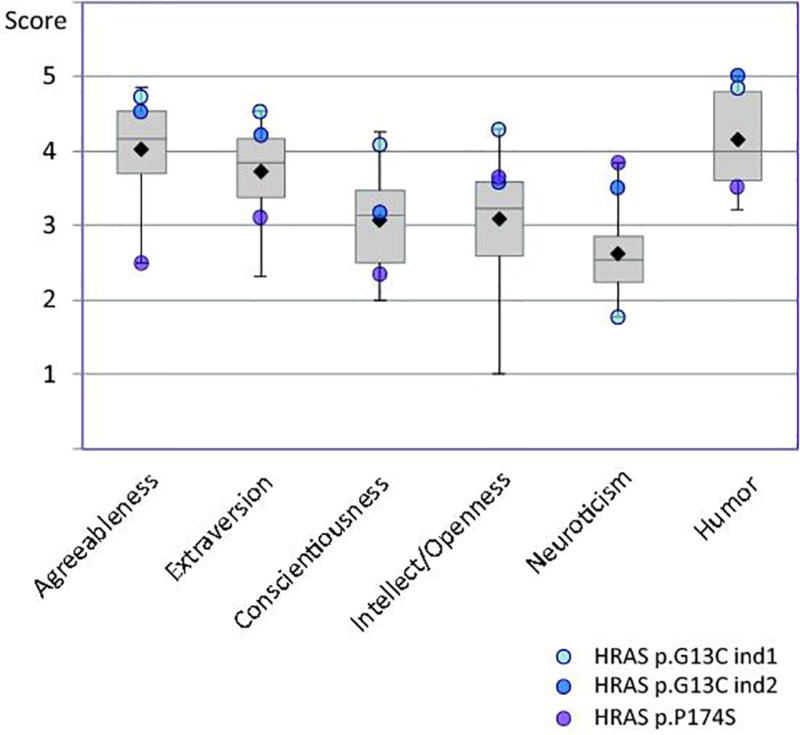
We observed considerable variation for each personality component within each RASopathy. Knowledge of specific disorder mutations within-syndrome has proven critical for understanding the expression variation of RASopathies in cognitive profiles, for example, indicating genotype-phenotype correlation with respect to pathogenic mutations (Cesarini et al. 2009). In our 135 participants, 48 had a known molecular diagnosis (20 CFC, 17 CS, 2 NF1 and 9 NS, Supp. Table IV). Most CS subjects carry an HRAS p.G12S mutation (14/17). We observed that two CS individuals with the HRAS p.G13D mutation scored higher than the CS mean or had the maximum score in agreeableness, extraversion, conscientiousness, openness, and humor. Another CS subject with HRAS p.P174S mutation showed the reverse profile with a high score in neuroticism and low scores in the other traits (Supp. fig. 2). No remarkable patterns by mutation were observed for NS or CFC. Unfortunately, these few observations are insufficient for a rigorous genotype-phenotype analysis.
Correlation between siblings
We next wanted to assess the contribution of background genetics to within-disorder variation, distinct from the effects of a RASopathy mutation. Thus, we measured correlation between RASopathy affected-unaffected sibling pairs for each personality dimension. We expect that despite different mean scores between affected and unaffected siblings, traits with high polygenic heritability in the presence of a RASopathy will show strong sibling correlation.
Agreeableness, extraversion, conscientiousness and openness were found correlated between affected subjects and their siblings to a greater extent for the NF1 and the NS groups than for CFC and CS. In particular, openness was found to be highly correlated between subjects with NF1 and their siblings (R2=0.62, p=0.007) (Supp. fig. 3.a). Neuroticism showed no correlation for CFC and NF1 families, and showed modest (non-significant) estimates for NS (R2=0.19, p=0.115) and CS (R2=0.12, p=0.170) families. Only NS showed some correlation between siblings in sense of humor (R2=0.30, p=0.04), despite the greatest mean difference for this trait (see above) (Supp. fig. 3.b).
Personality profile, by trait
Big Five factors and sense of humor are components of a more holistic personality profile, which we analyzed as one multidimensional trait to determine whether specific personality profiles can be associated with RASopathies. The multivariate analysis of variance obtained from the combined personality traits concluded that the RASopathy group and the sibling group demonstrated a significantly different personality profile (p<10−7). The MANOVA analysis utilizes the linear combination of component traits that maximizes the group differences. When analyzing the RASopathy groups together, no difference was observed amongst RASopathies. Correlation between traits is presented in Supp. Table V.
We next tried to confirm the subtler disparities within RASopathies we had observed for specific traits with pairwise MANOVA. When comparing only two groups to one another, the calculation is optimized for the two groups and can reveal differences which could not be observed among the four disorders. Here, we observed that the CS individuals expressed a different profile from NF1 (F6=3.582, p=0.005), and from NS (F6=2.881, p=0.017) (Supp. Table VI).
A principal component analysis calculated from each group and subgroup on the personality traits was used to define and visualize the differences observed between the personality profiles. Some consistent patterns were observed in the relationships between traits (Fig. 2.a and 2.b). However, a comparison between the coordinates of each group projected on a first principal component (Fig. 2.c.) confirmed a significant difference between the sibling group and the RASopathy group (F10=0.15, p<10−4). Each RASopathy also revealed a personality profile distinguishable from the control profile (CFC: F10=0.56, p < 10−3, CS: F10=0.93, p = 0.004, NF1: F10=0.01, p = 0.045, NS: F10=0.41, p = 0.011). No difference was found when individually comparing each RASopathy to the others, even pairwise, probably meaning that the disparities previously observed within RASopathies were not contributing to the major variance axes in the global questionnaire results (Supp. Table VII).
Figure 2.
Personality profile, by questions
Although the Big Five are usually considered as independent factors, with each question contributing equally to a trait, we do not know whether these underlying assumptions remain valid within these syndromic conditions. When looking at questions independently instead of summarized traits, differences driven by specific questions within each trait could become evident. Using a PCA to define a personality profile without averaging the questions into personality traits was an attempt to reveal similarities and differences between the RASopathy groups in a more granular way, and confirmed a significant difference between case and control coordinates on the first principal component (p < 10−4) (Supp. fig. 4, Supp. Table VIII).
To visualize how each question contributes to the difference observed between cases and controls, the p-values of a student test were plotted on a logarithmic scale. Interestingly, agreeableness, extraversion and openness showed two distinct groups of questions, one group showing little or no difference from controls, and a subset of questions showing high significance (10−11 < p < 10−6) (Fig. 2.d), which could be evidence for a specific aspect of these traits being affected by RASopathies (Supp. file 1). Every significant question was coherent with the result obtained for the trait (case scores were higher in neuroticism, and lower in other traits), except for one question in agreeableness, where cases scored nominally higher than controls (p = 0.031), although not significant after Bonferroni correction. This item addressed how the individual perceived others, and a high score was given if the subject thinks others are good and honest. In this case, where it is an outlier compared with other questions about general agreeableness, it may indicate instead gullibility or a behavioral feature of potential concern. This possibility provides a good example of how thorough analysis of personality in even larger genetic syndrome datasets could be informative about specific traits.
DISCUSSION
Our aim in this study was to determine whether RASopathies are associated with a distinct personality profile, implicating the Ras/MAPK pathway in the biology of personality. Using a variety of analytical approaches, we identified significant differences between subjects with RASopathies and control siblings on individual personality traits, on a multivariate personality profile, and by principal component analyses of personality data. Across each of these levels of analysis, the four RASopathy groups showed significant similarity to each other, indicating the existence of a cohesive Ras/MAPK driven personality profile.
The personality profile of RASopathies
Although the differences between RASopathy and control siblings were highly significant and consistent across analyses, they showed specificity in the aspects of personality affected. While extraversion, conscientiousness and openness showed an evident shift from the control scores, sense of humor was only slightly different compared with controls, and neuroticism did not show any significant difference across our analyses. Neuroticism (or emotional instability) has been associated with dysthymia, major depression and anxiety (Bienvenu et al. 2004). Although individuals with a RASopathy have been reported to be at higher risk for anxiety disorders, especially for CS (Axelrad et al. 2011) and NF1 (Wang et al. 2012), our observation suggests that anxiety within RASopathies is not the result of a general shift in personality, so may indicate a distinct subtype of anxiety not driven by neuroticism.
In a previous study utilizing a different questionnaire to evaluate the Big Five in 44 NF1 subjects compared with unaffected controls (Prinzie et al. 2003), a very similar profile to our observation emerged (extraversion and conscientiousness were much lower compared with controls, openness and neuroticism were modestly different from controls, and agreeableness was similar). Such a highly consistent pattern shows the robustness of this type of analysis. Minor differences in the level of significance found for each comparison could have arisen by differences in power due to sample size or differences in the age distribution, known to impact neuroticism in particular (Magan et al. 2014) (Ibáñez et al. 2016).
Despite the ostensible common personality profile across RASopathies, some unique features among RASopathies were noted. We consistently found lower scores on humor for NS, and a significantly distinct personality profile between CS subjects and NF1 or NS subjects documented by the two-way MANOVA. However, those differences did not contribute to the major axes of variation in the personality profile, thus did not have a large effect on overall similarity of profiles across RASopathies. The modest inter-RASopathy differences did not follow a pattern consistent with overall severity (medical, cognitive, dysmorphism) of the four syndromes, with NF1 and NS generally having milder presentations and CS and CFC more severe manifestations, with sense of humor being a key example where CS was most similar to controls and NS most different. Although the intelligence quotient (IQ) was not available for all our participants, we know that no correlation has been observed between IQ and any of Big Five factors in a study comparing two populations of low and high IQ (Waiyavutti, Johnson, et Deary 2012), or for instance in the particular case of NF1 (Prinzie et al. 2003). These observations suggest that personality can be evaluated independently from intellectual ability. This result would have been extremely interesting to confirm in our own population of subjects, and we regret that we could not obtain IQ data. In our study, individuals with CS scored higher in agreeableness (p = 0.035) and relatively higher in sense of humor (p = 0.09, non-significant) than the other RASopathies. Those results indeed support the independence of personality traits evaluated by the BFQ and sense of humor from other clinical manifestations of the RASopathies.
In fact, our results for sense of humor in CS were striking, in that affected individuals had scores indistinguishable from the control group (p = 0.293) in contrast to the other features evaluated. The correlation between siblings for that trait was low (R = 0.016, p = 0.950), potentially suggesting that CS mutations independently influence sense of humor. Further studies including more individuals and using a more thorough evaluation of humor, using more complete tools such as the Humor Styles Questionnaire, might lead to novel insights into sense of humor in CS.
The analysis of each question showing a significant difference between RASopathies and controls (Supp. File 1) can provide a better insight into the specific needs and strengths that specialists can consider in order to improve patient care, patient experience, and educational opportunities. For instance, children with RASopathies might be explicitly taught to notice others’ struggles, and encouraged to propose their help, which could also contribute to their own wellbeing and social adjustment. Teachers could be encouraged to utilize the individual’s will to learn and discover for engagement in order to instill additional self-confidence. Clinicians could relate to their patients via humor in order to build trust and form strong provider relationships.
Heritability
Twin studies showed evidence for some heritability of the Big Five factors, ranging from 41% for neuroticism, to 61% for openness (Jang, Livesley, et Vemon 1996) (N. Martin et al. 2000). The influence of a shared environment has proved to be negligible for neuroticism (Lake et al. 2000), and the trait tended to be more driven by additive and dominant genetic effects than familial influence (Eaves et al. 1999). Our study provides evidence that high-impact genetic mutations can influence personality and sense of humor.
Further, sibling correlation supports a polygenic contribution to some traits even in the presence of dominant mutation. Significant correlations between siblings for openness in NF1 and sense of humor in NS were observed in our study, notably in the syndromes with milder clinical severity. The relatively low correlations we found between siblings for the other personality traits and RASopathies might be due to low power, considering the small sample sizes for complex trait analysis. Alternatively, lack of sibling correlation could indicate an epistatic effect of the RASopathy mutation obscuring polygenic contribution to these traits found in the general population.
Genotype-phenotype correlation
A remaining question we were unable to address in this study is the impact of allelic heterogeneity within each syndrome. Only a subset of the subjects in our study (48 of 135) had molecular diagnostic information, and power was additionally limited by the distribution of mutations. Only two NF1 mutations were known, and for CS and CFC one common mutation accounts for most individuals, so not enough variation is present. NS is complicated by the many genes and many alleles that can be pathogenic. Despite low power to perform conclusive analyses, several CS individuals carrying rare mutations exhibited notably distinct profiles, so larger future studies may confirm the impact of specific disorder mutations.
Limitations
In order to maximize sample size, we have relied upon a single, relatively short questionnaire for this study and do not have multiple independent assessments. We also encountered classical limitations (Mash et Johnston 1983) related to questionnaires filled out by parents (as opposed to a single examiner) including debatable objectivity, differences in individual perception of a personality trait, and the difficulty for a parent in comparing a child with a chronic condition to an unaffected sibling. RASopathy symptoms and needs can both influence the way others interact with an individual and vary over time, which makes the comparison with controls and at different ages more challenging. In addition, personality itself can evolve or change over time, so the specific characteristics we noted may be influenced by the age distribution of our subjects. However, as the control siblings were well-matched for age, we expect the group differences we observed to be robust and valid.
In order to reduce the cumbersomeness of the questionnaire, only a subset of the humor styles questionnaire was used, thus although the subset was validated compared with the larger set, our interpretations with respect to humor may be less informed compared with other traits. Based on results of previous studies (Waiyavutti, Johnson, et Deary 2012), IQ has not been collected or considered as a confounding factor here. However, IQ data might have been useful to confirm that personality and IQ were not correlated in this study. The recruitment of patients affected with rare diseases (and a sibling for some analyses) limits our sample sizes and thus power to detect modest effects. This small sample size could have limited the significance of some results, especially for the PCA performed on the set of 69 questions.
Unique features of the study
Our study had a number of novel elements. First, in addition to personality, we evaluated sense of humor using a standardized measure. To our knowledge, this is the first study to evaluate sense of humor as an important adaptive trait in individuals with genetic syndromes. Second, we not only evaluated single-gene disorders, but we chose to group and compare syndromes affecting different components of the Ras/MAPK pathway to define how a critical signaling pathway could play a role in the development of personality. This could be particularly informative because RASopathies are thought to have gain-of-function in the Ras/MAPK pathway, so might have more specific consequences than loss-of-function monogenic disorders. Finally, in addition to evaluating each personality and humor trait in isolation, our multivariate approaches allowed us to describe a multidimensional personality profile for a comprehensive perspective, refined by an analysis of the results for each of the 69 questions.
CONCLUSIONS
Individuals with a mutation in the Ras/MAPK pathway present a common personality profile, which could lead to a better understanding of the physiological underpinnings of personality. Our strong and consistent results suggest that utilizing Mendelian genetic disorders with known single-gene mutations is a powerful approach to identifying genetic determinants of a personality profile. As these syndrome-related genes are now identified as contributing to agreeableness, openness, extraversion, conscientiousness and humor (Vinkhuyzen et al. 2012) (Power et Pluess 2015), pathway-based analyses investigating the potential contribution of common variation in the Ras/MAPK pathway to personality and humor in the general population could be fruitful. Moreover, understanding the specific personality profile for a given disorder, even within RASopathies, could lead to better support, adapted teaching and inclusive education for individuals with genetic syndromes, as well as more holistic genetic counseling for families.
Supplementary Material
Acknowledgments
We are grateful to Carol Mathews for helpful discussion, Dina Bseiso and Brigid Adviento for subject recruitment and data entry, and Marion Belloni for contribution to statistical analysis. We appreciate the generous participation of each family in this study. We also thank NF, Inc., Children’s Tumor Foundation, CFC International, Costello Kids, Costello Syndrome Family Support Network, and Noonan Foundation and for their contribution to our recruitment efforts. This work was supported by DP2 OD007449 (LAW), an IMHRO/Staglin Family Assistant Professorship (LAW), and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases R01 AR062165 (KAR).
Footnotes
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- Abe Yu, Aoki Yoko, Kuriyama Shinichi, Kawame Hiroshi, Okamoto Nobuhiko, Kurosawa Kenji, Ohashi Hirofumi, et al. Prevalence and Clinical Features of Costello Syndrome and Cardio-Facio-Cutaneous Syndrome in Japan: Findings from a Nationwide Epidemiological Survey. American Journal of Medical Genetics Part A. 2012;158A(5):1083–94. doi: 10.1002/ajmg.a.35292. [DOI] [PubMed] [Google Scholar]
- Acosta Maria T, Gioia Gerard A, Silva Alcino J, et al. Neurofibromatosis Type 1: New Insights into Neurocognitive Issues. Current Neurology and Neuroscience Reports. 2006;6(2):136–43. doi: 10.1007/s11910-996-0036-5. [DOI] [PubMed] [Google Scholar]
- Adviento Brigid, Corbin Iris L, Widjaja Felicia, Desachy Guillaume, Enrique Nicole, Rosser Tena, Risi Susan, et al. Autism traits in the RASopathies. Journal of medical genetics. 2014;51(1):10–20. doi: 10.1136/jmedgenet-2013-101951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri Paolo, Piccini Giorgia, Caciolo Cristina, Perrino Francesca, Gambardella Maria Luigia, Mallardi Maria, Cesarini Laura, et al. Behavioral Profile in RASopathies. American Journal of Medical Genetics Part A. 2014;164(4):934–42. doi: 10.1002/ajmg.a.36374. [DOI] [PubMed] [Google Scholar]
- Allport Gordon W, Odbert Henry S, et al. Trait-Names: A Psycho-Lexical Study. Psychological Monographs. 1936;47(1):i. doi: 10.1037/h0093360. [DOI] [Google Scholar]
- Aoki Yoko, Niihori Tetsuya, Banjo Toshihiro, Okamoto Nobuhiko, Mizuno Seiji, Kurosawa Kenji, Ogata Tsutomu, et al. Gain-of-Function Mutations in RIT1 Cause Noonan Syndrome, a RAS/MAPK Pathway Syndrome. American Journal of Human Genetics. 2013;93(1):173–80. doi: 10.1016/j.ajhg.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Yoko, Niihori Tetsuya, Kawame Hiroshi, Kurosawa Kenji, Ohashi Hirofumi, Tanaka Yukichi, Filocamo Mirella, et al. Germline Mutations in HRAS Proto-Oncogene Cause Costello Syndrome. Nature Genetics. 2005;37(10):1038–40. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- Avrahamy Hamutal, Pollak Yehuda, Shriki-Tal Liron, Genstil Larry, Hirsch Harry J, Gross-Tsur Varda, Benarroch Fortu, et al. A Disease Specific Questionnaire for Assessing Behavior in Individuals with Prader-Willi Syndrome. Comprehensive Psychiatry. 2015;58:189–97. doi: 10.1016/j.comppsych.2014.12.005. (avril): https://doi.org/10.1016/j.comppsych.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Axelrad Marni E, Glidden Rochelle, Nicholson Linda, Gripp Karen W, et al. Adaptive Skills, Cognitive, and Behavioral Characteristics of Costello Syndrome. American Journal of Medical Genetics Part A. 2004;128A(4):396–400. doi: 10.1002/ajmg.a.30140. [DOI] [PubMed] [Google Scholar]
- Axelrad Marni E, Schwartz David D, Fehlis Julie, Hopkins Elizabeth, Stabley Deborah L, Church Katia Sol, Gripp Karen W, et al. Longitudinal Course of Cognitive, Adaptive and Behavioral Characteristics in Costello Syndrome. American journal of medical genetics. Part A. 2009;149A(12):2666–72. doi: 10.1002/ajmg.a.33126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrad Marni E, Schwartz David D, Katzenstein Jennifer M, Hopkins Elizabeth, Gripp Karen W, et al. Neurocognitive, Adaptive, and Behavioral Functioning of Individuals with Costello Syndrome: A Review. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 2011;157C(2):115–22. doi: 10.1002/ajmg.c.30299. [DOI] [PubMed] [Google Scholar]
- Barbaranelli Claudio, Caprara Gian Vittorio, Rabasca Annarita, Pastorelli Concetta, et al. A questionnaire for measuring the Big Five in late childhood. Personality and Individual Differences. 2003;34(4):645–64. doi: 10.1016/S0191-8869(02)00051-X. [DOI] [Google Scholar]
- Barrett Paul, Eysenck Sybil, et al. The assessment of personality factors across 25 countries. Personality and Individual Differences. 1984;5(6):615–32. doi: 10.1016/0191-8869(84)90110-7. [DOI] [Google Scholar]
- Bienvenu O Joseph, Samuels Jack F, Costa Paul T, Reti Irving M, Eaton William W, Nestadt Gerald, et al. Anxiety and Depressive Disorders and the Five-Factor Model of Personality: A Higher- and Lower-Order Personality Trait Investigation in a Community Sample. Depression and Anxiety. 2004;20(2):92–97. doi: 10.1002/da.20026. [DOI] [PubMed] [Google Scholar]
- Calboli Federico CF, Tozzi Federica, Galwey Nicholas W, Antoniades Athos, Mooser Vincent, Preisig Martin, Vollenweider Peter, et al. A Genome-Wide Association Study of Neuroticism in a Population-Based Sample. PLOS ONE. 2010;5(7):e11504. doi: 10.1371/journal.pone.0011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprara Gian Vittorio, Barbaranelli Claudio, Borgogni Laura, Perugini Marco, et al. The “big five questionnaire”: A new questionnaire to assess the five factor model. Personality and Individual Differences. 1993;15(3):281–88. doi: 10.1016/0191-8869(93)90218-R. [DOI] [Google Scholar]
- Cesarini Laura, Alfieri Paolo, Pantaleoni Francesca, Vasta Isabella, Cerutti Marta, Petrangeli Valentina, Mariotti Paolo, et al. Cognitive Profile of Disorders Associated with Dysregulation of the RAS/MAPK Signaling Cascade. American Journal of Medical Genetics Part A. 2009;149A(2):140–46. doi: 10.1002/ajmg.a.32488. [DOI] [PubMed] [Google Scholar]
- Constantino John N, Zhang Yi, Holzhauer Kieran, Sant Sayli, Long Kyna, Vallorani Alicia, Malik Leena, Gutmann David H, et al. Distribution and Within-Family Specificity of Quantitative Autistic Traits in Patients with Neurofibromatosis Type I. The Journal of Pediatrics. 2015;167(3):621–626. e1. doi: 10.1016/j.jpeds.2015.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Paul T, McCrae Robert R, et al. Bridging the Gap with the Five-Factor Model. Personality Disorders. 2010;1(2):127–30. doi: 10.1037/a0020264. [DOI] [PubMed] [Google Scholar]
- Di Nuovo Santo, Buono Serafino, et al. Behavioral Phenotypes of Genetic Syndromes with Intellectual Disability: Comparison of Adaptive Profiles. Psychiatry Research. 2011;189(3):440–45. doi: 10.1016/j.psychres.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Digman JM. Five Robust Trait Dimensions: Development, Stability, and Utility. Journal of Personality. 1989;57(2):195–214. doi: 10.1111/j.1467-6494.1989.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Digman JM. Personality Structure: Emergence of the Five-Factor Model. Annual Review of Psychology. 1990;41(1):417–40. doi: 10.1146/annurev.ps.41.020190.002221. [DOI] [Google Scholar]
- Eaves Lindon, Heath Andrew, Martin Nicholas, Maes Hermine, Neale Michael, Kendler Kenneth, Kirk Katherine, Corey Linda, et al. Comparing the biological and cultural inheritance of personality and social attitudes in the Virginia 30 000 study of twins and their relatives. Twin Research and Human Genetics. 1999;2(02):62–80. doi: 10.1375/twin.2.2.62. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. The Scientific Study of Personality. British Journal of Statistical Psychology. 1953;6(1):44–52. doi: 10.1111/j.2044-8317.1953.tb00132.x. [DOI] [Google Scholar]
- Garg Shruti, Green Jonathan, Leadbitter Kathy, Emsley Richard, Lehtonen Annukka, Evans D Gareth, Huson Susan M, et al. Neurofibromatosis Type 1 and Autism Spectrum Disorder. Pediatrics. 2013;132(6):e1642–48. doi: 10.1542/peds.2013-1868. [DOI] [PubMed] [Google Scholar]
- Garg Shruti, Lehtonen Annukka, Huson Susan M, Emsley Richard, Trump Dorothy, Evans D Gareth, Green Jonathan, et al. Autism and Other Psychiatric Comorbidity in Neurofibromatosis Type 1: Evidence from a Population-Based Study. Developmental Medicine and Child Neurology. 2013;55(2):139–45. doi: 10.1111/dmcn.12043. [DOI] [PubMed] [Google Scholar]
- Garg Shruti, Plasschaert Ellen, Descheemaeker Mie-Jef, Huson Susan, Borghgraef Martine, Vogels Annick, Evans D Gareth, Legius Eric, Green Jonathan, et al. Autism Spectrum Disorder Profile in Neurofibromatosis Type I. Journal of Autism and Developmental Disorders. 2015;45(6):1649–57. doi: 10.1007/s10803-014-2321-5. [DOI] [PubMed] [Google Scholar]
- Goldberg Lewis R. An alternative “description of personality”: The Big-Five factor structure. Journal of Personality and Social Psychology. 1990;59(6):1216–29. doi: 10.1037/0022-3514.59.6.1216. [DOI] [PubMed] [Google Scholar]
- Gosch Angela, Pankau Rainer, et al. Personality Characteristics and Behaviour Problems in Individuals of Different Ages with Williams Syndrome. Developmental Medicine & Child Neurology. 1997;39(8):527–33. doi: 10.1111/j.1469-8749.1997.tb07481.x. [DOI] [PubMed] [Google Scholar]
- Hérault J, Perrot A, Barthélémy C, Büchler M, Cherpi C, Leboyer M, Sauvage D, Lelord G, Mallet J, Müh JP, et al. Possible Association of c-Harvey-Ras-1 (HRAS-1) Marker with Autism. Psychiatry Research. 1993;46(3):261–67. doi: 10.1016/0165-1781(93)90094-w. [DOI] [PubMed] [Google Scholar]
- Hyman Shelley L, Shores Arthur, North Kathryn N, et al. The Nature and Frequency of Cognitive Deficits in Children with Neurofibromatosis Type 1. Neurology. 2005;65(7):1037–44. doi: 10.1212/01.wnl.0000179303.72345.ce. [DOI] [PubMed] [Google Scholar]
- Ibáñez Manuel I, Viruela Ana M, Mezquita Laura, Moya Jorge, Villa Helena, Camacho Laura, Ortet Generós, et al. An Investigation of Five Types of Personality Trait Continuity: A Two-Wave Longitudinal Study of Spanish Adolescents from Age 12 to Age 15. Frontiers in Psychology. 2016;7:512. doi: 10.3389/fpsyg.2016.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James Lucy, Fox Claire, et al. The development of a humor styles questionnaire for younger children. HUMOR. 2016;29(4):555–582. doi: 10.1515/humor-2016-0042. [DOI] [Google Scholar]
- Jang Kerry L, Livesley W John, Vemon Philip A, et al. Heritability of the Big Five Personality Dimensions and Their Facets: A Twin Study. Journal of Personality. 1996;64(3):577–92. doi: 10.1111/j.1467-6494.1996.tb00522.x. [DOI] [PubMed] [Google Scholar]
- Kawame Hiroshi, Matsui Mihoko, Kurosawa Kenji, Matsuo Mari, Masuno Mitsuo, Ohashi Hirofumi, Fueki Noboru, et al. Further Delineation of the Behavioral and Neurologic Features in Costello Syndrome. American Journal of Medical Genetics. Part A. 2003;118A(1):8–14. doi: 10.1002/ajmg.a.10236. [DOI] [PubMed] [Google Scholar]
- Kim H-N, Kim B-H, Cho J, Ryu S, Shin H, Sung J, Shin C, et al. Pathway Analysis of Genome-Wide Association Datasets of Personality Traits. Genes, Brain and Behavior. 2015;14(4):345–56. doi: 10.1111/gbb.12212. [DOI] [PubMed] [Google Scholar]
- Klein-Tasman Bonita P, Mervis Carolyn B, et al. Distinctive Personality Characteristics of 8-, 9-, and 10-Year-Olds with Williams Syndrome. Developmental Neuropsychology. 2003;23(1–2):269–90. doi: 10.1080/87565641.2003.9651895. [DOI] [PubMed] [Google Scholar]
- Koolen DA, Sharp AJ, Hurst JA, Firth HV, Knight SJL, Goldenberg A, Saugier-Veber P, et al. Clinical and Molecular Delineation of the 17q21.31 Microdeletion Syndrome. Journal of Medical Genetics. 2008;45(11):710–20. doi: 10.1136/jmg.2008.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolen David A, Kramer Jamie M, Neveling Kornelia, Nillesen Willy M, Moore-Barton Heather L, Elmslie Frances V, Toutain Annick, et al. Mutations in the Chromatin Modifier Gene KANSL1 Cause the 17q21.31 Microdeletion Syndrome. Nature Genetics. 2012;44(6):639–41. doi: 10.1038/ng.2262. [DOI] [PubMed] [Google Scholar]
- Lake RI, Eaves LJ, Maes HH, Heath AC, Martin NG, et al. Further Evidence against the Environmental Transmission of Individual Differences in Neuroticism from a Collaborative Study of 45,850 Twins and Relatives on Two Continents. Behavior Genetics. 2000;30(3):223–33. doi: 10.1023/a:1001918408984. [DOI] [PubMed] [Google Scholar]
- Lammert Marga, Friedman Jan M, Kluwe Lan, Mautner Victor F, et al. Prevalence of Neurofibromatosis 1 in German Children at Elementary School Enrollment. Archives of Dermatology. 2005;141(1):71–74. doi: 10.1001/archderm.141.1.71. [DOI] [PubMed] [Google Scholar]
- Lepri Francesca, De Luca Alessandro, Stella Lorenzo, Rossi Cesare, Baldassarre Giuseppina, Pantaleoni Francesca, Cordeddu Viviana, et al. SOS1 Mutations in Noonan Syndrome: Molecular Spectrum, Structural Insights on Pathogenic Effects, and Genotype-Phenotype Correlations. Human Mutation. 2011;32(7):760–72. doi: 10.1002/humu.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt Pat, Campbell Daniel B, et al. The Genetic and Neurobiologic Compass Points toward Common Signaling Dysfunctions in Autism Spectrum Disorders. The Journal of Clinical Investigation. 2009;119(4):747–54. doi: 10.1172/JCI37934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Min-Tzu, Hinds David A, Tung Joyce Y, Franz Carol, Fan Chun-Chieh, Wang Yunpeng, Smeland Olav B, et al. Genome-Wide Analyses for Personality Traits Identify Six Genomic Loci and Show Correlations with Psychiatric Disorders. Nature Genetics. 2017;49(1):152–56. doi: 10.1038/ng.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough E, Rodgers J, Janes E, Little K, Riby DM, et al. Parent Insights into Atypicalities of Social Approach Behaviour in Williams Syndrome. Journal of Intellectual Disability Research: JIDR. 2016 doi: 10.1111/jir.12279. avril. https://doi.org/10.1111/jir.12279. [DOI] [PubMed]
- Magan Dipti, Mehta Manju, Sarvottam Kumar, Yadav Raj Kumar, Pandey RM, et al. Age and Gender Might Influence Big Five Factors of Personality: A Preliminary Report in Indian Population. Indian Journal of Physiology and Pharmacology. 2014;58(4):381–88. [PubMed] [Google Scholar]
- Martin N, Goodwin G, Fairburn C, Wilson R, Allison D, Cardon LR, Flint J, et al. A Population-Based Study of Personality in 34,000 Sib-Pairs. Twin Research: The Official Journal of the International Society for Twin Studies. 2000;3(4):310–15. [PubMed] [Google Scholar]
- Martin Rod A, Puhlik-Doris Patricia, Larsen Gwen, Gray Jeanette, Weir Kelly, et al. Individual differences in uses of humor and their relation to psychological well-being: Development of the Humor Styles Questionnaire. Journal of Research in Personality. 2003;37(1):48–75. doi: 10.1016/S0092-6566(02)00534-2. [DOI] [Google Scholar]
- Martinelli Simone, De Luca Alessandro, Stellacci Emilia, Rossi Cesare, Checquolo Saula, Lepri Francesca, Caputo Viviana, et al. Heterozygous Germline Mutations in the CBL Tumor-Suppressor Gene Cause a Noonan Syndrome-like Phenotype. The American Journal of Human Genetics. 2010;87(2):250–57. doi: 10.1016/j.ajhg.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash EJ, Johnston C, et al. Parental Perceptions of Child Behavior Problems, Parenting Self-Esteem, and Mothers’ Reported Stress in Younger and Older Hyperactive and Normal Children. Journal of Consulting and Clinical Psychology. 1983;51(1):86–99. doi: 10.1037//0022-006x.51.1.86. [DOI] [PubMed] [Google Scholar]
- Mendiburo-Seguel Andrés, Páez Darío, Martínez-Sánchez Francisco, et al. Humor Styles and Personality: A Meta-Analysis of the Relation between Humor Styles and the Big Five Personality Traits. Scandinavian Journal of Psychology. 2015;56(3):335–40. doi: 10.1111/sjop.12209. [DOI] [PubMed] [Google Scholar]
- Moor Marleen HM de, Costa Paul T, Terracciano Antonio, Krueger Robert F, de Geus Eco JC, Toshiko Tanaka, Penninx Brenda WJH, et al. Meta-analysis of genome-wide association studies for personality. Molecular psychiatry. 2012;17(3):337–49. doi: 10.1038/mp.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris Stephanie M, Acosta Maria T, Garg Shruti, Green Jonathan, Huson Susan, Legius Eric, North Kathryn N, et al. Disease Burden and Symptom Structure of Autism in Neurofibromatosis Type 1: A Study of the International NF1-ASD Consortium Team (INFACT) JAMA Psychiatry. 2016 doi: 10.1001/jamapsychiatry.2016.2600. octobre. https://doi.org/10.1001/jamapsychiatry.2016.2600. [DOI] [PMC free article] [PubMed]
- Muris Peter, Meesters Cor, Diederen Rufa, et al. Psychometric properties of the Big Five Questionnaire for Children (BFQ-C) in a Dutch sample of young adolescents. Personality and Individual Differences. 2005;38(8):1757–69. doi: 10.1016/j.paid.2004.11.018. [DOI] [Google Scholar]
- Ng Rowena, Järvinen Anna, Bellugi Ursula, et al. Toward a Deeper Characterization of the Social Phenotype of Williams Syndrome: The Association between Personality and Social Drive. Research in Developmental Disabilities. 2014;35(8):1838–49. doi: 10.1016/j.ridd.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niihori Tetsuya, Aoki Yoko, Narumi Yoko, Neri Giovanni, Cavé Hélène, Verloes Alain, Okamoto Nobuhiko, et al. Germline KRAS and BRAF Mutations in Cardio-Facio-Cutaneous Syndrome. Nature Genetics. 2006;38(3):294–96. doi: 10.1038/ng1749. [DOI] [PubMed] [Google Scholar]
- Norman Warren T. 2800 Personality Trait Descriptors: Normative Operating Characteristics for a University Population. University of Michigan, Department of Psychology; 1967. [Google Scholar]
- Pinto Dalila, Pagnamenta Alistair T, Klei Lambertus, Anney Richard, Merico Daniele, Regan Regina, Conroy Judith, et al. Functional Impact of Global Rare Copy Number Variation in Autism Spectrum Disorder. Nature. 2010;466(7304):368–72. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasschaert Ellen, Descheemaeker Mie-Jef, Van Eylen Lien, Noens Ilse, Steyaert Jean, Legius Eric, et al. Prevalence of Autism Spectrum Disorder Symptoms in Children with Neurofibromatosis Type 1. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2015;168B(1):72–80. doi: 10.1002/ajmg.b.32280. [DOI] [PubMed] [Google Scholar]
- Power RA, Pluess M, et al. Heritability Estimates of the Big Five Personality Traits Based on Common Genetic Variants. Translational Psychiatry. 2015;5(7):e604. doi: 10.1038/tp.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinzie P, Descheemaeker MJ, Vogels A, Cleymans T, Haselager GJT, Curfs LMG, Hellinckx W, et al. Personality Profiles of Children and Adolescents with Neurofibromatosis Type 1. American Journal of Medical Genetics. Part A. 2003;118A(1):1–7. doi: 10.1002/ajmg.a.10003. [DOI] [PubMed] [Google Scholar]
- Rauen Katherine A. The RASopathies. Annual review of genomics and human genetics. 2013;14:355–69. doi: 10.1146/annurev-genom-091212-153523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Viciana Pablo, Tetsu Osamu, Tidyman William E, Estep Anne L, Conger Brenda A, Cruz Molly Santa, McCormick Frank, Rauen Katherine A, et al. Germline Mutations in Genes within the MAPK Pathway Cause Cardio-Facio-Cutaneous Syndrome. Science (New York, N.Y.) 2006;311(5765):1287–90. doi: 10.1126/science.1124642. [DOI] [PubMed] [Google Scholar]
- Ruch Willibald. The Sense of Humor: Explorations of a Personality Characteristic. Walter de Gruyter; 2007. [Google Scholar]
- Samuelsson B, Riccardi VM, et al. Neurofibromatosis in Gothenburg, Sweden. III. Psychiatric and Social Aspects. Neurofibromatosis. 1989;2(2):84–106. [PubMed] [Google Scholar]
- Segel-Karpas Dikla, Lachman Margie E, et al. Social Contact and Cognitive Functioning: The Role of Personality. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2016 doi: 10.1093/geronb/gbw079. août. https://doi.org/10.1093/geronb/gbw079. [DOI] [PMC free article] [PubMed]
- Tartaglia M, Zampino G, Gelb BD, et al. Noonan Syndrome: Clinical Aspects and Molecular Pathogenesis. Molecular Syndromology. 2010;1(1):2–26. doi: 10.1159/000276766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano Antonio, Sanna Serena, Uda Manuela, Deiana Barbara, Usala Gianluca, Busonero Fabio, Maschio Andrea, et al. Genome-wide association scan for five major dimensions of personality. Molecular psychiatry. 2010;15(6):647–56. doi: 10.1038/mp.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidyman William E, Rauen Katherine A, et al. Pathogenetics of the RASopathies. Human Molecular Genetics. 2016 doi: 10.1093/hmg/ddw191. juillet, ddw191. https://doi.org/10.1093/hmg/ddw191. [DOI] [PMC free article] [PubMed]
- Vernon Philip A, Martin Rod A, Schermer Julie Aitken, Mackie Ashley, et al. A behavioral genetic investigation of humor styles and their correlations with the Big-5 personality dimensions. Personality and Individual Differences. 2008;44(5):1116–25. doi: 10.1016/j.paid.2007.11.003. [DOI] [Google Scholar]
- Vinkhuyzen AaE, Pedersen NL, Yang J, Lee SH, Magnusson PKE, Iacono WG, McGue M, et al. Common SNPs Explain Some of the Variation in the Personality Dimensions of Neuroticism and Extraversion. Translational Psychiatry. 2012;2(4):e102. doi: 10.1038/tp.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukasović Tena, Bratko Denis, et al. Heritability of Personality: A Meta-Analysis of Behavior Genetic Studies. Psychological Bulletin. 2015;141(4):769–85. doi: 10.1037/bul0000017. [DOI] [PubMed] [Google Scholar]
- Waiyavutti Chakadee, Johnson Wendy, Deary Ian J, et al. Do Personality Scale Items Function Differently in People with High and Low IQ? Psychological Assessment. 2012;24(3):545–55. doi: 10.1037/a0026266. [DOI] [PubMed] [Google Scholar]
- Walsh Karin S, Vélez Jorge I, Kardel Peter G, Imas Daniel M, Muenke Maximilian, Packer Roger J, Castellanos Francisco X, Acosta Maria T, et al. Symptomatology of Autism Spectrum Disorder in a Population with Neurofibromatosis Type 1. Developmental Medicine and Child Neurology. 2013;55(2):131–38. doi: 10.1111/dmcn.12038. [DOI] [PubMed] [Google Scholar]
- Wang Daphne L, Smith Kelly B, Esparza Sonia, Leigh Fawn A, Muzikansky Alona, Park Elyse R, Plotkin Scott R, et al. Emotional Functioning of Patients with Neurofibromatosis Tumor Suppressor Syndrome. Genetics in Medicine: Official Journal of the American College of Medical Genetics. 2012;14(12):977–82. doi: 10.1038/gim.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Ya, Alshikho Mohamad J, Herbert Martha R, et al. Pathway Network Analyses for Autism Reveal Multisystem Involvement, Major Overlaps with Other Diseases and Convergence upon MAPK and Calcium Signaling. PLoS ONE. 2016;11(4) doi: 10.1371/journal.pone.0153329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widiger TA, Costa PT, et al. Personality and Personality Disorders. Journal of Abnormal Psychology. 1994;103(1):78–91. doi: 10.1037//0021-843x.103.1.78. [DOI] [PubMed] [Google Scholar]
- Widiger Thomas A, Costa Paul T, et al. Integrating Normal and Abnormal Personality Structure: The Five-Factor Model. Journal of Personality. 2012;80(6):1471–1506. doi: 10.1111/j.1467-6494.2012.00776.x. [DOI] [PubMed] [Google Scholar]
- Wingbermuehle Ellen, Egger Jos, van der Burgt Ineke, Verhoeven Willem, et al. Neuropsychological and Behavioral Aspects of Noonan Syndrome. Hormone Research. 2009;72(Suppl 2):15–23. doi: 10.1159/000243774. (décembre): https://doi.org/10.1159/000243774. [DOI] [PubMed] [Google Scholar]
- Wingbermühle E, Roelofs RL, van der Burgt I, Souren PM, Verhoeven WMA, Kessels RPC, Egger JIM, et al. Cognitive Functioning of Adults with Noonan Syndrome: A Case-Control Study. Genes, Brain, and Behavior. 2012;11(7):785–93. doi: 10.1111/j.1601-183X.2012.00821.x. [DOI] [PubMed] [Google Scholar]
- Zöller ME, Rembeck B, et al. A Psychiatric 12-Year Follow-up of Adult Patients with Neurofibromatosis Type 1. Journal of Psychiatric Research. 1999;33(1):63–68. doi: 10.1016/s0022-3956(98)00052-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


