Abstract
Advances in therapies have led to prolonged survival from many previously lethal health threats in children, notably among prematurely born babies and those with congenital heart disease. Evidence for catch‐up growth is common in these children, but in many cases the adult phenotype is never achieved. A translational animal model is required in which specific tissues can be studied over a reasonable time interval. We investigated the impact of postnatal hypoxia (HY) (12%O2 (HY12) or 10% O2 (HY10)) on growth in rats relative to animals raised in room air. Subgroups had access to running wheels following the HY period. Growth was fully compensated in adult HY12 rats but not HY10 rats. The results of this study indicate that neonatal hypoxia can be a useful model for the elucidation of mechanisms that mediate successful catch‐up growth following neonatal insults and identify the critical factors that prevent successful catch‐up growth.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓ Many gaps still exist in our understanding of the mechanisms of catch‐up and/or compensatory growth,28 despite their clear importance in determining the long‐term consequences of chronic childhood diseases. This gap in knowledge is a major obstacle to clinicians attempting to find useful biomarkers of catch‐up growth and, eventually, to develop novel therapies and interventions.
WHAT QUESTION DID THE STUDY ADDRESS?
✓ This study discussed the development of a translational animal model to enable adequate exploration of the molecular pathways involved in successful and unsuccessful catch‐up growth.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
✓ Our study identifies a degree and duration of early‐life hypoxia exposure which attenuates catch‐up growth leading to an impaired adult phenotype.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE
✓ Finding the distinguishing molecular pathways in muscle and bone that differentiate the 10% from the 12% hypoxia exposure may lead to novel insights into the mechanisms that control the general process of compensatory growth and are likely to have clinical relevance for children with chronic disease and disability.
The periods of rapid growth in humans (postnatal and through the pubertal growth spurt) are acutely sensitive to relatively brief and even acute episodes of disease or deleterious environment perturbations such as hypoxia, imbalance of energy intake and energy expenditure, medications, or severe psychosocial stress.1, 2, 3, 4 Growth rates under these conditions can be slowed or stopped completely. When the perturbation ceases, growth rates can resume at an accelerated pace, constituting the phenomenon of catch‐up growth.5 Advances in therapies have led to prolonged survival from many previously lethal health threats in children, notably among prematurely born babies and those with congenital heart disease. Evidence for catch‐up growth is common in these children once the hypoxia (either from primary lung diseases or impaired tissue delivery of oxygen) is ameliorated, but in many cases the adult phenotype is never achieved.6, 7, 8, 9
Essential mechanisms (including as basic a factor as sex) that govern catch‐up growth are still poorly understood, nor is it clear why catch‐up growth in some cases leads to a largely normal adult phenotype, while in others, the catch‐up growth is incomplete. This gap in knowledge is a major obstacle to clinicians attempting to find useful biomarkers of catch‐up growth and, eventually, to develop novel therapies and interventions. To adequately explore the molecular pathways involved in successful and unsuccessful catch‐up growth, a translational animal model is required in which specific tissues can be studied over a reasonable time interval, and few such models exist. We report here the development of a translational animal model in which a relatively brief, early‐life hypoxia exposure inhibited growth acutely and was sufficiently robust such that subsequent catch‐up growth after the hypoxia exposure was terminated was incomplete, resulting in an abnormal adult phenotype.
Energy balance, quantified by the equation linking energy intake to energy expenditure, is a key factor in growth during infancy and childhood. Reduced energy intake (caloric deprivation) has been a typical model of growth impairment in experimental models.10 Less focus has been placed on the other side of the energy balance equation, namely, energy expenditure, and there are increasing data that an imbalance in the energy equation whether by caloric restriction or by excessive energy expenditure can alter metabolism and growth factors such as insulin‐like growth factor 1 (IGF‐1).11
The ability of physical activity to stimulate growth in a variety of tissues has led to the hypothesis that exercise could mitigate some of the growth effects seen in children with chronic diseases.12 But exercise involves increased energy expenditure and at what point the beneficial anabolic effects of exercise are diminished by an imbalance of energy expenditure and intake remains poorly understood, particularly in the context of the accelerated growth rate that is key to the catch‐up growth phenomenon. The translational rat model, through experimental manipulation of physical activity and exercise, also permitted us to examine the impact of varying levels of physical activity on the effectiveness of catch‐up growth. Children are the most naturally physical active human beings,13 and the degree to which a growth‐impaired child (e.g., those suffering from diseases like chronic lung disease or survivors of childhood cancer) can and should participate in exercise programs remains a major conundrum for parents and healthcare providers. Accordingly, we included a study arm designed to investigate the longer‐term impact of exercise on the effectiveness of catch‐up growth following the acute, growth‐inhibiting, hypoxic exposure.
METHODS
The protocols used in this study were approved by the University of California, Irvine Institutional Animal Care and Use Committee. Pregnant Sprague–Dawley rats were purchased from Charles River (Wilmington, MA). Immediately postpartum, the litters were randomly cross‐fostered and sex‐balanced. At postpartum day 2, litters randomized to the hypoxia treatment groups (HY) were culled to four pups per litter and housed, with the dam, in standard cages placed in a normobaric chamber (Biospherix, Parish, NY). The small litter size was adopted to ensure adequate nutrition and minimize the maternal stress associated with full litter size in the hypoxic environment.14 The litters randomized to the normoxic room air (RA) control groups were also culled to four pups and housed in standard cages in the same room as the chamber; RA, n = 17 male, n = 18, female.
Hypoxia protocol
To induce hypoxia, a feedback controller senses O2 levels in the chamber and feeds N2 into the chamber to maintain the preset level of O2.15 The 12% hypoxia exposure was maintained from day 2 postpartum until day 21. The 10% protocol was imposed at day 2 and the animals returned to RA on day 12 to limit the mortality caused by this more stressful treatment. The chamber used in this study allows exchange with the environment so that pressure remains unchanged. Humidity and CO2 were monitored and regulated.
Animals in the 12% O2 (HY12) treatment groups were removed from the hypoxia chamber at day 21 (n = 26, male, n = 19, female). On day 22, all groups were weaned. At this timepoint the two groups: normoxic room air controls (RA) and HY12 were further subdivided into sedentary or exercise (Ex) cohorts. The exercise cohort groups were designated: RA + Ex and HY12 + Ex and had a final minimum n of 6 animals.
Rats randomized to the 10% O2 (HY10; n = 14, male, n = 14 female) treatment groups were removed from the chamber at day 12 and maintained, with the dam, in RA until weaning at day 22. At this time, the exercise subgroups (RA + Ex and HY10 + Ex, minimum n = 5/sex) were housed in the presence of running wheels.
Exercise protocol
Animals randomized to the exercise groups were housed in cages that included running wheels at weaning (∼day 22). Initially, the devices provided were mouse running wheels, which are easily accessed by young rats. These running wheels were not instrumented for data collection. At day 30 postpartum, the animals in the exercise groups were single housed in cages with running wheels suitable for young and adult rats. These devices were instrumented to allow for the quantitation of voluntary running activity. Running activity was quantified as daily running distance in km.
Verification of pulmonary hypertension
Following the preliminary data collection from the above groups, a persistent right ventricular hypertrophy was observed. We postulated that this was an indicator of pulmonary hypertension. To confirm this, an additional investigation arm was added to evaluate pulmonary artery pressure. Prior to sacrifice, pulmonary artery pressure was measured using a fluid‐filled catheter that was inserted into the pulmonary artery.16 Briefly, rats were anesthetized using ketamine/xylazine/acepromazine (50/4/1 mg/kg, respectively). The right anterior external jugular vein isolated and a Silastic catheter (0.64 mm OD, 0.32 mm ID) with introducer inserted into the right ventricle through the jugular vein while pressure was recorded using a Biopac system (Goleta, CA). The catheter was then manipulated into the pulmonary artery, which has a characteristic pressure waveform. The introducer was then removed, and the catheter sutured in place and exteriorized behind the neck. The catheter was flushed with 0.2 ml of heparin (1,000 U) and occluded with wire plugs. Pulmonary artery pressure data collection was completed 24 hours postsurgery in resting, awake rats. Rats from this study arm were not included in further analyses.
Tissue collection and analysis
The study was terminated on day 65 postpartum. The rats were euthanized using pentosol solution (100 mg/kg). All animals were weighed and body length measured. After the induction of deep anesthesia, but prior to the cessation of breathing, blood was collected from the left ventricle using cardiac puncture through the diaphragm using a heparinized syringe. The chest was then opened and the ventricles were removed and weighed. The soleus, plantaris, and medial gastrocnemius (MG) muscles of both legs were dissected free of connective tissue and weighed. The tibia of the left leg was dissected free and the length measured.
Determination of complete blood count and differential white blood count
On day 65 postpartum, whole blood was collected in 4 ml EDTA purple top tubes (Becton Dickinson, San Jose, CA) and processed on the Hemavet 950FS (Drew Scientific, Waterbury, CT) as per the manufacturers guidelines. The HemaVet 950FS Multi‐trol validation beads were used to perform a quality control and assure that the machine was ready for analysis of samples. Each animal complete blood count (CBC) with differential analysis was performed using the Rat measurement button on the HemaVet; all values reported are within the range of the quality control sheet.
Blood sampling and analysis
Aliquoted plasma was stored at −80°C until assayed. Circulating IGF‐1 and IGF‐Binding Protein 3 levels were measured using commercially available enzyme‐linked immunosorbent assay (ELISA) kits manufactured by R&D Systems (Minneapolis, MN).
Statistical analysis
Between‐group analysis was conducted using a one‐way analysis of variance (ANOVA) with Bonferroni's multiple comparisons test posttest (PRISM, Graphpad, San Diego, CA). Graphical representations of the data include standard error of the mean (SEM). Tabular data include mean ± standard error. For all statistical tests the significance level was set at 0.05. Exercise had no effect on measures of body size (mass, tibia length, body length). Accordingly, the exercise data were combined with that of the sedentary groups for most analyses.
RESULTS
Effects of early‐life hypoxia on attainment of adult phenotype
As we previously reported,15 exposure to hypoxia during the early postnatal period resulted in dramatic growth deficits. Figure 1 shows that at day 11 postpartum, rat pups exposed to 12% O2 were ∼26% smaller than the RA animals. The pups maintained at 10% O2 were 50% smaller than the RA controls at this timepoint. At 65 days of age, both the male and female rats that had been exposed to the 12% O2 environment had attained body mass similar to the RA animals (Figure 1). In contrast, the rats exposed to 10% O2 were significantly smaller (–23% male, –20% female) than the RA rats (Figure 1). In the HY10 animals, growth deficits were also evident in body length (–5% male, –7% female) and in the length of the tibia (–6% male, –8%) female (Table 1). The two control groups, RA12, raised along with the HY12, n = 36, and RA10, raised along with HY10, n = 36, were handled in every way the same. This was reflected in all of the data collected (i.e., no significant differences). As a result, the RA10 and RA12 group data were combined for data presentation.
Figure 1.
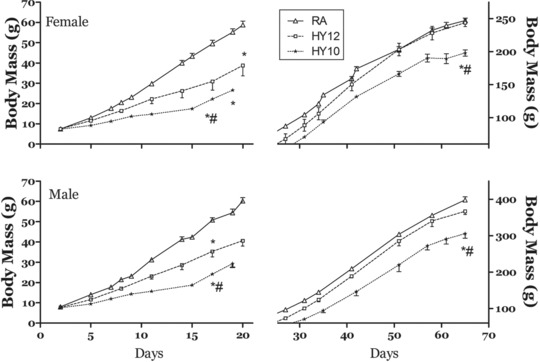
Growth curves demonstrating the effects of exposure to hypoxic (HY) conditions postpartum on rats. Rats exposed to 12% O2 (HY12) for 20 days exhibited successful catch‐up growth relative to normoxic rats raised in room air (RA). The animals exposed to 10% O2 (Hy10) for 10 days had more dramatic reductions in early growth and failed to attain full growth relative to the other groups. At 65 days of age, rats exposed to 12% O2 postpartum did not differ in body mass from the RA animals. The HY10 rats were significantly smaller. Data points are means ± SE. N = 6–18 for various groups. (*P < 0.05 vs. RA, #P < 0.05 vs. HY12).
Table 1.
Tibia length (mm) and body length (cm) at 65 days of age
| Tibia length (mm) | Body length (cm) | |||
|---|---|---|---|---|
| Group | Female | Male | Female | Male |
| RA | 33.3 ± 0.2 | 36.6 ± 0.2 | 21.0 ± 0.3 | 23.2 ± 0.1 |
| HY12 | 33.0 ± 0.3 | 36.8 ± 0.6 | 20.5 ± 0.1 | 22.8 ± 0.2 |
| HY10 | 30.7 ± 0.3a, b | 34.4 ± 0.5a, b | 19.5 ± 0.1a | 22.0 ± 0.2a, b |
Data are means ± SE.
P < 0.05 vs. RA.
P < 0.05 vs. HY12.
Effect of access to running wheels
As previously observed,17 female Sprague–Dawley rats with unlimited access to running wheels spontaneously exercise to a far greater degree than do male rats. In this study, the female rats as a group exercised 2.6‐fold more than the males (Figure 2). In both males and females, there was no significant difference in the amount of voluntary running between HY12 and RA groups. In the female rats exposed to 10% hypoxia, exercise was reduced by 20% (P < 0.05) compared with the female rats in the RA group. Voluntary running exercise had no effect on the magnitude of catch‐up growth; there were no exercise‐related differences in the adult phenotypes (e.g., measure of body size) among the rats exposed to early‐life hypoxia or controls.
Figure 2.
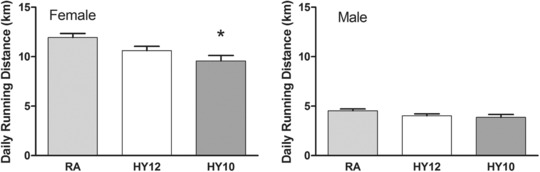
The mean daily voluntary running distances (km) of rats exposed to room air (RA) or hypoxia (HY12, HY10). (*P < 0.05 vs. RA). N = 6 RA/HY12, N = 5 HY10. Daily average from age 30 to 65 days.
There were distinct sex‐related effects of voluntary running. In female rats from both the hypoxia exposed and control environments, increased exercise led to a relative (muscle mass/body mass) increase in the mass of the plantaris muscles (PLN), in those most involved in wheel running. In the male animals, only the HY10 + Ex males showed greater PLN muscle mass than the nonexercised, RA controls (Table 2). These changes ranged from 12–17% greater PLN mass in the groups with access to running wheels. There were no significant differences in the magnitude of this change between treatments. There were no significant differences in the relative mass of the soleus or medial gastrocnemius muscles across sexes, groups, or treatments (data not shown).
Table 2.
Plantaris muscle mass normalized to body mass expressed as mg/g in male and female rats at 65 days of age
| Group | Female | Male |
|---|---|---|
| RA | 0.94 ± 0.01 | 0.86 ± 0.02 |
| HY12 | 0.93 ± 0.02 | 0.90 ± 0.02 |
| HY10 | 1.00 ± 0.01 | 0.96 ± 0.02 |
| RA‐Ex | 1.08 ± 0.04a, b | 0.96 ± 0.04 |
| HY12‐Ex | 1.10 ± 0.04a, b | 0.94 ± 0.03 |
| HY10‐Ex | 1.11 ± 0.05a, b | 1.01 ± 0.03a |
Data are means ± SE.
P < 0.05 vs. RA.
P < 0.05 vs. HY10 and HY12.
Effects on heart size and pulmonary artery pressure
As noted in our earlier publication,15 20‐day hypoxia exposure resulted in marked cardiac hypertrophy (RV ∼fivefold, LV ∼twofold) at 21 days of age. In the current study, we found that this RV adaptation persists into adulthood in both males and females, but to a lesser degree. For example, in females RV relative mass was 46% and 43% larger in HY12 and HY10, respectively, compared with RA (P < 0.05) (Figure 3). In females, the RV hypertrophy was exacerbated by voluntary running wheel exercise (Figure 3). The hypoxia treatment did not appear to have a substantial impact on LV mass in adult rats (Figure 4). Voluntary wheel running exercise did result in some LV hypertrophy, particularly in female rats (Figure 4); however, this appeared to be independent of the treatment history of the animals.
Figure 3.
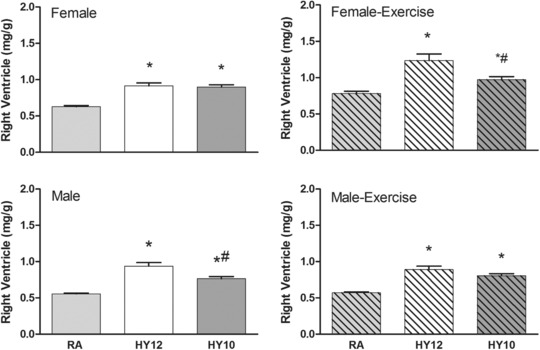
Effects of neonatal hypoxia on the relative mass (mg/g body) of the right ventricles in adulthood. Neonatal hypoxia resulted in persistent RV hypertrophy at 65 days of age in both sexes and both treatments. (*P < 0.05 vs. RA; #P < 0.05 vs. HY12). Bars are means ± SE, n = 5–8 rats/group.
Figure 4.
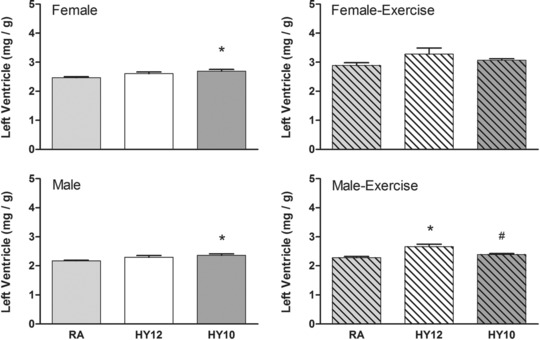
Effects of neonatal hypoxia on the relative mass (mg/g body) of the left ventricles in adulthood. Voluntary running exercise was associated with LV hypertrophy in all females and the HY12 males. (*P < 0.05 vs. RA; #P < 0.05 vs. HY12).
With regard to cardiovascular responses, there were no significant differences noted between treatment groups or sexes in the heart rates of awake, resting rats (data not shown). Both male and female rats exposed to hypoxia as neonates exhibited pulmonary hypertension as adults (e.g., for mean arterial pressure (MAP), female RA 18.4 ± 1.2; HY12 30.9 ± 0.5; HY10 29.7 ± 0.3 mmHg). Voluntary running exercise did not significantly increase or reduce the magnitude of the pulmonary arterial hypertension in either sex (e.g., female MAP HY‐Ex 29.9 ± 0.4 mmHg).
Effects on CBC, IGF‐1, and IGF‐1 BP‐3
None of the treatments had significant impacts on the constituent elements of the CBD. At 65 days of age, the circulating levels of IGF‐1 and IGF binding protein‐3 were similar in both the RA and HY groups (data not shown).
DISCUSSION
A translational rat model of incomplete catch‐up growth: Possible mechanisms
We succeeded in identifying a specific duration and level of hypoxia exposure perturbation early in life in which the catch‐up growth response occurred, but was incomplete long after normoxia was restored. In the bulk of chronic conditions that lead to impaired adult phenotype, the perturbation (e.g., nutritional, inflammatory, oxygen delivery) is imposed early in life but persists throughout the growth period.18, 19 In our model, the growth‐impairing stimulus, hypoxia, occurred only for a short period early in life and the animals then grew in a normoxic environment. Despite the rapid initial phase of compensatory growth upon return to room air seen in both groups (Figure 1), animals exposed to 10% O2 during the immediate neonatal period failed to attain the adult body size phenotype observed in the controls (Figure 1, Table 1). This animal model of incomplete catch‐up growth following an acute, postnatal exposure to hypoxia may prove useful in gaining insight into the molecular pathways that govern the still incompletely understood phenomenon of catch‐up and compensatory growth.
We anticipated that the incomplete catch‐up growth would have been accompanied by lower levels of circulating IGF‐1. This was not the case. In both rodent and human studies, growth rates and circulating IGF‐1 are highly correlated.20, 21 In previous studies, our group showed that both hypoxia and reduced caloric intake independently led to impaired growth and reduced circulating IGF‐1 in young rats,15 but after the hypoxia exposure, compensatory growth was complete and circulating IGF‐1 in the adult animals was no different from controls.
In humans, constitutive short stature is a complex and heterogeneous group of syndromes.22, 23 It is found in children with both low and normal IGF‐1, the latter categorized as “IGF‐1 resistant,” in contrast to mechanisms in which the growth hormone→IGF‐1 axis is impaired, leading to reduced growth with abnormalities in circulating IGF‐1. Baron et al. in a recent comprehensive review23 outlined the mechanisms (both related and unrelated to the classic GH→IGF‐1) axis that could play a role in impaired growth through genetic, genomic, and epigenetic effects on the bone growth plate. In our rat model, in addition to body weight, both body length and tibial length were reduced in all rats exposed to the HY10 protocol, suggesting, in fact, that the long‐term effect of the early‐life hypoxia exposure had specifically impaired the bone growth plate.
We reviewed the current literature to identify which of the signaling pathways identified by Baron et al. were also sensitive to hypoxia. As shown in Table 3, we found that many of the known regulatory pathways were sensitive to hypoxia exposure through specific genes. This framework may ultimately prove useful in uncovering the mechanisms responsible for the long‐term effects of early‐life hypoxia on subsequent growth.
Table 3.
Examples of hypoxia sensitive genes in signaling and hormonal pathways identified recently by Baron et al.23 to play essential roles in bone growth
| Mechanism | Hypoxia‐sensitive gene | Effect |
|---|---|---|
| CNP signaling | NPR2 | VEGFA receptor NPR2 plays a role in chondrocyte development and seems to be related to hypoxia‐inducible growth factor‐α43 |
| FGF signaling | FGFR3 | Hypoxia induces FGFR3 signaling.44 FGFR3 plays a role in human achondroplasia45 |
| Cartilage matrix | ACAN | Hypoxia upregulates ACAN, a member of the aggrecan/versican proteoglycan family. The encoded protein is an integral part of the extracellular matrix in cartilagenous tissue46 |
| Transcription | SOX9 | Hypoxia increases nuclear‐expressed MIF (macrophage migration inhibitory factor) which acts as a transcriptional regulator by interacting with the promoter of SOX9.47 MIF plays a role in fracture healing48 |
| Epigenetic control | DNMT3A | Hypoxia mimetic deferoxamine influences expression of histone acetylation‐ and DNA methylation‐associated genes in osteoblasts49 |
| Thyroid hormone signaling | PAX8 | This gene plays a role in the mechanisms through which thyroid hormones modulate bone growth50 |
Effect of physical activity
Female rats exhibited much greater volumes of spontaneous running than did males (Figure 2); thus, to the extent that the training effect is related to the amount of exercise, the females would be expected to have a greater response than the males. Consistent with this was the observation that the relative size of the plantaris muscle was greater in all of the female groups (both the two hypoxia groups and the controls) that had access to running wheels compared with the female rats that did not have running wheel access (Table 2). Daily running distance was smaller in the HY10 group of females, but this did not seem to influence the effect of the generally increased running on plantaris muscle mass. An interesting but as yet unexplained finding was that in the male rats that had access to running wheels, only those exposed to 10% hypoxia demonstrated a relative increase in the plantaris muscle mass. We observed this despite the fact that the daily running distance in males was much less than that observed in the females, and no exercise effect was found in the 12% hypoxia or room air groups. In none of the groups was increased exercise associated with reduced growth. In fact, exercise had no effect on measures of body size (mass, tibia length, and body length).
Persistent effects of early‐life hypoxia exposure
In addition to the sustained impact of early‐life 10% hypoxia exposure leading to incomplete catch‐up growth, there was an additional persistent effect that we observed in both the 10% and 12% hypoxia exposed animals. RV hypertrophy, presumably resulting from hypoxia induced pulmonary vasoconstriction24 leading to persistent pulmonary hypertension, continued into adulthood (Figure 3). It is well established that hypoxia exposure in the absence of inflammatory lung disease in neonatal rats rapidly leads to RV hypertrophy.15, 25 The current study shows the ominous persistence of this hypertrophy and of pulmonary hypertension into adulthood, long after the initial exposure had ended. Our results extend those of Keith et al.26 in 2000 (RV hypertrophy and pulmonary hypertension) and Lumbroso and Joseph27 in 2009 (RV hypertrophy) who reported on antenatal hypoxia exposure in the pregnant dam. Collectively, these studies showed that abnormalities persist later in life after this early‐life hypoxia exposure was ended. In the 12% hypoxia‐exposed animals, there appeared to be no other sustained effects of the early‐life hypoxia as evidenced by those rats achieving the same adult body mass as did the control animals. Growth is impaired in children with persistent pulmonary hypertension, particularly when it occurs in younger children and accompanying congenital heart disease.7
Exercise did not worsen the degree of pulmonary hypertension, but did result in a generalized cardiac hypertrophy, most notably, in the females with high exercise volumes, affecting the size of both the right and left ventricles.
Summary and relevance to clinical translation science
Many gaps still exist in our understanding of the mechanisms of catch‐up and/or compensatory growth28 despite their clear importance in determining the long‐term consequences of chronic childhood diseases. The traditional research interest in compensatory growth has focused on malnutrition and endocrine disorders.29 However, there is a growing body of research on the long‐term growth effects of hypoxia spurred, in part, by the increasing number of premature babies that survive chronic lung disease of prematurity, and by the recognition that disease or deleterious environmental factors (like hypoxia) occurring during critical periods of growth and development will impact health across the lifespan.30, 31 Earlier theories of whole‐organism, homeostatically controlled regulation of growth32 have, under the weight of experimental evidence given way to a (still rudimentary) model of compensatory growth in which tissue‐ and organ‐specific proliferative capacities, rather than some whole‐body set points, ultimately control the compensatory response to growth‐inhibiting diseases or noxious stimuli.
Our data suggest that prior hypoxia exposure did not inhibit the ability of physical activity to stimulate skeletal muscle hypertrophy in rats that were allowed to run. Moreover, although access to running wheels was associated with increased left and right ventricular size, there was no worsening of the pulmonary hypertension that began with the hypoxia exposure much earlier in life.
From a clinical translational perspective, the long‐term effects of early‐life exposures are particularly associated with conditions that impair oxygen flow and availability to the tissues such as congenital heart disease,33, 34 childhood lung disease,35 blood diatheses (e.g., sickle‐cell anemia),36 and lung disease of prematurity.37 We found that a relatively well‐tolerated level of hypoxia exposure early in life caused persistent effects in adulthood long after the hypoxia exposure was terminated. Although interspecies comparisons are never perfect, the levels of hypoxia likely induced by our exposure to FiO2 of 10% can be extrapolated to the care of neonates. Premature babies are the most likely to suffer prolonged periods of hypoxemia. Although data from neonatal rats are sparse, studies in adult rats suggest that our model would have led to an oxygen saturation of 65–70%.38, 39 These levels are certainly well below the therapeutic O2 saturation (i.e., between 85–95%) currently used in many neonatal intensive care units.40 Moreover, hypoxemia at this level can result in long‐term injury to the brain in the rat.41 The latter, through effects, perhaps, on the hypothalamic pituitary axis, could contribute to the growth abnormalities we observed.
Our study identifies a degree and duration of early‐life hypoxia exposure that attenuates catch‐up growth leading to an impaired adult phenotype. Finding the distinguishing molecular pathways in muscle and bone that differentiate the 10% from the 12% hypoxia exposure may lead to novel insights into the mechanisms that control the general process of compensatory growth and are likely to have clinical relevance for children with chronic disease and disability. Targeting molecular pathways that are known to be particularly responsive to hypoxia and involved broadly in metabolism and growth (e.g., hypoxia‐inducible factor42) is likely to generate mechanistic information using our model in future studies.
Conflict of Interest
The authors declare no competing interests for this work.
Acknowledgment
The authors thank Cherryl Nugas and Paul Bodell for technical assistance.
Funding
This work was supported in part by NIH ‐ 2P01HD048721, and the UCI Institute for Clinical and Translational Science (CTSA grant) UL1 TR001414.
Author Contributions
S.R.‐A., D.M.C., and G.R.A. wrote the article; S.R.‐A., D.M.C., and G.R.A. designed the research; F.P.Z., D.M.N., F.H. and G.R.A. performed the research; S.R.‐A., F.P.Z., F.H. and G.R.A. analyzed the data.
References
- 1. Moromisato, D.Y. , Moromisato, M.Y. , Brasel, J.A. & Cooper, D.M. Effect of growth hormone therapy in mitigating hypoxia‐induced and food restriction‐induced growth retardation in the newborn rat. Crit. Care Med. 27, 2234–2238 (1999). [DOI] [PubMed] [Google Scholar]
- 2. Ahmed, S.F. & Savendahl, L. Promoting growth in chronic inflammatory disease: Lessons from studies of the growth plate. Horm. Res. 72(Suppl 1), 42–47 (2009). [DOI] [PubMed] [Google Scholar]
- 3. Munoz‐Hoyos, A. et al Psychosocial dwarfism: Psychopathological aspects and putative neuroendocrine markers. Psychiatry Res. 188, 96–101 (2011). [DOI] [PubMed] [Google Scholar]
- 4. Perkins, J.M. , Subramanian, S.V. , Davey Smith, G. & Ozaltin, E. Adult height, nutrition, and population health. Nutr. Rev. 74, 149–165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stevens, A. et al Insights into the pathophysiology of catch‐up compared with non‐catch‐up growth in children born small for gestational age: An integrated analysis of metabolic and transcriptomic data. Pharmacogenomics J. 14, 376–384 (2014). [DOI] [PubMed] [Google Scholar]
- 6. Wood, N.S. et al The EPICure study: Growth and associated problems in children born at 25 weeks of gestational age or less. Arch. Dis. Child. Fetal Neonatal Ed. 88, F492–F500 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ploegstra, M.J. et al Growth in children with pulmonary arterial hypertension: A longitudinal retrospective multiregistry study. Lancet Respir. Med. 4, 281–290 (2016). [DOI] [PubMed] [Google Scholar]
- 8. Begić, H. & Tahirović, H. The impact of delayed cardiac surgery on the postnatal growth of children with congenital heart disease in Bosnia and Herzegovina. Coll. Antropol. 37, 507–513 (2013). [PubMed] [Google Scholar]
- 9. Hasan, B.S. et al Somatic growth after fontan and mustard palliation. Congen. Heart Dis. 3, 330–335 (2008). [DOI] [PubMed] [Google Scholar]
- 10. Pando, R. et al Bone quality is affected by food restriction and by nutrition‐induced catch‐up growth. J. Endocrinol. 223, 227–239 (2014). [DOI] [PubMed] [Google Scholar]
- 11. Nemet, D. et al Negative energy balance plays a major role in the IGF‐I response to exercise training. J. Appl. Physiol. 96, 276–282 (2004). [DOI] [PubMed] [Google Scholar]
- 12. Stalnaker, K.A. & Poskey, G.A. Osteopenia of prematurity: Does physical activity improve bone mineralization in preterm infants? Neonatal Netw. 35, 95–104 (2016). [DOI] [PubMed] [Google Scholar]
- 13. Mielgo‐Ayuso, J. et al Physical activity patterns of the Spanish population are mostly determined by sex and age: Findings in the ANIBES study. PLoS One 11, e0149969 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Del Duca, D. et al Association of neonatal hypoxia with lasting changes in left ventricular gene expression: An animal model. J. Thorac. Cardiovasc. Surg. 138, 538–546 (2009). [DOI] [PubMed] [Google Scholar]
- 15. Radom‐Aizik, S. et al Growth inhibition and compensation in response to neonatal hypoxia in rats. Pediatr. Res. 74, 111–120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McNabb, L.J. & Baldwin, K.M. Hemodynamic and metabolic effects of exercise in Crotalaria‐induced pulmonary hypertension in rats. J. Appl. Physiol. 57, 1829–1833 (1984). [DOI] [PubMed] [Google Scholar]
- 17. Eikelboom, R. & Mills, R. A microanalysis of wheel running in male and female rats. Physiol. Behav. 43, 625–630 (1988). [DOI] [PubMed] [Google Scholar]
- 18. Tu, J. , Cheung, W.W. & Mak, R.H. Inflammation and nutrition in children with chronic kidney disease. World J. Nephrol. 5, 274–282 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sederquist, B. , Fernandez‐Vojvodich, P. , Zaman, F. & Savendahl, L. Recent research on the growth plate: Impact of inflammatory cytokines on longitudinal bone growth. J. Mol. Endocrinol. 53, T35–44 (2014). [DOI] [PubMed] [Google Scholar]
- 20. Yakar, S. et al Circulating levels of IGF‐1 directly regulate bone growth and density. J. Clin. Invest. 110, 771–781 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Puche, J.E. & Castilla‐Cortázar, I. Human conditions of insulin‐like growth factor‐I (IGF‐I) deficiency. J. Transl. Med. 10, 224–224 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Argente, J. Challenges in the management of short stature. Horm. Res. Paediatr. 85, 2–10 (2016). [DOI] [PubMed] [Google Scholar]
- 23. Baron, J. et al Short and tall stature: A new paradigm emerges. Nat. Rev. Endocrinol. 11, 735–746 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dunham‐Snary, K.J. et al Hypoxic pulmonary vasoconstriction: From molecular mechanisms to medicine. Chest 151, 181–192 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Azar, N. et al Cardiac growth patterns in response to chronic hypoxia in a neonatal rat model mimicking cyanotic heart disease. Exp. Clin. Cardiol. 8, 189–194 (2003). [PMC free article] [PubMed] [Google Scholar]
- 26. Keith, I.M. , Tjen‐A‐Looi, S. , Kraiczi, H. & Ekman, R. Three‐week neonatal hypoxia reduces blood CGRP and causes persistent pulmonary hypertension in rats. Am. J. Physiol. 279, H1571–H1578 (2000). [DOI] [PubMed] [Google Scholar]
- 27. Lumbroso, D. & Joseph, V. Impaired acclimatization to chronic hypoxia in adult male and female rats following neonatal hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R421–R427 (2009). [DOI] [PubMed] [Google Scholar]
- 28. Martin, A. , Connelly, A. , Bland, R.M. & Reilly, J.J. Health impact of catch‐up growth in low‐birth weight infants: Systematic review, evidence appraisal, and meta‐analysis. Matern. Child Nutr. 13(1) (2017). https://doi.org/10.1111/mcn.12297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Finkielstain, G. , Lui, J. & Baron, J. Catch‐up growth: cellular and molecular mechanisms. World Rev. Nutr. Diet. 106, 100–104 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Farahani, R. et al Differential effects of chronic intermittent and chronic constant hypoxia on postnatal growth and development. Pediatr. Pulmonol. 43, 20–28 (2008). [DOI] [PubMed] [Google Scholar]
- 31. Cameron, N. & Demerath, E. Critical periods in human growth and their relationship to diseases of aging. Am. J. Phys. Anthropol. Suppl 35, 159–184 (2002). [DOI] [PubMed] [Google Scholar]
- 32. Tanner, J. Regulation of growth in size in mammals. Nature 199, 845–850 (1963). [DOI] [PubMed] [Google Scholar]
- 33. Tamayo, C. , Manlhiot, C. , Patterson, K. , Lalani, S. & McCrindle, B.W. Longitudinal evaluation of the prevalence of overweight/obesity in children with congenital heart disease. Can. J. Cardiol. 31, 117–123 (2015). [DOI] [PubMed] [Google Scholar]
- 34. Ryan, T. & Jefferies, J. Managing heart failure in adults with congenital heart disease. Curr. Treat Options Cardiovasc. Med. 17, 5 (2015). [DOI] [PubMed] [Google Scholar]
- 35. Tai, A. et al Outcomes of childhood asthma to the age of 50 years. J. Allergy Clin. Immunol. 133, 1572–1578.e1573 (2014). [DOI] [PubMed] [Google Scholar]
- 36. Wang, Y. et al Mortality of New York children with sickle cell disease identified through newborn screening. Genet. Med. 17, 452–459 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mourani, P.M. et al Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 191, 87–95 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Julien, C. et al Measuring hemoglobin oxygen saturation during graded hypoxic hypoxia in rat striatum. Anesth. Analg. 102, 565–570 (2006). [DOI] [PubMed] [Google Scholar]
- 39. Morgan, B.J. , Adrian, R. , Bates, M.L. , Dopp, J.M. & Dempsey, J.A. Quantifying hypoxia‐induced chemoreceptor sensitivity in the awake rodent. J. Appl. Physiol. (1985). 117, 816–824 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Manja, V. , Saugstad, O.D. & Lakshminrusimha, S. Oxygen saturation targets in preterm infants and outcomes at 18–24 months: A systematic review. Pediatrics 139, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ment, L.R. , Schwartz, M. , Makuch, R.W. & Stewart, W.B. Association of chronic sublethal hypoxia with ventriculomegaly in the developing rat brain. Brain Res. Dev. Brain Res. 111, 197–203 (1998). [DOI] [PubMed] [Google Scholar]
- 42. Wigerup, C. , Pahlman, S. & Bexell, D. Therapeutic targeting of hypoxia and hypoxia‐inducible factors in cancer. Pharmacol. Ther. 164, 152–169 (2016). [DOI] [PubMed] [Google Scholar]
- 43. Zelzer, E. et al VEGFA is necessary for chondrocyte survival during bone development. Development 131, 2161–2171 (2004). [DOI] [PubMed] [Google Scholar]
- 44. Blick, C. et al Hypoxia regulates FGFR3 expression via HIF‐1alpha and miR‐100 and contributes to cell survival in non‐muscle invasive bladder cancer. Br. J. Cancer. 109, 50–59 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li, J. & Dong, S. The signaling pathways involved in chondrocyte differentiation and hypertrophic differentiation. Stem Cells Int. 2016, 2470351 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yodmuang, S. , Marolt, D. , Marcos‐Campos, I. , Gadjanski, I. & Vunjak‐Novakovic, G. Synergistic effects of hypoxia and morphogenetic factors on early chondrogenic commitment of human embryonic stem cells in embryoid body culture. Stem Cell Rev. 11, 228–241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yao, Y. et al MIF plays a key role in regulating tissue‐specific chondro‐osteogenic differentiation fate of human cartilage endplate stem cells under hypoxia. Stem Cell Rep. 7, 249–262 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kobayashi, T. et al Impaired fracture healing in macrophage migration inhibitory factor‐deficient mice. Osteoporos. Int. 22, 1955–1965 (2011). [DOI] [PubMed] [Google Scholar]
- 49. Vrtacnik, P. , Marc, J. & Ostanek, B. Hypoxia mimetic deferoxamine influences the expression of histone acetylation‐ and DNA methylation‐associated genes in osteoblasts. Connect. Tissue Res. 56, 228–235 (2015). [DOI] [PubMed] [Google Scholar]
- 50. Garreta, E. , Melo, E. , Navajas, D. & Farre, R. Low oxygen tension enhances the generation of lung progenitor cells from mouse embryonic and induced pluripotent stem cells. Physiol. Rep. 2, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


