Abstract
The solute carrier (SLC) SLC16 gene family comprises 14 members and encodes for monocarboxylate transporters (MCTs), which mediate the absorption and distribution of monocarboxylic compounds across plasma membranes. As the knowledge about their physiological function, activity, and regulation increases, their involvement and contribution to cancer and other diseases become increasingly evident. Moreover, promising opportunities for therapeutic interventions by directly targeting their endogenous functions or by exploiting their ability to deliver drugs to specific organ sites emerge.
MONOCARBOXYLATE TRANSPORTER FAMILY
The monocarboxylate transporters (MCTs) belong to the solute carrier (SLC) transporter superfamily and are encoded by the members of the SLC16 gene family. Based on sequence homology, the MCT family comprises 14 members.1 A phylogenetic tree of the MCT family members is shown in Figure 1. It is important to note that the MCT and SLC16 numbering do not coincide and that, as of 1998 (after publication of Wilson et al.2), several MCT members were renamed after their initial identification. Correct annotation of MCT and corresponding SLC16 nomenclature can be found in Figure 1.
Figure 1.
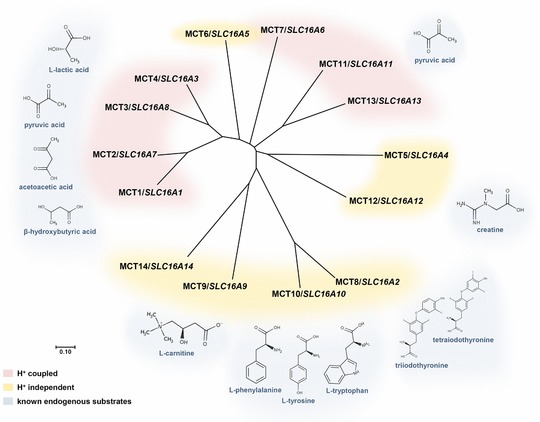
Predicted phylogenetic tree of the monocarboxylate transporter (MCT) family members and corresponding known endogenous substrates. The phylogenetic tree was generated using MEGA7 (http://www.megasoftware.net/) and sequence alignments were performed using the multiple sequence alignment tool clustal omega (https://www.ebi.ac.uk/Tools/msa/clustalo/). Transporters mediating H+‐coupled or H+‐independent substrate transport are highlighted in red and yellow, respectively. Next to the transporters, the main known endogenous substrates are depicted and marked in blue. For MCT5–7, MCT13, and MCT14, no substrates have been identified in humans yet.
STRUCTURE OF MCT TRANSPORTERS
The topology model for all 14 members is predicted to comprise 12 transmembrane domains and less conserved intracellular C‐termini and N‐termini as well as a large loop between transmembrane domains 6 and 7 based on hydrophobicity plots.3 Although MCTs are primarily localized at the plasma membrane, the transport proteins are not glycosylated post‐translationally.4 Instead, several members have been shown to depend on the association with a highly glycosylated ancillary protein for correct targeting and functional expression at the plasma membrane. MCT1, MCT3, MCT4,3 MCT11,5 and MCT126 were demonstrated to preferably interact with the transmembrane glycoprotein CD147, also known as basigin or EMMPRIN, whereas MCT2 has been shown to form a complex with a closely related glycoprotein gp70, known as embigin.3 Both basigin and embigin belong to the immunoglobulin superfamily and consist of a single transmembrane domain and two to three extracellular immunoglobulin domains depending on the splice variant.3 MCT8 was the first member to show that it does not need an ancillary protein but is expressed as homodimer.1 Concerning the remaining members (MCT5–7, MCT9, 10, 13, and 14), it is unknown to date whether a glycosylated ancillary protein is necessary for functional expression and activity.
SUBSTRATES OF MCT TRANSPORTERS
The name of the transporter family is derived from the endogenous substrates of the first identified and still best described family members MCT1/SLC16A1, MCT2/SLC16A7, MCT3/SLC16A8, and MCT4/SLC16A3, which are responsible for the H+‐coupled transport of short chain monocarboxylates, primarily L‐lactate, pyruvate, or ketone bodies (Figure 1) with varying substrate affinities.3 Other members, whose transport substrates were characterized later, were shown to mediate H+‐independent translocation of more hydrophobic monocarboxylates, such as thyroid hormones (MCT8/SLC16A2), carnitine (MCT9/SLC16A9), aromatic amino acids (MCT10/SLC16A10), or creatine (MCT12/SLC16A12; Figure 1).7 Far less is known about the remaining family members up to now, although the number of studies to characterize the remaining orphan MCTs is increasing. For instance, for MCT6 transport of xenobiotics, such as loop diuretics (e.g., bumetanide), the antidiabetic drug nateglinide or the uricosuric agent probenecid was described.8, 9 In addition, in contrast to the typical MCT substrates, dietary flavonoids were recently proposed as substrate for MCT6, with additional inhibiting effect,10 suggesting distinct substrate specificities compared with MCT1–MCT4. The endogenous substrates of MCT6, however, are not identified so far. Human MCT7 has not yet been systematically characterized. Mct7/slc16a6 from zebrafish has been demonstrated to selectively export ketone bodies from the liver, especially β‐hydroxybutyrate, and transgenic expression of human SLC16A6 rescued a mutant slc16a6 zebrafish phenotype with hepatic steatosis, indicating a similar function for human MCT7.11 MCT11 has recently been shown to mediate H+‐coupled transport of pyruvate, indicating similar substrate specificities as MCT1–MCT4; however, transport of other monocarboxylates was not tested. Thus, other endogenous or exogenous substrates of MCT11 have not been identified by now.5 Human MCT5, MCT13, and MCT14 remain orphan transporters with yet unknown functions and substrate specificities. Interestingly, it has been reported that murine MCT14 does not seem to transport the typical substrates of other MCT family members, such as lactate or amino acids.12 Based on sequence conservation and the presence of key residues required for H+‐coupled transport, MCT13 is likely to be an H+‐coupled transporter, unlike MCT5 and MCT14, which are predicted to mediate H+‐independent transport of substrates (Figure 1).3 Untargeted metabolomic approaches13 (e.g., using carriers of genetic variants or knockout mice), represent promising tools to support the identification of endogenous substrates of the remaining orphan transporters in the near future.14
TISSUE DISTRIBUTION AND ENDOGENOUS FUNCTION OF MCT TRANSPORTERS
The major differences between the 14 MCT isoforms, despite the distinct substrate affinities and specificities, are their tissue distribution and intracellular localization as well as the regulation of expression. Although the expression profiles partly overlap and some substrates are shared, the tissue distribution and distinct substrate affinities correspond to the particular metabolic need of the respective tissue in which they are expressed (Figure 2). Thus, MCTs enable substrate flux covering the full range of substrate concentrations occurring in these tissues under normal physiological conditions and in response to changing energy and nutrient demands.15
Figure 2.
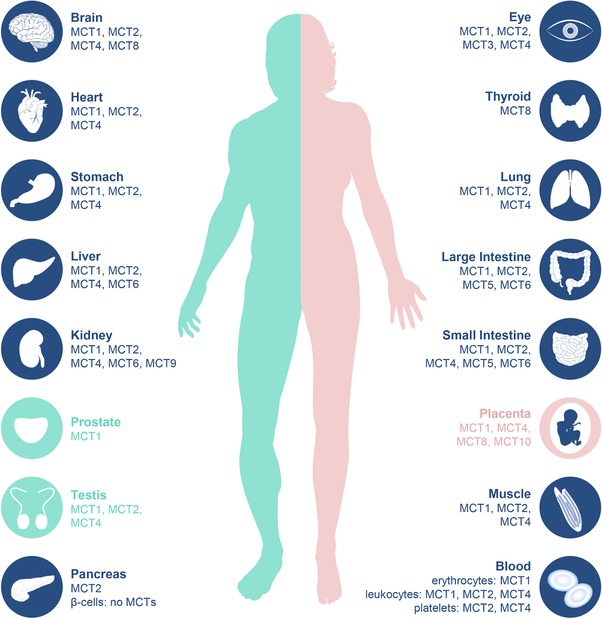
Tissue distribution of monocarboxylate transporter (MCT) isoforms. Expression of individual MCT isoforms based on published human protein expression data in different organs according to current knowledge.
The tissue distribution of MCT isoforms is most extensively studied in rodent tissues but species differences have been observed to varying degrees.16 Although the tissue distribution of the individual human MCT isoforms has been studied extensively on the level of mRNA expression (e.g., by Price et al.4), knowledge of functional expression on protein level is of major importance to evaluate their precise physiological role within the tissues they are expressed. Figure 2 summarizes the tissue distribution of MCT isoforms based on published data on human protein expression, according to current knowledge.
Most studies evaluating human MCT protein expression have been performed for MCT1, MCT2, and MCT4. As mediators of H+‐coupled translocation of L‐lactate, pyruvate, and ketone bodies across cell membranes, MCT1–MCT4 support the maintenance of an energy balance and pH homeostasis and enable metabolic cooperation between different tissues with distinct energetic profiles. In particular, the tissue distribution of MCT1–MCT4 enables a metabolic coupling in which lactate, pyruvate, or ketone bodies produced in one tissue can be taken‐up and used by another. MCT1, a transporter with moderate substrate affinities, facilitates either influx or efflux of the substrates depending on substrate and H+‐concentration gradients and is ubiquitously expressed in human tissues.17 It is expressed in tissues that rely on efflux or uptake of energy metabolites based on the energetic profile but also in the gut to support the absorption of short chain fatty acids, such as acetate, propionate, or butyrate.15 In contrast, MCT2 is a high affinity transporter with a more restricted protein expression pattern. It is expressed in tissues that depend on substrate uptake as fuel for oxidative phosphorylation or as substrate for gluconeogenesis or lipogenesis and is, therefore, mainly expressed in neurons, testis, liver, kidneys, and adipose tissue. MCT2 protein expression was also found in the pancreas, heart, colon, and stomach.17 MCT3 expression is confined to the retinal pigment epithelium where it is primarily responsible for the efflux of L‐lactate from the retina.18 MCT4 is a low affinity but high capacity transporter with L‐ lactate as the main substrate. The transporter is primarily responsible for L‐lactate export from highly glycolytic cells and, therefore, shows wider tissue distribution with peak expression levels e.g., in glycolytic white muscle fibers, leukocytes, or astrocytes. Human placenta, kidneys, and small intestine were also demonstrated to express MCT4.17, 19
Apart from shuttling metabolites between different tissues with particular energy demands, MCT1–MCT4, with lactate being their quantitatively most important substrate, are attributed a major role in lactate shuttling between different cell types within the same tissue (Figure 3). The concept of metabolic cooperation via lactate shuttling between cell types of the same organ has been described for skeletal muscle, in which highly glycolytic fast‐twitch white muscle fibers extrude lactate via MCT4, which is taken up by oxidative slow‐twitch red muscle fibers by MCT1 (Figure 3).15 A rapid exchange of lactate has also been established for different cell types of the brain, known as the astrocyte‐neuron lactate shuttle hypothesis.20 Here, glycolytic astrocytes provide energy via lactate extrusion by MCT1 and MCT4 to oxidative neurons, which express the high affinity uptake transporter MCT2 (Figure 3). Similar cooperation between cell types of the same tissue has been demonstrated to be important for visual function and spermatogenesis (Figure 3).16
Figure 3.
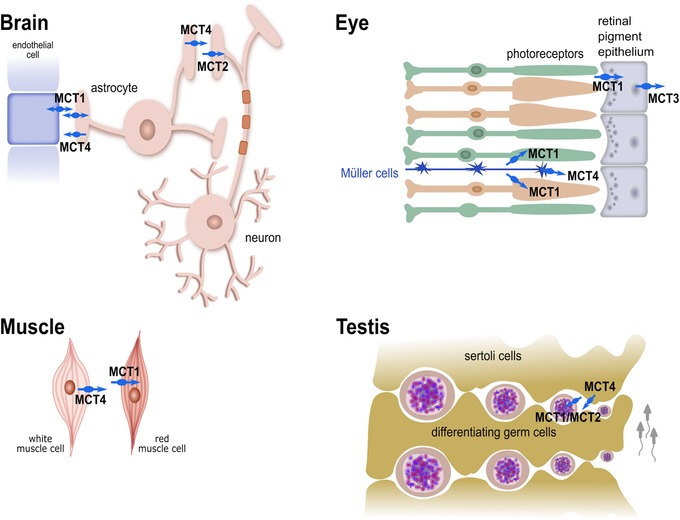
Intracellular lactate shuttling between different cell types of specific organs. Focus on lactate shuttling on cellular level in: (1) the brain, between endothelial cells, glycolytic astrocytes, and oxidative neurons; (2) the eyes, between glycolytic Müller cells and oxidative photoreceptors; (3) the muscles, between lactate producing white muscle cells and lactate consuming red muscle cells; and (4) in testis, between lactate providing Sertoli cells and lactate consuming differentiating germ cells. MCT, monocarboxylate transporter.
Only limited systematic studies providing human protein expression data are available for the remaining MCT isoforms. The thyroid hormone transporter MCT8 and the amino acid transporter MCT10 seem to be widely expressed in human tissues. MCT8 expression seems to be of major importance in the brain and placenta for fetal development.21 Human MCT5 and MCT6 protein were found to be expressed in the human intestine (note: Gill et al.22 uses former MCT numbering). Expression of MCT6 in the human intestine was confirmed by Kohyama et al.8 Moreover, Jones et al.10 suggest high MCT6 expression in hepatocytes and intermediate levels in the kidneys and colon (abstracts.aaps.org, AAPS2016, 07M0330). Protein expression of human MCT9 was found in the proximal tubular epithelial cell of the kidneys.23
Based on human mRNA expression data, MCT11 is highly expressed in the thyroid, liver, and salivary gland.24 Peak gene expression levels of MCT12 were observed in the eyes, lungs, kidneys, and testis.25 Although a first study provides evidence for MCT14 protein expression in the mouse central nervous system (CNS) and kidneys,26 no human data are available to date. In addition, human MCT7 and MCT13 protein tissue distribution has not yet been elucidated. For selected MCT transporters, human protein expression data can also be found in the Human Protein Atlas (https://www.proteinatlas.org/).
IMPORTANCE AND CLINICAL RELEVANCE OF MCTs IN DISEASES
Owing to their importance in shuttling metabolites used as respiratory fuels or as precursors and intermediates in many metabolic pathways between different cell types and tissues, MCTs are majorly involved in the maintenance of a metabolic homeostasis in response to – possibly rapidly – changing demands. Accordingly, aberrant expression, activity, and function of MCTs confound metabolic homeostasis with detrimental consequences contributing to a number of clinical manifestations ranging from mild disorders to severe diseases.
MCTs IN CANCER
Role of MCT1, MCT2, and MCT4
The important role of MCT1, MCT2, and MCT4 in cancer is mainly ascribed to the lactate shuttling between cells with distinct energy demands. In cancer, a metabolic cooperation is hypothesized to occur either between hypoxic glycolytic and oxygenated oxidative tumor cells, between hypoxic tumor and endothelial cells, or between oxidative tumor and glycolytic stromal cells.27 Export of lactate from highly glycolytic tumor (or stromal) cells ensures the progression of glycolysis and prevents intracellular acidification. The extracellular lactate serves not only as a respiratory fuel for oxidative cells, but, together with the H+, contributes to an acidic tumor microenvironment and is meanwhile recognized as an important signaling molecule promoting migration, angiogenesis, and immunosuppression.27 The phenomenon of intratumoral lactate shuttling has been described for several solid tumors and is suggested to occur commonly across cancer types.28 Accordingly, aberrant expression of MCT1, MCT2, and/or MCT4 has been demonstrated e.g., in colon cancer, glioblastoma, breast cancer, prostate cancer, pancreatic cancer, or clear cell renal cell carcinoma.29, 30 A comprehensive overview of aberrant MCT1, MCT2, and MCT4 expression in different cancer entities is provided by Pinheiro et al.31 With this important impact on lactate metabolism in tumor cells, MCT1, MCT2, and MCT4 have been proposed as diagnostic biomarkers or prognostic factors for cancer outcome and survival. For instance, increasing MCT1 expression is observed along with tumor aggressiveness e.g., in cervical cancer or is described as a prognostic marker in invasive breast cancer.32, 33 In prostate cancer, MCT2 has been proposed as a biomarker for disease diagnosis, because the transporter is not expressed in normal prostate tissue, an increase in expression can be observed in adjacent non‐neoplastic tissue and strong MCT2 expression is found in primary prostate tumor tissue samples.34 The lactate exporter MCT4, which is overexpressed in glycolytic tumor cells, is a promising candidate for cancer prognosis in various cancer entities, such as pancreatic cancer,35 clear cell renal cell carcinoma,29 or hepatocellular carcinoma.36 A recent meta‐analysis corroborates that increased MCT4 expression is linked to poor patient outcome across five different cancer entities.37
Role of other MCT isoforms
The role of other MCT isoforms in cancer is barely addressed in the literature, which is why their relevance for cancer development or progression is largely elusive so far. However, substantial differences of gene expression levels in various cancer entities can be observed also for the other MCT isoforms. Figure 4 illustrates the gene expression of the individual MCT isoforms in selected cancer entities using data from The Cancer Genome Atlas (TCGA). Strikingly, the gene expression of MCT12 is most frequently downregulated in tumors compared with non‐neoplastic tissues in the examined cancer entities. Only few genomewide gene expression profiling studies are available, indicating altered expression of MCT isoforms e.g., in hepatocellular carcinoma,38 thyroid cancer,39 adrenocortical cancer,40 or ovarian cancer41 but the precise molecular mechanisms behind altered MCT expression are not revealed. Given that tumor growth is a highly energy consuming process and tumors demonstrate metabolic reprogramming and considerable metabolic plasticity, other MCT isoforms presumably play an important role in cancer development and progression as suppliers of nutrients or exporters of metabolic end products. On the other hand, downregulation of MCTs might occur in order to shut down dispensable metabolic pathways in favor of cell growth, proliferation, and metastasis.
Figure 4.
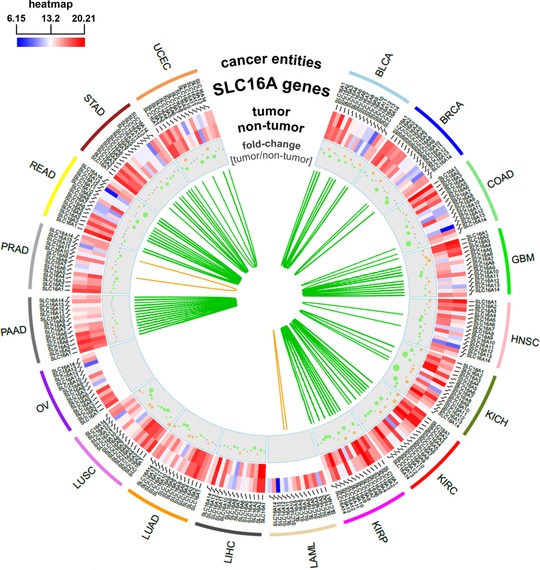
Differential gene expression of monocarboxylate transporter (MCT) isoforms in different cancer entities of The Cancer Genome Atlas (TCGA). The circos plot shows a heat‐map of gene expression of the individual members of the solute carrier (SLC)16 gene family in different cancer entities in tumors and corresponding normal tissues using gene expression data from TCGA (http://cancergenome.nih.gov/). Furthermore, fold‐changes (log2) of expression levels in tumors compared with normal tissues are represented by grey, orange, or green dots (grey: comparable expression levels in tumors and normal tissues; orange: higher expression in tumors; and green: higher expression in normal tissues). The size of the dots depends on the absolute value of fold‐changes (log2). The green and orange lines depict Spearman correlation coefficients (rS > 0.7) between individual SLC16 isoforms in normal (green) and tumor (orange) tissue. BLCA, bladder cancer; BRCA, breast cancer; COAD, colon cancer; GBM, glioblastoma; HNSC, head and neck cancer; KICH, kidney chromophobe; KIRC, kidney clear cell carcinoma; KIRP, kidney papillary carcinoma; LAML, acute myeloid leukemia; LIHC, liver cancer; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; OV, ovarian cancer; PAAD, pancreatic cancer; PRAD, prostate cancer; READ, rectal cancer; STAD, stomach cancer; UCEC, endometrioid cancer.
MCTS IN OTHER DISEASES
Fatigue
The first condition associated with a lactate transporter defect that was later proposed to be caused by a mutation in the SLC16A1 gene, was reported as rare cryptic exercise intolerance or fatigue syndrome, manifested through muscle cramping and chest pain, caused by impaired lactate removal after intense exercise in otherwise healthy people.42 Whether the mutations or rather impaired interactions with the ancillary protein CD147/basigin are responsible for the MCT1 deficiency was questioned afterward since the in vitro experiments could not prove the reduced transporter activity of the mutated isoform compared with wildtype MCT1.15
Exercise induced hyperinsulinism
Sound evidence exists for implications of activating SLC16A1 promoter mutations with hereditary exercise induced hyperinsulinism (EIHI) and hypoglycemia.43 This inherited disorder is caused by failed silencing of MCT1 in pancreatic β‐cells, leading to pyruvate and lactate uptake, which is normally suppressed in these cells, and subsequent metabolic oxidation. The increased ATP levels generated by the oxidation trigger inappropriate insulin secretion and, hence hypoglycemia during anaerobic exercise in individuals carrying one of the mutations. Very recently, a novel intragenic heterozygous mutation in the SLC16A1 gene was found in an infant with congenital hyperinsulinism and persistent hypoglycemia. Although functional studies have not been performed yet, the missense variant is predicted to be damaging and causes disease.44
Inflammatory bowel disease
Because MCT1 is also involved in intestinal absorption of short chain fatty acids, supplying energy substrates for colonic cells, deregulation is also suggested to affect colonic tissue homeostasis and might be involved in inflammatory bowel diseases. For instance, impaired butyrate oxidation has been associated with active mucosal inflammation. Apart from reduced butyrate oxidation itself, impaired butyrate uptake, most likely as a consequence of decreased MCT1 expression, was found in patients with active ulcerative colitis.45, 46 Moreover, density analyses from an open access genomewide association studies database revealed a dense cluster of associations for SLC16A7 (encoding for MCT2) variants with Crohn disease and high‐density lipoprotein (HDL) cholesterol levels, although functional validation is completely missing so far.47
METABOLIC DISEASES
Obesity
MCTs are not only involved in maintaining energy homeostasis within particular tissues, but also contribute to an energetic balance in the whole body. In this respect, they can act as fuel sensors impacting food intake and body weight. Altered MCT expression levels in key tissues in response to metabolic status, especially in the brain, muscles, liver, intestine, pancreas, and adipose tissue are suggested to be important determinants with respect to whole body energy balance.48 As comprehensively reviewed by Carneiro et al.,48 considerable alterations in MCT1, MCT2, or MCT4 expression in these tissues are found in accordance to metabolic states (e.g., in obese or fasting subjects). Although, again, most studies refer to rodent or in vitro models, alterations were also confirmed in human tissues. For instance, MCT4 was found to be overexpressed in muscle of obese subjects but decreased after weight loss.48 Most striking results were achieved using a heterozygous transgenic MCT1 knockout mouse model (MCT1+/−; MCT1−/− homozygote knockout is lethal), which has been demonstrated to be resistant to diet‐induced obesity.49 In addition to reduced food intake under high‐sugar and high‐fat diets, nutrient absorption in the intestine was shown to be decreased, lipid accumulation in adipose tissue and the liver was decreased or absent, respectively, and glucose intolerance or insulin resistance were not observed in MCT1+/− as compared with MCT1 wildtype mice.49 Elucidation of the precise mechanisms and the contribution of other MCT isoforms to body weight regulation might reveal interesting novel druggable pathways or targets for prevention of obesity and resultant diseases.
Gout
Gout is a metabolic disease caused by elevated serum uric acid (SUA) levels. In a meta‐analysis of 14 genomewide association studies50 as well as in replication studies51, 52 a single‐nucleotide polymorphism (SNP; rs12356193) within the SLC16A9 gene was identified as a factor contributing to the variability of SUA levels. The variant correlated also to DL‐carnitine and propionyl‐L‐carnitine levels, which, in turn, were strongly associated with SUA. Carnitine was later experimentally validated to be a substrate of MCT9, whereas urate was demonstrated to be not transported by MCT9.53 Therefore, rs12356193 is suggested to have an indirect effect on urate excretion through aberrant carnitine levels.50 A contribution of the missense variant rs2242206 (close to the previously identified intronic variant) to the risk of gout could be found only for renal overload gout in Japanese individuals, in contrast to renal underexcretion gout54 or all gout susceptibility in Europeans,51 indicating impaired intestinal urate excretion. The functional mechanisms and the clinical relevance of MCT9 and its contribution to the variability of SUA and the susceptibility to resulting diseases, especially gout, are, therefore, not fully clarified and need further investigations. Interestingly, Jansen et al.55 identified that the SLC16A9 gene variant rs12356193 was associated with lower plasma carnitine levels and led to enhanced proliferative capacity of CD8 T‐cells and improved response to peginterferon alfa‐2a/adefovir treatment in patients with chronic hepatitis B.
Type 2 diabetes mellitus
A genomewide association study in a Latin American population with patients with type 2 diabetes mellitus (T2DM) and nondiabetic controls identified a novel locus reaching genomewide significance spanning the genes SLC16A11 and SLC16A13.24 The risk haplotype consists of five SNPs, whereby the tag‐SNP for the risk haplotype rs17767867 is found most frequently in Native American populations (∼50%), at intermediate frequency in Asians (∼10%), but occurs rarely in Europe or Africa and might explain, in part, the higher prevalence of T2DM in Mexican Americans.24 The five SNP haplotype comprises a synonymous as well as four missense variants in SLC16A11, of which one was predicted to be damaging, indicating that SLC16A11, encoding for MCT11, is the causal gene for the link with T2DM. Simultaneously, Hara et al.55 identified rs312457, an intronic variant within the SLC16A13 gene, in East Asian populations, which is in linkage disequilibrium with the five SNP haplotypes found in Mexicans. A study investigating Mexican families with children <18 years with T2DM found a link between a SLC16A11 variant (rs13342232) and early onset of the disease.57 Evidence supporting the assumption that SLC16A11 is a causal gene contributing to T2DM pathogenesis was just recently provided on a functional level.5 The risk haplotype has been demonstrated to dose‐dependently decrease SLC16A11 gene expression in the liver and disturb the interaction of MCT11 with CD147, reducing the functional expression at the plasma membrane. Knockdown of MCT11/SLC16A11 in in vitro experiments using primary human hepatocytes led to an increase in acylcarnitines, diacylglycerols, and triacylglycerols i.e., changes in fatty acid and lipid metabolism consistent with those observed in insulin resistance and T2DM.5 Therefore, more detailed investigations of exact mechanisms and further systematic characterization of the transporter is needed.
Interestingly, transcriptome analyses in a cohort of Malaysian patients with T2DM revealed overexpression of MCT4/SLC16A3 in patients with different stages of diabetic nephropathy compared with patients without renal impairment.58 Large‐scale studies in other populations and functional validation are needed to understand the association of MCT4 with susceptibility of diabetic nephropathy.
Ketoacidosis
Apart from the fatigue syndrome, a further manifestation of MCT1 deficiency was found in children who had recurrent ketoacidosis. The affected children were identified to harbor inactivating homozygous, but also heterozygous mutations in the SLC16A1 gene, which lead to loss of MCT1 function.59 Because ketolytic defects were ruled out, a defect in ketone body utilization during catabolic stress (e.g., induced by infections or fasting) due to reduced uptake of ketone bodies in extrahepatic tissues via MCT1, and, hence, acid‐base imbalance, is suggested as the cause of the disorder in these patients. So far, unknown additional triggers accompanying heterozygous MCT1 mutations are expected to contribute to symptom manifestation. The finding was supported by a case report of affected half‐siblings with heterozygous MCT1 mutations inherited from their mother, present also in the patients’ half‐sister who were both asymptomatic, corroborating that heterozygous mutations can induce recurrent ketoacidosis, but are not necessarily sufficient.60
Diseases of the central and peripheral nervous system
Some patients with recurrent ketoacidosis caused by MCT1 deficiency also had moderate intellectual disability, epilepsy, or migraine headaches,59 indicating consequences also for the CNS. Although it remains unknown whether these symptoms were direct effects of MCT1 deficiency in the CNS or were caused by repeated ketoacidosis, an involvement of MCT1 or other MCTs is not unreasonable. The brain is highly dependent on continuous energy supply and disturbed shuttling of energy substrates between the different brain cell types may have detrimental consequences for the highly complex and tightly regulated brain physiology and energy homeostasis.61 Most studies investigating diseases of the CNS and/or the peripheral nervous system and the relevance of MCTs were performed in rodent models. However, the findings allow insights and first assumptions of the functional role of MCTs, which are to be validated in appropriate human model systems.
With respect to cognitive functions, it could be demonstrated in rat models that downregulation of MCT1, but also MCT4, in glial cells, and/or MCT2 in neurons prevented learning and long‐term memory formation.61 Cognitive impairment due to decreased energy metabolism was also found in the pathophysiology of Alzheimer disease in rats. Indeed, decreased lactate content accompanied by reduced MCT2 expression was demonstrated in the cerebral cortex and hippocampus in a rat model of the disease.61 Changes in rat MCT expression were also observed in response to oxygen deprivation, for instance, due to ischemic stroke.62 In contrast, in a mouse model of Parkinson disease, which is also characterized by compromised energy metabolism, no alterations of MCT expression were found.61
Although functional evidence is missing, there are first indications supporting the assumption that deregulated MCT expression and impaired metabolic interactions contribute to human neurological disorders. Decreased MCT1 protein expressions in human microvessels of the hippocampus as well as a redistribution of MCT2 have been suggested to be involved in the pathophysiology of drug‐resistant temporal lobe epilepsy.63 In addition, MCT4 protein expression levels were shown to be significantly decreased in temporal lobe epileptic foci excised from patients with intractable epilepsy.64 Besides altered supply of monocarboxylates, the exact functional relevance of MCTs in epilepsy is unknown to date, just like the question whether the detected altered MCT expression is a cause or a consequence of epileptic seizures.63 Reduced MCT1 expression in oligodendrocytes in the spinal cord is proposed to be involved in neurodegeneration in patients with amyotrophic lateral sclerosis. Here, lactate release from oligodendrocytes via MCT1 has been shown to be critical to metabolically support neuron and axon functions.65 Furthermore, overexpression of MCT1 was found in astrocytes within active white matter lesions of patients with multiple sclerosis, whereas decreased axonal MCT2 expression was found in inactive lesions, presumably leading to insufficient energy supply and progressive neurodegeneration.66
OPHTHALMOLOGICAL DISEASES
Age‐related macular degeneration
A meta‐analysis of the AMD gene consortium revealed seven new loci associated with age‐related macular degeneration reaching genomewide significance levels, including a variant in the SLC16A8 gene, which was independently associated with risk of disease.67 A large genomewide association study including over 16,000 patients with age‐related macular degeneration and almost 18,000 controls confirmed the association of the splice variant in SLC16A8 (MCT3) with strongly increased disease risk. A direct involvement in disease pathology is suggested and seems plausible given the organ confined expression of MCT3 in the human retinal pigment epithelium and the importance of appropriate energy substrate shuttling between the different ocular cell types (Figure 3).68
Juvenile and age‐related cataracts
Juvenile cataracts accompanied by microcornea and renal glucosuria were found to be associated with a nonsense mutation in SLC16A12 in a Swiss family, which leads to a truncated form of MCT12 that is retained in the endoplasmic reticulum.6, 25 Although physiological functions or the substrate specificity of the transporter was unknown at the time, the speculated involvement in maintenance of homeostasis in the lens was presumed to be of importance also in age‐related cataracts. Indeed, in a subsequent study, mutations in the 5ʹUTR of SLC16A12, leading to altered translational efficiency, were associated with the age‐related onset of the disease.69 In the meantime, creatine has been identified as a substrate of MCT12. Although the precise function of MCT12 in creatine metabolism is not yet elucidated, detrimental consequences of MCT12 deficiency are not surprising, because creatine represents a major energy buffer, providing rapid ATP supply during high energy demand, and an antioxidant, protecting cells from oxidative stress. In a study re‐evaluating the original family, the cause for renal glucosuria accompanying juvenile cataract in all but three family members, was attributed to a second independent heterozygous mutation in the glucose transporter SGLT2/SLC5A2 rather than the mutation in SLC16A12. Interestingly, this study revealed an indirect link between the SLC16A12 mutation and reduced guanidinoacetate levels, which is a precursor of creatine but not a substrate of MCT12.70 Thus, there are still many open questions regarding the exact role and function of MCT12 in the kidneys.
Male infertility
As previously mentioned, lactate shuttling between different cell types is required during spermatogenesis. Whereas lactate is released from Sertoli cells, which express MCT4, it is taken up as fuel by MCT1 and MCT2 expressing germ cells (Figure 3). Indeed, genetic variants in the 3ʹUTR of the SLC16A7 gene, encoding for MCT2, have been associated with male infertility in Korean men.71 The genetic variant is speculated to cause decreased MCT2 expression during spermatogenesis.71
Rheumatoid arthritis
Although the investigated study cohort was small, synovial fluid of patients with active rheumatoid arthritis (RA) was demonstrated to have lower pH‐ and increased lactate levels accompanied by increased MCT4 gene and protein expression in synovial fibroblasts compared with patients with osteoarthritis.72 The siRNA‐mediated knockdown of MCT4 reduced proliferation of synovial fibroblasts of patients with RA in vitro and reduced severity of disease in mice with collagen‐induced arthritis.72 With respect to RA treatment, a missense variant rs3763980 in the SLC16A7 gene, which correlated with gene expression in vitro, was found to be linked to methotrexate response in juvenile idiopathic arthritis (JIA). The association could be validated in an independent cohort of patients with JIA, but was not found in adult patients with RA.73 Because MCT2 has so far not been shown to directly mediate methotrexate transport, the precise mechanism of methotrexate (MTX) response and the relevance of MCT2 remain unclear.73
West Nile virus infection
An important association independent from energy or metabolic homeostasis was identified for SLC16A4, which encodes for MCT5 (formerly referred to as MCT4), with West Nile virus infections.74 In an in vitro RNA interference screen to investigate virus host cell interactions, knockdown of SLC16A4 was demonstrated to enhance West Nile virus infection. In this context, functional MCT5 expression is proposed to be a resistance factor for viral replication by delaying transition of endocytosed West Nile virus particles into the replication phase.74
Allan‐Herndon‐Dudley syndrome
The Allan‐Herndon‐Dudley syndrome (AHDS) is a rare hereditary disease, affecting almost exclusively male subjects, that is characterized by severe X‐linked psychomotor retardation, severe neurological impairments, including muscle hypotonia and hypoplasia, accompanied by high serum triiodothyronine (T3) levels. In 2004, loss‐of‐function mutations within the SLC16A2 gene, located on the X chromosome, encoding for the thyroid hormone transporter MCT8, have been identified as the monogenetic cause of AHDS.75, 76 Thyroid hormones play essential roles in growth and development of various tissues, especially the brain, and rely on cellular uptake to fulfill their physiological functions.77 Impaired uptake of T3 across the blood‐brain barrier (BBB) and into neurons already during embryonic brain development, which is normally mediated via the highly specific thyroid hormone transporter MCT8, leads to the observed neurological defects. Since the initial finding in 2004, over 80 MCT8 mutations across the entire coding region have been described in more than 100 families with AHDS.78 Interestingly, the effect of the mutations on MCT8 activity and function correlates with the severity of the observed variation of AHDS phenotypes.77
Hepatic steatosis
An imbalance of accumulation and removal of triglycerides in hepatocytes characterizes hepatic steatosis, and represents the first pathological stage of fatty liver disease. Although functional investigations have yet only been performed in zebrafish,11 this study provides first evidence for a contribution of MCT7 in hepatic lipid metabolism by secreting ketone bodies, especially β‐hydroxybutyrate. Zebrafish mutants with hepatic steatosis were identified to harbor a loss‐of‐function mutation in the slc16a6 locus, which causes lipid accumulation in fasted livers. Most interestingly, the steatotic phenotype could be rescued by transgenic expression of the human SLC16A6 orthologue, indicating a similar function for the human MCT7 isoform. However, the significance and contribution of MCT7 to hepatic lipid metabolism in humans needs experimental validation in appropriate model systems. A first indication for an involvement of MCT10 in the pathophysiology of nonalcoholic fatty liver disease (NAFLD) was found in a transcriptome analysis by Lake et al.,79 who showed downregulation of SLC16A10 gene expression along with NAFLD progression.79
Barely anything is known about the contribution and clinical relevance of the remaining human MCT isoforms in disease pathophysiology. However, as comprehensive characterization of the transporters, including identification of their endogenous substrates and elucidation of their tissue distribution based on functional protein expression levels progress, precise molecular mechanisms will be elucidated and involvement in pathological pathways are expected to become apparent.
EPIGENETIC REGULATION OF MCT FAMILY MEMBERS
The expression and function of transport proteins is not only affected by genetic variation, but also by nongenetic factors, such as age, gender, or disease state, regulatory factors, like transcription factors, or by epigenetic mechanisms.80 Regarding regulation of MCT expression, again, mainly MCT1, MCT2, and MCT4 were examined. The regulation of MCT gene expression has to be tissue‐specific and, if necessary, very rapid in order to enable adaption to changes in activity or different metabolic states. Transcriptional or post‐transcriptional mechanisms of MCT regulation have been identified and summarized in a comprehensive review.15 In line with its expression in highly glycolytic and hypoxic tissues, MCT4 possesses hypoxia response elements within its promoter region and the transcription is upregulated by the hypoxia‐inducible factor (HIF) 1.81 In contrast, MCT1 is regulated by transcription factors, such as MYC, PGC‐1α, or p53 or post‐transcriptionally by miRNA‐29, which is involved in the cell‐type specific inhibition of MCT1 expression in pancreatic β‐cells.15, 82
Epigenetic regulation is based on DNA modifications, which do not change the DNA sequence and include histone modifications and DNA methylation. Regulation of MCT expression by promoter DNA methylation has been described for MCT2 in prostate cancer. It was suggested that hypomethylation of an internal promoter region and hypermethylation of an upstream promoter leads to the expression of an alternative MCT2 isoform, differing only in the 5ʹUTR, which leads to MCT2 overexpression compared with benign tissue.83 MCT4 expression has been shown to be regulated by DNA methylation in clear cell renal cell carcinoma, whereby a single CpG site within the SLC16A3 promoter was identified to be a prognostic marker for patient survival.29 Of note, promoter DNA methylation of the ancillary protein CD147 did not have an influence on protein expression.84 Furthermore, in patients with temporal lobe epilepsy, cortical MCT4 expression levels were significantly lower at least partially due to SLC16A3 promoter methylation.64
THERAPEUTIC APPROACHES
Importance of MCTs for drug delivery
Besides endogenous substrates, some MCTs have been identified to contribute to the disposition of xenobiotic compounds, including drugs. Because MCTs are localized in the gastrointestinal tract, liver, and kidneys, as well as the BBB, their expression is suggested to have an impact on drug pharmacokinetics by influencing intestinal absorption, tissue distribution, or renal reabsorption of substrate drugs. Whereas for MCT1, MCT2, MCT4, and MCT6, several xenobiotic substrates have been identified, the implication of other MCT isoforms in drug transport is not well understood. Mainly in vivo and in vitro studies with rodent MCTs and some in vitro studies with human MCTs demonstrated the transport of, for example, γ‐hydroxybutyrate, 4‐phenylbutyrate, valproic acid, nicotinic acid, salicylic acid, some β‐lactam antibiotics, or selected statins.85 The expression of MCT1 at the BBB is, therefore, of specific clinical relevance, as it is suggested to contribute to the delivery of drugs for treatment of neurological diseases, such as 4‐phenylbutyrate, nicotinic acid, or valproic acid, to the brain. The expression of MCT1 in the intestine can be exploited to improve the bioavailability of substrate drugs. For instance, XP13512, a prodrug of gabapentin, was specifically designed as MCT1 substrate to improve the bioavailability of the pharmacologically active metabolite.86 Similarly, the prodrug arbaclofen placarbil (XP19986) has improved the bioavailability of the active drug R‐baclofen due to better absorption from the colon, which was found to rely on MCT1‐mediated transport.87
Because MCT isoforms are overexpressed on tumor cells, the transporters represent attractive targets to specifically deliver chemotherapeutics to cancer tissue. The efficacy of the anticancer drug 3‐bromopyruvate has been demonstrated to depend on MCT1‐mediated uptake into the tumor cells (Figure 5 a). A genomewide genetic screen identified MCT1 to be the main determinant of 3‐bromopyruvate sensitivity in human cancer cell lines and tumor xenografts.88 A recent study from Lopes‐Coelho et al.89 confirms that MCT1 expression mediates 3‐bromopyruvate transport in vitro using AML cell lines and suggests MCT1 expression as a predictive biomarker for response to 3‐bromopyruvate treatment.
Figure 5.
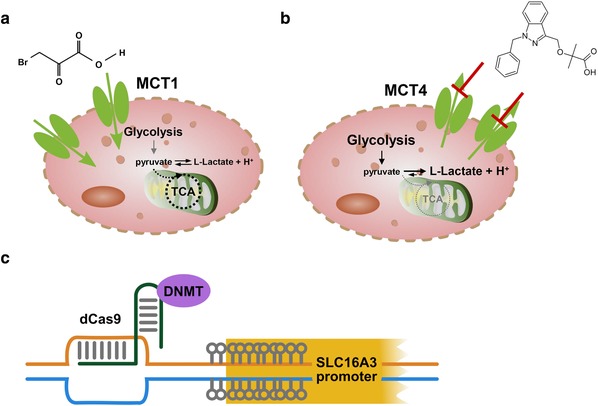
Therapeutic approaches involving isoforms monocarboxylate transporter (MCT)1 and MCT4. (a) MCT1 expression can be exploited for targeted drug delivery (e.g., of 3‐bromopyruvate), into MCT1 expressing tumor cells. (b) MCT4 represents an attractive target for inhibition in tumor cells. Compounds, such as bindarit or modified derivatives, represent promising candidates. (c) Targeted DNA methylation of the hypomethylated SLC16A3 promoter, mediated, for example, by a CRISPR‐dCas9‐DNMT construct, might represent an alternative approach to restore normal MCT4 expression levels in tumor cells.
MCT‐mediated adverse drug reactions
On the downside of drug delivery to target organ sites, MCT‐mediated distribution of substrate drugs can induce unintended adverse drug reactions. Thus, penetration of the BBB by statins can induce sleep disturbances as unwanted side effects.90 In addition, depending on the substrate concentrations, the statins can act as competitive MCT inhibitors. In this way, inhibition of MCT4, especially by lipophilic statins, is proposed to be involved in statin‐induced cytotoxicity and muscle pain.91 A very recent study demonstrates a novel association between a genetic variant in the SLC16A5 gene, encoding for MCT6, and cisplatin‐induced hearing loss.92 The common genetic synonymous variant, rs4788863, in the coding region of SLC16A5, which is predicted to alter the rate of codon usage, exerted a protective effect on cisplatin‐induced ototoxicity in patients with testicular cancer. Although the endogenous function of MCT6 is unknown so far, this study indicates a role of MCT6 for hearing. In addition, the protective effect of impaired MCT6 function can be exploited by combinatorial treatment of cisplatin with cimetidine as inhibitor, which did not compromise the anticancer effect of cisplatin in vivo in a mouse model but prevented ototoxicity.92
MCTs as drug targets
Understanding of molecular mechanisms in which MCTs are involved and how altered transport may lead to disease pathology helps to develop therapeutic interventions restoring normal physiological metabolism or interfering with pathophysiological metabolic pathways. The use of MCT inhibitors represents an attractive opportunity to elucidate precise mechanisms and to eventually therapeutically target altered MCT function. A number of MCT inhibitors, such as p‐chloromercuribenzenesulfonate (pCMBS), stilbene disulfonates, cyanocinnamates, quercetin, phloretin, or statins, have been described.3 However, these compounds are unspecific and cannot be used to determine the individual role of MCT isoforms or to particularly inhibit one specific altered MCT in a pathophysiological pathway. Still, unspecific MCT inhibitors have been demonstrated to exert therapeutic effects. Especially in cancer, MCT1, MCT2, and MCT4 are considered attractive targets to inhibit lactate shuttling and thereby interfering with tumor energy metabolism.93 For instance, the anticancer drug lonidamine is especially effective in sensitizing tumor cells to other chemotherapeutic agents and this activity can be attributed, in part, to impaired lactate transport by unspecific inhibition of MCT1, MCT2, and MCT4.94
In the meantime, several specific inhibitors, targeting only MCT1 and MCT2, have been developed and their efficacy has been investigated in the preclinical setting. The MCT1/2‐inhibitor AR‐C155858 has been found to inhibit T‐lymphocyte activation by blocking lactate efflux and, therefore, has an immunosuppressive effect.95 A second class of more potent MCT1/2 inhibitors has been designed, and one of these, AZD3965, is currently tested in phase I/II clinical trials for treatment of patients with advanced prostate cancer, gastric cancer, or diffuse large B‐cell lymphoma (NCT01791595). Preclinical studies in patients with small cell lung cancer indicate that this compound might also be effective in this cancer entity.96 Due to the overexpression of MCT4, especially in hypoxic tumor regions, it has long been searched for an MCT4‐specific inhibitor. AstraZeneca developed a specific and effective MCT4 inhibitor (AZ93), but no preclinical data were published so far.27 Just recently, first data of an MCT4‐specific inhibitor, namely bindarit, were published by Futagi et al.97 Bindarit, already known as an anti‐inflammatory agent inhibiting the production of selected cytokines, such as CCL2, as discussed in Futagi et al.,97 is a potent and highly selective noncompetitive MCT4 inhibitor, because it is ∼15 times more selective for MCT4 than for MCT1. Therefore, bindarit is suggested to be a good pharmacological tool to decipher particularly the physiological role of MCT4. Further improvement is expected by refining the structure to increase the inhibitory function, which might help to use the compound as an anticancer agent (Figure 5 b)97 and may also reduce the effect of suppressing the transcription of selected cytokines. An alternative approach to revert MCT4 overexpression to normal physiological levels, apart from isoform‐specific inhibition, is to interfere with promoter DNA methylation.98 The observed hypomethylated SLC16A3 promoter region in clear cell renal cell carcinoma might be specifically targeted by, for example, CRISPR/dCas9‐mediated remethylation using DNA methyltransferases, thereby decreasing MCT4 overexpression (Figure 5 c).29, 98
Despite all the efforts, knockdown or inhibition studies have demonstrated that, due to the functional redundancy of MCT1 and MCT4, selective inhibition of either one of the isoforms may induce tumor growth arrest or may increase chemosensitivity and radiosensitivity but is not sufficient to induce tumor cell death.27 Even simultaneous inhibition of MCT1 and MCT4 can ultimately induce a metabolic shift toward oxidative phosphorylation and survival of tumor cells. In order to tackle this metabolic plasticity, rational drug combinations, including MCT1 and MCT4 inhibitors, as well as inhibitors of oxidative phosphorylation, such as metformin or phenformin, are considered the most promising approach to run the tumor cells into a “metabolic catastrophe,” leading to tumor cell death.27, 99
With respect to other MCT isoforms, data regarding their usefulness, not to mention their clinical relevance as therapeutic targets, are limited. The recently identified and characterized function of MCT11 and its contribution to T2DM, suggests MCT11 as a new drug target for the treatment of T2DM.100 In addition, first evidence from in vitro studies shows that the impaired MCT12 function caused by selected genetic variants, leading to juvenile and age‐related cataracts, can be rescued by exogenous expression of the chaperone CD147.101 Although clinical relevance remains to be elucidated, the study highlights the importance of CD147 for functional MCT expression.
CONCLUSION
The involvement of MCTs in key metabolic pathways, such as glycolysis, fatty acid, or lipid metabolism, and not least their importance for lactate shuttling, implies detrimental consequences of altered expression or function to the pathophysiology of mild disorders or severe diseases. Better understanding of the precise molecular mechanisms involving MCTs is expected to uncover novel targets and approaches for therapeutic intervention. Research into most MCT isoforms is just starting out as their importance emerges from (combined) genomewide, transcriptome‐wide, proteome‐wide, or metabolome‐wide association studies. Moreover, due to overlapping substrate specificities, it can be expected that there is cooperation between members of the MCT (SLC16) transporter family and ABC or other SLC transporters to enable coordinate vectorial transport of substrates across plasma membranes. Altered expression or function of either one of the involved transporters can be assumed to hamper substrate flux and contribute to accumulation of substances and, hence, the pathophysiology of diseases. Thus, systematic characterization of the so far insufficiently investigated MCT transporters, further validation of preliminary findings, and elucidation of involved molecular mechanisms are absolutely necessary in the near future and are expected to provide promising new insights into disease pathophysiology.
Conflict of Interest
The authors declared no competing interests for this work.
Acknowledgments
The authors thank Stefan Winter and Bernd Borstel for drafting the figures. We would also like to thank TCGA initiative, all tissue donors, and investigators who contributed to the acquisition and analyses of the samples used in this study. Information about TCGA and the investigators and institutions that constitute the TCGA research network can be found at http://cancergenome.nih.gov/.
Source of Funding
This work was supported by the Robert Bosch Stiftung, Stuttgart, Germany, the Federal Ministry for Education and Research (BMBF, Berlin, Germany, FKZ 031L0037), the Horizon 2020‐PHC‐2015 grant U‐PGx 668353, the ICEPHA Graduate School Tuebingen‐Stuttgart, and Deutsche Krebshilfe FKZ 70112564.
References
- 1. Halestrap, A.P. The SLC16 gene family – structure, role and regulation in health and disease. Mol. Aspects Med. 34, 337–349 (2013). [DOI] [PubMed] [Google Scholar]
- 2. Wilson, M.C. et al Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. J. Biol. Chem. 273, 15920–15926 (1998). [DOI] [PubMed] [Google Scholar]
- 3. Halestrap, A.P. The monocarboxylate transporter family ‐ structure and functional characterization. IUBMB Life 64, 1–9 (2012). [DOI] [PubMed] [Google Scholar]
- 4. Price, N.T. , Jackson, V.N. & Halestrap, A.P. Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochem. J. 329 (Pt 2), 321–328 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rusu, V. et al Type 2 diabetes variants disrupt function of SLC16A11 through two distinct mechanisms. Cell 170, 199–212 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castorino, J.J. et al Juvenile cataract‐associated mutation of solute carrier SLC16A12 impairs trafficking of the protein to the plasma membrane. Invest. Ophthalmol. Vis. Sci. 52, 6774–6784 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones, R.S. & Morris, M.E. Monocarboxylate transporters . Therapeutic targets and prognostic factors in disease. Clin. Pharmacol. Ther. 100, 454–463 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kohyama, N. , Shiokawa, H. , Ohbayashi, M. , Kobayashi, Y. & Yamamoto, T . Characterization of monocarboxylate transporter 6. Expression in human intestine and transport of the antidiabetic drug nateglinide. Drug Metab. Dispos. 41, 1883–1887 (2013). [DOI] [PubMed] [Google Scholar]
- 9. Murakami, Y. et al Functional characterization of human monocarboxylate transporter 6 (SLC16A5). Drug Metab. Dispos. 33, 1845–1851 (2005). [DOI] [PubMed] [Google Scholar]
- 10. Jones, R.S. , Parker, M.D. & Morris, M.E. Quercetin, morin, luteolin, and phloretin are dietary flavonoid inhibitors of monocarboxylate transporter 6. Mol. Pharm. 14, 2930–2936 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hugo, S.E. , Cruz‐Garcia, L. , Karanth, S. , Anderson, R.M. , Stainier, D.Y. & Schlegel, A . A monocarboxylate transporter required for hepatocyte secretion of ketone bodies during fasting. Genes Dev. 26, 282–293 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Knöpfel, T. , Atanassoff, A. , Hernando, N. , Biber, J. & Wagner, C.A. Renal localization and regulation by dietary phosphate of the MCT14 orphan transporter. PLoS One 12, e0177942 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leuthold, P. et al Comprehensive metabolomic and lipidomic profiling of human kidney tissue. A platform comparison. J. Proteome Res. 16, 933–944 (2017). [DOI] [PubMed] [Google Scholar]
- 14. Song, I.S. et al Pharmacogenetics meets metabolomics: discovery of tryptophan as a new endogenous OCT2 substrate related to metformin disposition. PLoS One 7, e36637 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Halestrap, A.P. & Wilson, M.C. The monocarboxylate transporter family—role and regulation. IUBMB Life 64, 109–119 (2012). [DOI] [PubMed] [Google Scholar]
- 16. Merezhinskaya, N. & Fishbein, W.N. Monocarboxylate transporters. Past, present and future. Histol. Histopathol. 24, 243–264 (2009). [DOI] [PubMed] [Google Scholar]
- 17. Fishbein, W.N. , Merezhinskaya, N. & Foellmer, J.W. Relative distribution of three major lactate transporters in frozen human tissues and their localization in unfixed skeletal muscle. Muscle Nerve 26, 101–112 (2002). [DOI] [PubMed] [Google Scholar]
- 18. Philp, N.J. , Wang, D. , Yoon, H. & Hjelmeland, L.M. Polarized expression of monocarboxylate transporters in human retinal pigment epithelium and ARPE‐19 cells. Invest. Ophthalmol. Vis. Sci. 44, 1716–1721 (2003). [DOI] [PubMed] [Google Scholar]
- 19. Settle, P. et al Polarized lactate transporter activity and expression in the syncytiotrophoblast of the term human placenta. Placenta 25, 496–504 (2004). [DOI] [PubMed] [Google Scholar]
- 20. Pellerin, L. & Magistretti, P.J. Sweet sixteen for ANLS. J. Cereb. Blood Flow Metab. 32, 1152–1166 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chan, S.Y. et al MCT8 expression in human fetal cerebral cortex is reduced in severe intrauterine growth restriction. J. Endocrinol. 220, 85–95 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gill, R.K. et al Expression and membrane localization of MCT isoforms along the length of the human intestine. Am. J. Physiol. Cell Physiol. 289, C846–C852 (2005). [DOI] [PubMed] [Google Scholar]
- 23. Habuka, M. et al The kidney transcriptome and proteome defined by transcriptomics and antibody‐based profiling. PLoS One 9, e116125 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Williams, A.L. et al Sequence variants in SLC16A11 are a common risk factor for type 2 diabetes in Mexico. Nature 506, 97–101 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kloeckener‐Gruissem, B. et al Mutation of solute carrier SLC16A12 associates with a syndrome combining juvenile cataract with microcornea and renal glucosuria. Am. J. Hum. Genet. 82, 772–779 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roshanbin, S. et al Histological characterization of orphan transporter MCT14 (SLC16A14) shows abundant expression in mouse CNS and kidney. BMC Neurosci. 17, 43 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marchiq, I. & Pouyssegur, J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J. Mol. Med. 94, 155–171 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lyssiotis, C.A. & Kimmelman, A.C. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 27, 863–875 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fisel, P. et al DNA methylation of the SLC16A3 promoter regulates expression of the human lactate transporter MCT4 in renal cancer with consequences for clinical outcome. Clin. Cancer Res. 19, 5170–5181 (2013). [DOI] [PubMed] [Google Scholar]
- 30. Baltazar, F. et al Monocarboxylate transporters as targets and mediators in cancer therapy response. Histol. Histopathol. 29, 1511–1524 (2014). [DOI] [PubMed] [Google Scholar]
- 31. Pinheiro, C. , Longatto‐Filho, A. , Azevedo‐Silva, J. , Casal, M. , Schmitt, F.C. & Baltazar, F . Role of monocarboxylate transporters in human cancers. State of the art. J. Bioenerg. Biomembr. 44, 127–139 (2012). [DOI] [PubMed] [Google Scholar]
- 32. Pinheiro, C. et al Increasing expression of monocarboxylate transporters 1 and 4 along progression to invasive cervical carcinoma. Int. J. Gynecol. Pathol. 27, 568–574 (2008). [DOI] [PubMed] [Google Scholar]
- 33. Johnson, J.M. et al MCT1 in invasive ductal carcinoma. Monocarboxylate metabolism and aggressive breast cancer. Front. Cell Dev. Biol. 5, 27 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pértega‐Gomes, N. et al Monocarboxylate transporter 2 (MCT2) as putative biomarker in prostate cancer. Prostate 73, 763–769 (2013). [DOI] [PubMed] [Google Scholar]
- 35. Baek, G. et al MCT4 defines a glycolytic subtype of pancreatic cancer with poor prognosis and unique metabolic dependencies. Cell Rep. 9, 2233–2249 (2014). [DOI] [PubMed] [Google Scholar]
- 36. Gao, H.J. et al Monocarboxylate transporter 4 predicts poor prognosis in hepatocellular carcinoma and is associated with cell proliferation and migration. J. Cancer Res. Clin. Oncol. 141, 1151–1162 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bovenzi, C.D. et al Prognostic indications of elevated MCT4 and CD147 across cancer types: a meta‐analysis. Biomed. Res. Int. 2015, 242437 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nwosu, Z.C. et al Identification of the consistently altered metabolic targets in human hepatocellular carcinoma. Cell. Mol. Gastroenterol. Hepatol. 4, 303–323 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Badziong, J. et al Differential regulation of monocarboxylate transporter 8 expression in thyroid cancer and hyperthyroidism. Eur. J. Endocrinol. 177, 243–250 (2017). [DOI] [PubMed] [Google Scholar]
- 40. Fernandez‐Ranvier, G.G. et al Identification of biomarkers of adrenocortical carcinoma using genomewide gene expression profiling. Arch. Surg. 143, 841–846 (2008). [DOI] [PubMed] [Google Scholar]
- 41. Elsnerova, K. et al Gene expression of membrane transporters. Importance for prognosis and progression of ovarian carcinoma. Oncol. Rep. 35, 2159–2170 (2016). [DOI] [PubMed] [Google Scholar]
- 42. Merezhinskaya, N. , Fishbein, W.N. & Davis, J.I. , Foellmer, J.W. Mutations in MCT1 cDNA in patients with symptomatic deficiency in lactate transport. Muscle Nerve 23, 90–97 (2000). [DOI] [PubMed] [Google Scholar]
- 43. Otonkoski, T. et al Physical exercise‐induced hypoglycemia caused by failed silencing of monocarboxylate transporter 1 in pancreatic beta cells. Am. J. Hum. Genet. 81, 467–474 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tosur, M. & Jeha, G.S. A novel intragenic SLC16A1 mutation associated with congenital hyperinsulinism. Glob. Pediatr. Health 4, 2333794X17703462 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thibault, R. et al Down‐regulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation. Gastroenterology 133, 1916–1927 (2007). [DOI] [PubMed] [Google Scholar]
- 46. Planell, N. et al Transcriptional analysis of the intestinal mucosa of patients with ulcerative colitis in remission reveals lasting epithelial cell alterations. Gut 62, 967–976 (2013). [DOI] [PubMed] [Google Scholar]
- 47. Johnson, A.D. & O'Donnell, C.J. An open access database of genome‐wide association results. BMC Med. Genet. 10, 6 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carneiro, L. & Pellerin, L . Monocarboxylate transporters. New players in body weight regulation. Obes. Rev. 16, 55–66 (2015). [DOI] [PubMed] [Google Scholar]
- 49. Lengacher, S. et al Resistance to diet‐induced obesity and associated metabolic perturbations in haploinsufficient monocarboxylate transporter 1 mice. PLoS One 8, e82505 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kolz, M. et al Meta‐analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 5, e1000504 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Köttgen, A. et al Genome‐wide association analyses identify 18 new loci associated with serum urate concentrations. Nat. Genet. 45, 145–154 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van der Harst, P. et al Replication of the five novel loci for uric acid concentrations and potential mediating mechanisms. Hum. Mol. Genet. 19, 387–395 (2010). [DOI] [PubMed] [Google Scholar]
- 53. Suhre, K. et al Human metabolic individuality in biomedical and pharmaceutical research. Nature 477, 54–60 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nakayama, A. et al Common missense variant of monocarboxylate transporter 9 (MCT9/SLC16A9) gene is associated with renal overload gout, but not with all gout susceptibility. Hum. Cell 26, 133–136 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jansen, L. et al HBsAg loss in patients treated with peginterferon alfa‐2a and adefovir is associated with SLC16A9 gene variation and lower plasma carnitine levels. J. Hepatol. 61, 730–737 (2014). [DOI] [PubMed] [Google Scholar]
- 56. Hara, K. et al Genome‐wide association study identifies three novel loci for type 2 diabetes. Hum. Mol. Genet. 23, 239–246 (2014). [DOI] [PubMed] [Google Scholar]
- 57. Miranda‐Lora, A.L. , Cruz, M. , Molina‐Díaz, M. , Gutiérrez, J. , Flores‐Huerta, S. & Klünder‐Klünder, M . Associations of common variants in the SLC16A11, TCF7L2, and ABCA1 genes with pediatric‐onset type 2 diabetes and related glycemic traits in families. A case‐control and case‐parent trio study. Pediatr. Diabetes 18, 824–831 (2017). [DOI] [PubMed] [Google Scholar]
- 58. Lokman, F.E. et al Gene expression profiling in ethnic Malays with type 2 diabetes mellitus, with and without diabetic nephropathy. J. Nephrol. 24, 778–789 (2011). [DOI] [PubMed] [Google Scholar]
- 59. van Hasselt, P. M. et al Monocarboxylate transporter 1 deficiency and ketone utilization. N. Engl. J. Med. 371, 1900–1907 (2014). [DOI] [PubMed] [Google Scholar]
- 60. Balasubramaniam, S. et al Heterozygous monocarboxylate transporter 1 (MCT1, SLC16A1) deficiency as a cause of recurrent ketoacidosis. JIMD Rep. 29, 33–38 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pérez‐Escuredo, J. et al Monocarboxylate transporters in the brain and in cancer. Biochim. Biophys. Acta 1863, 2481–2497 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang, F. , Vannucci, S.J. , Philp, N.J. & Simpson, I.A. Monocarboxylate transporter expression in the spontaneous hypertensive rat. Effect of stroke. J. Neurosci. Res. 79, 139–145 (2005). [DOI] [PubMed] [Google Scholar]
- 63. Lauritzen, F. , Eid, T. & Bergersen, L.H. Monocarboxylate transporters in temporal lobe epilepsy. Roles of lactate and ketogenic diet. Brain Struct. Funct. 220, 1–12 (2015). [DOI] [PubMed] [Google Scholar]
- 64. Liu, B. et al Decreased astroglial monocarboxylate transporter 4 expression in temporal lobe epilepsy. Mol. Neurobiol. 50, 327–338 (2014). [DOI] [PubMed] [Google Scholar]
- 65. Lee, Y. et al Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nijland, P.G. et al Cellular distribution of glucose and monocarboxylate transporters in human brain white matter and multiple sclerosis lesions. Glia 62, 1125–1141 (2014). [DOI] [PubMed] [Google Scholar]
- 67. Fritsche, L.G. et al Seven new loci associated with age‐related macular degeneration. Nat. Genet. 45, 433–439 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fritsche, L.G. et al A large genome‐wide association study of age‐related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 48, 134–143 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zuercher, J. et al Alterations of the 5’untranslated region of SLC16A12 lead to age‐related cataract. Invest. Ophthalmol. Vis. Sci. 51, 3354–3361 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dhayat, N. et al Mutation in the monocarboxylate transporter 12 gene affects guanidinoacetate excretion but does not cause glucosuria. J. Am. Soc. Nephrol. 27, 1426–1436 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lee, J. , Lee, D. R. & Lee, S . The genetic variation in monocarboxylic acid transporter 2 (MCT2) has functional and clinical relevance with male infertility. Asian J. Androl. 16, 694–697 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fujii, W. et al Monocarboxylate transporter 4, associated with the acidification of synovial fluid, is a novel therapeutic target for inflammatory arthritis. Arthritis Rheumatol. 67, 2888–2896 (2015). [DOI] [PubMed] [Google Scholar]
- 73. Lima, A. , Bernardes, M. , Medeiros, R. , Medeiros, R. & Seabra, V. Pharmacogenomics of methotrexate membrane transport pathway: can clinical response to methotrexate in rheumatoid arthritis be predicted? Int. J. Mol. Sci. 16, 13760–13780 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Krishnan, M.N. et al RNA interference screen for human genes associated with West Nile virus infection. Nature 455, 242–245 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Friesema, E.C. et al Association between mutations in a thyroid hormone transporter and severe X‐linked psychomotor retardation. Lancet 364, 1435–1437 (2004). [DOI] [PubMed] [Google Scholar]
- 76. Dumitrescu, A. M. , Liao, X.H. , Best, T.B. , Brockmann, K. & Refetoff, S . A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am. J. Hum. Genet. 74, 168–175 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Visser, W.E. , Friesema, E.C. , Jansen, J. & Visser, T.J. Thyroid hormone transport in and out of cells. Trends Endocrinol. Metab. 19, 50–56 (2008). [DOI] [PubMed] [Google Scholar]
- 78. Groeneweg, S. , Visser, W.E. & Visser, T.J. Disorder of thyroid hormone transport into the tissues. Best. Pract. Res. Clin. Endocrinol. Metab. 31, 241–253 (2017). [DOI] [PubMed] [Google Scholar]
- 79. Lake, A.D. et al Branched chain amino acid metabolism profiles in progressive human nonalcoholic fatty liver disease. Amino Acids 47, 603–615 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fisel, P. , Nies, A.T. , Schaeffeler, E. & Schwab, M . The importance of drug transporter characterization to precision medicine. Expert Opin. Drug Metab. Toxicol. 13, 361–365 (2017). [DOI] [PubMed] [Google Scholar]
- 81. Ullah, M.S. , Davies, A.J. & Halestrap, A.P. The plasma membrane lactate transporter MCT4, but not MCT1, is up‐regulated by hypoxia through a HIF‐1alpha‐dependent mechanism. J. Biol. Chem. 281, 9030–9037 (2006). [DOI] [PubMed] [Google Scholar]
- 82. Pullen, T.J. , Khan, A.M. , Barton, G. , Butcher, S.A. , Sun, G. & Rutter, G.A. Identification of genes selectively disallowed in the pancreatic islet. Islets 2, 89–95 (2010). [DOI] [PubMed] [Google Scholar]
- 83. Pertega‐Gomes, N. et al Epigenetic and oncogenic regulation of SLC16A7 (MCT2) results in protein over‐expression, impacting on signalling and cellular phenotypes in prostate cancer. Oncotarget 6, 21675–21684 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fisel, P. et al MCT4 surpasses the prognostic relevance of the ancillary protein CD147 in clear cell renal cell carcinoma. Oncotarget 6, 30615–30627 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Enerson, B.E. & Drewes, L.R. Molecular features, regulation, and function of monocarboxylate transporters. Implications for drug delivery. J. Pharm. Sci. 92, 1531–1544 (2003). [DOI] [PubMed] [Google Scholar]
- 86. Cundy, K.C. et al XP13512 [(+/‐)‐1‐([(alpha‐isobutanoyloxyethoxy)carbonyl] aminomethyl)‐1‐cyclohexane acetic acid], a novel gabapentin prodrug: I. Design, synthesis, enzymatic conversion to gabapentin, and transport by intestinal solute transporters. J. Pharmacol. Exp. Ther. 311, 315–323 (2004). [DOI] [PubMed] [Google Scholar]
- 87. Demirbilek, H. , Rahman, S.A. , Buyukyilmaz, G.G. & Hussain, K . Diagnosis and treatment of hyperinsulinaemic hypoglycaemia and its implications for paediatric endocrinology. Int. J. Pediatr. Endocrinol. 2017, 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Birsoy, K. et al MCT1‐mediated transport of a toxic molecule is an effective strategy for targeting glycolytic tumors. Nat. Genet. 45, 104–108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lopes‐Coelho, F. et al Monocarboxylate transporter 1 (MCT1), a tool to stratify acute myeloid leukemia (AML) patients and a vehicle to kill cancer cells. Oncotarget 8, 82803–82823 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Vijay, N. & Morris, M.E. Role of monocarboxylate transporters in drug delivery to the brain. Curr. Pharm. Des. 20, 1487–1498 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kikutani, Y. et al Involvement of monocarboxylate transporter 4 expression in statin‐induced cytotoxicity. J. Pharm. Sci. 105, 1544–1549 (2016). [DOI] [PubMed] [Google Scholar]
- 92. Drögemöller, B.I. et al Association between SLC16A5 genetic variation and cisplatin‐induced ototoxic effects in adult patients with testicular cancer. JAMA Oncol. 3, 1558–1562 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Doherty, J.R. & Cleveland, J.L. Targeting lactate metabolism for cancer therapeutics. J. Clin. Invest. 123, 3685–3692 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nancolas, B. et al The anti‐tumour agent lonidamine is a potent inhibitor of the mitochondrial pyruvate carrier and plasma membrane monocarboxylate transporters. Biochem. J. 473, 929–936 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Murray, C.M. et al Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat. Chem. Biol. 1, 371–376 (2005). [DOI] [PubMed] [Google Scholar]
- 96. Polański, R. et al Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin. Cancer Res. 20, 926–937 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Futagi, Y. , Kobayashi, M. , Narumi, K. , Furugen, A. & Iseki, K . Identification of a selective inhibitor of human monocarboxylate transporter 4. Biochem. Biophys. Res. Commun. 495, 427–432 (2018). [DOI] [PubMed] [Google Scholar]
- 98. Fisel, P. , Schaeffeler, E. & Schwab, M. DNA Methylation of ADME Genes. Clin. Pharmacol. Ther. 99, 512–527 (2016). [DOI] [PubMed] [Google Scholar]
- 99.Emami Riedmaier, A. , Fisel, P. , Nies, A.T. , Schaeffeler, E. & Schwab, M . Metformin and cancer: from the old medicine cabinet to pharmacological pitfalls and prospects. Trends Pharmacol. Sci. 34, 126–135 (2013). [DOI] [PubMed] [Google Scholar]
- 100. Stadler, L.K. J. & Farooqi, I.S. A new drug target for type 2 diabetes. Cell 170, 12–14 (2017). [DOI] [PubMed] [Google Scholar]
- 101. Stäubli, A. et al Abnormal creatine transport of mutations in monocarboxylate transporter 12 (MCT12) found in patients with age‐related cataract can be partially rescued by exogenous chaperone CD147. Hum. Mol. Genet. 26, 4203–4214 (2017). [DOI] [PubMed] [Google Scholar]


