Natalizumab (NTZ) was the first approved humanized monoclonal antibody in highly active relapsing remitting MS (RRMS). Because of the mechanism of inhibiting the migration of immune cells through the blood-brain barrier into the CNS, NTZ is associated with an increased risk of progressive multifocal leukoencephalopathy (PML) by the John Cunningham virus (JCV). Infections with other neurotropic viruses are rarely reported.1,2 We present a case of rapid retinal necrosis induced by varicella zoster virus (VZV) in a patient with RRMS under long-term NTZ treatment.
Case report
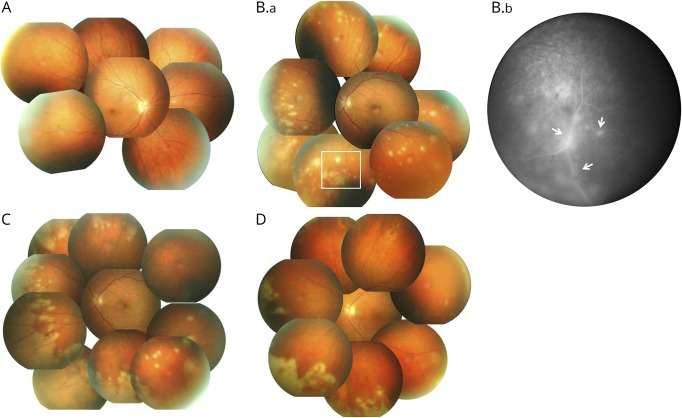
A 46-year-old Caucasian man diagnosed with RRMS in 2001 was initially treated with interferon beta-1a. After 2 relapses, therapy was switched to NTZ in 2006. Subsequently, the patient presented neither clinical nor radiographic disease activity until January 2018. With the exception of a treatment interruption between 2014 and 2017, he received a total of 127 NTZ infusions. In January 2018, progressive visual loss over 2 days occurred in his left eye. During clinical examination, visual acuity was only 0.2. Apart from a residual subtle paresis of the left leg, no other new neurologic deficits were present. Brain imaging was comparable to a scan acquired 6 months prior, with no signs of new inflammatory lesions. Unexpectedly, ophthalmologic examination showed a retinal vasculitis and focal infiltration in the outer part of the retina (figure 1, A–C). CSF measurement revealed a pleocytosis of 122 cells/μL, positive CSF oligoclonal bands, elevated protein levels of 564 mg/L, albumin quotient (CSF/serum) of 9.1, lactate of 2.0 mmol/L, and isolated VZV intrathecal antibody index of 30.2 (<1.5). PCR amplification of VZV-DNA was positive in CSF (8,175 copies/mL), whereas other microbiologic CSF and serologic analyses were without pathologic findings. We diagnosed VZV-associated retinal vasculitis and retinitis and initiated IV treatment with acyclovir (3 × 900 mg/d for 16 days). Two days after starting acyclovir treatment, retinal infiltration progressed to acute retinal necrosis. Therapy was escalated using intravitreal ganciclovir injections (2 mg twice, at days 1 and 4 after progression) (figure 1B) and oral prednisolone (50 mg/d) after negative CSF results for bacterial pathogens. This therapy hindered progression of retinal necrosis, and visual acuity of the left eye improved to 0.5. Fundoscopy showed regressive retinal necrosis with beginning repigmentation of VZV lesions (figure 1C). After 14 days, CSF parameters improved to 47 cells/μL and 97.5 copies/mL of VZV-DNA. Acyclovir was switched to oral application (800 mg twice daily) for long-term control of retinal vasculitis. Teriflunomide treatment was started 2 months after the last NTZ infusion to prevent future relapses.
Figure. Fundoscopic examination of the VZV-infected left eye over time.
(A) Healthy right eye and (B.a) affected left eye during initial fundoscopy. Multiple focal infiltrations in the peripheral retina are present in the left eye. (B.b) The fluorescent angiography (white box of B.a) illustrates the (peri)-vasculitis as exudates around retinal vessels resulting in white sheathing (arrows). The follow-up examination (C) after 3 days indicates progression of infiltration due to acute retinal necrosis. Regression of retinal necrosis and beginning of repigmentation of several infiltrates are observed 24 days after initiation of antiviral treatment (D).
Discussion
Owing to the NTZ-associated PML incidence due to JCV, NTZ-treated RRMS patients are closely monitored by serologic and radiologic means. Other neurotropic virus infections have rarely been identified as critical pathogens in this context, both in clinical trials and in postmarketing observations.3 However, several authors have described VZV-induced meningitis2 and CNS vasculitis4 with associated retinitis in patients treated with NTZ,5 which highlights the potential role of other neurotropic virus infections aside from PML.
In contrast to previous reports about VZV reactivation, our patient presented neither with clinical signs of meningeal involvement, nor was he showing any evidence of cerebral vasculitis apart from retinitis, despite the highly elevated CSF cell count. Others have reported patients with vasculitis-mediated ischemic stroke or encephalitis with surprisingly normal5 or only slightly elevated CSF cell levels.4 It is tempting to speculate that the short time span between clinical onset and treatment initiation (3 days) prevented further cerebral affection. Fortunately, our patient could be saved from developing amaurosis, possibly because of an early and aggressive systemic and additional local antiviral treatment. An early concomitant drug therapy should therefore be considered in future cases, showing progression to retinal necrosis after initiation of IV antiviral treatment.
Moreover, without close ophthalmologic examination, the isolated vision loss could have easily been misdiagnosed as an optic neuritis, a typical syndrome in patients with RRMS. High doses of steroids are commonly indicated in this scenario6 with possible detrimental consequences to the patient's eye sight.
Of interest, NTZ-treated MS patients have a significant increase in serologic anti–VZV-IgG levels compared with healthy controls, indicating a tendency toward a subclinical VZV reactivation before clinical manifestation.7 Consequently, this could imply an increased risk of VZV infections similar to the elevated risk of PML under long-term NTZ treatment.
In addition to the diagnosis of MS relapse or PML, physicians should consider other opportunistic infections when new neurologic deficits present under long-term NTZ treatment.
Author contributions
Marc Pawlitzki has access to all the data and takes responsibility for the data, accuracy of the data analysis and interpretation of the data, and drafting the manuscript for intellectual content. Jan Teuber: design and conceptualization of the case and revising the manuscript for intellectual content. Christin Campe: design and conceptualization of the case and revising the manuscript for intellectual content and language improvement. Markus Wagner: design and conceptualization of the study and revising the manuscript for intellectual content. Claudia Schuart: design and conceptualization of the case and revising the manuscript for intellectual content. Friedemann Paul: critical revision of the manuscript for important intellectual content. Daniel Bittner: study supervision.
Study funding
No targeted funding reported.
Disclosure
M. Pawlitzki received speaker honoraria from Roche, Genzyme, and Novartis and travel/accommodation/meeting expenses from Novartis, Biogen Idec, Genzyme, and Merck Serono. J. Teuber received research support from the German National Academic Foundation. C. Campe, M. Wagner, and C. Schuart report no disclosures. F. Paul has received honoraria and research support from Alexion, Bayer, Biogen, Chugai, Merck Serono, Novartis, Genyzme, MedImmune, Shire, Teva, and Sanofi/Aventis; serves on the scientific advisory boards of Alexion, MedImmune, and Novartis; served as an academic editor of PLoS ONE and an associate editor of Neurology: Neuroimmunology & Neuroinflammation; consulted for Sanofi Genzyme, BiogenIdec, MedImmune, Shire, and Alexion; and has received funding from Deutsche Forschungsgemeinschaft (DFG Exc 257), Bundesministerium für Bildung und Forschung (Competence Network Multiple Sclerosis), Guthy Jackson Charitable Foundation, EU Framework Program 7, National Multiple Sclerosis Society of the USA, Arthur Arnstein Stiftung Berlin, and Arthur Arnstein Foundation Berlin. D. Bittner has received honoraria from Bristol-Myers Squibb. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/NN.
References
- 1.Schippling S, Kempf C, Büchele F, et al. JC virus granule cell neuronopathy and GCN-IRIS under natalizumab treatment. Ann Neurol 2013;74:622–626. [DOI] [PubMed] [Google Scholar]
- 2.Fine AJ, Sorbello A, Kortepeter C, Scarazzini L. Central nervous system herpes simplex and varicella zoster virus infections in natalizumab-treated patients. Clin Infect Dis 2013;57:849–852. [DOI] [PubMed] [Google Scholar]
- 3.Williamson EML, Berger JR. Central nervous system infections with immunomodulatory therapies. Continuum (Minneap Minn) 2015;21:1577–1598. [DOI] [PubMed] [Google Scholar]
- 4.Mulero P, Auger C, Parolin L, et al. Varicella-zoster meningovasculitis in a multiple sclerosis patient treated with natalizumab. Mult Scler 2018;24:358–360. [DOI] [PubMed] [Google Scholar]
- 5.Kobeleva X, Wegner F, Brunotte I, Dadak M, Dengler R, Stangel M. Varicella zoster-associated retinal and central nervous system vasculitis in a patient with multiple sclerosis treated with natalizumab. J Neuroinflammation 2014;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galetta SL, Villoslada P, Levin N, et al. Acute optic neuritis: unmet clinical needs and model for new therapies. Neurol Neuroimmunol Neuroinflamm 2015;2:e135 doi: 10.1212/NXI.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohlmann R, Salmen A, Chan A, et al. Serological evidence of increased susceptibility to varicella-zoster virus reactivation or reinfection in natalizumab-treated patients with multiple sclerosis. Mult Scler 2015;21:1823–1832. [DOI] [PubMed] [Google Scholar]